Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preperation of Catalysts
2.2. Characterization of Catalysts
2.3. Catalyst Test of Catalysts
3. Results
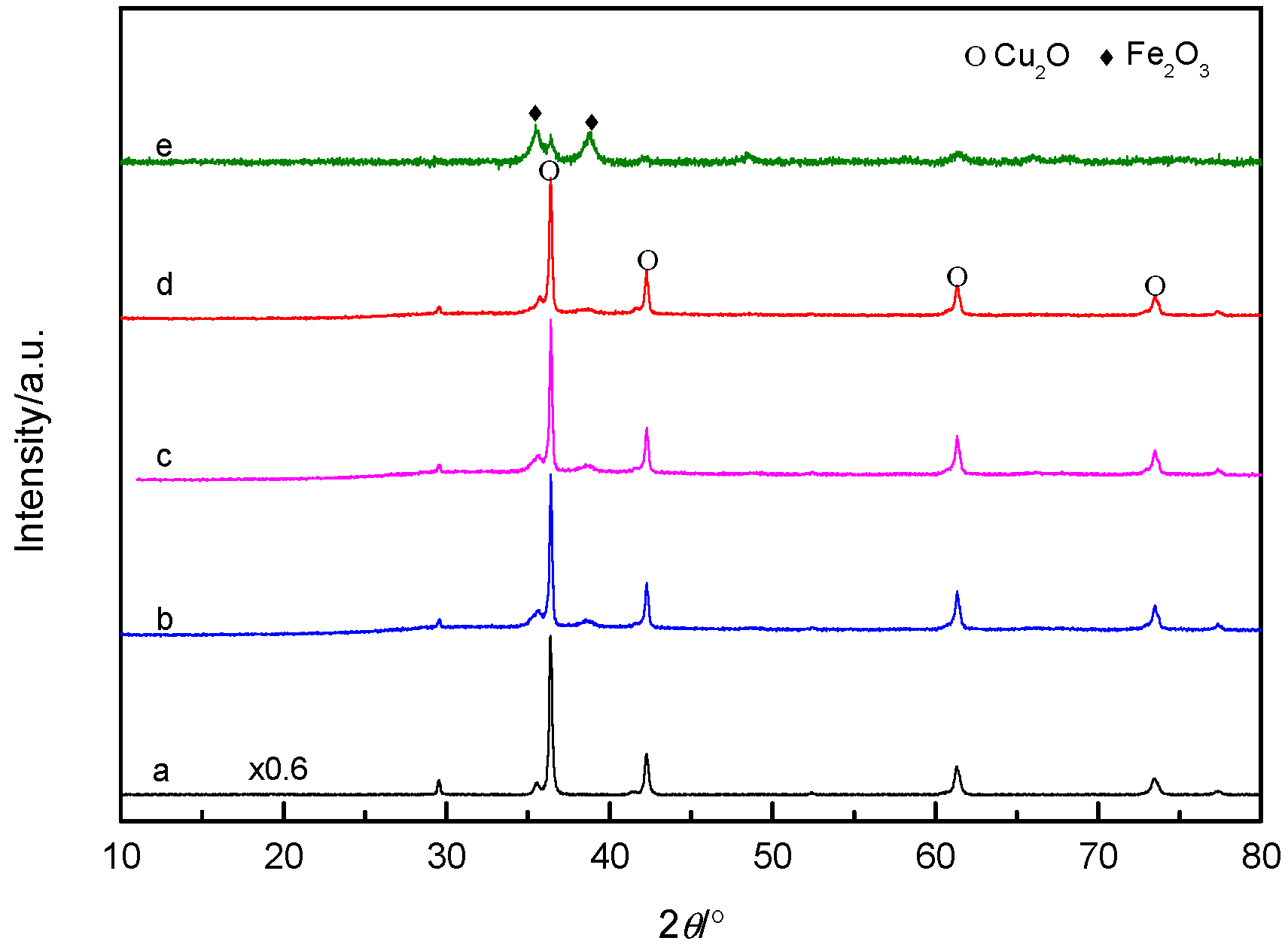
3.1. Structure Analysis of Catalysts
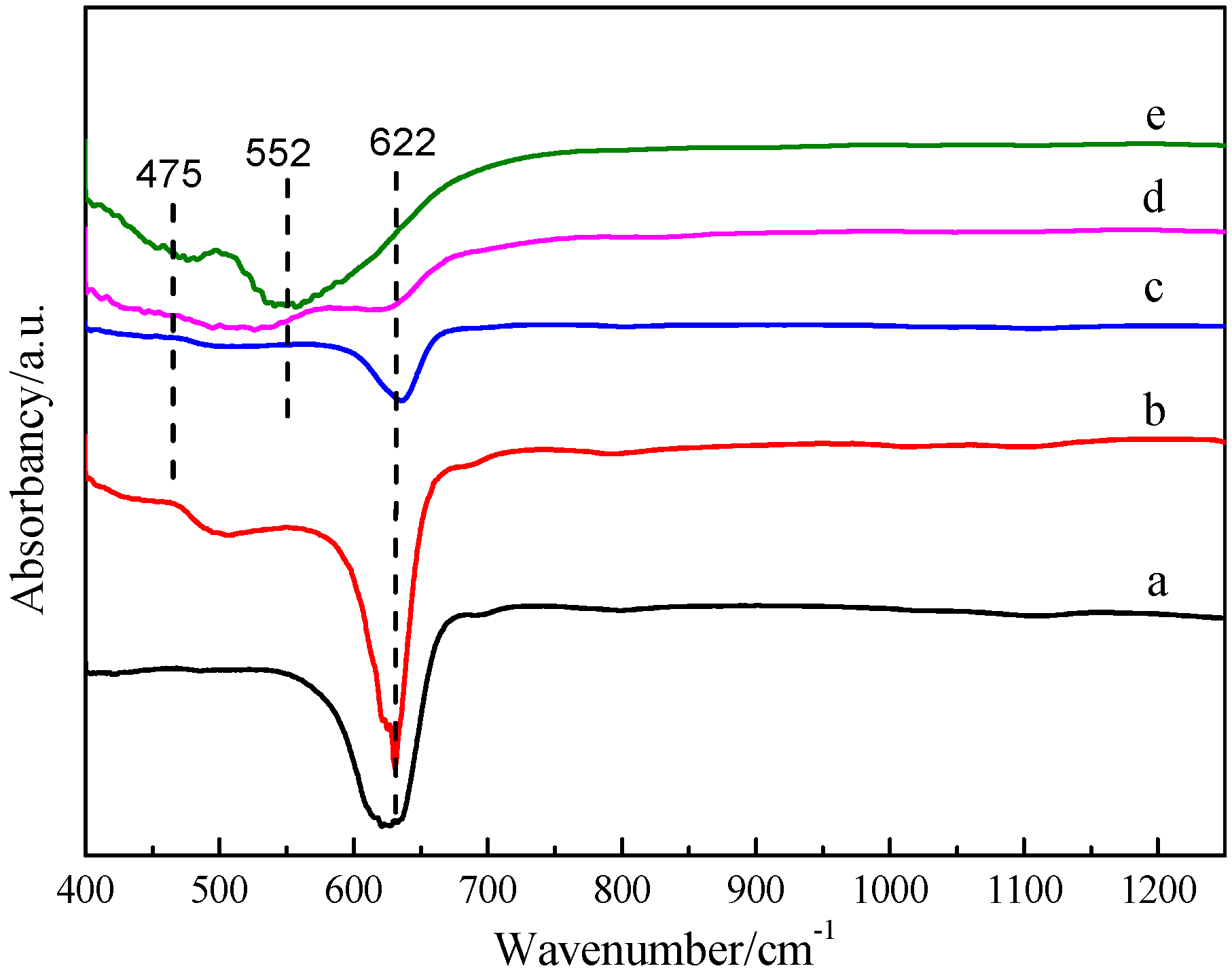
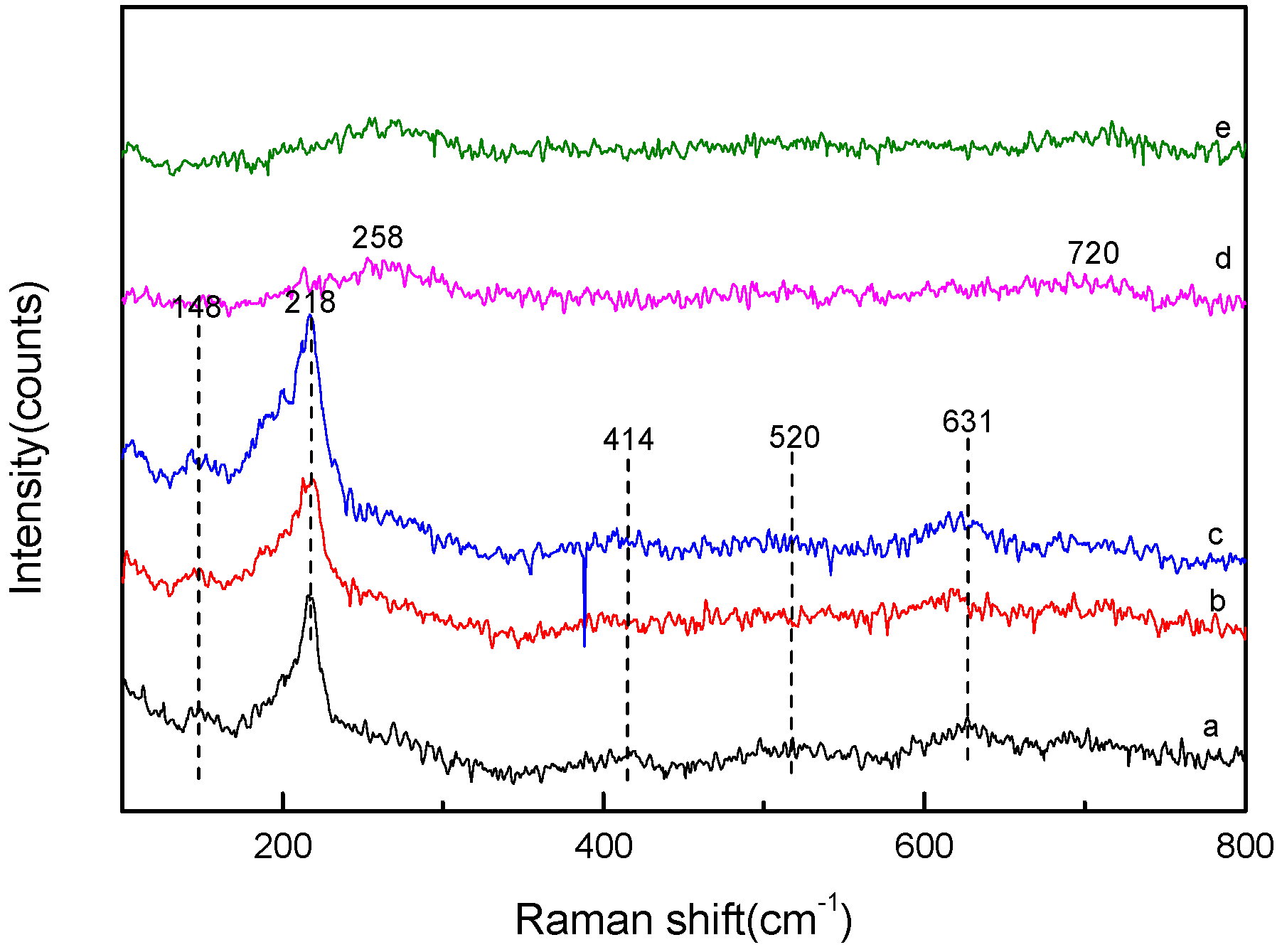
3.2. FT-IR Analysis and Raman Analysis
3.3. Morphological Analysis
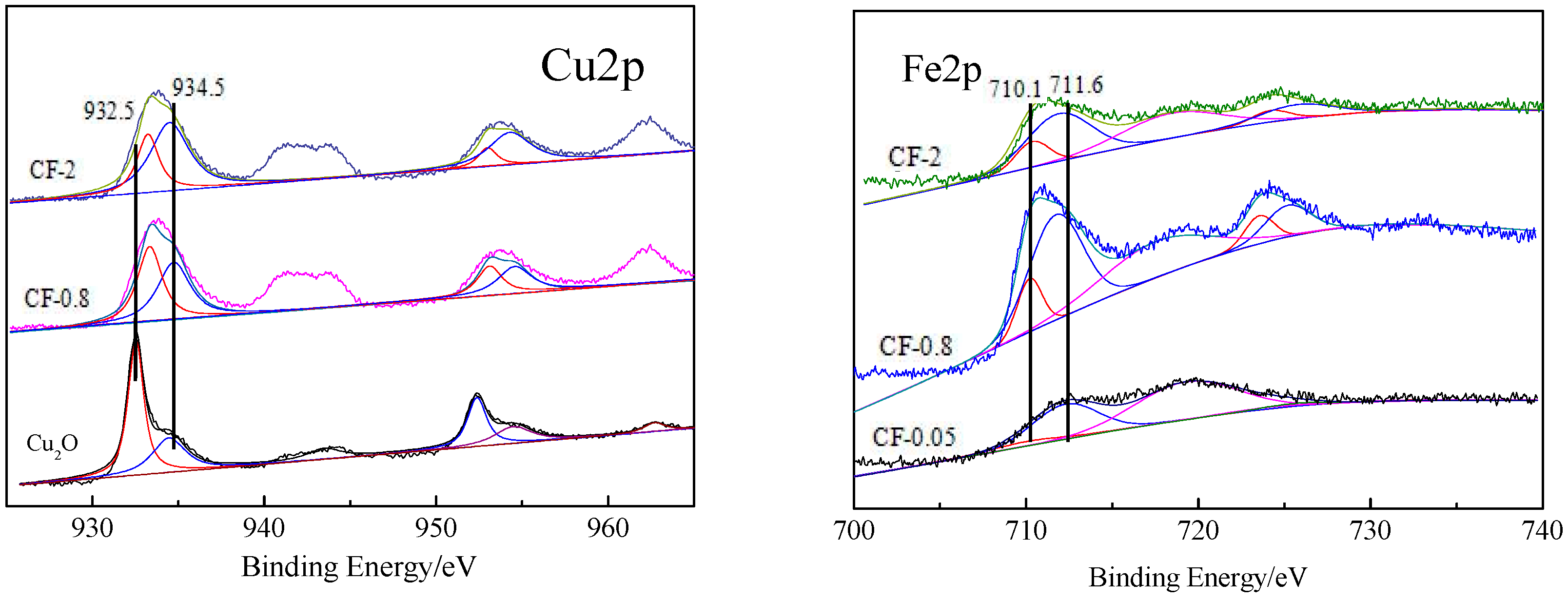
3.4. Surface Composition and Valence Analysis of the Catalyst
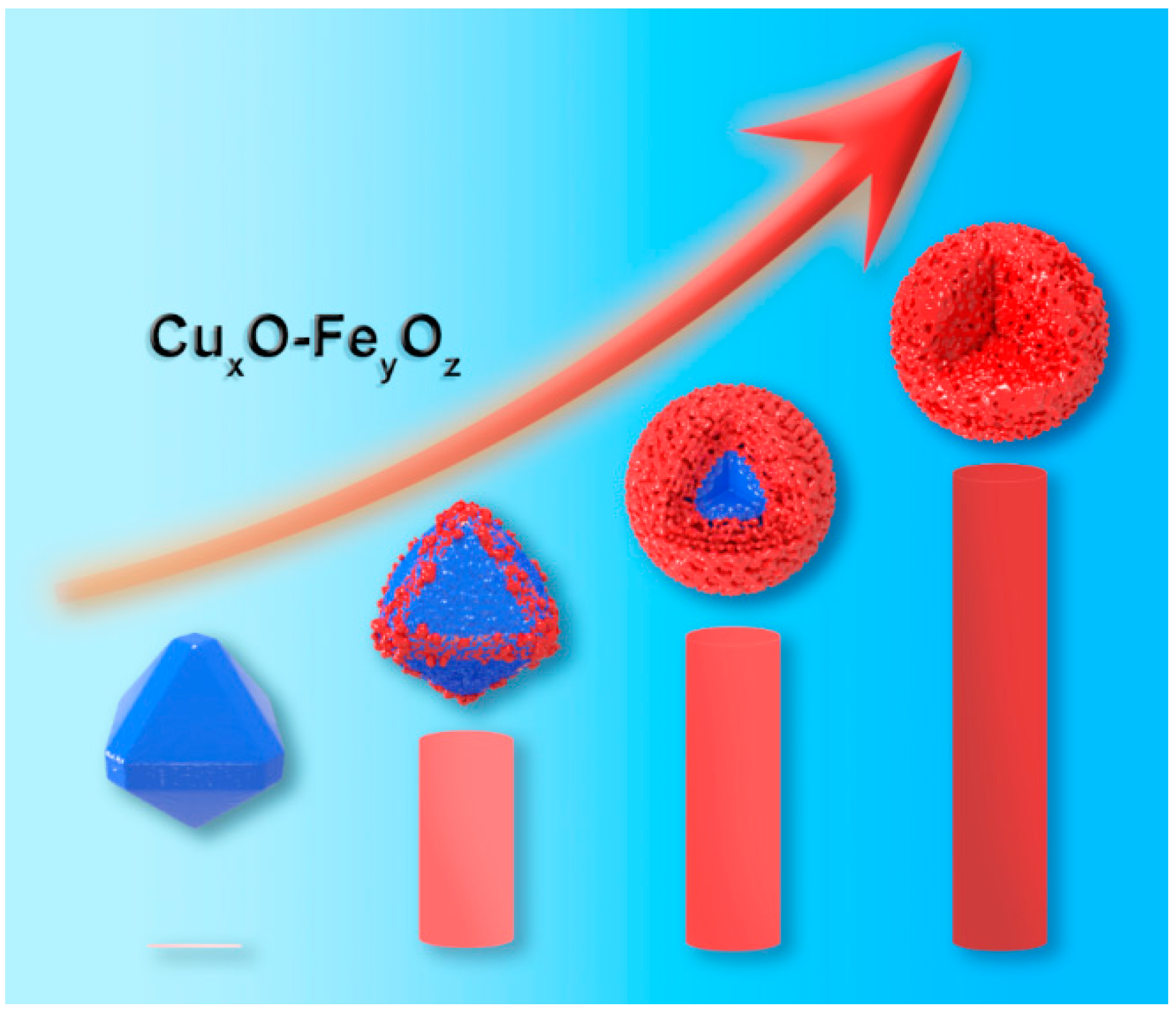
3.5. Discussion on the Formation Mechanism of CuxO-FeyOz Structure
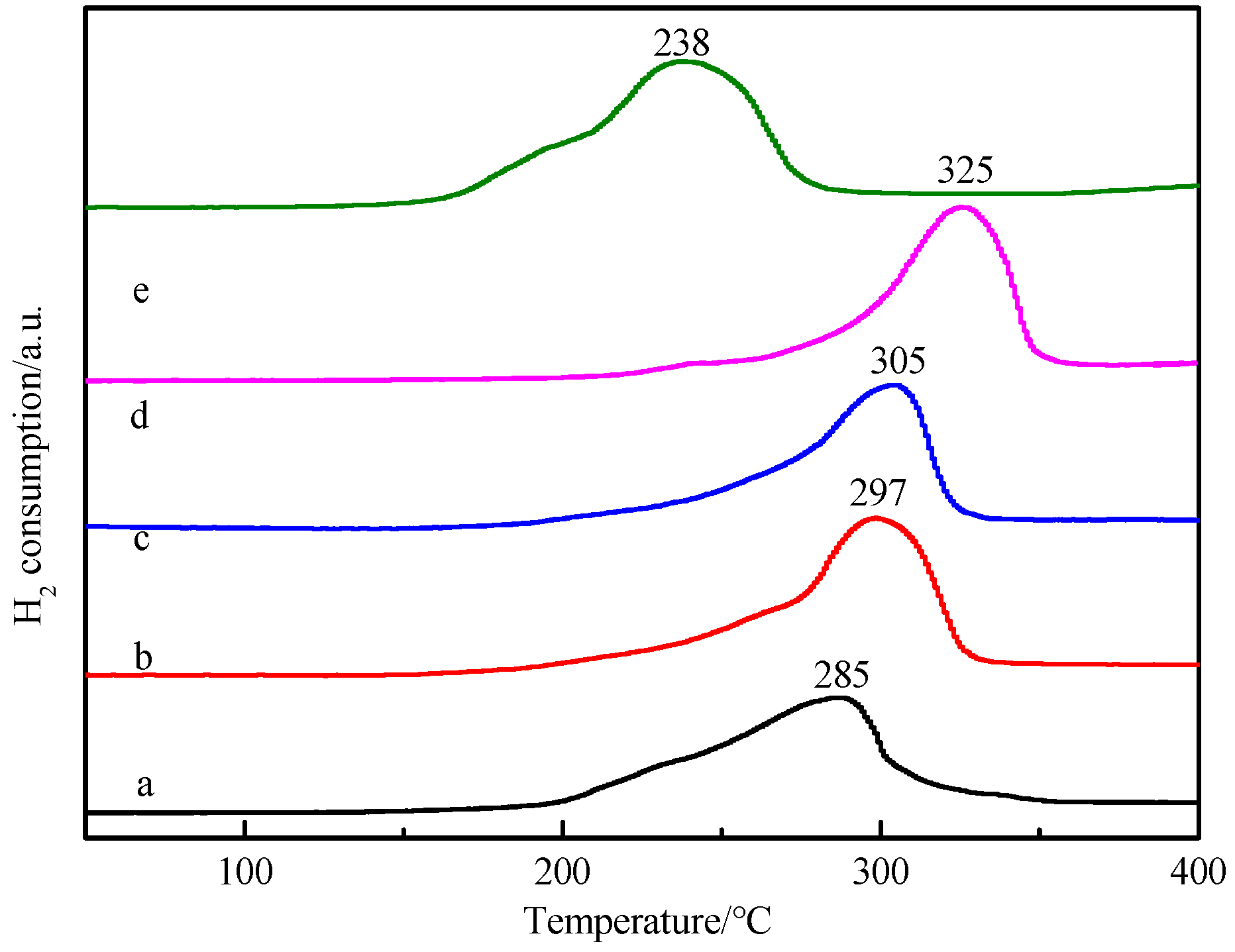
3.6. Analysis of Reduction Behavior of the Catalyst
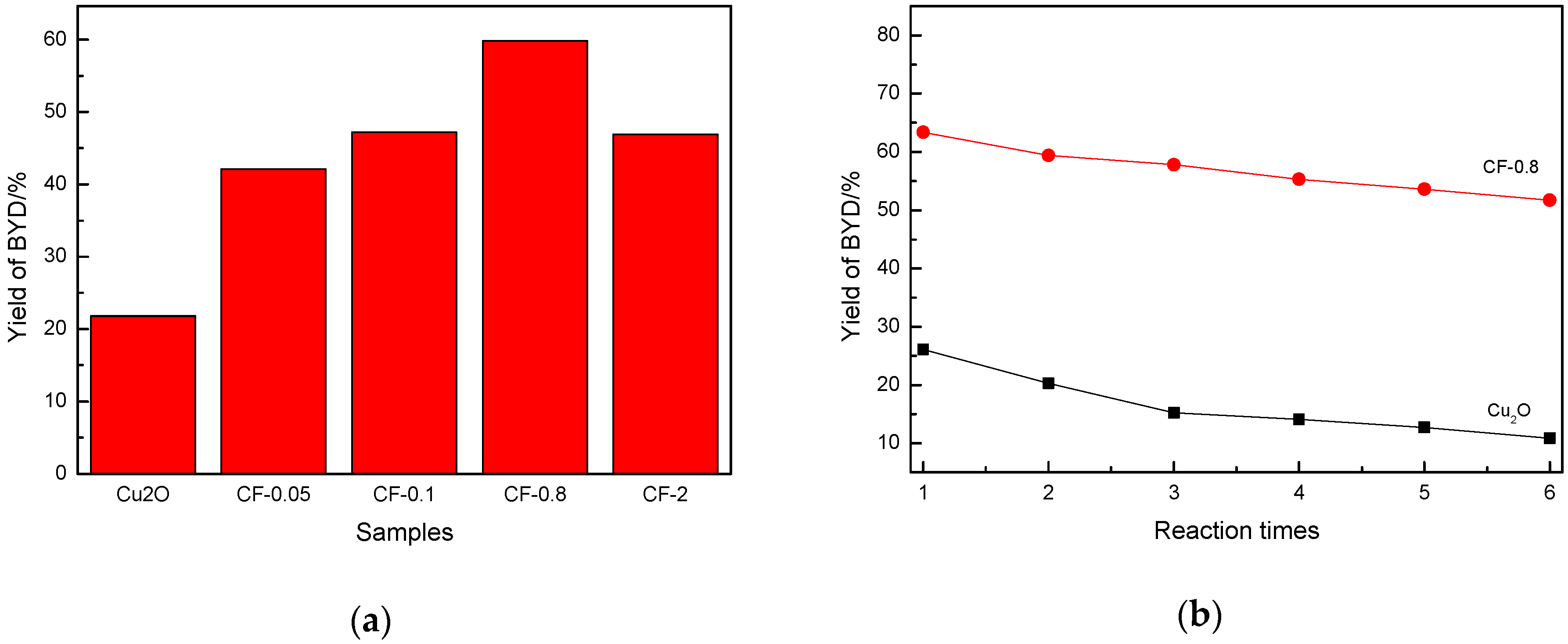
3.7. Evaluation Results of Catalyst Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanielyan, S.; Schmidt, S.; Marin, N.; Alvez, G.; Augustine, R. Selective hydrogenation of 2-butyne-1,4-diol to 1,4-butanediol over particulate Raney® nickel catalysts. Top. Catal. 2010, 53, 1145–1149. [Google Scholar] [CrossRef]
- Nadgeri, J.M.; Telkar, M.M.; Rode, C.V. Hydrogenation activity and selectivity behavior of supported palladium nanoparticles. Catal. Commun. 2008, 9, 441–446. [Google Scholar] [CrossRef]
- Trotus, I.T.; Zimmermann, T.; Schüth, F. Catalytic reactions of acetylene: A feedstock for the chemical industry revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Y.; Gao, C.; Wang, Y.; Sun, Z.; Liang, X. Study on deactivation of Ni/Al2O3 catalyst for liquid phase hydrogenation of crude 1,4-butanediol aqueous solution. Chem. Eng. J. 2012, 181–182, 501–507. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Ikushima, Y.; Arai, M. Hydrogenation of 2-butyne-1,4-diol in supercritical carbon dioxide promoted by stainless steel reactor wall. Catal. Today 2004, 93–95, 439–443. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, H.; Gao, C.; Zhao, Y. Heterogeneous CaO-ZrO2 acid-base bifunctional catalysts for vapor-phase selective dehydration of 1,4-butanediol to 3-buten-1-ol. Appl. Catal. A Gen. 2013, 466, 233–239. [Google Scholar] [CrossRef]
- Li, H.T.; Ban, L.J.; Wang, Z.P.; Meng, P.F.; Zhang, Y.; Wu, R.F.; Zhao, Y.X. Regulation of Cu species in CuO/SiO2 and its structural evolution in ethynylation reaction. Nanomaterials 2019, 9, 842. [Google Scholar] [CrossRef]
- Yang, G.F.; Li, H.T.; Zhang, H.X.; Wang, Z.P.; Liu, L.L.; Zhao, Y.X. Effect of NaOH concentration on structure and catalytic performance of Cu2O for formaldehyde ethynylation. J. Mol. Catal. 2016, 30, 540–546. [Google Scholar]
- Eugene, V.H.; Pispacaway, N.J. Ethynylation Catalyst and Process for Production Alkynols by Low Pressure Reactions. US 3920759, 18 November 1975. [Google Scholar]
- Zak, D.J. Butynediol Production. US 4085151, 18 April 1978. [Google Scholar]
- Fremont, J.M. Preparation of Butynediol Using Bismuth Modified Spheroidal Malachite. U.S. Patent 4127,734, 28 November 1978. [Google Scholar]
- Haas, T.; Jaeger, B.; Weber, R.; Mitchell, S.F.; King, C.F. New diol process: 1,3-propanediol and 1,4-butanediol. Appl. Catal. A Gen. 2005, 280, 83–88. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Zhang, H.X.; Li, H.T. Preparation of core-shell CuO-Bi2O3@meso-SiO2 catalyst and its catalytic performance for formaldehyde ethynylation. Ind. Catal. (China) 2015, 23, 344–348. [Google Scholar]
- Luo, M.; Li, H.T.; Ma, Z.Q.; Wang, J.J.; Zhao, Y.X. Researches on activation process of CuO-Bi2O3 /SiO2-MgO catalyst in formaldehyde ethynylation reaction. Ind. Catal. (China) 2014, 22, 363–368. [Google Scholar]
- Zheng, Y.; Sun, Z.J.; Wang, Y.Z.; Li, H.T.; Wang, S.A.; Luo, M.; Zhao, J.L.; Zhao, Y.X. Preparation of CuO-Bi2O3/SiO2-MgO catalyst and its ethynylation performance. J. Mol. Catal. (China) 2012, 26, 233–238. [Google Scholar]
- Wang, J.J.; Li, H.T.; Ma, Z.Q.; Wang, Z.P.; Guo, J.Y.; Zhao, Y.X. Preparation of magnetic CuO-Bi2O3/Fe3O4-SiO2-MgO catalyst and its catalytic performance for formaldehyde ethynylation. J. Chem. Ind. Eng. 2015, 66, 2098–2104. [Google Scholar]
- Wang, Z.P.; Niu, Z.Z.; Hao, Q.A.; Ban, L.J.; Li, H.T.; Zhao, Y.X.; Jiang, Z. Enhancing the ethynylation performance of CuO-Bi2O3 nanocatalysts by tuning Cu-Bi interactions and phase structures. Catalysts 2019, 9, 35. [Google Scholar] [CrossRef]
- Li, H.T.; Niu, Z.Z.; Yang, G.F.; Zhang, H.X.; Wang, Z.P.; Zhao, Y.X. Support effect of Cu2O/TiO2 employed in formaldehyde ethynylation. J. Chem. Ind. Eng. 2018, 69, 2512–2518. [Google Scholar]
- Wang, Z.P.; Ban, L.J.; Meng, P.F.; Li, H.T.; Zhao, Y.X. Ethynylation of Formaldehyde over Binary Cu-Based Catalysts: Study on Synergistic Effect between Cu+ Species and Acid/Base Sites. Nanomaterials 2019, 9, 1038. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Hao, Q.A.; Wang, Z.P.; Ban, L.J.; Meng, P.F. Study on catalytic of CuO-ZnO catalyst prepared by different precipitants. Ind. Catal. (China) 2019, 33, 124–131. [Google Scholar]
- Lakhera, S.K.; Venkataramana, R.; Watts, A.; Anpo, M.; Neppolian, B. Facile synthesis of Fe2O3/Cu2O nanocomposite and its visible light photocatalytic activity for the degradation of cationic dyes. Res. Chem. Intermed. 2017, 43, 5091–5102. [Google Scholar] [CrossRef]
- Jiang, H.; Dai, Y.; Hu, Y.; Chen, W.; Li, C. Nanostructured ternary nanocomposite of rGO/CNTs/MnO2 for high-rate supercapacitors. ACS Sustain. Chem. Eng. 2014, 2, 70–74. [Google Scholar] [CrossRef]
- Pal, J.; Ganguly, M.; Dutta, S.; Mondal, C.; Negishi, Y.; Pal, T. Hierarchical Au-CuO nanocomposite from redox transformation reaction for surface enhanced Raman scattering and clock reaction. CrystEngComm 2014, 16, 883–893. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, D.; Li, C.M.; Zhang, M.; Lei, D.; Yin, X.; Chen, L.; Li, Q.; Wang, Y.; Wang, T. Magnetite/Graphene composites: Microwave irradiation synthesis and enhanced cycling and rate performances for lithium ion batteries. J. Mater. Chem. 2010, 20, 5538–5543. [Google Scholar]
- Zhao, Z.; Liu, J.; Cui, F.; Feng, H.; Zhang, L. One-pot synthesis of tunable Fe3O4–MnO2 core–shell nanoplates and their applications for water purification. J. Mater. Chem. 2012, 22, 9052–9057. [Google Scholar] [CrossRef]
- Choi, Y.; Hong, S.; Liu, L.; Kim, S.K.; Park, S. Galvanically replaced hollow Au–Ag nanospheres: Study of their surface plasmon resonance. Langmuir 2012, 28, 6670–6676. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.; Mondal, C.; Sasmal, A.K.; Ganguly, M.; Negishi, Y.; Pal, T. Account of nitroarene reduction with size- and facet-controlled CuO–MnO2 nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 9173–9184. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, Z.; Yao, W.; Wang, P.; Yu, S.; Wang, X. Decorating of Ag and CuO on Cu nanoparticles for enhanced high catalytic activity to the degradation of organic pollutants. Langmuir 2017, 33, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Kaviyarasan, K.; Anandan, S.; Mangalaraja, R.V.; Sivasankar, T.; Ashokkumar, M. Sonochemical synthesis of Cu2O nanocubes for enhanced chemiluminescence applications. Ultrason. Sonochem. 2016, 29, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Lakheraa, S.K.; Watts, A.; Haffeez, H.Y.; Neppolian, B. Interparticle double charge transfer mechanism of heterojunction α-Fe2O3/Cu2O mixed oxide catalysts and its visible light photocatalytic activity. Catal. Today 2018, 300, 58–70. [Google Scholar] [CrossRef]
- Huang, K. The long wave modes of the Cu2O Lattice. Zeitschrift für Physik 1963, 171, 213–225. [Google Scholar] [CrossRef]
- Dawson, P.; Hargreave, M.M.; Wilkinson, G.R. The dielectric and lattice vibrational spectrum of cuprous oxide. J. Phys. Chem. Solids 1973, 34, 2201–2208. [Google Scholar] [CrossRef]
- Singhal, A.; Pai, M.R.; Rao, R.; Pillai, K.T.; Lieberwirth, I.; Tyagi, A.K. Copper(I) oxide nanocrystals-one step synthesis, characterization, formation mechanism and photocatalytic properties. Eur. J. Inorg. Chem. 2013, 14, 2640–2651. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Sheremet, E.; Deckert-Gaudig, T.; Chaneac, C.; Hietschold, M.; Deckert, V.; Zahn, D.R. Surface- and tip-enhanced Raman spectroscopy reveals spin-waves in iron oxide nanoparticles. Nanoscale 2015, 7, 9545–9551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bersani, D.; Lottici, P.P.; Montenero, A. A micro-Raman study of iron-titanium oxides obtained by sol-gel synthesis. J. Mater. Sci. 2000, 35, 4301–4305. [Google Scholar] [CrossRef]
- Huang, W.C.; Lyu, L.M.; Yang, Y.C.; Huang, M.H. Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Tian, Y.L.; Zhang, J.D. Construction of p-n heterojunction film of Cu2O/a-Fe2O3 for efficiently photoelectrocatalytic degradation of oxytetracycline. J. Colloid Interface Sci. 2018, 526, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.H.; Zhang, X.M.; Xu, J.; Han, Y.F. Effects of preparation methods on the activity of CuO/CeO2 catalysts for CO oxidation. Front. Chem. Sci. Eng. 2017, 11, 603–612. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhang, L.; Fang, W.X.; Ren, J.; Li, Y.Y.; Yao, H.C.; Wang, J.S.; Li, Z.J. Enhanced photoreduction CO2 activity over direct z-scheme α-Fe2O3/Cu2O heterostructures under visible light lrradiation. ACS Appl. Mater. Interfaces 2015, 7, 8631–8639. [Google Scholar] [CrossRef]
- Wilson, D.; Langell, M.A. XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Kuo, C.H.; Huang, M.H. Facile synthesis of Cu2O nanocrystals with systematic shape evolution from cubic to octahedral structures. J. Phys. Chem. C 2008, 112, 18355–18360. [Google Scholar] [CrossRef]
- Ho, J.Y.; Huang, M.H. Synthesis of submicrometer-sized Cu2O crystals with morphological evolution from cubic to hexapod structures and their comparative photocatalytic activity. J. Phys. Chem. C 2009, 113, 14159–14164. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.C.; Kim, A.; Kim, A.Y.; Lee, H.J.; Song, H.; Park, K.H. Cu2O nanocube-catalyzed cross-coupling of aryl halides with phenols via ullmann coupling. Eur. J. Inorg. Chem. 2009, 28, 4219–4223. [Google Scholar] [CrossRef]
- Colacino, E.; Villebrun, L.; Martinez, J.; Lamaty, F. PEG3400-Cu2O-Cs2CO3: An efficient and recyclable microwave-enhanced catalytic system for ligand-free ullmann arylation of indole and benzimidazol. Tetrahedron 2010, 66, 3730–3735. [Google Scholar] [CrossRef]
- Kiss, J.; Óvári, L.; Oszkó, A.; Pótári, G.; Tóth, M.; Baán, K.; Erdóhelyi, A. Structure and reactivity of Au-Rh bimetallic clusters on titanate nanowires, nanotubes and TiO2(110). Catal. Today 2012, 181, 163–170. [Google Scholar] [CrossRef]
- Óvári, L.; Berkó, A.; Balázs, N.; Majzik, Z.; Kiss, J. Formation of Rh-Au Core-Shell Nanoparticles on TiO2(110) Surface Studied by STM and LEIS. Langmuir 2010, 26, 2167–2175. [Google Scholar] [CrossRef]
- Zhu, H.; Du, M.L.; Wang, Y.; Wang, L.N.; Zou, M.L.; Zhang, M.; Fu, Y.Q. A new strategy for the surface-free-energy-distribution induced selective growth and controlled formation of Cu2O-Au hierarchical heterostructures with a series of morphological evolutions. J. Mater. Chem. A 2013, 1, 919–929. [Google Scholar] [CrossRef]
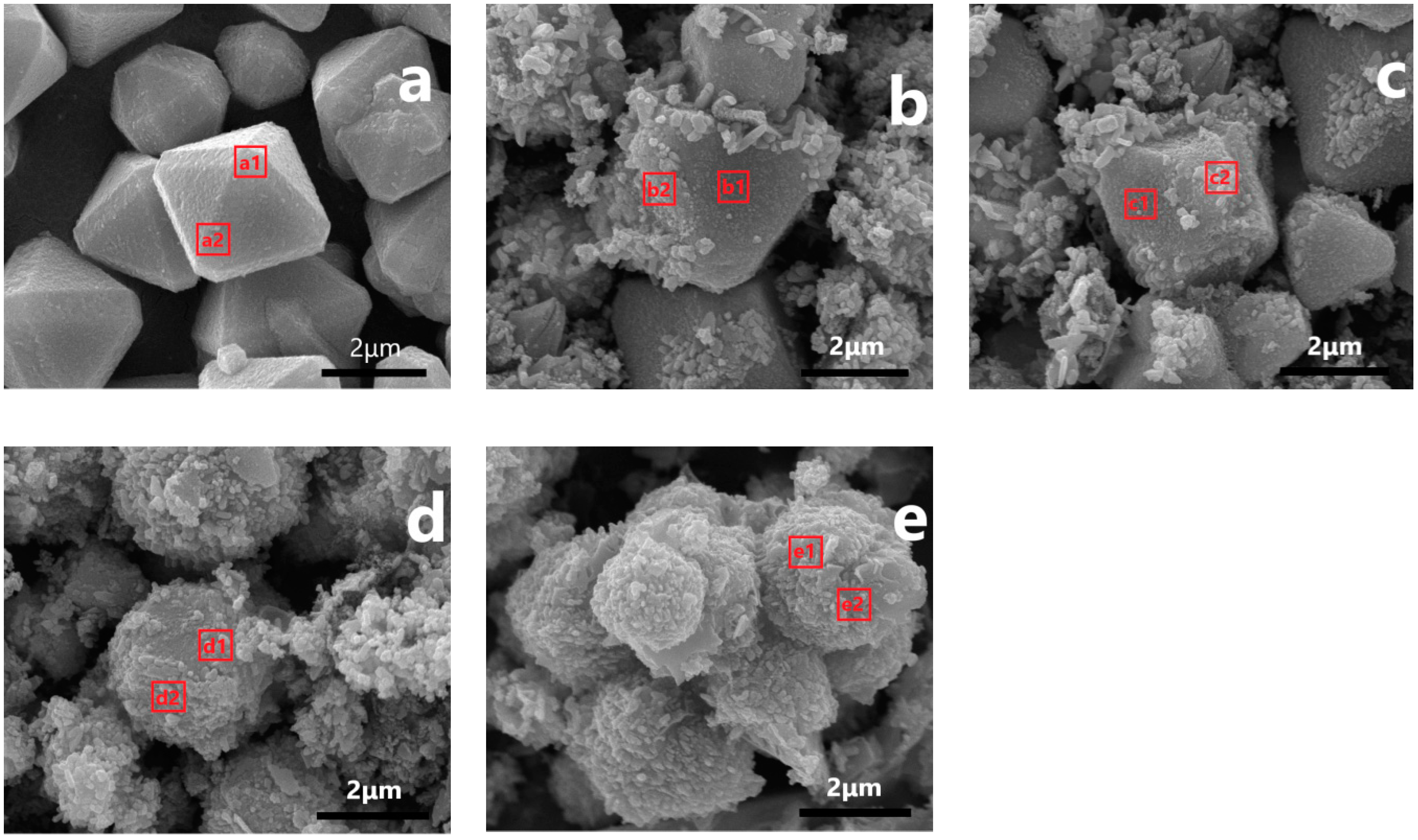
| Catalysts | Region | AT% | ||
|---|---|---|---|---|
| OK | FeK | CuK | ||
| Cu2O | a1 | 7.67 | 0 | 92.33 |
| a2 | 8.97 | 0 | 91.03 | |
| CF-0.05 | b1 | 7.85 | 0 | 92.15 |
| b2 | 13.91 | 10.46 | 75.63 | |
| CF-0.1 | c1 | 8.67 | 0 | 91.33 |
| c2 | 17.36 | 14.84 | 67.80 | |
| CF-0.8 | d1 | 29.33 | 35.74 | 34.92 |
| d2 | 29.12 | 35.97 | 34.91 | |
| CF-2 | e1 | 33.23 | 41.96 | 24.81 |
| e2 | 34.12 | 41.72 | 24.16 | |
| Catalysts | Fe(eV) | Fe2+/Fe3+ (Atomic) | Cu(eV) | Cu2+/Cu+ (Atomic) | Fe/Cu (Atomic) | ||
|---|---|---|---|---|---|---|---|
| Fe2+2p3/2 | Fe3+2p3/2 | Cu+2p3/2 | Cu2+2p3/2 | ||||
| Cu2O | - | - | - | 932.4 | 934.7 | 0.31 | - |
| CF-0.05 | 710.1 | 711.6 | 0.07 | - | - | - | 0.26 |
| CF-0.8 | 710.1 | 711.6 | 0.26 | 932.8 | 934.7 | 0.83 | 0.79 |
| CF-2 | 710.1 | 711.6 | 0.32 | 932.8 | 934.7 | 2.3 | 1.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ban, L.; Niu, Z.; Huang, X.; Meng, P.; Han, X.; Zhang, Y.; Zhang, H.; Zhao, Y. Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde. Nanomaterials 2019, 9, 1301. https://doi.org/10.3390/nano9091301
Li H, Ban L, Niu Z, Huang X, Meng P, Han X, Zhang Y, Zhang H, Zhao Y. Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde. Nanomaterials. 2019; 9(9):1301. https://doi.org/10.3390/nano9091301
Chicago/Turabian StyleLi, Haitao, Lijun Ban, Zhuzhu Niu, Xin Huang, Pingfan Meng, Xudong Han, Yin Zhang, Hongxi Zhang, and Yongxiang Zhao. 2019. "Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde" Nanomaterials 9, no. 9: 1301. https://doi.org/10.3390/nano9091301
APA StyleLi, H., Ban, L., Niu, Z., Huang, X., Meng, P., Han, X., Zhang, Y., Zhang, H., & Zhao, Y. (2019). Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde. Nanomaterials, 9(9), 1301. https://doi.org/10.3390/nano9091301





