Zingiber officinale Extract (ZOE) Incorporated with Layered Double Hydroxide Hybrid through Reconstruction to Preserve Antioxidant Activity of ZOE against Ultrasound and Microwave Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pristine MgFe–CO3–LDH
2.3. Preparation of ZOE–LDH Hybrid
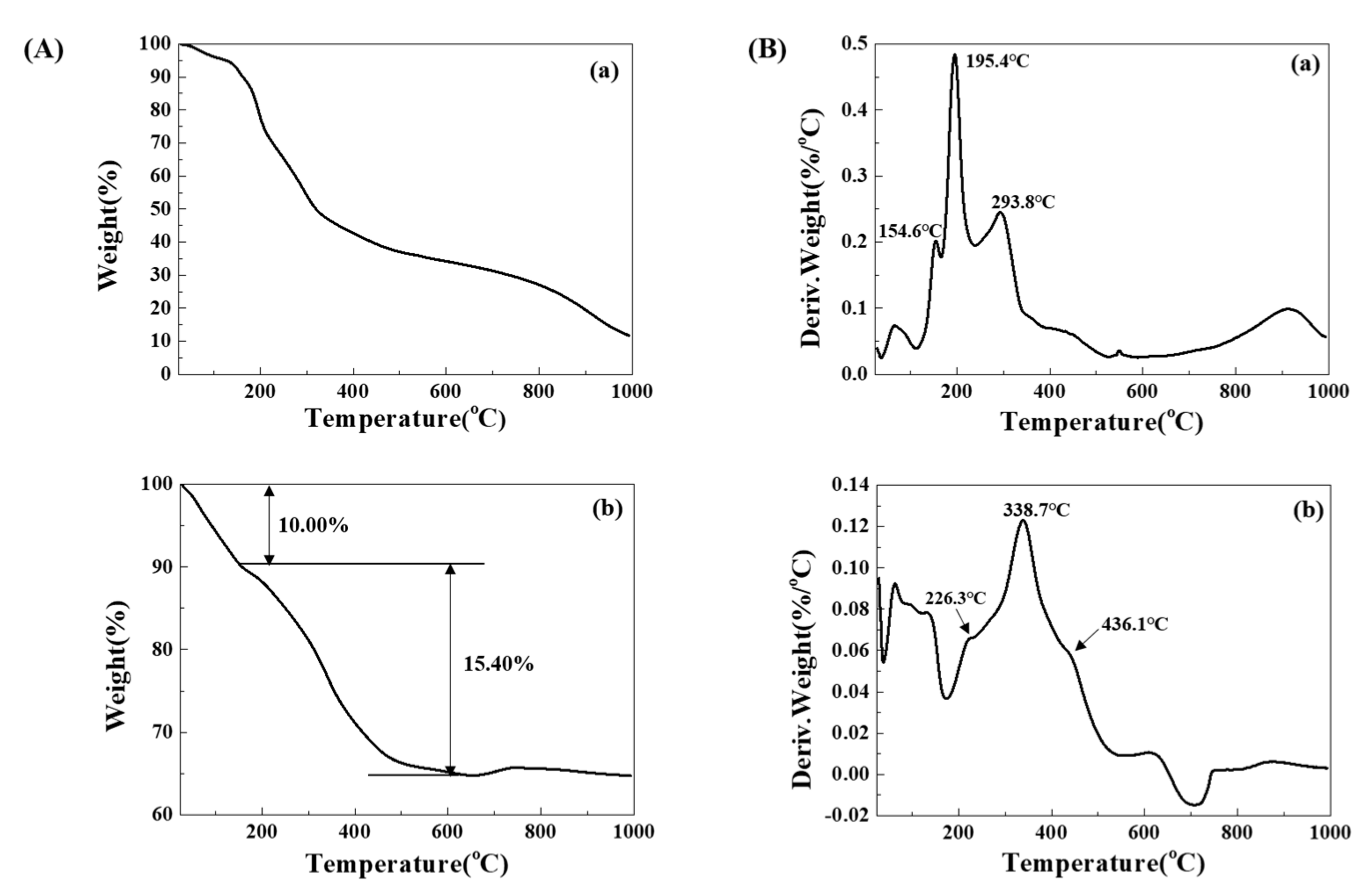
2.4. Characterization
2.5. Radical Scavenging Activity
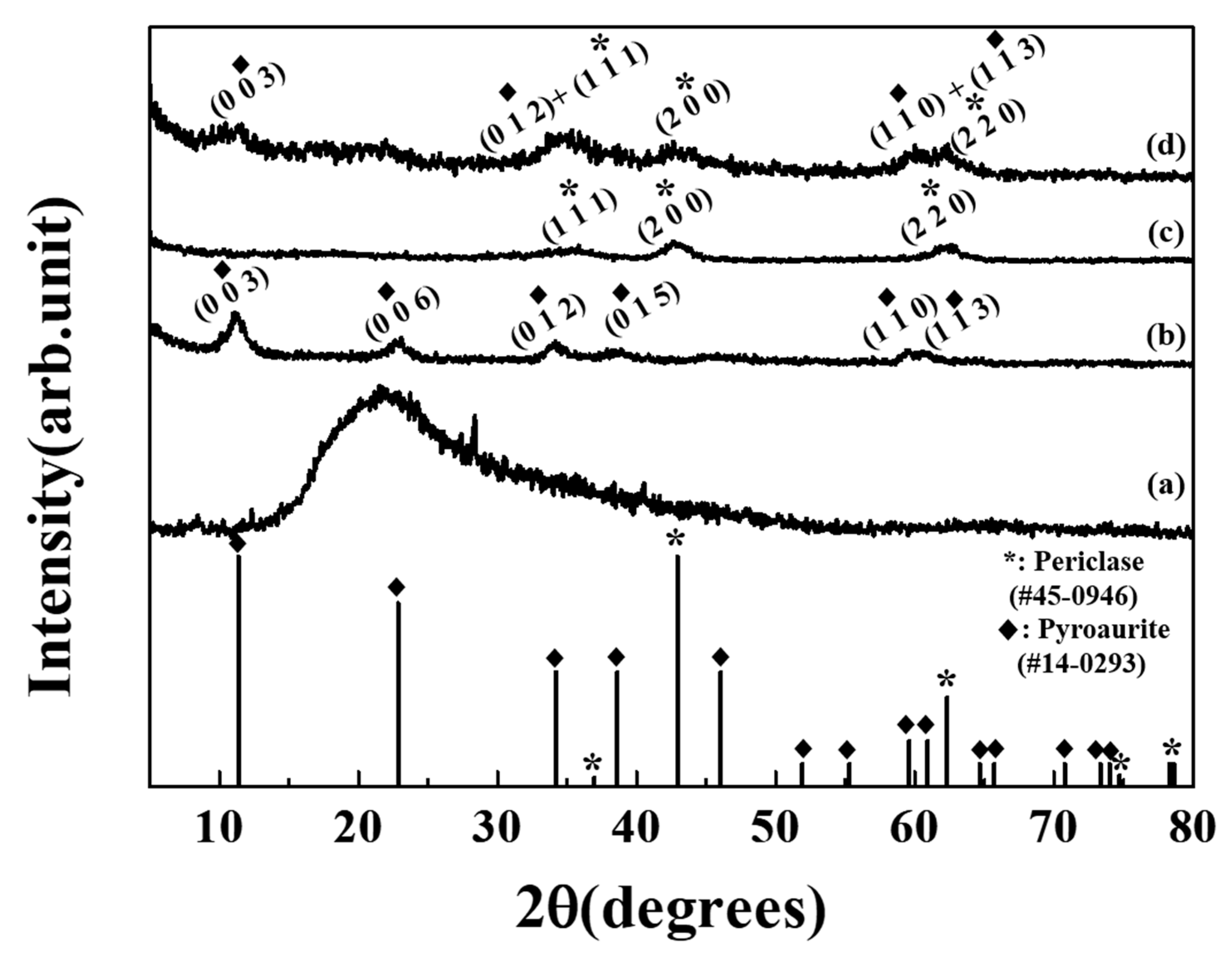
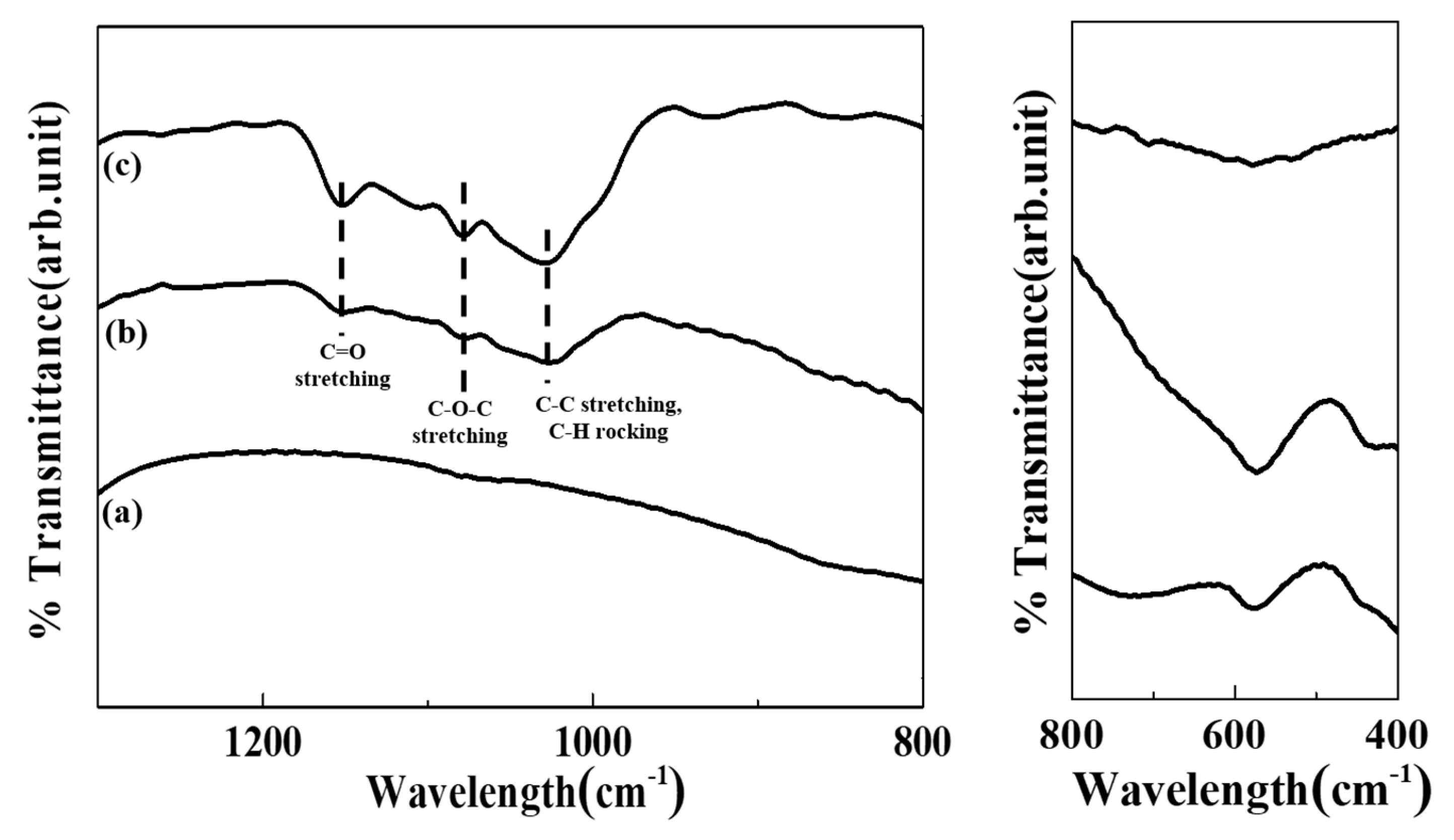
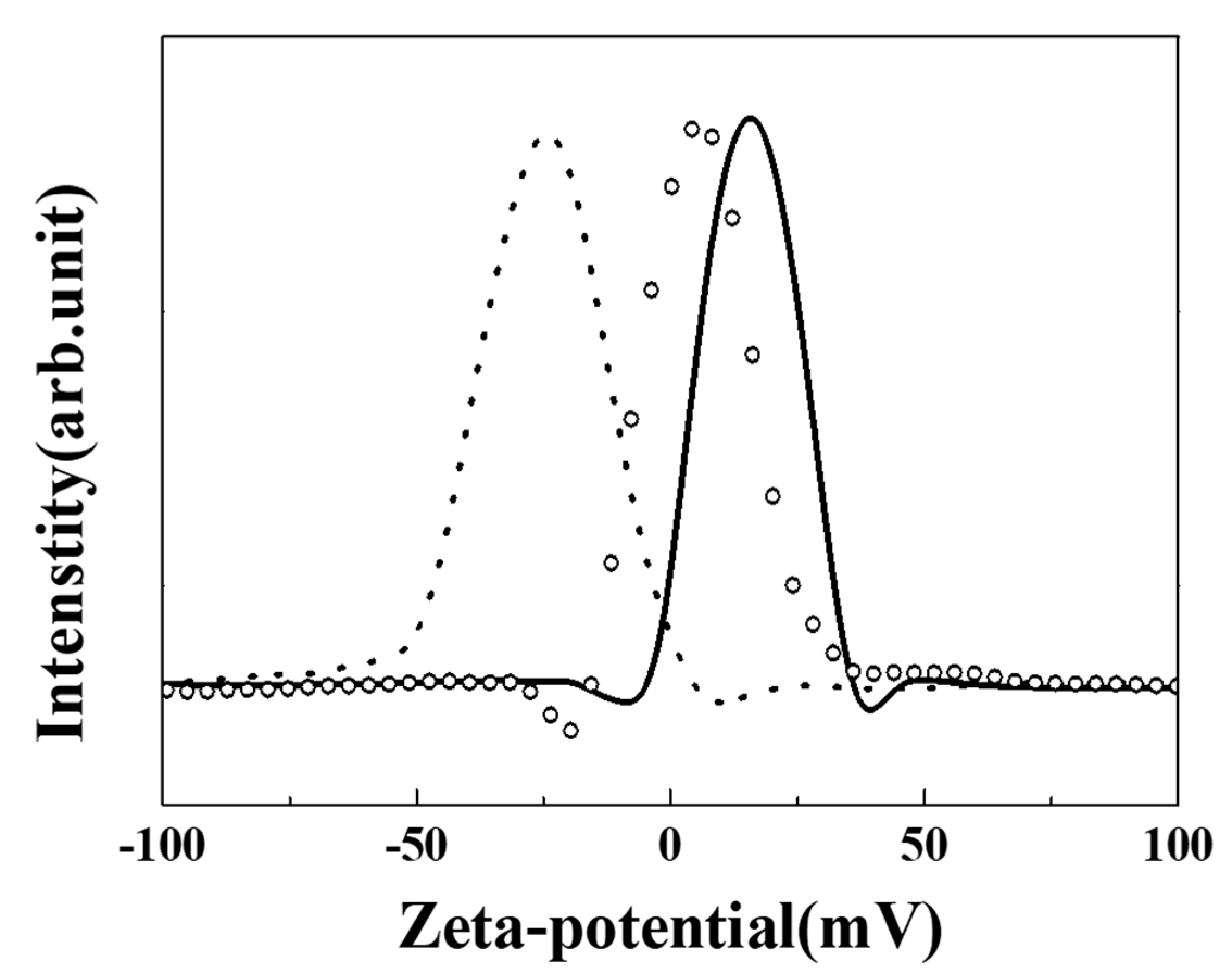
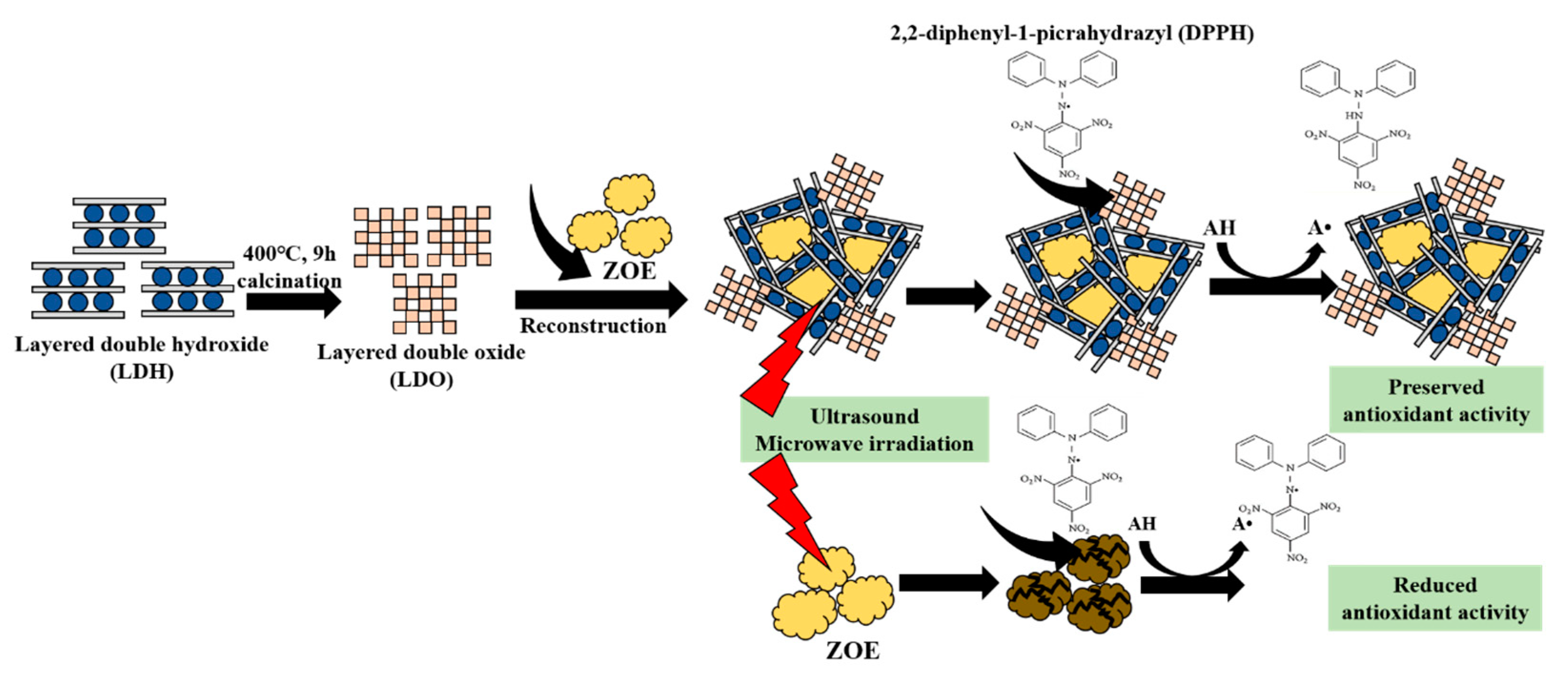
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Morakinyo, A.; Adeniyi, O.; Arikawe, A. Effects of Zingiber Officinale on Reproductive Functions in the Male Rat. Afr. J. Biomed. Res. 2010, 11, 329–334. [Google Scholar] [CrossRef]
- Stoilova, I.; Krastanov, A.; Stoyanova, A.; Denev, P.; Gargova, S. Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem. 2007, 102, 764–770. [Google Scholar] [CrossRef]
- El-Ghorab, A.H.; Nauman, M.; Anjum, F.M.; Hussain, S.; Nadeem, M. A Comparative Study on Chemical Composition and Antioxidant Activity of Ginger (Zingiber officinale) and Cumin (Cuminum cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Fazaeli, M.; Yousefi, S.; Emam-Djomeh, Z. Investigation on the effects of microwave and conventional heating methods on the phytochemicals of pomegranate (Punica granatum L.) and black mulberry juices. Food Res. Int. 2013, 50, 568–573. [Google Scholar] [CrossRef]
- Fang, X.; Mark, G.; Von Sonntag, C. OH radical formation by ultrasound in aqueous solutions Part I: The chemistry underlying the terephthalate dosimeter. Ultrason. Sonochem. 1996, 3, 57–63. [Google Scholar] [CrossRef]
- Bremner, D.H.; Burgess, A.E.; Chand, R. The Chemistry of Ultrasonic Degradation of Organic Compounds. Curr. Org. Chem. 2011, 15, 168–177. [Google Scholar] [CrossRef]
- Sun, J.; Mei, Z.; Tang, Y.; Ding, L.; Jiang, G.; Zhang, C.; Sun, A.; Bai, W. Stability, Antioxidant Capacity and Degradation Kinetics of Pelargonidin-3-glucoside Exposed to Ultrasound Power at Low Temperature. Molecules 2016, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Nakilcioglu-Taş, E.; Ötleş, S. Degradation kinetics of bioactive compounds and antioxidant capacity of Brussels sprouts during microwave processing. Int. J. Food Prop. 2017, 20, S2798–S2809. [Google Scholar] [CrossRef]
- Haq, N.; Muhammad Aslam, S.; Aziz ur Rehman, A.; Sidra Tul, M.; Saima, M. Phytochemical Composition and Antioxidant Potential of Oven Heated and Microwave Treated Ginger (Zingiber officinale Roscoe). Free Radic. Antioxid. 2018, 8. [Google Scholar] [CrossRef]
- Abbas, K.A.; Saleh, A.M.; Saeed, M.; MohdAzhan, N. The recent advances in the nanotechnology and its applications in food processing: A review. J. Food Agric. Environ. 2009, 7, 14–17. [Google Scholar]
- LeMarchand, C.; Gref, R.; Couvreur, P.; Patrick, C. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004, 58, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef]
- Choy, J.-H.; Jung, J.-S.; Oh, J.-M.; Park, M.; Jeong, J.; Kang, Y.-K.; Han, O.-J. Layered double hydroxide as an efficient drug reservoir for folate derivatives. Biomaterials 2004, 25, 3059–3064. [Google Scholar] [CrossRef]
- Wei, M.; Shi, S.; Wang, J.; Li, Y.; Duan, X. Studies on the intercalation of naproxen into layered double hydroxide and its thermal decomposition by in situ FT-IR and in situ HT-XRD. J. Solid State Chem. 2004, 177, 2534–2541. [Google Scholar] [CrossRef]
- Gao, X.; Chen, L.; Xie, J.; Yin, Y.; Chang, T.; Duan, Y.; Jiang, N. In vitro controlled release of vitamin C from Ca/Al layered double hydroxide drug delivery system. Mater. Sci. Eng. C 2014, 39, 56–60. [Google Scholar] [CrossRef]
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Publishers: Hauppauge, NY, USA, 2001. [Google Scholar]
- Gao, Y.; Wu, J.; Wang, Q.; Wilkie, C.A.; O’Hare, D. Flame retardant polymer/layered double hydroxide nanocomposites. J. Mater. Chem. A 2014, 2, 10996. [Google Scholar] [CrossRef]
- Ramaraj, B.; Yoon, K.R. Thermal and physicomechanical properties of ethylene-vinyl acetate copolymer and layered double hydroxide composites. J. Appl. Polym. Sci. 2008, 108, 4090–4095. [Google Scholar] [CrossRef]
- Oh, J.-M.; Kwak, S.-Y.; Choy, J.-H. Intracrystalline structure of DNA molecules stabilized in the layered double hydroxide. J. Phys. Chem. Solids 2006, 67, 1028–1031. [Google Scholar] [CrossRef]
- Choy, J.-H.; Son, Y.-H. Intercalation of vitamer into LDH and their controlled release properties. Bull. Korean Chem. Soc. 2004, 25, 122–126. [Google Scholar]
- Kim, B.-K.; Gwak, G.-H.; Okada, T.; Oh, J.-M. Effect of particle size and local disorder on specific surface area of layered double hydroxides upon calcination-reconstruction. J. Solid State Chem. 2018, 263, 60–64. [Google Scholar] [CrossRef]
- Brown, G. Crystal Structures of Clay Minerals and Their X-ray Identification; The Mineralogical Society of Great Britain and Ireland: London, UK, 1982; Volume 5. [Google Scholar]
- Pallon, L.K.H.; Olsson, R.T.; Pourrahimi, A.M.; Hedenqvist, M.S.; Hoang, A.T.; Gubanski, S.; Gedde, U.W.; Liu, D. Formation and the structure of freeze-dried MgO nanoparticle foams and their electrical behaviour in polyethylene. J. Mater. Chem. A 2015, 3, 7523–7534. [Google Scholar] [CrossRef][Green Version]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction; Prentice Hall: Upper Saddle River, NJ, USA, 2001; Volume 3. [Google Scholar]
- Kang, H.; Kim, H.-J.; Yang, J.-H.; Kim, T.-H.; Choi, G.; Paek, S.-M.; Choi, A.-J.; Choy, J.-H.; Oh, J.-M. Intracrystalline structure and release pattern of ferulic acid intercalated into layered double hydroxide through various synthesis routes. Appl. Clay Sci. 2015, 112, 32–39. [Google Scholar] [CrossRef]
- Devi, R.R.; Umlong, I.M.; Raul, P.K.; Das, B.; Banerjee, S.; Singh, L. Defluoridation of water using nano-magnesium oxide. J. Exp. Nanosci. 2014, 9, 512–524. [Google Scholar] [CrossRef]
- Srivastava, V.; Sharma, Y.C.; Sillanpää, M. Green synthesis of magnesium oxide nanoflower and its application for the removal of divalent metallic species from synthetic wastewater. Ceram. Int. 2015, 41, 6702–6709. [Google Scholar] [CrossRef]
- Benito, P.; Labajos, F.; Mafra, L.; Rocha, J.; Rives, V. Carboxylate-intercalated layered double hydroxides aged under microwave–hydrothermal treatment. J. Solid State Chem. 2009, 182, 18–26. [Google Scholar] [CrossRef]
- Du, B.; Guo, Z.; Fang, Z. Effects of organo-clay and sodium dodecyl sulfonate intercalated layered double hydroxide on thermal and flame behaviour of intumescent flame retarded polypropylene. Polym. Degrad. Stab. 2009, 94, 1979–1985. [Google Scholar] [CrossRef]
- Ding, Y.; Gui, Z.; Zhu, J.; Hu, Y.; Wang, Z. Exfoliated poly(methyl methacrylate)/MgFe-layered double hydroxide nanocomposites with small inorganic loading and enhanced properties. Mater. Res. Bull. 2008, 43, 3212–3220. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Evans, D.G.; Duan, X. Stoichiometric Synthesis of Pure MFe2O4(M = Mg, Co, and Ni) Spinel Ferrites from Tailored Layered Double Hydroxide (Hydrotalcite-Like) Precursors. Chem. Mater. 2004, 16, 1597–1602. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, H.; Chen, J.; Ao, Q. FTIR, XRD and SEM Analysis of Ginger Powders with Different Size. J. Food Process. Preserv. 2015, 39, 2017–2026. [Google Scholar] [CrossRef]
- Devi, A.; Das, V.K.; Deka, D. Ginger extract as a nature based robust additive and its influence on the oxidation stability of biodiesel synthesized from non-edible oil. Fuel 2017, 187, 306–314. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, G.J.; Kang, J.-H.; Kim, H.-J.; Kim, T.-I.; Oh, J.-M. Anticancer Drug-Incorporated Layered Double Hydroxide Nanohybrids and Their Enhanced Anticancer Therapeutic Efficacy in Combination Cancer Treatment. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Luo, T.; Yuan, Y. Controllable synthesis of Mg–Fe layered double hydroxide nanoplates with specific Mg/Fe ratios and their effect on adsorption of As(v) from water. New J. Chem. 2014, 38, 4427. [Google Scholar] [CrossRef]
- Kang, H.; Kim, M.; Feng, Q.; Lin, S.; Wei, K.; Li, R.; Choi, C.J.; Kim, T.-H.; Li, G.; Oh, J.-M.; et al. Nanolayered hybrid mediates synergistic co-delivery of ligand and ligation activator for inducing stem cell differentiation and tissue healing. Biomaterials 2017, 149, 12–28. [Google Scholar] [CrossRef]
- Yu, B.; Benning, C. Anionic lipids are required for chloroplast structure and function in Arabidopsis. Plant J. 2003, 36, 762–770. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, G.J.; Choi, A.-J.; Kim, T.-H.; Kim, T.-I.; Oh, J.-M. Layered Double Hydroxide Nanomaterials Encapsulating Angelica gigas Nakai Extract for Potential Anticancer Nanomedicine. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Elmoubarki, R.; Mahjoubi, F.Z.; Elhalil, A.; Tounsadi, H.; Abdennouri, M.; Sadiq, M.; Qourzal, S.; Zouhri, A.; Barka, N. Ni/Fe and Mg/Fe layered double hydroxides and their calcined derivatives: Preparation, characterization and application on textile dyes removal. J. Mater. Res. Technol. 2017, 6, 271–283. [Google Scholar] [CrossRef]
- Murase, H.; Yasuda, H.; Nakahira, A. Effect of High Magnetic Field on Ferrite Materials Obtained by Calcination of Layered Double Hydroxide. Mater. Trans. 2007, 48, 2877–2882. [Google Scholar] [CrossRef]
- Goutelle, S.; Maurin, M.; Rougier, F.; Barbaut, X.; Bourguignon, L.; Ducher, M.; Maire, P. The Hill equation: A review of its capabilities in pharmacological modelling. Fundam. Clin. Pharmacol. 2008, 22, 633–648. [Google Scholar] [CrossRef] [PubMed]

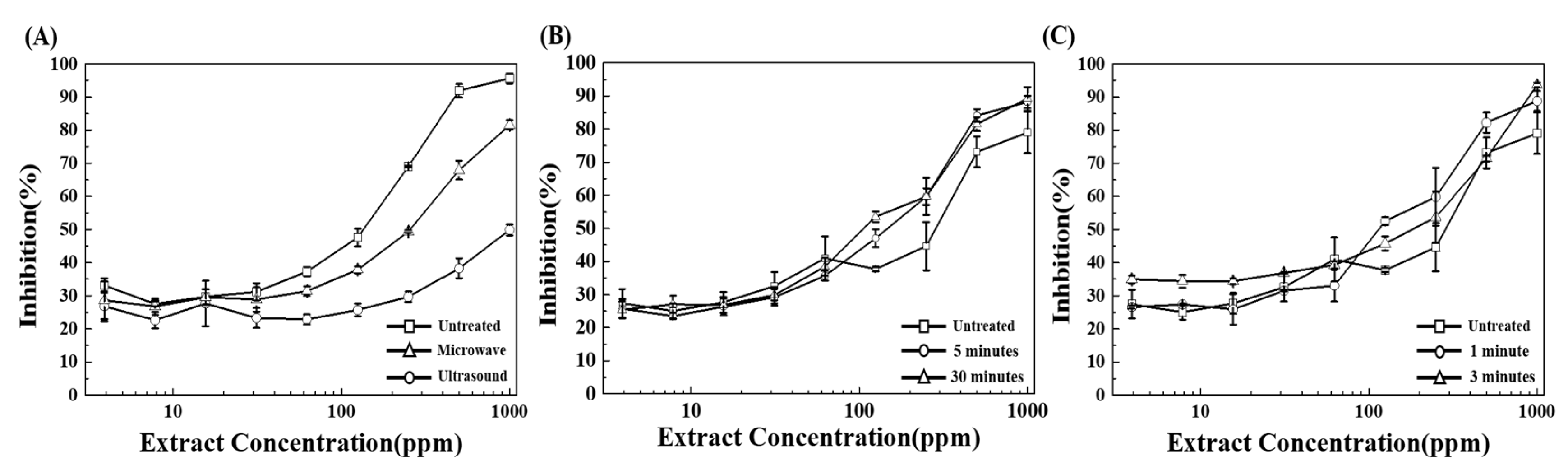
| Irradiated Conditions | IC50 (ppm) | |
|---|---|---|
| ZOE | ZOE–LDH Hybrid | |
| Untreated | 201.81 ± 13.59 | 349.17 ± 47.50 |
| Ultrasound for 5 min | - | 270.65 ± 28.78 a |
| Ultrasound for 30 min | 1003.29 ± 79.82 | 252.46 ± 12.47 a |
| Microwave irradiation for 1 min | 353.73 ± 29.93 | 259.36 ± 25.42 b |
| Microwave irradiation for 3 min | - | 231.82 ± 19.00 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Lee, S.-B.; Choi, A.-J.; Oh, J.-M. Zingiber officinale Extract (ZOE) Incorporated with Layered Double Hydroxide Hybrid through Reconstruction to Preserve Antioxidant Activity of ZOE against Ultrasound and Microwave Irradiation. Nanomaterials 2019, 9, 1281. https://doi.org/10.3390/nano9091281
Kim H-J, Lee S-B, Choi A-J, Oh J-M. Zingiber officinale Extract (ZOE) Incorporated with Layered Double Hydroxide Hybrid through Reconstruction to Preserve Antioxidant Activity of ZOE against Ultrasound and Microwave Irradiation. Nanomaterials. 2019; 9(9):1281. https://doi.org/10.3390/nano9091281
Chicago/Turabian StyleKim, Hyoung-Jun, Su-Bin Lee, Ae-Jin Choi, and Jae-Min Oh. 2019. "Zingiber officinale Extract (ZOE) Incorporated with Layered Double Hydroxide Hybrid through Reconstruction to Preserve Antioxidant Activity of ZOE against Ultrasound and Microwave Irradiation" Nanomaterials 9, no. 9: 1281. https://doi.org/10.3390/nano9091281
APA StyleKim, H.-J., Lee, S.-B., Choi, A.-J., & Oh, J.-M. (2019). Zingiber officinale Extract (ZOE) Incorporated with Layered Double Hydroxide Hybrid through Reconstruction to Preserve Antioxidant Activity of ZOE against Ultrasound and Microwave Irradiation. Nanomaterials, 9(9), 1281. https://doi.org/10.3390/nano9091281




