Synthesis, Characterization and Dye Removal Behavior of Core–Shell–Shell Fe3O4/Ag/Polyoxometalates Ternary Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Measurements
2.2. Synthesis of Fe3O4/Ag
2.3. Synthesis of POMs
2.4. Synthesis of Fe3O4/Ag/POMs
2.5. Dye Removal Experiment
3. Results and Discussion
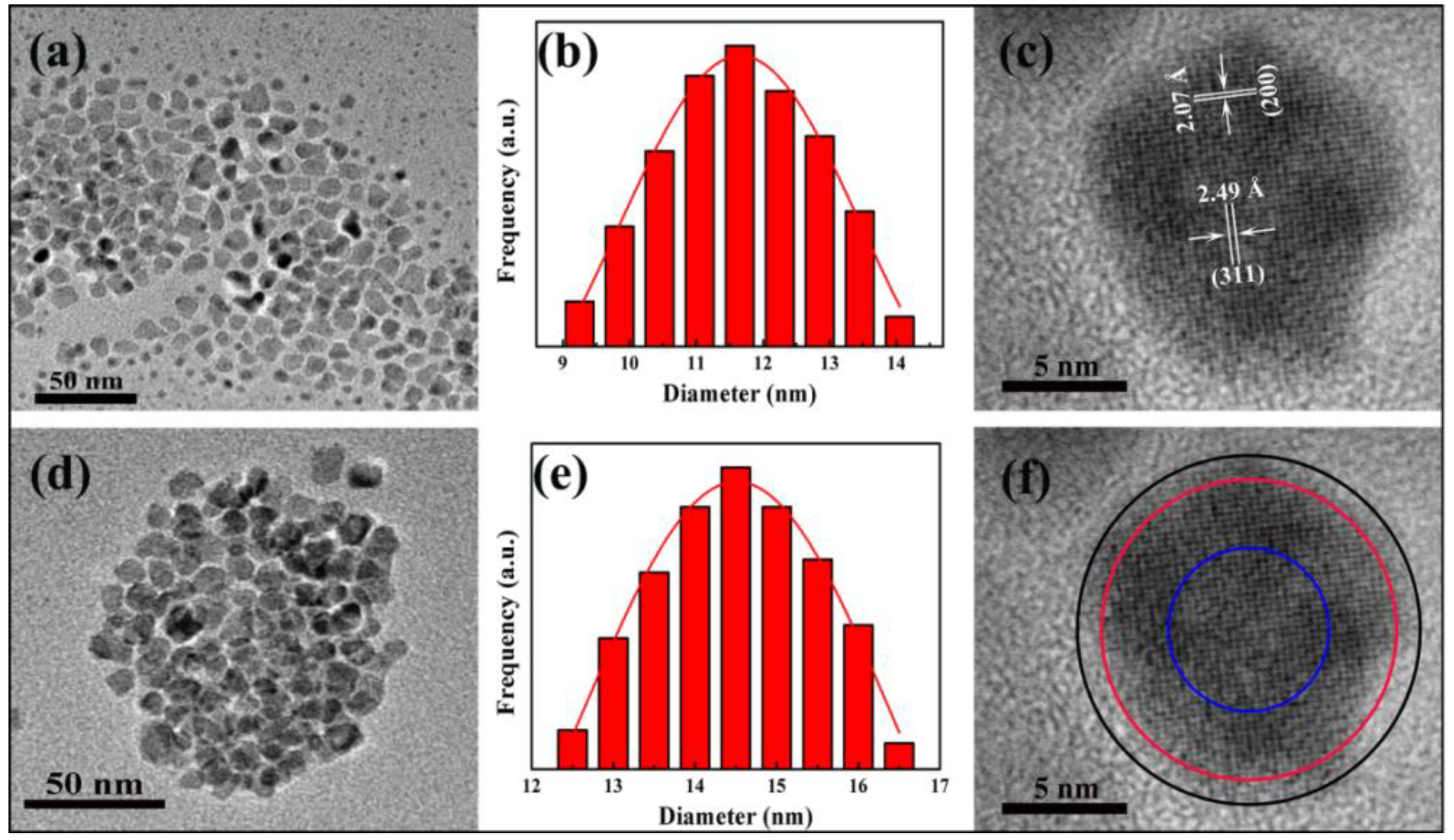
3.1. Morphology and Monodispersity of Fe3O4/Ag/POMs Nanocomposites
3.2. XPS Spectra
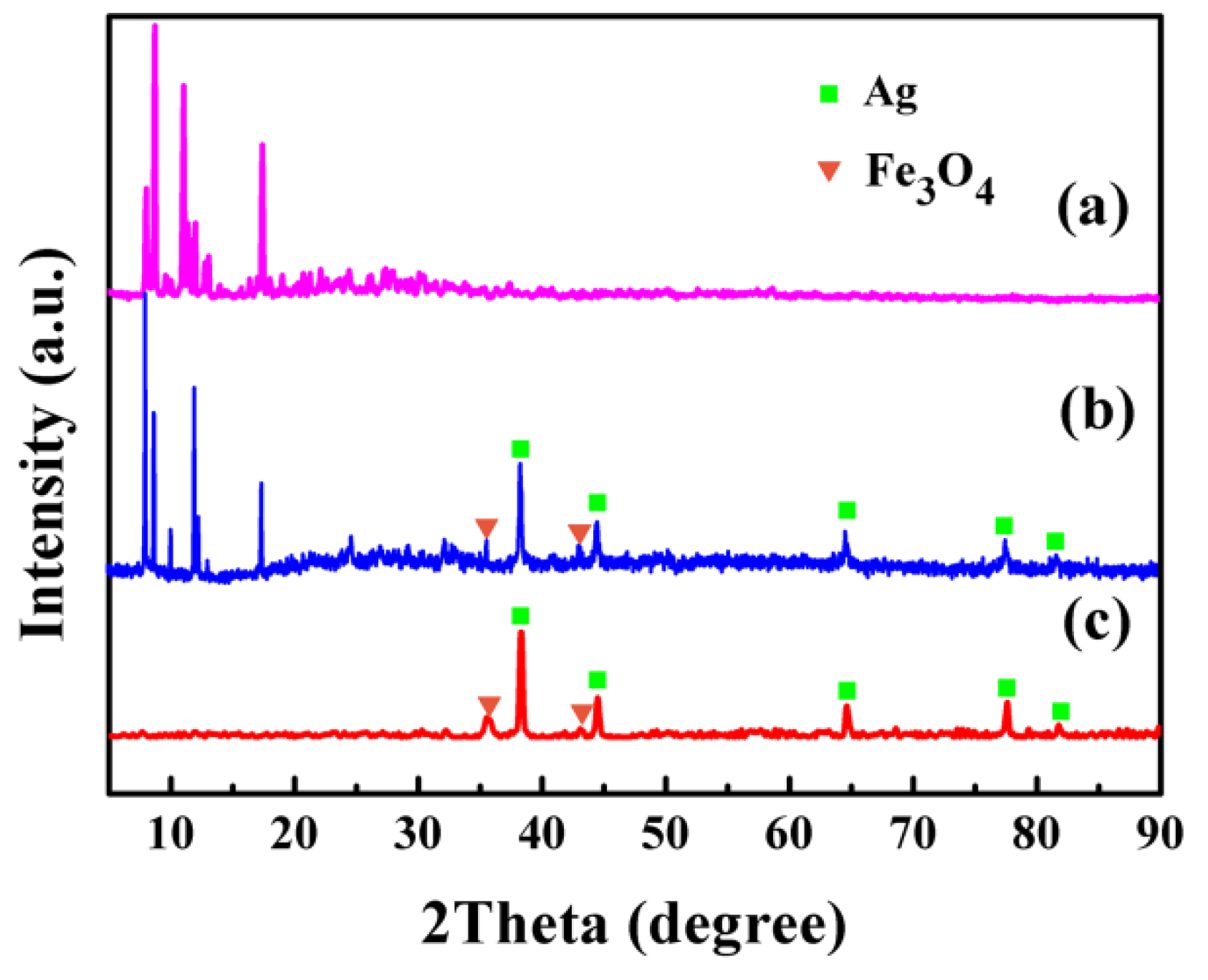
3.3. XRD Patterns
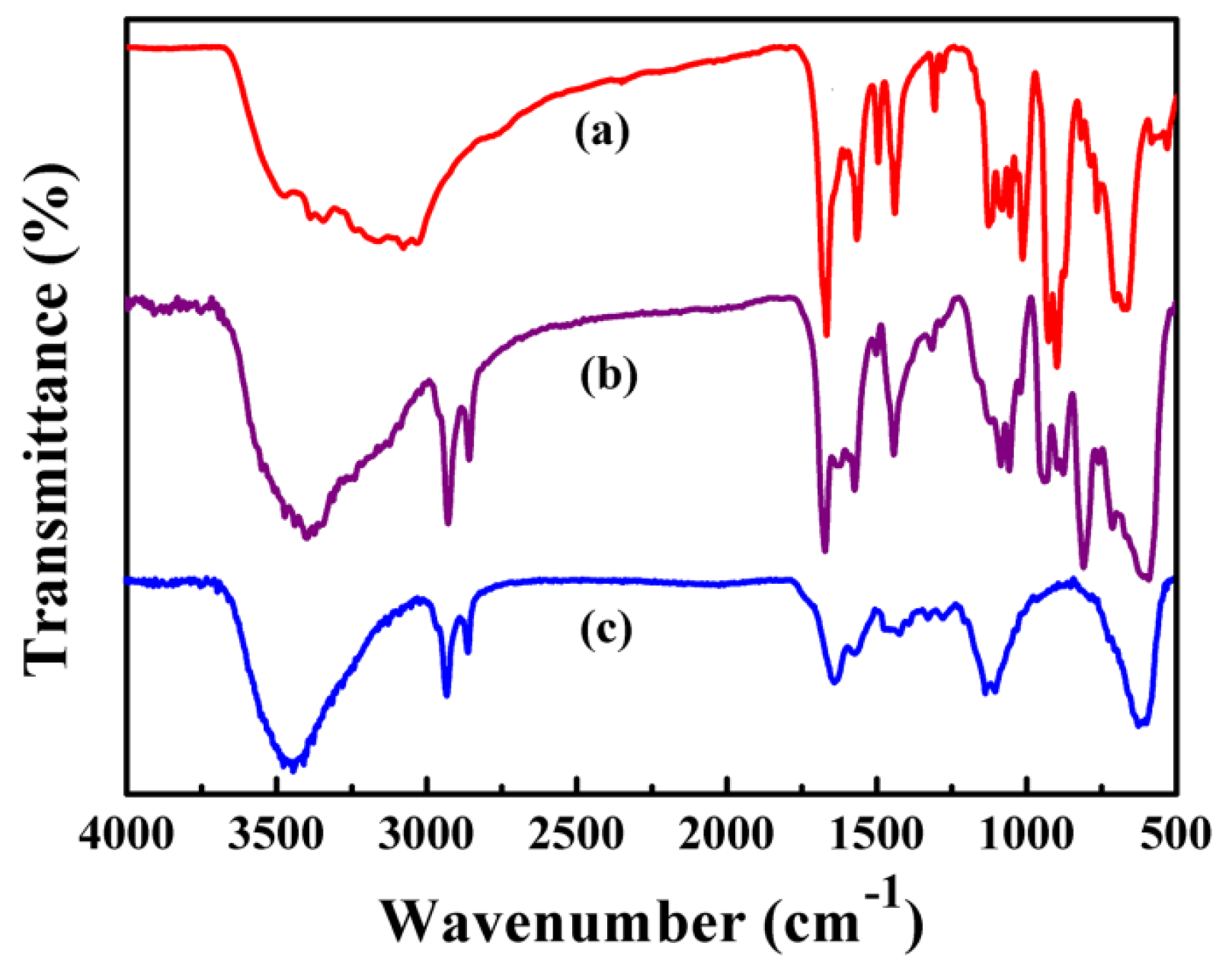
3.4. FTIR Spectroscopy
3.5. UV–Vis Spectroscopy
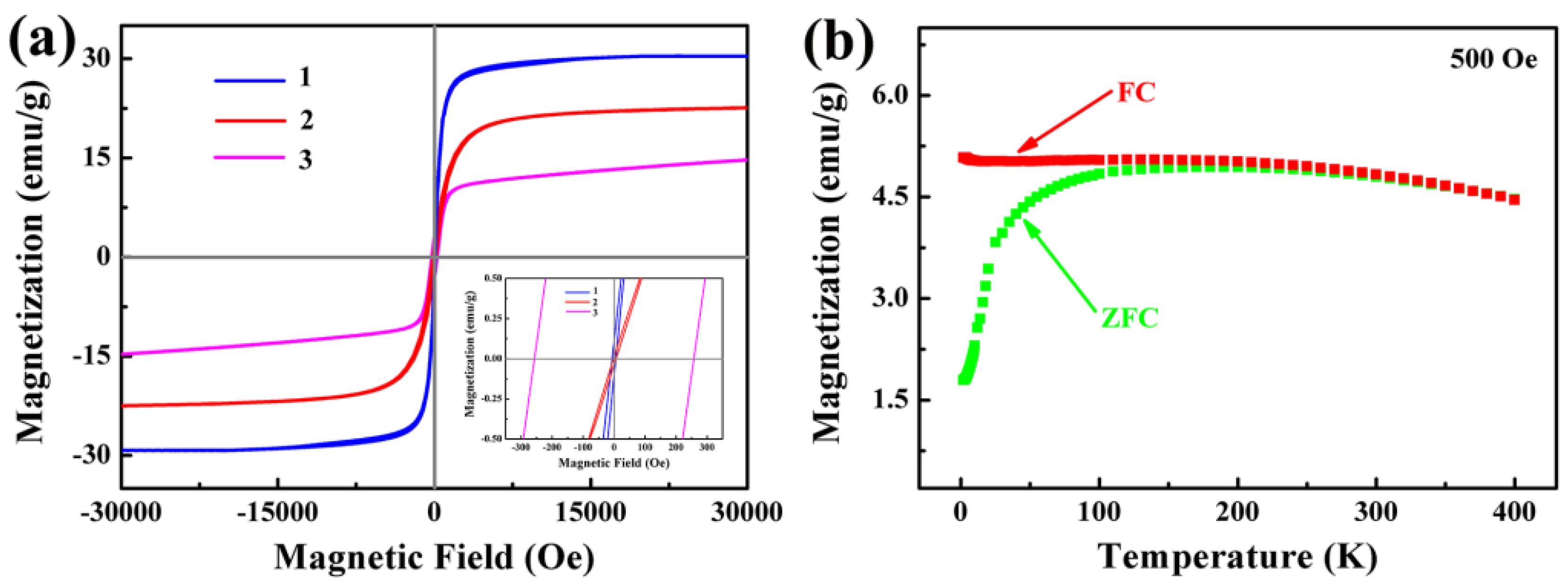
3.6. Magnetic Properties of Fe3O4/Ag/POMs Nanocomposites
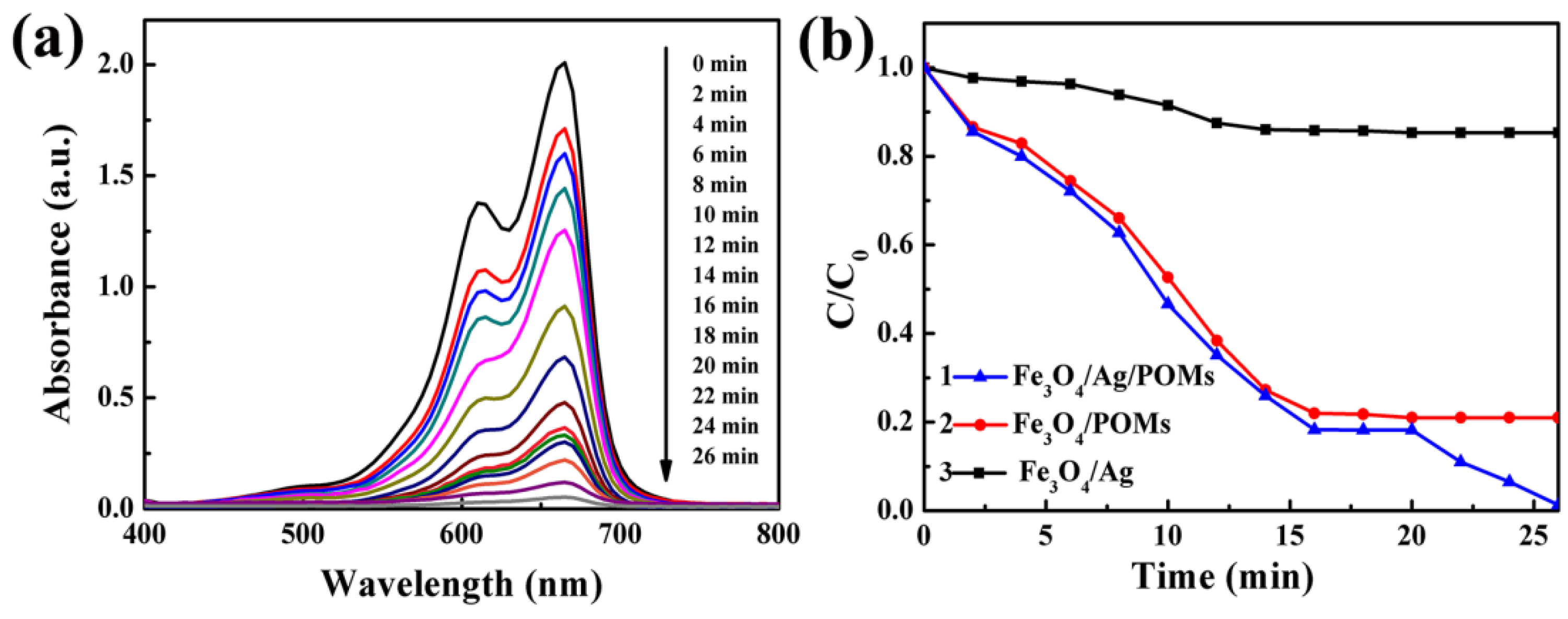
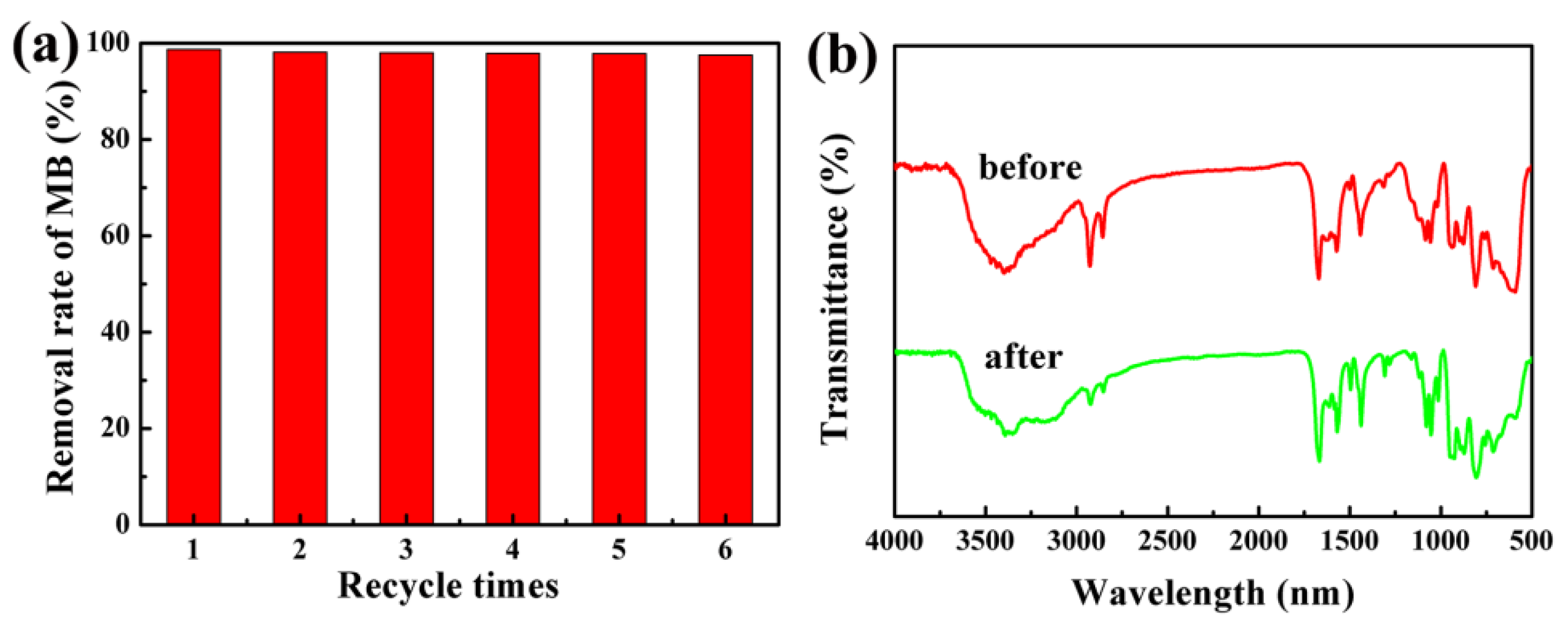
3.7. Dye Removal Activity of the Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, S.; Han, S.I.; Jung, D.; Hwang, H.J.; Lim, C.; Bae, S.; Park, O.K.; Tschabrunn, C.M.; Lee, M.; Bae, S.Y.; et al. Highly conductive, stretchable and biocompatible Ag-Au core-sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 2018, 1, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, Y.; Zhang, Y.; Feng, X.; Zhao, X.; Tan, H. Polyoxometalate/TiO2/Ag composite nanofibers with enhanced photocatalytic performance under visible light. Appl. Catal. B Environ. 2018, 221, 280–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Nsabimana, A.; Munyentwali, A.; Han, C.; Li, M.; Guo, L. Green and facile synthesis of an Au nanoparticles@polyoxometalate/ordered mesoporous carbon tri-component nanocomposite and its electrochemical applications. Biosens. Bioelectron. 2015, 66, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Yao, Q.; Yan, F.; Cui, C.; Sun, M.; Zhang, H. Facile and green decoration of Pd nanoparticles on macroporous carbon by polyoxometalate with enhanced electrocatalytic ability. RSC Adv. 2016, 6, 39618–39626. [Google Scholar] [CrossRef]
- Zhuang, Y.F.; Cao, X.Y.; Zhang, J.N.; Ma, Y.Y.; Shang, X.X.; Lu, J.X.; Yang, S.L.; Zheng, K.; Ma, Y.M. Monomer casting nylon/graphene nanocomposite with both improved thermal conductivity and mechanical performance. Compos. Part A Appl. Sci. Manuf. 2019, 120, 49–55. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. Part B Eng. 2019, 162, 538–568. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Graphene/semiconductor nanocomposites (GSNs) for heterogeneous photocatalytic decolorization of wastewaters contaminated with synthetic dyes: A review. Appl. Catal. B Environ. 2014, 160, 307–324. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, L.; Li, S. Review of electrical properties for polypropylene based nanocomposite. Compos. Commun. 2018, 10, 221–225. [Google Scholar] [CrossRef]
- Mensah, B.; Gupta, K.C.; Kim, H.; Wang, W.; Jeong, K.U.; Nah, C. Graphene-reinforced elastomeric nanocomposites: A review. Polym. Test. 2018, 68, 160–184. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, E.; Park, Y.; Kim, J.; Ryu, W.; Rho, J.; Kim, K. Photodeposited metal-semiconductor nanocomposites and their applications. J. Materiom. 2018, 4, 83–94. [Google Scholar] [CrossRef]
- Walz, F. The Verwey transition-a topical review. J. Phys. 2002, 14, R285. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Guo, B.; Wu, H.; Shao, N.; Li, D.; Liu, J.; Dang, L.; Wang, C.; Li, H.; Li, S.; et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference–based bone anabolic strategy. Nat. Med. 2015, 21, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Jędrzak, A.; Grześkowiak, B.F.; Coy, E.; Wojnarowicz, J.; Szutkowski, K.; Jurga, S.; Jesionowski, T.; Mrówczyński, R. Dendrimer based theranostic nanostructures for combined chemo-and photothermal therapy of liver cancer cells in vitro. Colloid. Surface. B 2019, 173, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Liang, Y.; Zhang, Z.C.; Ren, G.H.; Liu, Y.J.; Wu, S.S.; Shen, J. Ag@Fe3O4@C nanoparticles for multi-modal imaging-guided chemo-photothermal synergistic targeting for cancer therapy. Anal. Chim. Acta 2019. [Google Scholar] [CrossRef]
- Shao, H.Q.; Hou, P.; Wang, X.H.; Liu, X.; Li, X.M.; Liu, H.L.; Wu, J.H. Facile synthesis and magnetic properties of hybrid-phase iron oxide nanoparticles by polymer-supported nanoemulsion. Sci. Adv. Mater. 2014, 6, 1708–1715. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; He, Y.X.; Qin, Q.D.; Wang, F.C.; Wang, W.K.; Luo, Y.M. The synthesis of Cu/Fe/Fe3O4 catalyst through the aqueous solution ball milling method assisted by high-frequency electromagnetic field. Superlattice. Microst. 2018, 118, 123–129. [Google Scholar]
- Xin, T.; Ma, M.; Zhang, H.; Gu, J.; Wang, S.; Liu, M.; Zhang, Q. A facile approach for the synthesis of magnetic separable Fe3O4@TiO2, core–shell nanocomposites as highly recyclable photocatalysts. Appl. Surf. Sci. 2014, 288, 51–59. [Google Scholar] [CrossRef]
- Zhan, J.; Zhang, H.; Zhu, G. Magnetic photocatalysts of cenospheres coated with Fe3O4/TiO2 core/shell nanoparticles decorated with Ag nanopartilces. Ceram. Int. 2014, 40, 8547–8559. [Google Scholar] [CrossRef]
- Cheng, L.; Ruan, W.; Zou, B.; Liu, Y.; Wang, Y. Chemical template-assisted synthesis of monodisperse rattle-type Fe3O4@C hollow microspheres as drug carrier. Acta Biomater. 2017, 58, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wen, L.; Svec, F.; Tan, T.; Lv, Y. Magnetic AuNP@Fe3O4 nanoparticles as reusable carriers for reversible enzyme immobilization. Chem. Eng. J. 2016, 286, 272–281. [Google Scholar] [CrossRef]
- Kale, M.J.; Avanesian, T.; Christopher, P. Direct Photocatalysis by plasmonic nanostructures. ACS Catal. 2017, 4, 116–128. [Google Scholar] [CrossRef]
- Liu, T.; Li, B.; Hao, Y.; Han, F.; Zhang, L.; Hu, L. A general method to diverse silver/mesoporous-metal-oxide nanocomposites with plasmon-enhanced photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 378–388. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Z.; Pavaskar, P.; Hung, W.H.; Cronin, S.B. Plasmonic enhancement of photocatalytic decomposition of methyl orange under visible light. J. Catal. 2011, 277, 149–153. [Google Scholar] [CrossRef]
- Cushing, K.S.; Li, J.T.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.J.; Li, M.; Bristow, A.D.; Wu, N.Q. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Xu, B.; Li, Y.D.; Gao, Y.Q.; Liu, S.; Lv, D.; Zhao, S.J.; Gao, H.; Yang, G.Q.; Li, N.; Ge, L. Ag-AgI/Bi3O4Cl for efficient visible light photocatalytic degradation of methyl orange: The surface plasmon resonance effect of Ag and mechanism insight. Appl. Catal. B Environ. 2019, 246, 140–148. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, X.Y.; Fan, W.Y.; Guo, S.; Zhang, Y.J.; Liu, Y.; Chen, L.; Jung, Y.M. SERS study of Ag/FeS/4-MBA interface based on the SPR effect. Spectrochim. Acta. A 2019, 219, 147–153. [Google Scholar] [CrossRef]
- Karimi, A.; Kazeminezhad, I.; Azizi, S. Ag/αFe2O3-rGO novel ternary nanocomposites: Synthesis, characterization, and photocatalytic activity. Ceram. Int. 2019, 45, 3441–3448. [Google Scholar] [CrossRef]
- Song, D.; Yang, R.; Wang, C.; Xiao, R.; Long, F. Reusable nanosilver-coated magnetic particles for ultrasensitive SERS-based detection of malachite green in water samples. Sci. Rep. 2016, 6, 22870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.J.; Wei, Q.; Du, B.; Wu, D.; Li, H.; Yan, L.G.; Ma, H.M.; Zhang, Y. Label-free immunosensor for the detection of kanamycin using Ag@Fe3O4 nanoparticles and thionine mixed graphene sheet. Biosens. Bioelectron. 2013, 48, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Amarjargal, A.; Tijing, L.D.; Im, I.T.; Kim, C.S. Simultaneous preparation of Ag/Fe3O4 core-shell nanocomposites with enhanced magnetic moment and strong antibacterial and catalytic properties. Chem. Eng. J. 2013, 226, 243–254. [Google Scholar] [CrossRef]
- Zhu, S.H.; Gao, X.Q.; Dong, F.; Zhu, Y.L.; Zheng, H.Y.; Li, Y.W. Design of a highly active silver-exchanged phosphotungstic acid catalyst for glycerol esterification with acetic acid. J. Catal. 2013, 306, 155–163. [Google Scholar] [CrossRef]
- Yu, B.; Zou, B.; Hu, C.W. Recent applications of polyoxometalates in CO2 capture and transformation. J. CO2 Util. 2018, 26, 314–322. [Google Scholar] [CrossRef]
- Müller, A.; Petters, F.; Pope, M.T.; Gatteschi, D. Polyoxometalates: Very large Clusters-nanoscale magnets. Chem. Rev. 1998, 98, 239–272. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Gao, R.; Lu, S.; Liu, D.; Tang, Y.; Guo, Z. Water-compatible magnetic imprinted nanoparticles served as solid-phase extraction sorbents for selective determination of trace 17beta-estradiol in environmental water samples by liquid chromatography. J. Chromatogr. A 2015, 1396, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.H.; Li, N.; Cao, M.H.; Hu, C.W. Three-dimensional Ag/POM/Cu2O tricomponent nanohybrids with enhanced visible-light photocatalytic activity. Mater. Lett. 2013, 99, 68–71. [Google Scholar] [CrossRef]
- Jing, F.F.; Liang, R.W.; Xiong, J.H.; Chen, R.; Zhang, S.Y.; Li, Y.H.; Wu, L. MIL-68(Fe) as an efficient visiblelight-driven photocatalyst for the treatment of a simulated waste-water contain Cr(VI) and Malachite Green. Appl. Catal. B 2017, 206, 9–15. [Google Scholar] [CrossRef]
- Fang, N.; Ji, Y.M.; Li, C.Y.; Wu, Y.Y.; Ma, C.G.; Liu, H.L.; Li, M.X. Synthesis and adsorption properties of [Cu(L)2(H2O)]H2[Cu(L)2(P2Mo5O3)]·4H2O/Fe3O4 nanocomposites. RSC Adv. 2017, 7, 25325–25333. [Google Scholar] [CrossRef]
- Li, C.; Guan, Z.; Ma, C.; Fang, N.; Liu, H.; Li, M. Bi-phase dispersible Fe3O4/Ag core–shell nanoparticles: Synthesis, characterization and properties. Inorg. Chem. Commun. 2017, 84, 246–250. [Google Scholar] [CrossRef]
- Thomas, J.; Ramanan, A. Phosphomolybdate cluster based solids mediated by transition metal complexes. Inorg. Chim. Acta 2011, 372, 243–249. [Google Scholar] [CrossRef]
- Đaković, M.; Popović, Z.; Giester, G.; Rajić-Linarić, M. Thiocyanate complexes of the group 12 metals with pyridine-2-carboxamide: Synthesis and structural characterization. Polyhedron 2008, 27, 210–222. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Pal’yanova, G.A.; Tomashevich, Y.V.; Vishnyakova, E.A.; Vorobyev, S.A.; Kokh, K.A. XPS and Ag L3-edge XANES characterization of silver and silver–gold sulfoselenides. J. Phys. Chem. Solids 2018, 116, 292–298. [Google Scholar] [CrossRef]
- Zilm, M.E.; Staruch, M.; Jain, M.; Wei, M. An intrinsically magnetic biomaterial with tunable magnetic properties. J. Mater. Chem. B 2014, 2, 7176–7185. [Google Scholar] [CrossRef]
- Liu, H.L.; Wu, J.H.; Min, J.H.; Lee, J.H.; Kim, Y.K. Monosized core–shell Fe3O4 (Fe)/Au multifunctional nanocrystals. J. Nanosci. Nanotechnol. 2009, 9, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, B.; Gong, X.; Yang, Z.; Liu, Y. Selective reduction of nitrate to nitrogen gas by novel Cu2O-CuO@FeO composite combined with HCOOH under UV radiation. Chem. Eng. J. 2019, 359, 1195–1204. [Google Scholar] [CrossRef]
- Xu, J.L.; Tao, S.C.; Bao, L.Z.; Luo, J.M.; Zheng, Y.F. Effects of Mo contents on the microstructure, properties and cytocompatibility of the microwave sintered porous Ti-Mo alloys. Mater. Sci. Eng. C 2019, 97, 156–165. [Google Scholar] [CrossRef]
- Wang, X.H.; Liu, H.L.; Zhang, W.X.; Cheng, W.Z.; Liu, X.; Li, X.M.; Wu, J.H. Synthesis and characterization of polymer-coated AgZnO nanoparticles with enhanced photocatalytic activity. RSC Adv. 2014, 4, 44011–44017. [Google Scholar] [CrossRef]
- Li, Z.L.; Wang, Y.; Zhang, L.C.; Wang, J.P.; You, W.S.; Zhu, Z.M. Three molybdophosphates based on Strandberg-type anions and Zn(II)-H2biim/H2O subunits: Syntheses, structures and catalytic properties. Dalton Trans. 2014, 43, 5840–5846. [Google Scholar] [CrossRef]
- D’Cruz, B.; Samuel, J.; George, L. Characterization, non-isothermal decomposition kinetics and photocatalytic water splitting of green chemically synthesized polyoxoanions of molybdenum containing phosphorus as hetero atom. Thermochim. Acta 2014, 596, 29–36. [Google Scholar] [CrossRef]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Preparation of PMMA/GO and PMMA/GO-Fe3O4 nanocomposites for malachite green dye adsorption: Kinetic and thermodynamic studies. Compos. Part. B 2019, 167, 544–555. [Google Scholar] [CrossRef]
- Wei, L.H.; Wang, Z.L.; Zhao, J.W.; Wang, J.P. Synthesis and Structure Investigation of a Transition Metal Ion Bridging Bis (diphosphopentamolybdates), [H2BIIM]3[HBIIM]4[Mn(H2O)4(P2Mo5O23)2]·7H2O. Chin. J. Struct. Chem. 2010, 29, 784–788. [Google Scholar]
- Dong, Z.; Wu, M.; Wu, J.; Ma, Y.; Ma, Z. In situ synthesis of TiO2/SnOx-Au ternary heterostructures effectively promoting visiblelight photocatalysis. Dalton Trans. 2015, 44, 11901–11910. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.L.; Jiang, Z.C.; Hu, B.; Wang, B.H. γ-MPTMS modified nanometer-sized alumina micro-column separation and preconcentration of trace amounts of Hg, Cu, Au and Pd in biological, environmental and geological samples and their determination by inductively coupled plasma mass spectrometry. J. Anal. Atom. Spectrom. 2004, 19, 984–989. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, X.; Guan, M.; Zhao, C.; Huang, H.; Wang, C. Preparation of functionalized magnetic nanoparticulate sorbents for rapid extraction of biphenolic pollutants from environmental samples. J. Sep. Sci. 2009, 32, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, E.; Yamini, Y.; Moradi, M.; Esrafili, A. Polythiophene-coated Fe3O4 superparamagnetic nanocomposite: Synthesis and application as a new sorbent for solid-phase extraction. Anal. Chim. Acta 2013, 770, 68–74. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Modak, J.B.; Bhowal, A.; Datta, S. Extraction of dye from aqueous solution in rotating packed bed. J. Hazard. Mater. 2016, 304, 337–342. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Wang, J.; Bai, R. Formic acid enhanced effective degradation of methyl orange dye in aqueous solutions under UV–Vis irradiation. Water Res. 2016, 101, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xia, Q.; Gao, Y.Y.; Cheng, Q.X.; Ding, L.; Yang, B.; Tian, Q.; Ma, C.Y.; Sand, W.; Liu, Y.B. Anaerobic biodegradation and decolorization of a refractory acid dye by a forward osmosis membrane bioreactor. Environ. Sci. Water Res. Technol. 2018, 4, 272–280. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Xia, Q.; Lou, M.; Yang, B.; Tian, Q.; Liu, Y. Direct contact membrane distillation for the treatment of industrial dyeing wastewater and characteristic pollutants. Sep. Purif. Technol. 2018, 195, 83–91. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, S.; Li, C.; Tian, H.; Ma, C.; Liu, H.; Luo, J.; Li, M. Synthesis, Characterization and Dye Removal Behavior of Core–Shell–Shell Fe3O4/Ag/Polyoxometalates Ternary Nanocomposites. Nanomaterials 2019, 9, 1255. https://doi.org/10.3390/nano9091255
Zhan S, Li C, Tian H, Ma C, Liu H, Luo J, Li M. Synthesis, Characterization and Dye Removal Behavior of Core–Shell–Shell Fe3O4/Ag/Polyoxometalates Ternary Nanocomposites. Nanomaterials. 2019; 9(9):1255. https://doi.org/10.3390/nano9091255
Chicago/Turabian StyleZhan, Shixia, Chunyan Li, Heyun Tian, Chenguang Ma, Hongling Liu, Jie Luo, and Mingxue Li. 2019. "Synthesis, Characterization and Dye Removal Behavior of Core–Shell–Shell Fe3O4/Ag/Polyoxometalates Ternary Nanocomposites" Nanomaterials 9, no. 9: 1255. https://doi.org/10.3390/nano9091255
APA StyleZhan, S., Li, C., Tian, H., Ma, C., Liu, H., Luo, J., & Li, M. (2019). Synthesis, Characterization and Dye Removal Behavior of Core–Shell–Shell Fe3O4/Ag/Polyoxometalates Ternary Nanocomposites. Nanomaterials, 9(9), 1255. https://doi.org/10.3390/nano9091255





