Encapsulation of Few-Layer MoS2 in the Pores of Mesoporous Carbon Hollow Spheres for Lithium-Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of MoS2/MCHS Composite
2.2. Preparation of the MoS2/MCHS/S and MCHS/S Composites
2.3. Material Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
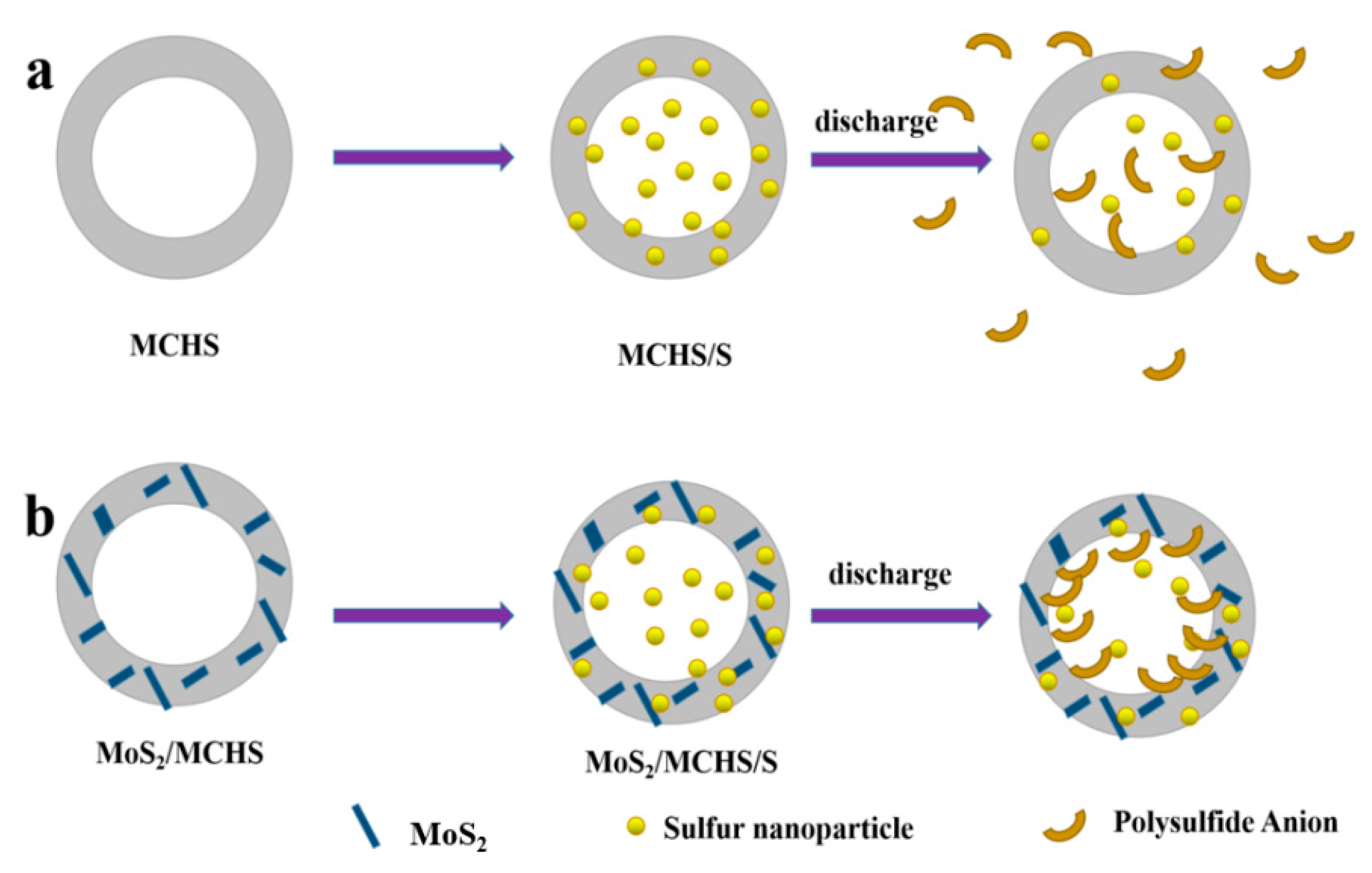
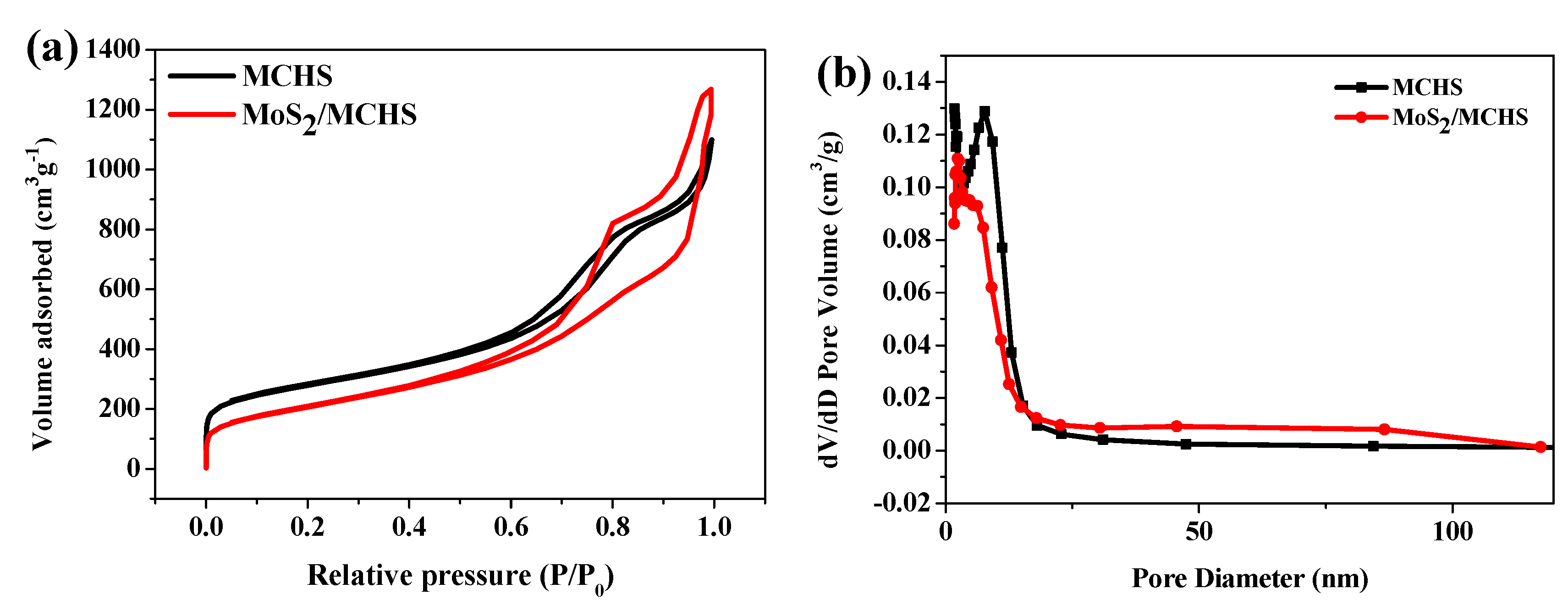
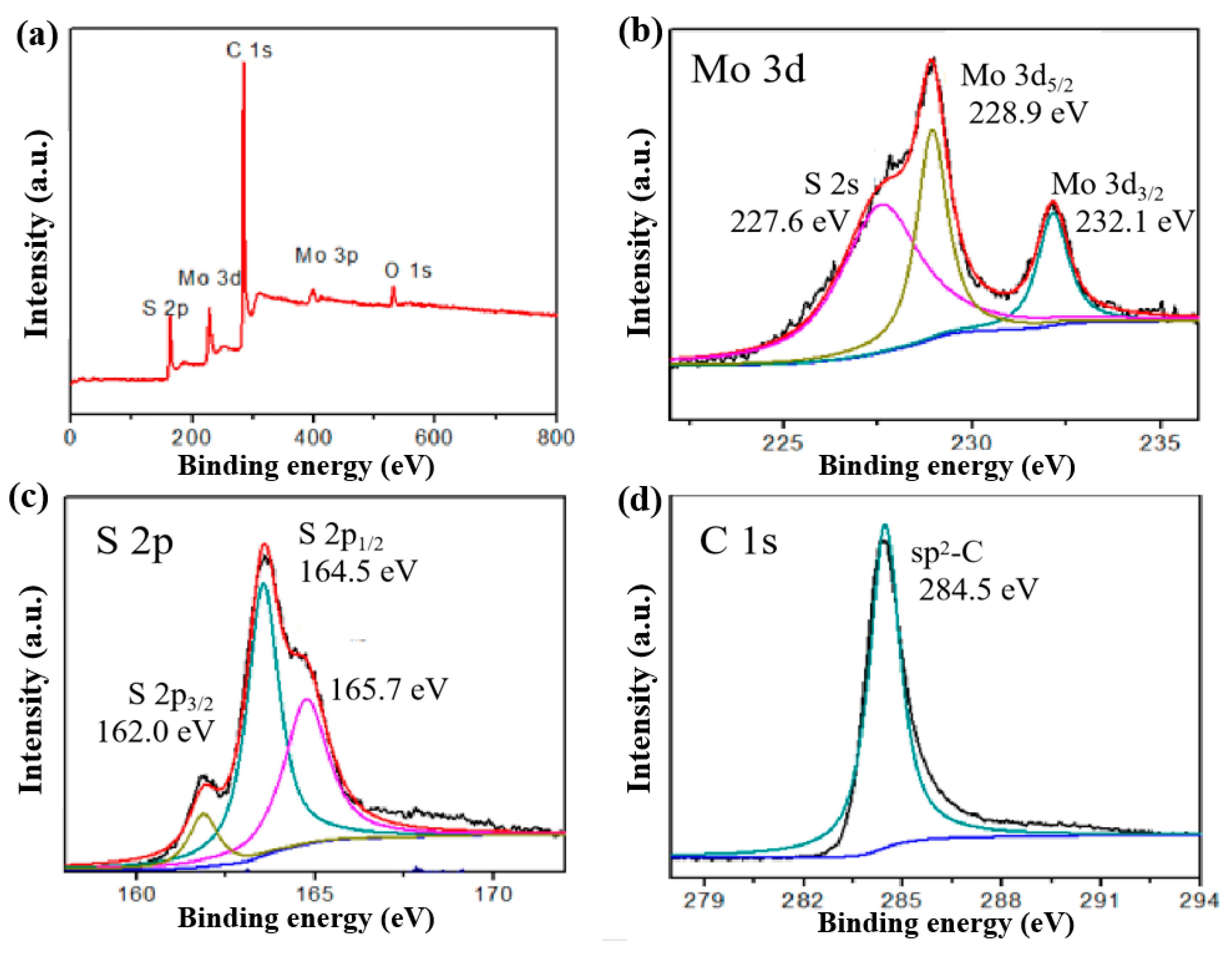
3.1. Structure and Morphology
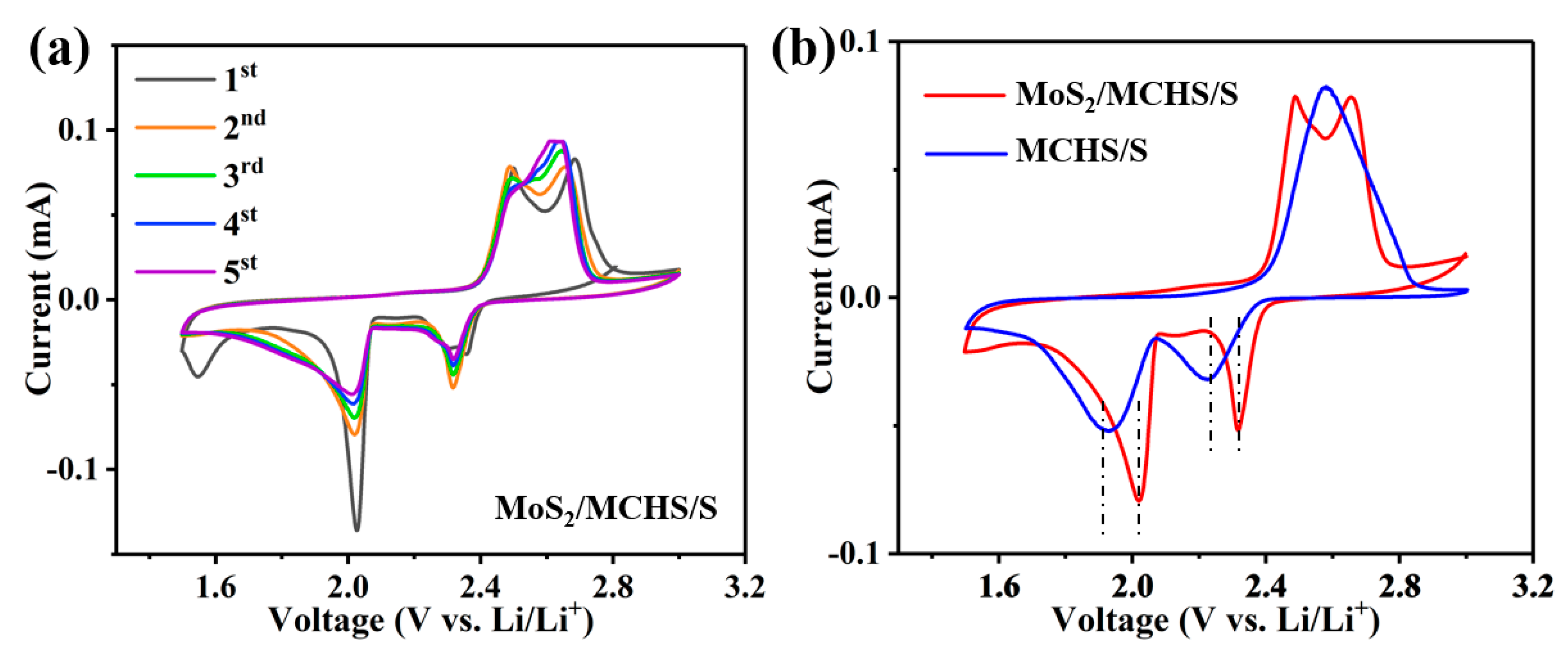
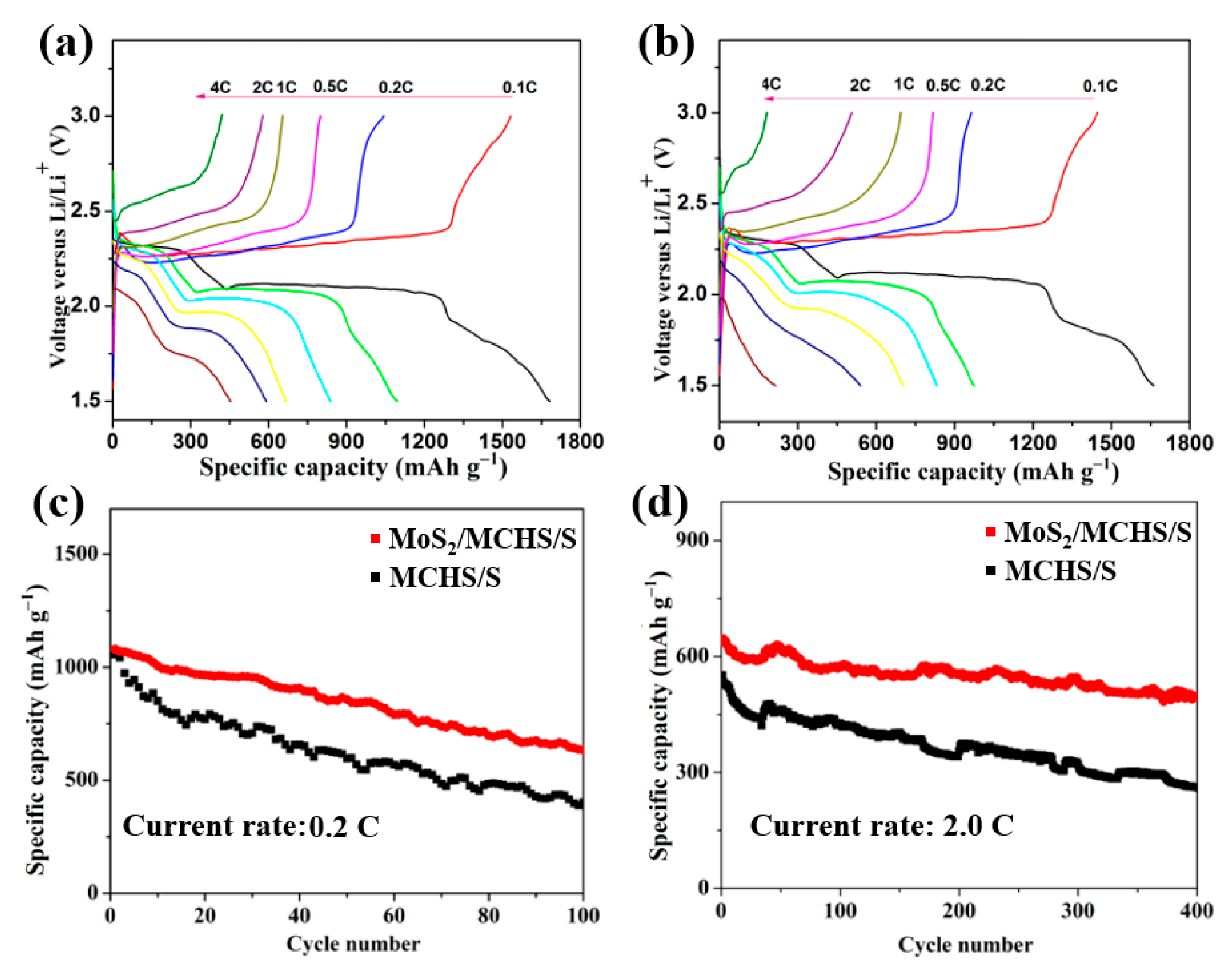
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, M.; Wang, Y.; Long, L.; Fu, X.; Sui, G.; Yang, X. An optimal carbon fiber interlayer integrated with bio-based gel polymer electrolyte enabling trapping-diffusion-conversion of polysulfides in lithium-sulfur batteries. Chem. Eng. J. 2019, 370, 1068–1076. [Google Scholar] [CrossRef]
- Wang, R.; Wang, K.; Gao, S.; Jiang, M.; Zhou, M.; Cheng, S.; Jiang, K. Rational design of yolk-shell silicon dioxide@hollow carbon spheres as advanced Li-S cathode hosts. Nanoscale 2017, 9, 14881–14887. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Oh, J.; Lee, Y.; Ryou, M. Effect of varying the ratio of carbon black to vapor-grown carbon fibers in the separator on the performance of Li–S batteries. Nanomaterials 2019, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Park, K.; Song, J.; Hong, J.; Zhou, L.; Mai, Y.W.; Huang, H.; Goodenough, J.B. Hollow carbon-nanotube/carbon-nanofiber hybrid anodes for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 16280–16283. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Tang, J.; Huang, H.; Liang, X.; Hart, C.; Tam, K.C.; Nazar, L.F. A nitrogen and sulfur dual-doped carbon derived from polyrhodanine@cellulose for advanced lithium-sulfur batteries. Adv. Mater. 2015, 27, 6021–6028. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Q.; Lian, C.; Yuan, Y.; Long, D. Promoting polythionate intermediates formation by oxygen-deficient manganese oxide hollow nanospheres for high performance lithium-sulfur batteries. Chem. Eng. J. 2019, 370, 556–564. [Google Scholar] [CrossRef]
- Fan, L.; Deng, N.; Yan, J.; Li, Z.; Kang, W.; Cheng, B. The recent research status quo and the prospect of electrolytes for lithium sulfur batteries. Chem. Eng. J. 2019, 369, 874–897. [Google Scholar] [CrossRef]
- Sun, F.; Wang, J.; Chen, H.; Qiao, W.; Ling, L.; Long, D. Bottom-Up Catalytic Approach towards Nitrogen-Enriched Mesoporous Carbons/Sulfur Composites for Superior Li-S Cathodes. Sci. Rep. 2013, 3, 2823. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Zhao, T.; Lu, J.; Wu, F.; Li, L.; Chen, J.; Tan, G.; Ye, Y.; Amine, K. Graphene-based three-dimensional hierarchical sandwich-type architecture for high-performance Li/S batteries. Nano Lett. 2013, 13, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Xie, K.; Xiong, S.; Hong, X. Analysis of Polysulfide Dissolved in Electrolyte in Discharge-Charge Process of Li-S Battery. J. Electrochem. Soc. 2012, 159, A421–A425. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Z.; Zhang, X.; Ren, G.; Wang, X.; Lai, Y.; Liu, Y.; Li, J. Synthesis of hierarchical porous honeycomb carbon for lithium-sulfur battery cathode with high rate capability and long cycling stability. Electrochim. Acta 2014, 137, 439–446. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, W.; Li, J.; Xu, Z.; Guan, L. Encapsulating MWNTs into hollow porous carbon nanotubes: A tube-in-tube carbon nanostructure for high-performance lithium-sulfur batteries. Adv. Mater. 2014, 26, 5113–5118. [Google Scholar] [CrossRef] [PubMed]
- Hwa, Y.; Seo, H.K.; Yuk, J.M.; Cairns, E.J. Freeze-Dried Sulfur-Graphene Oxide-Carbon Nanotube Nanocomposite for High Sulfur-Loading Lithium/Sulfur Cells. Nano Lett. 2017, 17, 7086–7094. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Carter, R.; Douglas, A.; Oakes, L.; Pint, C.L. Sulfur Vapor-Infiltrated 3D Carbon Nanotube Foam for Binder-Free High Areal Capacity Lithium-Sulfur Battery Composite Cathodes. ACS Nano 2017, 11, 4877–4884. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-B.; Huang, J.-Q.; Zhang, Q.; Peng, H.-J.; Zhao, M.-Q.; Wei, F. Aligned carbon nanotube/sulfur composite cathodes with high sulfur content for lithium–sulfur batteries. Nano Energy 2014, 4, 65–72. [Google Scholar] [CrossRef]
- Liang, X.; Wen, Z.; Liu, Y.; Zhang, H.; Jin, J.; Wu, M.; Wu, X. A composite of sulfur and polypyrrole–multi walled carbon combinatorial nanotube as cathode for Li/S battery. J. Power Sources 2012, 206, 409–413. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, P.; Yan, C.; Dong, X.; Zhang, X. Recent progress in polymer materials for advanced lithium-sulfur batteries. Prog. Polym. Sci. 2019, 90, 118–163. [Google Scholar] [CrossRef]
- Wang, M.; Xia, X.; Zhong, Y.; Wu, J.; Xu, R.; Yao, Z.; Wang, D.; Tang, W.; Wang, X.; Tu, J. Porous Carbon Hosts for Lithium-Sulfur Batteries. Chemistry 2019, 25, 3710–3725. [Google Scholar] [CrossRef]
- Fang, M.; Chen, Z.; Liu, Y.; Quan, J.; Yang, C.; Zhu, L.; Xu, Q.; Xu, Q. Design and synthesis of novel sandwich-type C@TiO2@C hollow microspheres as efficient sulfur hosts for advanced lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 1630–1638. [Google Scholar] [CrossRef]
- Xiao, D.; Li, Q.; Zhang, H.; Ma, Y.; Lu, C.; Chen, C.; Liu, Y.; Yuan, S. A sulfur host based on cobalt–graphitic carbon nanocages for high performance lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 24901–24908. [Google Scholar] [CrossRef]
- Pang, Q.; Nazar, L.F. Long-Life and High-Areal-Capacity Li-S Batteries Enabled by a Light-Weight Polar Host with Intrinsic Polysulfide Adsorption. ACS Nano 2016, 10, 4111–4118. [Google Scholar] [CrossRef]
- Cao, K.; Liu, H.; Li, Y.; Wang, Y.; Jiao, L. Encapsulating sulfur in δ-MnO2 at room temperature for Li-S battery cathode. Energy Storage Mater. 2017, 9, 78–84. [Google Scholar] [CrossRef]
- He, J.; Luo, L.; Chen, Y.; Manthiram, A. Yolk-Shelled C@Fe3O4 Nanoboxes as Efficient Sulfur Hosts for High-Performance Lithium-Sulfur Batteries. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chi, Z.X.; Mao, W.X.; Lv, R.W.; Cao, A.M.; Wan, L.J. One-nanometer-precision control of Al2O3 nanoshells through a solution-based synthesis route. Angew. Chem. Int. Ed. Engl. 2014, 53, 12776–12780. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and prospects of lithium–sulfur batteries. Acc. Chem. Res. 2012, 46, 1125–1134. [Google Scholar] [PubMed]
- Douglas, A.; Carter, R.; Oakes, L.; Share, K.; Cohn, A.P.; Pint, C.L. Ultrafine iron pyrite (FeS2) nanocrystals improve sodium–sulfur and lithium–sulfur conversion reactions for efficient batteries. ACS Nano 2015, 9, 11156–11165. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ma, L.; Cheng, B.; Chen, R.; Hu, Y.; Zhu, G.; Wang, Y.; Liang, J.; Tie, Z.; Liu, J.; et al. Metallic and polar Co9S8 inlaid carbon hollow nanopolyhedra as efficient polysulfide mediator for lithium−sulfur batteries. Nano Energy 2017, 38, 239–248. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, L.; He, X.; Zeinu, K.; Huang, L.; Zhu, X.; Hou, H.; Liu, B.; Hu, J.; Yang, J. Separator modified with N,S co-doped mesoporous carbon using egg shell as template for high performance lithium-sulfur batteries. Chem. Eng. J. 2017, 320, 178–188. [Google Scholar] [CrossRef]
- Wang, L.; Abraham, A.; Lutz, D.M.; Quilty, C.D.; Takeuchi, E.S.; Takeuchi, K.J.; Marschilok, A.C. Toward Environmentally Friendly Lithium Sulfur Batteries: Probing the Role of Electrode Design in MoS2-Containing Li–S Batteries with a Green Electrolyte. ACS Sustain. Chem. Eng. 2019, 7, 5209–5222. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Ge, X.; Ma, J.; Zhang, Z.; Li, Q.; Wang, C.; Yin, L. Reduced graphene oxide wrapped MOFs-derived cobalt-doped porous carbon polyhedrons as sulfur immobilizers as cathodes for high performance lithium sulfur batteries. Nano Energy 2016, 23, 15–26. [Google Scholar] [CrossRef]
- Song, J.; Xu, T.; Gordin, M.L.; Zhu, P.; Lv, D.; Jiang, Y.-B.; Chen, Y.; Duan, Y.; Wang, D. Nitrogen-Doped Mesoporous Carbon Promoted Chemical Adsorption of Sulfur and Fabrication of High-Areal-Capacity Sulfur Cathode with Exceptional Cycling Stability for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 1243–1250. [Google Scholar] [CrossRef]
- Tan, S.; Yang, Z.; Yuan, H.; Zhang, J.; Yang, Y.; Liu, H. MnO2-decorated graphene aerogel with dual-polymer interpenetrating network as an efficient hybrid host for Li-S batteries. J. Alloys Compd. 2019, 791, 483–489. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Zeng, H.; Xue, Y.; Chen, F.; Xue, Z.; Ye, Y.; Xie, X. Fast electrochemical kinetics and strong polysulfide adsorption by a highly oriented MoS2 nanosheet@N-doped carbon interlayer for lithium—Sulfur batteries. J. Mater. Chem. A 2019, 7, 7897–7906. [Google Scholar] [CrossRef]
- Zhang, H.; Noonan, O.; Huang, X.; Yang, Y.; Xu, C.; Zhou, L.; Yu, C. Surfactant-Free Assembly of Mesoporous Carbon Hollow Spheres with Large Tunable Pore Sizes. ACS Nano 2016, 10, 4579–4586. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Zhang, X.; You, T. Defect- and S-rich ultrathin MoS2 nanosheet embedded N-doped carbon nanofibers for efficient hydrogen evolution. J. Mater. Chem. A 2015, 3, 15927–15934. [Google Scholar] [CrossRef]
- Lin, H.; Yang, L.; Jiang, X.; Li, G.; Zhang, T.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium—Sulfur batteries. Energy Environ. Sci. 2017, 10, 1476–1486. [Google Scholar] [CrossRef]
- Xue, S.; Yao, S.; Jing, M.; Zhu, L.; Shen, X.; Li, T.; YiLiu, Z. Three-dimension ivy-structured MoS2 nanoflakes-embedded nitrogen doped carbon nanofibers composite membrane as free-standing electrodes for Li/polysulfides batteries. Electrochim. Acta 2019, 299, 549–559. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Jiang, Z.-H.; Lu, B.-R.; Liu, J.-T.; Cao, F.-H.; Li, H.; Yu, Z.-L.; Yu, S.-H. MoS2 nanoplates assembled on electrospun polyacrylonitrile-metal organic framework-derived carbon fibers for lithium storage. Nano Energy 2019, 61, 104–110. [Google Scholar] [CrossRef]
- Wu, J.; You, N.; Li, X.; Zeng, H.; Li, S.; Xue, Z.; Ye, Y.; Xie, X. SiO2@MoS2 core–shell nanocomposite layers with high lithium ion diffusion as a triple polysulfide shield for high performance lithium—Sulfur batteries. J. Mater. Chem. A 2019, 7, 7644–7653. [Google Scholar] [CrossRef]
- Walle, M.D.; Zeng, K.; Zhang, M.; Li, Y.; Liu, Y.-N. Flower-like molybdenum disulfide/carbon nanotubes composites for high sulfur utilization and high-performance lithium—Sulfur battery cathodes. Appl. Surf. Sci. 2019, 473, 540–547. [Google Scholar] [CrossRef]
- Yan, Y.; Shi, M.; Zou, Y.; Wei, Y.; Chen, L.; Fan, C.; Yang, R.; Xu, Y. Tunable hierarchical porous carbon aerogel/graphene composites cathode matrix for Li-S batteries. J. Alloys Compd. 2019, 791, 952–961. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhuang, Q.; Li, W.; Peng, H.; Li, G.; Zhang, Z. Encapsulation of Few-Layer MoS2 in the Pores of Mesoporous Carbon Hollow Spheres for Lithium-Sulfur Batteries. Nanomaterials 2019, 9, 1247. https://doi.org/10.3390/nano9091247
Zhao Y, Zhuang Q, Li W, Peng H, Li G, Zhang Z. Encapsulation of Few-Layer MoS2 in the Pores of Mesoporous Carbon Hollow Spheres for Lithium-Sulfur Batteries. Nanomaterials. 2019; 9(9):1247. https://doi.org/10.3390/nano9091247
Chicago/Turabian StyleZhao, Yunyan, Qianyu Zhuang, Wenda Li, Hongrui Peng, Guicun Li, and Zhonghua Zhang. 2019. "Encapsulation of Few-Layer MoS2 in the Pores of Mesoporous Carbon Hollow Spheres for Lithium-Sulfur Batteries" Nanomaterials 9, no. 9: 1247. https://doi.org/10.3390/nano9091247




