Biocompatibility of Cyclopropylamine-Based Plasma Polymers Deposited at Sub-Atmospheric Pressure on Poly (ε-caprolactone) Nanofiber Meshes
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of NFs and Plasma Polymer Deposition
2.2. XPS Analysis
2.3. SEM Imaging
2.4. Cell Culturing
2.5. Live/dead Assay by Fluorescence Imaging
2.6. MTT Assay
2.7. Examination of Cell Morphology by SEM
3. Results and Discussion
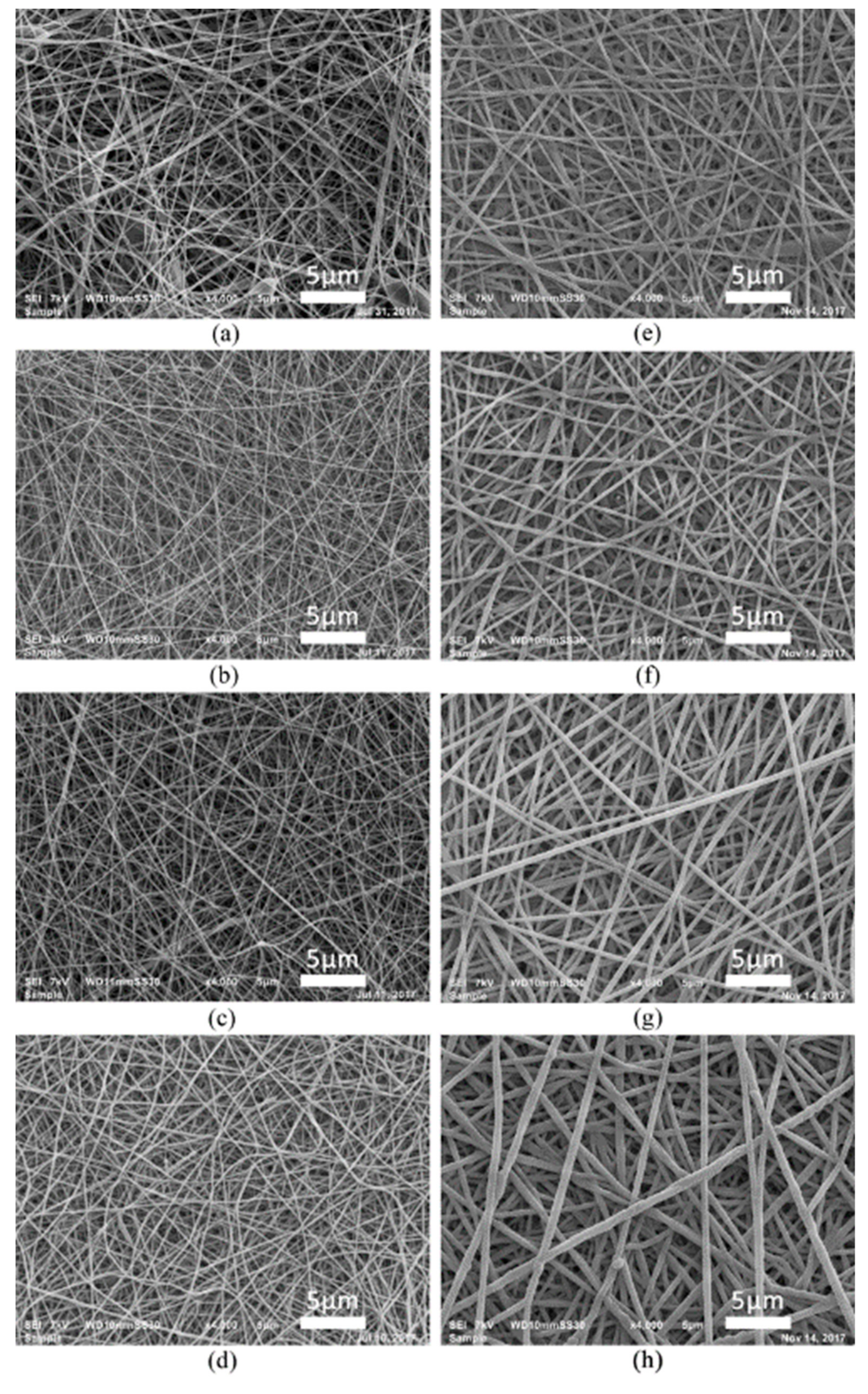
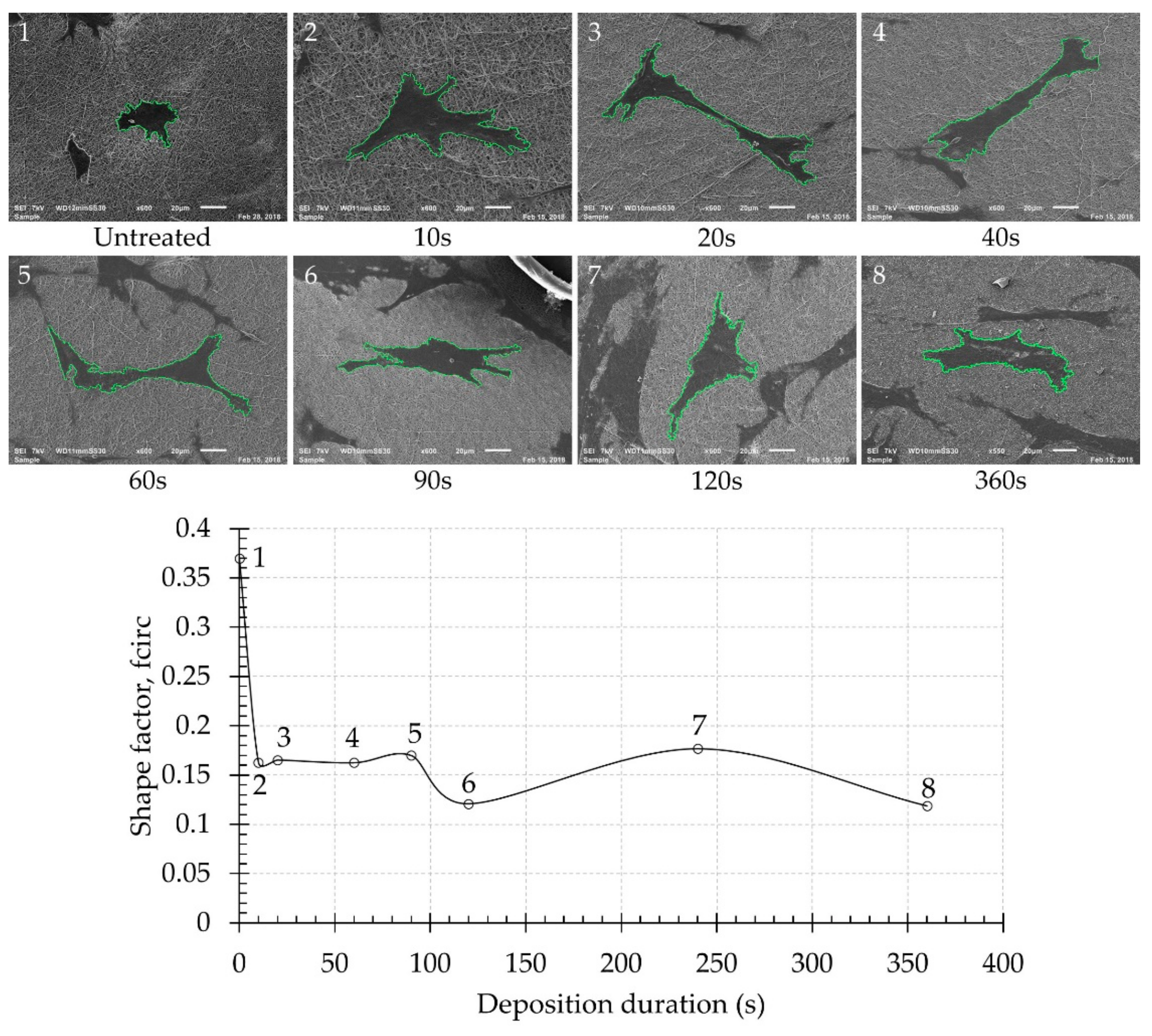
3.1. Surface Morphology
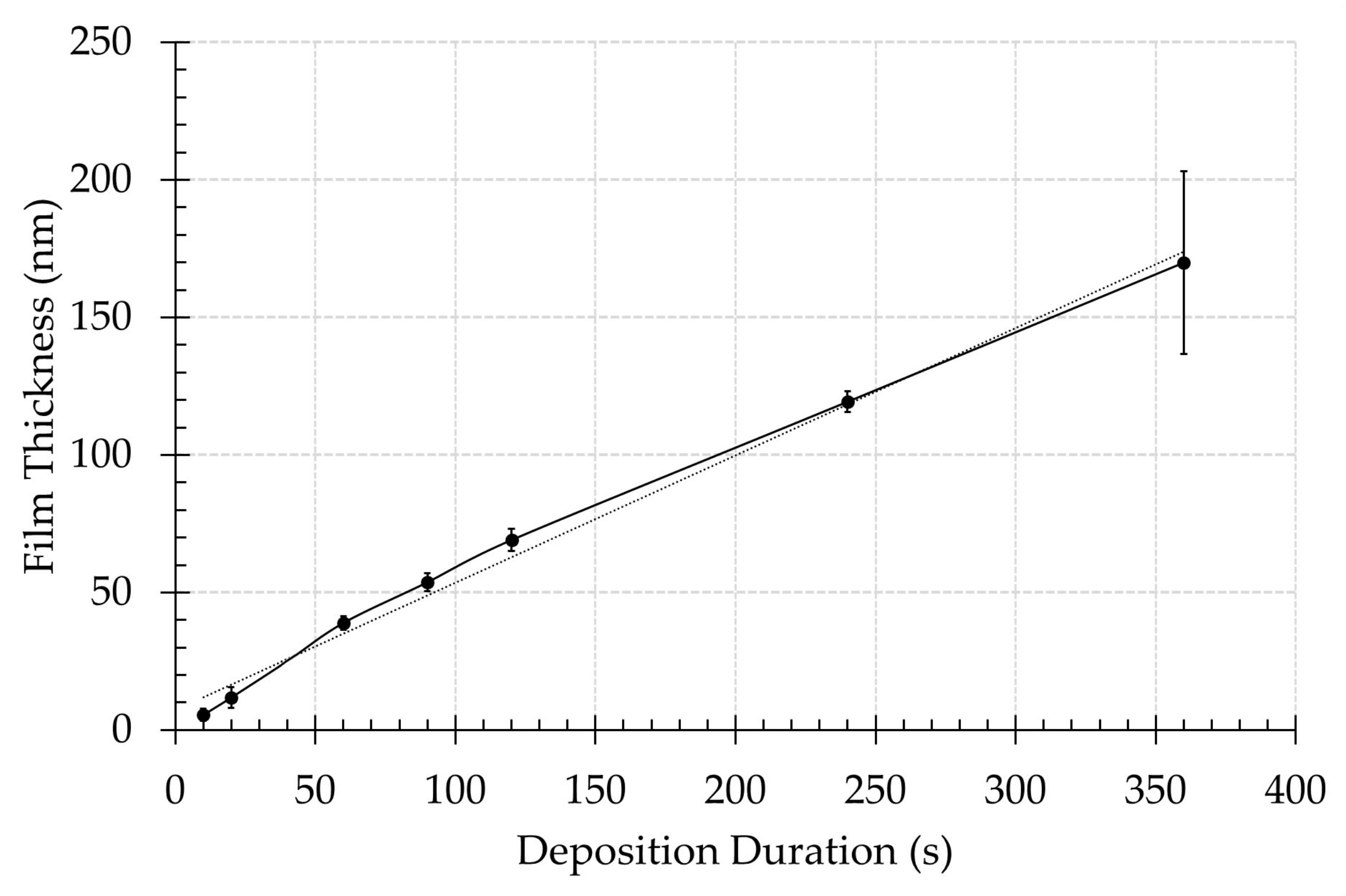
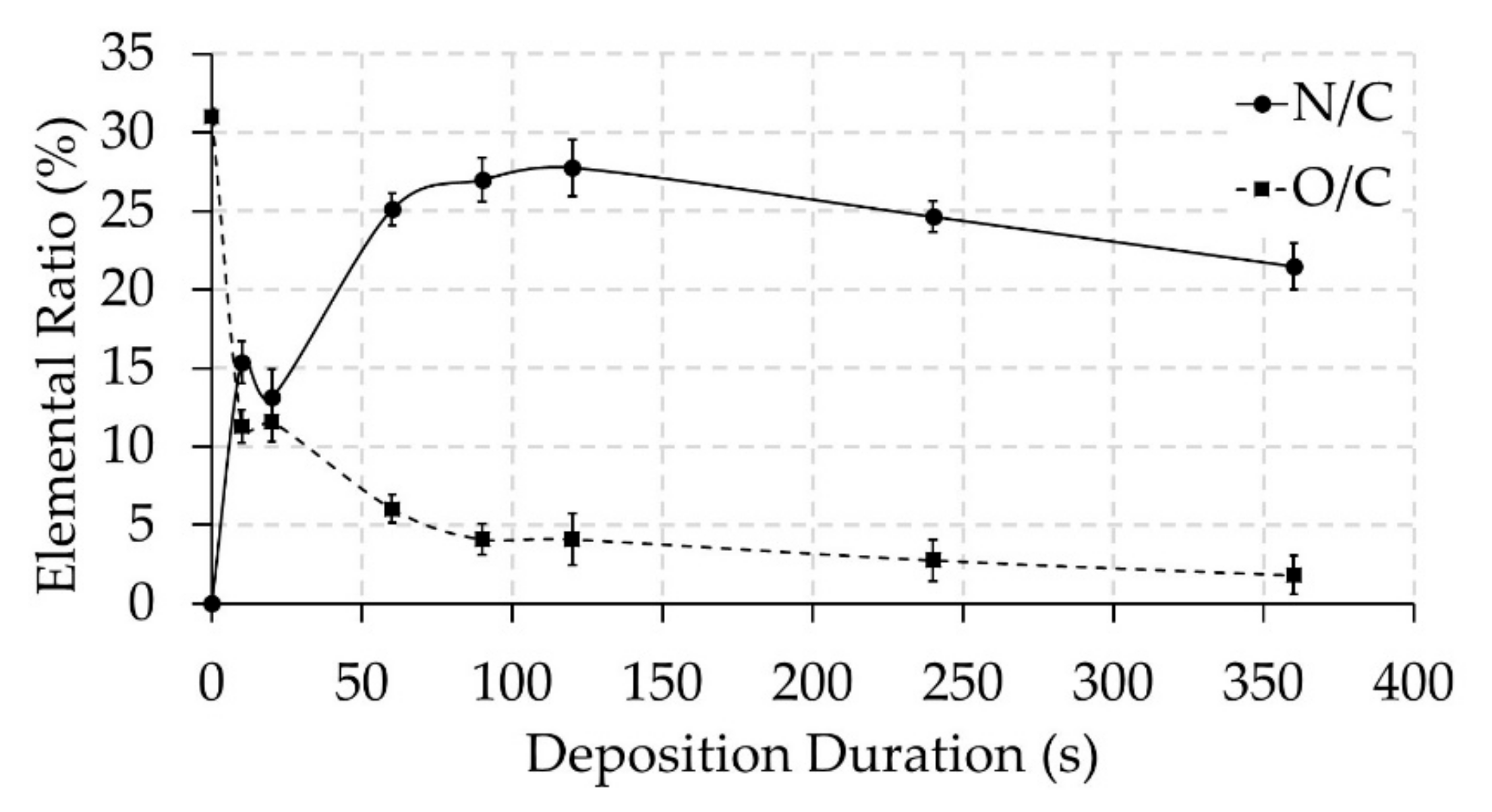
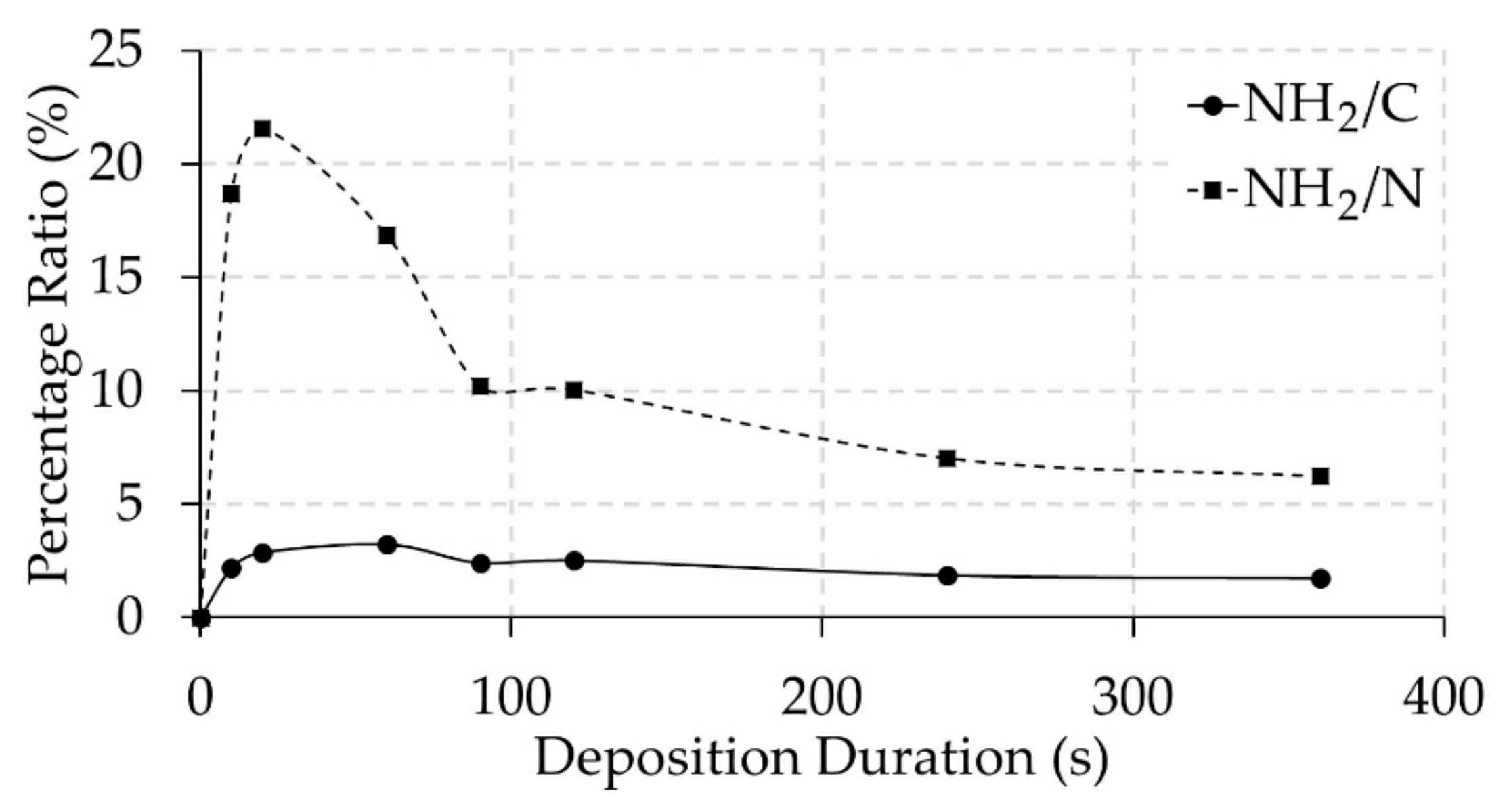
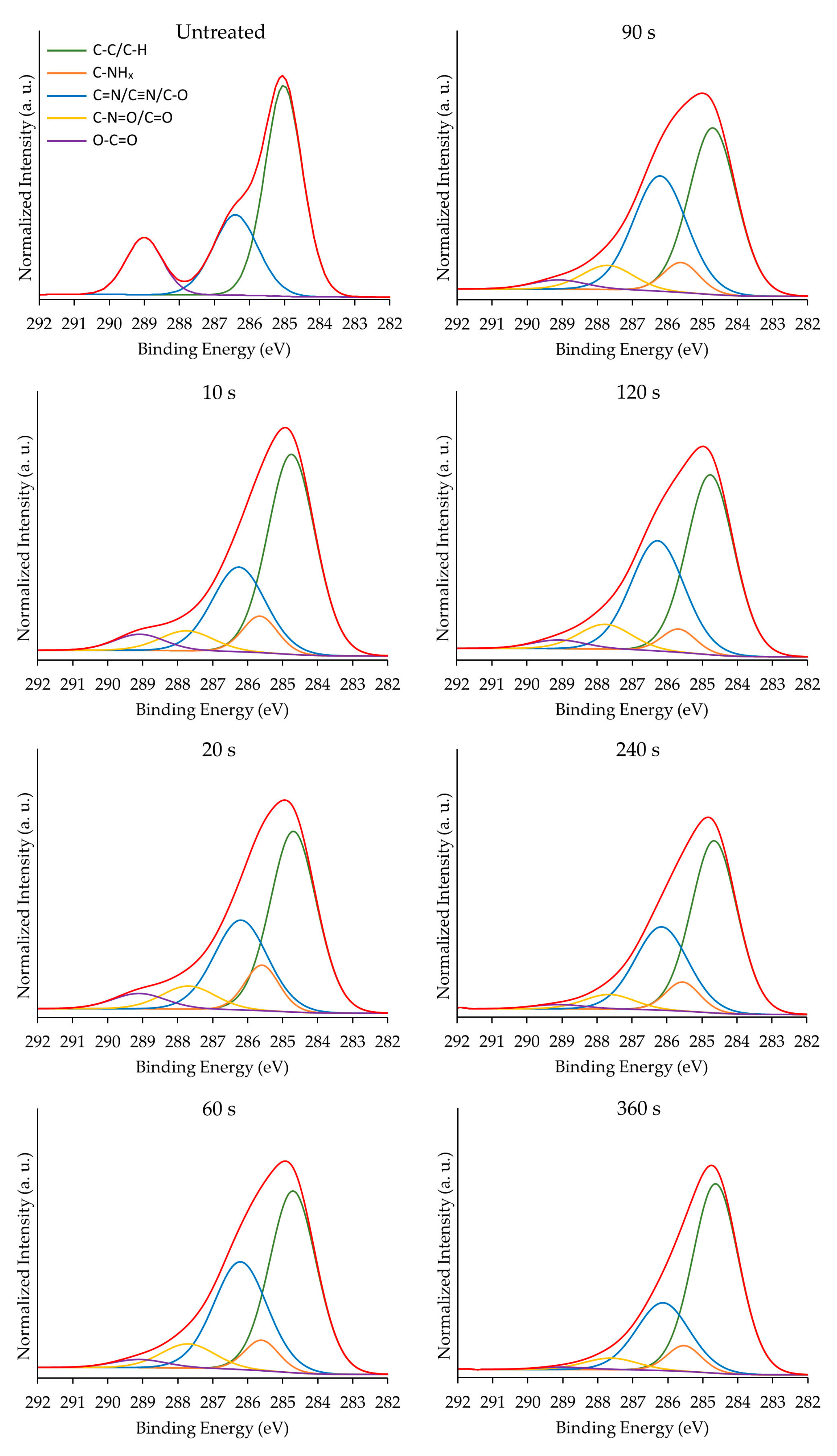
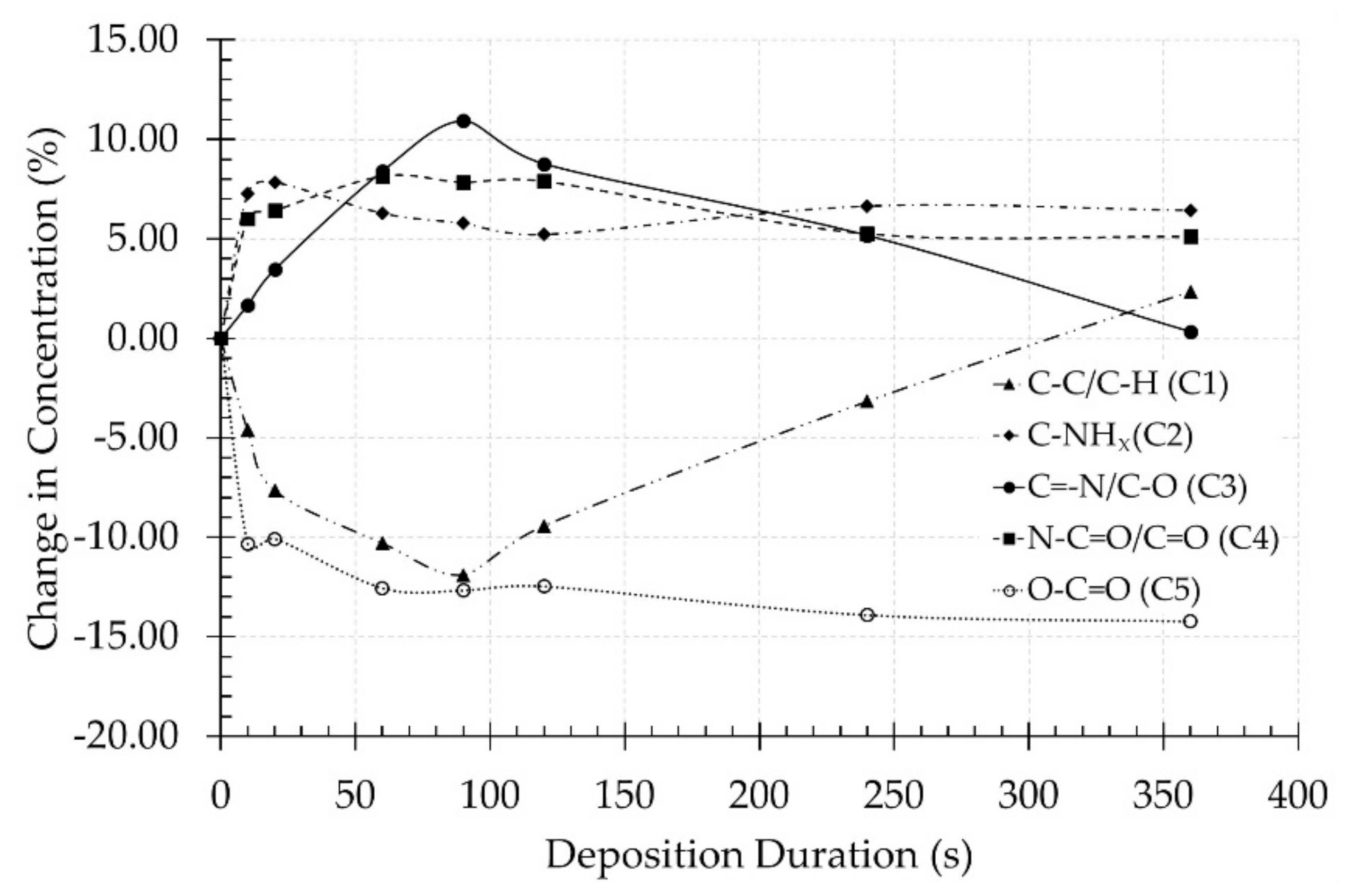
3.2. Surface Chemical Composition
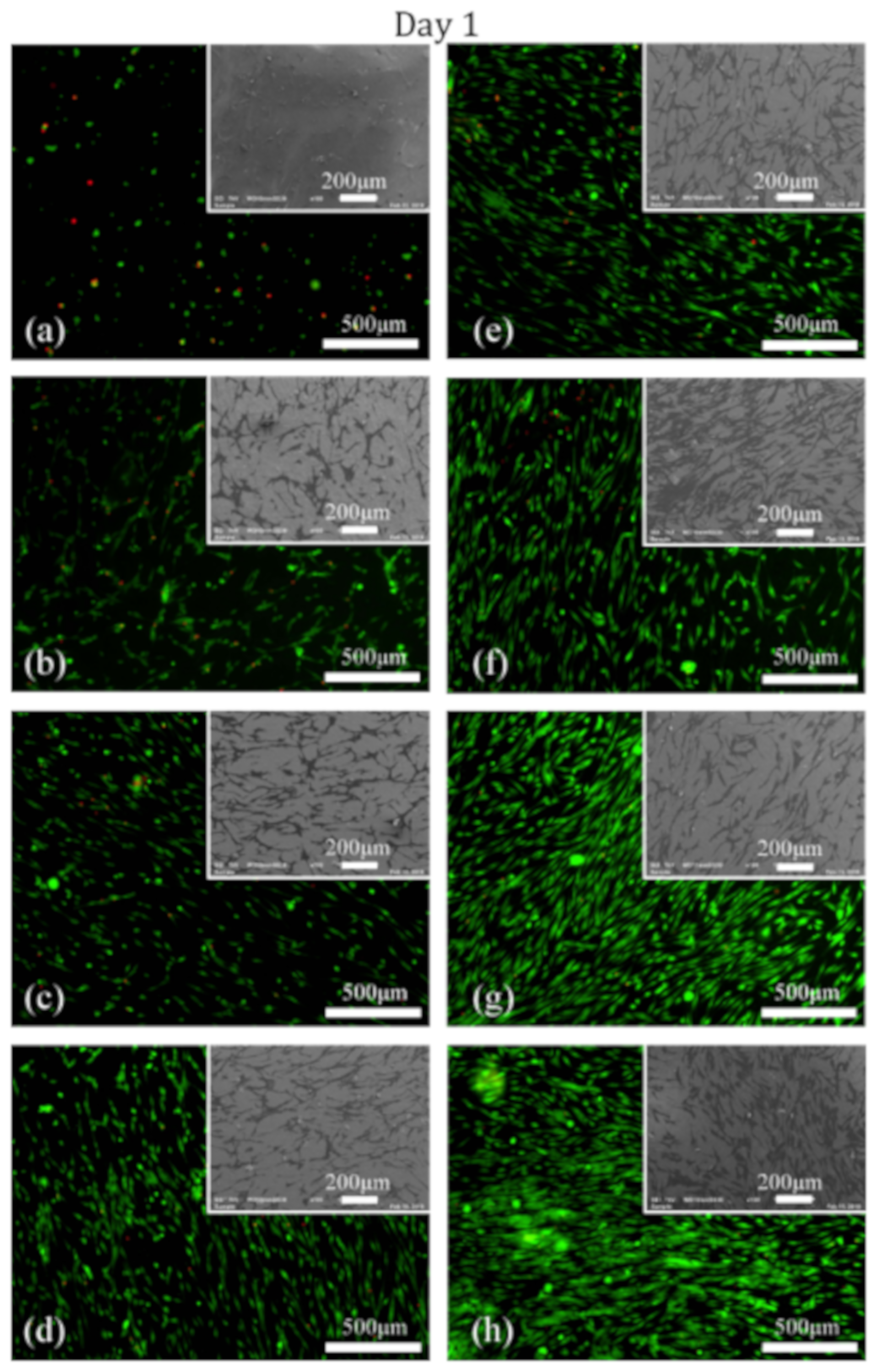
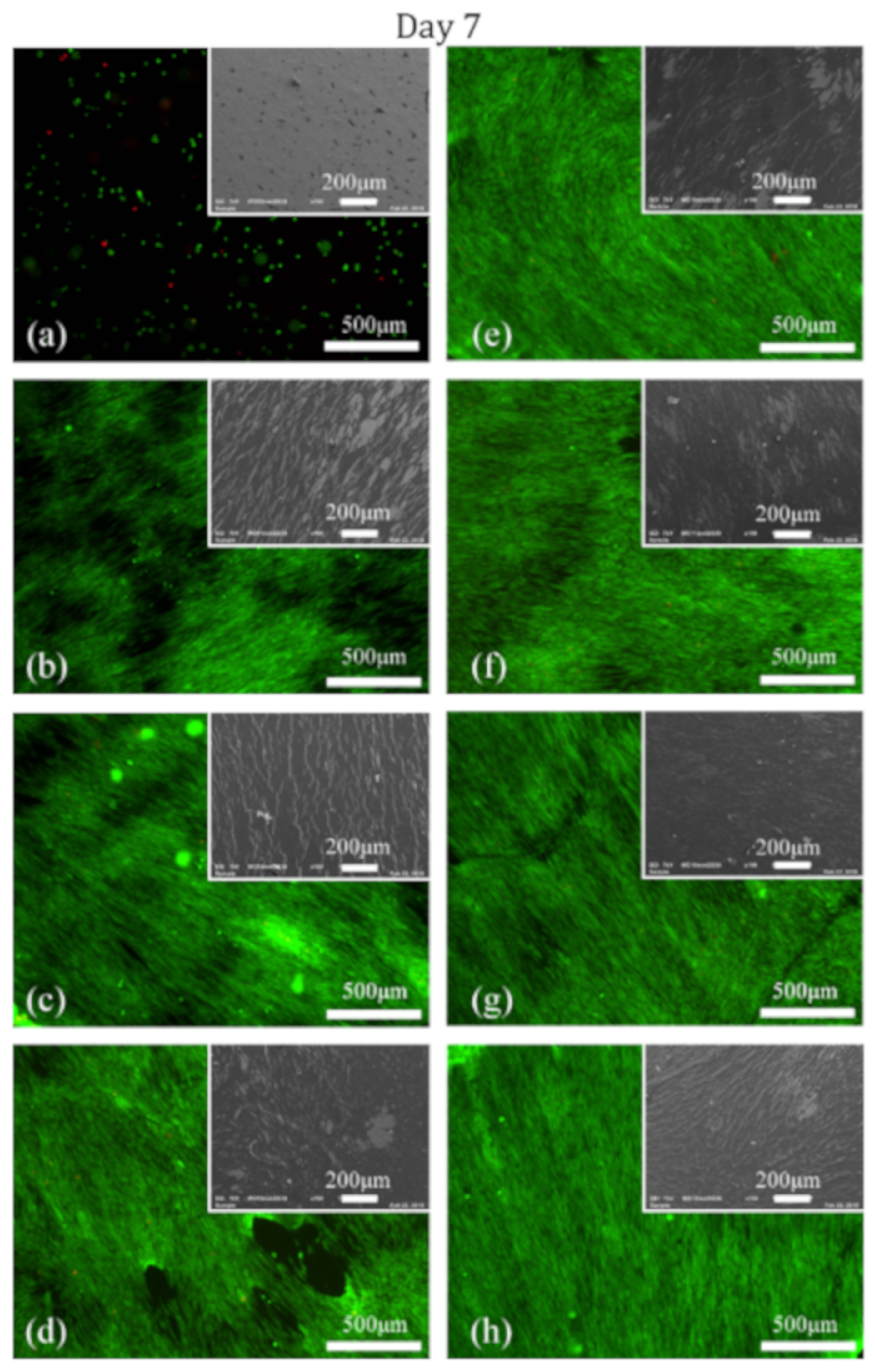
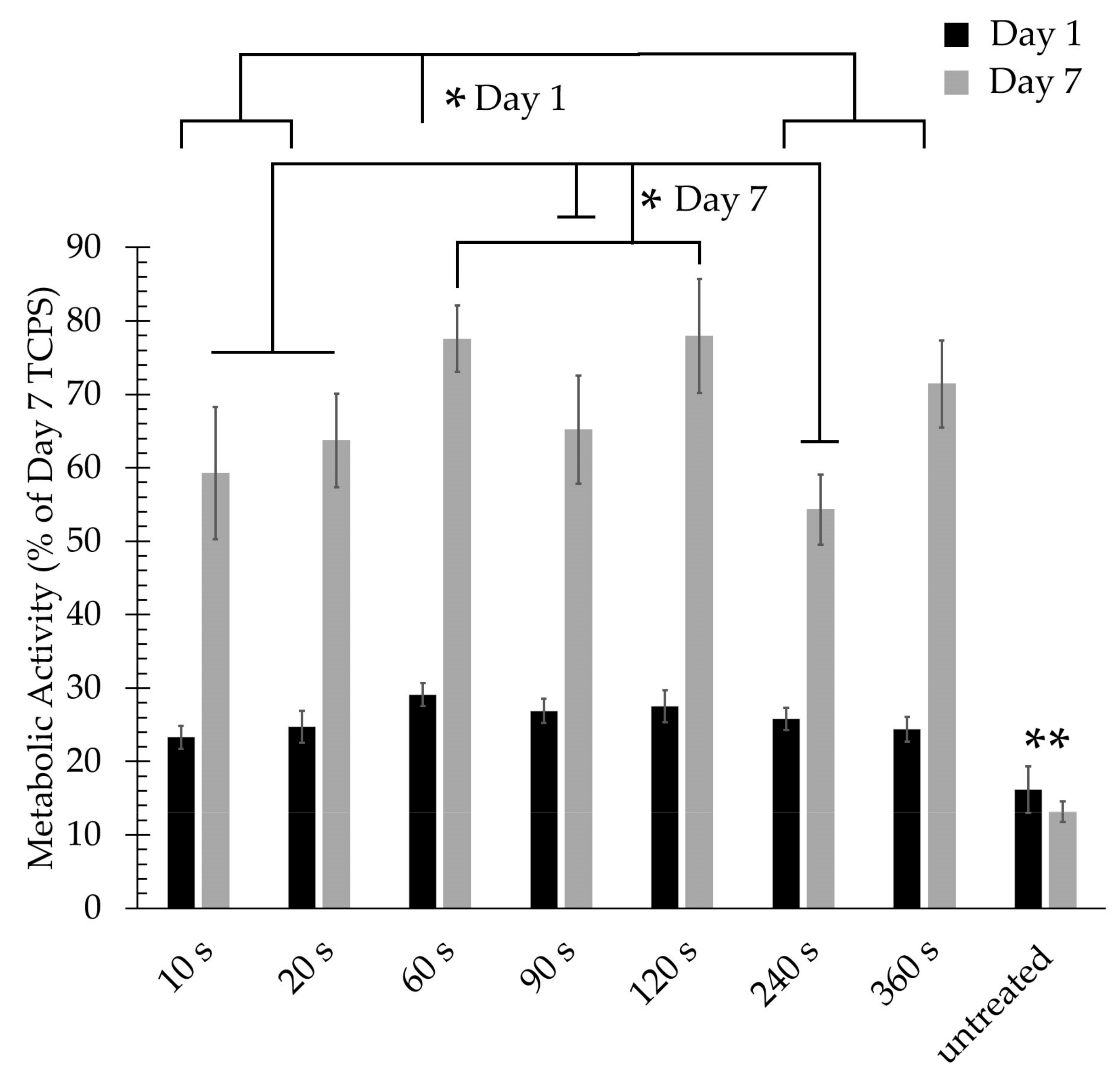
3.3. Cell Adhesion and Proliferation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ingber, D.E.; Mow, V.C.; Butler, D.; Niklason, L.; Huard, J.; Mao, J.; Yannas, I.; Kaplan, D.; Vunjak-Novakovic, G. Tissue Engineering and Developmental Biology: Going Biomimetic. Tissue Eng. 2006, 12, 3265–3283. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Martino, M.M.; Hubbell, J.A. Biomimetic materials in tissue engineering. Mater. Today 2010, 13, 14–22. [Google Scholar] [CrossRef]
- Asadian, M.; Onyshchenko, I.; Thukkaram, M.; Saadat, P.; Tabaei, E.; Van Guyse, J.; Cools, P.; Declercq, H.; Hoogenboom, R.; Morent, R.; et al. Effects of a dielectric barrier discharge (DBD) treatment on chitosan/polyethylene oxide nano fibers and their cellular interactions. Carbohydr. Polym. 2018, 201, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Asadian, M.; Onyshchenko, I.; Thiry, D.; Cools, P.; Declercq, H.; Snyders, R.; Morent, R.; Geyter, N. De Applied Surface Science Thiolation of polycaprolactone (PCL) nanofibers by inductively coupled plasma (ICP) polymerization: Physical, chemical and biological properties. Appl. Surf. Sci. 2019, 479, 942–952. [Google Scholar] [CrossRef]
- Asadian, M.; Dhaenens, M.; Onyshchenko, I.; De Waele, S.; Declercq, H.; Cools, P.; Devreese, B.; Deforce, D.; Morent, R.; De Geyter, N. Plasma Functionalization of Polycaprolactone Nanofibers Changes Protein Interactions with Cells, Resulting in Increased Cell Viability. ACS Appl. Mater. Interfaces 2018, 10, 41962–41977. [Google Scholar] [CrossRef] [PubMed]
- Sankar, D.; Shalumon, K.; Chennazhi, K.; Menon, D.; Jayakumar, R. Surface Plasma Treatment of Poly(caprolactone) Micro, Nano, and Multiscale Fibrous Scaffolds for Enhanced Osteoconductivity. Tissue Eng. Part A 2014, 20, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.M.; Choi, D.Y.; Jung, S.C.; Kim, B.H. Characteristics of Plasma Treated Electrospun Polycaprolactone (PCL) Nanofiber Scaffold for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2015, 15, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, K.Y.; Lee, S.J.; Park, K.E.; Park, W.H. Plasma-treated poly (lactic-co-glycolic acid) nanofibers for tissue engineering. Macromol. Res. 2007, 15, 238–243. [Google Scholar] [CrossRef]
- Zandén, C.; Voinova, M.; Gold, J.; Mörsdorf, D.; Bernhardt, I.; Liu, J. Surface characterisation of oxygen plasma treated electrospun polyurethane fibres and their interaction with red blood cells. Eur. Polym. J. 2012, 48, 472–482. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Dinarvand, P.; Seyedjafari, E.; Langroudi, L.; Adegani, F.J.; Soleimani, M. Enhanced reconstruction of rat calvarial defects achieved by plasma-treated electrospun scaffolds and induced pluripotent stem cells. Cell Tissue Res. 2013, 354, 849–860. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Rangkupan, R.; Jeeratawatchai, H.; Kanokpanont, S.; Damrongsakkul, S. Influences of physical and chemical crosslinking techniques on electrospun type A and B gelatin fiber mats. Int. J. Biol. Macromol. 2010, 47, 431–438. [Google Scholar] [CrossRef]
- Baek, H.S.; Park, Y.H.; Ki, C.S.; Park, J.-C.; Rah, D.K. Enhanced chondrogenic responses of articular chondrocytes onto porous silk fibroin scaffolds treated with microwave-induced argon plasma. Surf. Coat. Technol. 2008, 202, 5794–5797. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.Y.; Peng, J.; Peng, X.F.; Turng, L.S. Electrospun aligned poly (propylene carbonate) microfibers with chitosan nanofibers as tissue engineering scaffolds. Carbohydr. Polym. 2015, 117, 941–949. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Venugopal, J.; Chan, C.K.; Ramakrishna, S. Surface modified electrospun nanofibrous scaffolds for nerve tissue engineering. Nanotechnology 2008, 19, 455102. [Google Scholar] [CrossRef]
- Shkarina, S.; Shkarin, R.; Weinhardt, V.; Melnik, E.; Vacun, G.; Kluger, P.J.; Loza, K.; Epple, M.; Ivlev, S.I.; Baumbach, T.; et al. Author Correction: 3D biodegradable scaffolds of polycaprolactone with silicate-containing hydroxyapatite microparticles for bone tissue engineering: High-resolution tomography and in vitro study. Sci. Rep. 2018, 8, 17589. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface Modification of Polycaprolactone Membrane via Aminolysis and Biomacromolecule Immobilization for Promoting Cytocompatibility of Human Endothelial Cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef]
- Chen, F.; Lee, C.N.; Teoh, S. Nanofibrous modification on ultra-thin poly (e-caprolactone) membrane via electrospinning. Mater. Sci. Eng. C 2007, 27, 325–332. [Google Scholar] [CrossRef]
- Khosravi, A.; Ghasemi-Mobarakeh, L.; Mollahosseini, H.; Ajalloueian, F.; Rad, M.M.; Norouzi, M.R.; Jokandan, M.S.; Khoddami, A.; Chronakis, I.S. Immobilization of silk fibroin on the surface of PCL nanofibrous scaffolds for tissue engineering applications. J. Appl. Polym. Sci. 2018, 135, 46684. [Google Scholar] [CrossRef]
- Sahebalzamani, M.A.; Khorasani, M.T.; Joupari, M.D. Enhancement of Fibroblasts Outgrowth onto Polycaprolactone Nanofibrous Grafted by Laminin Protein Using Carbon Dioxide Plasma Treatment. Nano Biomed. Eng. 2017, 9, 191–198. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Z.; Yan, W.; Wang, S.; Zhang, S.; Jia, J. Preparation of collagen-coated electrospun nanofibers by remote plasma treatment and their biological properties. J. Biomater. Sci. Polym. Ed. 2007, 18, 1153–1164. [Google Scholar] [CrossRef]
- Jia, J.; Duan, Y.; Yu, J.; Lu, J. Preparation and immobilization of soluble eggshell membrane protein on the electrospun nanofibers to enhance cell adhesion and growth. J. Biomed. Mater. Res. Part A 2008, 86, 364–373. [Google Scholar] [CrossRef]
- Endothelial, I.; Spreading, C. Grafting of Gelatin on Electrospun Poly (caprolactone) Nanofibers to Improve Endothelial Cell Spreading and Proliferation and to Control Cell Orientation. Tissue Eng. 2005, 11, 1149–1158. [Google Scholar]
- De Valence, S.; Tille, J.C.; Chaâbane, C.; Gurny, R.; Bochaton-Piallat, M.L.; Walpoth, B.H.; Möller, M. Plasma treatment for improving cell biocompatibility of a biodegradable polymer scaffold for vascular graft applications. Eur. J. Pharm. Biopharm. 2013, 85, 78–86. [Google Scholar] [CrossRef]
- Martins, A.; Pinho, E.D.; Faria, S.; Pashkuleva, I.; Marques, A.P.; Reis, R.L.; Neves, N.M. Surface Modification of Electrospun Polycaprolactone Nanofiber Meshes by Plasma Treatment to Enhance Biological Performance. Small 2009, 5, 1195–1206. [Google Scholar] [CrossRef]
- Cheng, Q.; Lee, B.L.P.; Komvopoulos, K.; Yan, Z.; Li, S. Plasma Surface Chemical Treatment of Electrospun Poly(l-Lactide) Microfibrous Scaffolds for Enhanced Cell Adhesion, Growth, and Infiltration. Tissue Eng. Part A 2013, 19, 1188–1198. [Google Scholar] [CrossRef]
- Yan, D.; Jones, J.; Yuan, X.Y.; Xu, X.H.; Sheng, J.; Lee, J.C.M.; Ma, G.Q.; Yu, Q.S. Plasma treatment of electrospun PCL random nanofiber meshes (NFMs) for biological property improvement. J. Biomed. Mater. Res. A 2013, 101, 963–972. [Google Scholar] [CrossRef]
- Seyedjafari, E.; Soleimani, M.; Ghaemi, N.; Sarbolouki, M.N. Enhanced osteogenic differentiation of cord blood-derived unrestricted somatic stem cells on electrospun nanofibers. J. Mater. Sci. Mater. Med. 2011, 22, 165–174. [Google Scholar] [CrossRef]
- Bormashenko, E.; Chaniel, G.; Grynyov, R. Towards understanding hydrophobic recovery of plasma treated polymers: Storing in high polarity liquids suppresses hydrophobic recovery. Appl. Surf. Sci. 2013, 273, 549–553. [Google Scholar] [CrossRef]
- Manakhov, A.; Landová, M.; Medalová, J.; Michlíček, M.; Polčák, J.; Nečas, D.; Zajíčková, L. Cyclopropylamine plasma polymers for increased cell adhesion and growth. Plasma Process. Polym. 2016, 14, 1–12. [Google Scholar] [CrossRef]
- Manakhov, A.; Nečas, D.; Čechal, J.; Pavliňák, D.; Eliáš, M.; Zajíčková, L. Deposition of stable amine coating onto polycaprolactone nanofibers by low pressure cyclopropylamine plasma polymerization. Thin Solid Films 2015, 581, 7–13. [Google Scholar] [CrossRef]
- Heath, M.D.; Henderson, B.; Perkin, S. Ion-Specific Effects on the Interaction between Fibronectin and Negatively Charged Mica Surfaces. Langmuir 2010, 26, 5304–5308. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 5953–5957. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, N.; Shi, H.; Liu, J.; Shi, L.; Zhang, B.; Wang, H.; Ji, J.; Chu, P.K. Upregulation of BMSCs Osteogenesis by Positively-Charged Tertiary Amines on Polymeric Implants via Charge/iNOS Signaling Pathway. Sci. Rep. 2015, 5, 9369. [Google Scholar] [CrossRef]
- Sagitha, P.; Reshmi, C.; Sundaran, S.P.; Sujith, A. Recent advances in post-modification strategies of polymeric electrospun membranes. Eur. Polym. J. 2018, 105, 227–249. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Chan, K.V.; Onyshchenko, I.; Nikiforov, A.; Aziz, G.; Morent, R.; De Geyter, N. Plasma polymerization of cyclopropylamine with a sub-atmospheric pressure DBD. Eur. Polym. J. 2018, 103, 1–10. [Google Scholar] [CrossRef]
- Manakhov, A.; Zajíčková, L.; Eliáš, M.; Čechal, J.; Polčák, J.; Hnilica, J.; Bittnerová, Š.; Necas, D. Optimization of Cyclopropylamine Plasma Polymerization toward Enhanced Layer Stability in Contact with Water. Plasma Process. Polym. 2014, 11, 532–544. [Google Scholar] [CrossRef]
- Noel, S.; Liberelle, B.; Robitaille, L.; De Crescenzo, G. Quantification of Primary Amine Groups Available for Subsequent Biofunctionalization of Polymer Surfaces. Bioconjugate Chem. 2011, 22, 1690–1699. [Google Scholar] [CrossRef]
- Denis, L.; Marsal, P.; Olivier, Y.; Godfroid, T.; Lazzaroni, R.; Hecq, M.; Cornil, J.; Snyders, R. Deposition of Functional Organic Thin Films by Pulsed Plasma Polymerization: A Joint Theoretical and Experimental Study. Plasma Process. Polym. 2010, 7, 172–181. [Google Scholar] [CrossRef]
- Choukourov, A.; Biederman, H.; Slavinska, D.; Trchova, M.; Hollander, A. The influence of pulse parameters on film composition during pulsed plasma polymerization of diaminocyclohexane. Surf. Coat. Technol. 2003, 174, 863–866. [Google Scholar] [CrossRef]
- De Wever, O.; Hendrix, A.; De Boeck, A.; Westbroek, W.; Braems, G.; Emami, S.; Sabbah, M.; Gespach, C.; Bracke, M. Modeling and quantification of cancer cell invasion through collagen type I matrices. Int. J. Dev. Biol. 2010, 54, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Poinern, G.E.J.; Ali, N.; Berry, C.M.; Fawcett, D. Engineering a Biocompatible Scaffold with Either Micrometre or Nanometre Scale Surface Topography for Promoting Protein Adsorption and Cellular Response. Int. J. Biomater. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 34002. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, D.; Hossain, M.M.; Körner, E.; Balazs, D.J. Macroscopic Description of Plasma Polymerization. Plasma Process. Polym. 2007, 4, 229–238. [Google Scholar] [CrossRef]
- Girard-Lauriault, P.L.; Dietrich, P.M.; Gross, T.; Wirth, T.; Unger, W.E.S. Chemical Characterization of the Long-Term Ageing of Nitrogen-Rich Plasma Polymer Films under Various Ambient Conditions. Plasma Process. Polym. 2013, 10, 388–395. [Google Scholar] [CrossRef]
- Manakhov, A.; Michlíček, M.; Felten, A.; Pireaux, J.J.; Nečas, D.; Zajíčková, L. XPS depth profiling of derivatized amine and anhydride plasma polymers: Evidence of limitations of the derivatization approach. Appl. Surf. Sci. 2017, 394, 578–585. [Google Scholar] [CrossRef]
- Losic, D.; Cole, A.M.; Dollmann, B.; Vasilev, K.; Griesser, H.J. Surface modification of nanoporous alumina membranes by plasma polymerization. Nanotechnology 2008, 19, 245704. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, Q.; Bachhuka, A.; Vasilev, K. Surface Modification by Allylamine Plasma Polymerization Promotes Osteogenic Differentiation of Human Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2014, 6, 9733–9741. [Google Scholar] [CrossRef]
- Causa, F.; Battista, E.; Della Moglie, R.; Guarnieri, D.; Iannone, M.; Netti, P.A. Surface Investigation on Biomimetic Materials to Control Cell Adhesion: The Case of RGD Conjugation on PCL. Langmuir 2010, 26, 9875–9884. [Google Scholar] [CrossRef]
- Dubreuil, M.F.S.; Bongaers, E.M.; Lens, P.F. A Incorporation of amino moieties through atmospheric pressure plasma: Relationship between precursor structure and coating properties. Surf. Coat. Technol. 2011, 206, 1439–1448. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Q.; Cai, H.; Zhang, Y. The influences of processing parameters on structure of amine-containing film and its cell culture adsorption in pulsed DBD plasma. Thin Solid Films 2009, 517, 4268–4271. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. J. Chem. Educ. 1993, 70, A25. [Google Scholar]
- Tarasova, A.; Gengenbach, T.; Griesser, H.J.; Meagher, L.; Hamilton-Brown, P.; Hamilton-Brown, P. Colloid Probe AFM and XPS Study of Time-Dependent Aging of Amine Plasma Polymer Coatings in Aqueous Media. Plasma Process. Polym. 2008, 5, 175–185. [Google Scholar] [CrossRef]
- Tidwell, C.D.; Ertel, S.I.; Ratner, B.D.; Tarasevich, B.J.; Atre, S.; Allara, D.L. Endothelial Cell Growth and Protein Adsorption on Terminally Functionalized, Self-Assembled Monolayers of Alkanethiolates on Gold. Langmuir 1997, 13, 3404–3413. [Google Scholar] [CrossRef]
- Davidson, M.R.; Mitchell, S.A.; Bradley, R.H. UV-ozone modification of plasma-polymerised acetonitrile films for enhanced cell attachment. Colloids Surf. B Biointerfaces 2004, 34, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Manakhov, A.; Kedroňová, E.; Medalová, J.; Černochová, P.; Obrusník, A.; Michlíček, M.; Shtansky, D.V.; Zajíčková, L. Carboxyl-anhydride and amine plasma coating of PCL nanofibers to improve their bioactivity. Mater. Des. 2017, 132, 257–265. [Google Scholar] [CrossRef]
| Deposition Duration (s) | Before TFBA Derivatization | Incorporated Amount of F after TFBA Derivatization | ||
|---|---|---|---|---|
| C (%) | O (%) | N (%) | F (%) | |
| 0 | 76.3 ± 0.4 | 23.7 ± 0.4 | 0 | 0 |
| 10 | 79.0 ± 1.0 | 8.9 ± 0.2 | 12.1 ± 0.9 | 4.2 ± 0.4 |
| 20 | 80.2 ± 0.9 | 9.3 ± 0.9 | 10.5 ± 1.5 | 5.3 ± 0.4 |
| 60 | 76.3 ± 0.9 | 4.6 ± 0.4 | 19.1 ± 1.1 | 5.8 ± 0.5 |
| 90 | 76.3 ± 1.5 | 3.1 ± 0.6 | 20.6 ± 1.0 | 4.5 ± 1.2 |
| 120 | 75.9 ± 0.8 | 3.1 ± 1.1 | 21.0 ± 0.6 | 4.6 ± 0.5 |
| 240 | 78.5 ± 1.1 | 2.2 ± 0.6 | 19.3 ± 1.0 | 3.6 ± 0.8 |
| 360 | 81.1 ± 1.1 | 1.5 ± 0.5 | 17.4 ± 0.9 | 3.4 ± 0.4 |
| Deposition Duration (s) | C–C/C–H [C1] 285.0 eV | C–NHx [C2] 285.9 eV | C=N/C≡N/C–O [C3] 286.5 eV | N–C=O/C=O [C4] 288.0 eV | O–C=O [C5] 289.0 eV |
|---|---|---|---|---|---|
| 0 | 58.9 ± 0.9 | - | 25.8 ± 1.0 | - | 15.3 ± 0.4 |
| 10 | 54.3 ± 1.7 | 7.3 ± 1.1 | 27.5 ± 0.9 | 6.0 ± 0.4 | 5.0 ± 0.3 |
| 20 | 51.2 ± 1.5 | 9.0 ± 1.1 | 29.3 ± 1.5 | 6.4 ± 0.7 | 5.2 ± 0.5 |
| 60 | 48.6 ± 1.6 | 6.3 ± 1.2 | 34.3 ± 0.6 | 8.2 ± 0.7 | 2.7 ± 0.2 |
| 90 | 47.0 ± 1.6 | 5.8 ± 1.3 | 36.8 ± 2.2 | 7.9 ± 1.2 | 2.6 ± 0.3 |
| 120 | 49.4 ± 1.6 | 5.2 ± 0.9 | 34.6 ± 1.8 | 7.9 ± 0.3 | 2.8 ± 0.3 |
| 240 | 55.7 ± 1.9 | 6.7 ± 1.7 | 31.0 ± 3.6 | 5.3 ± 0.5 | 1.4 ± 0.3 |
| 360 | 61.2 ± 1.8 | 6.4 ± 0.6 | 26.2 ± 2.5 | 5.1 ± 0.5 | 1.1 ± 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.V.; Asadian, M.; Onyshchenko, I.; Declercq, H.; Morent, R.; De Geyter, N. Biocompatibility of Cyclopropylamine-Based Plasma Polymers Deposited at Sub-Atmospheric Pressure on Poly (ε-caprolactone) Nanofiber Meshes. Nanomaterials 2019, 9, 1215. https://doi.org/10.3390/nano9091215
Chan KV, Asadian M, Onyshchenko I, Declercq H, Morent R, De Geyter N. Biocompatibility of Cyclopropylamine-Based Plasma Polymers Deposited at Sub-Atmospheric Pressure on Poly (ε-caprolactone) Nanofiber Meshes. Nanomaterials. 2019; 9(9):1215. https://doi.org/10.3390/nano9091215
Chicago/Turabian StyleChan, Ke Vin, Mahtab Asadian, Iuliia Onyshchenko, Heidi Declercq, Rino Morent, and Nathalie De Geyter. 2019. "Biocompatibility of Cyclopropylamine-Based Plasma Polymers Deposited at Sub-Atmospheric Pressure on Poly (ε-caprolactone) Nanofiber Meshes" Nanomaterials 9, no. 9: 1215. https://doi.org/10.3390/nano9091215
APA StyleChan, K. V., Asadian, M., Onyshchenko, I., Declercq, H., Morent, R., & De Geyter, N. (2019). Biocompatibility of Cyclopropylamine-Based Plasma Polymers Deposited at Sub-Atmospheric Pressure on Poly (ε-caprolactone) Nanofiber Meshes. Nanomaterials, 9(9), 1215. https://doi.org/10.3390/nano9091215







