Photoluminescent Hydroxylapatite: Eu3+ Doping Effect on Biological Behaviour
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
2.3. Samples Notation
2.4. Morphological and Structural Characterization
2.5. Cellular Viability Assays
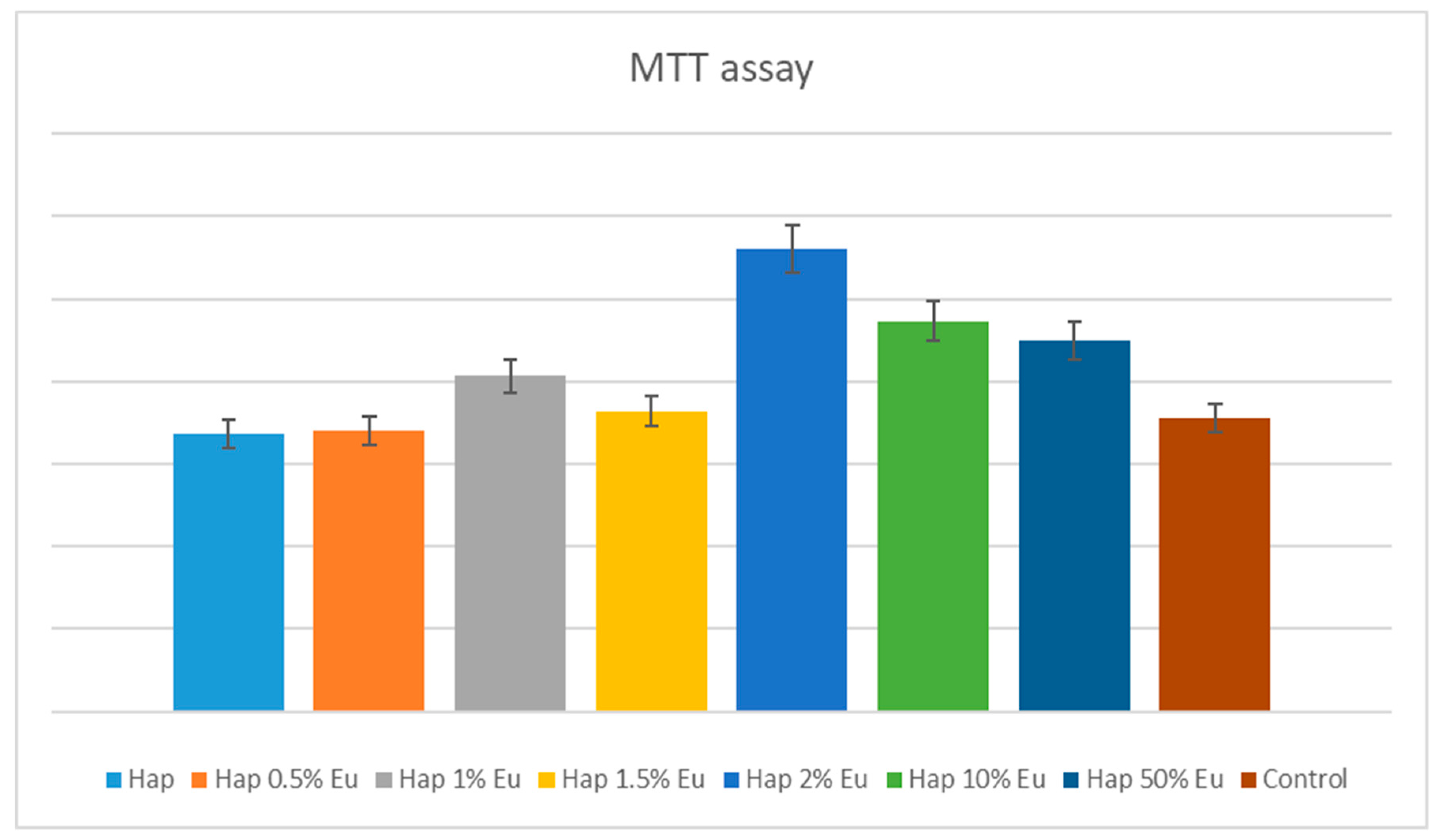
2.5.1. Quantitative In-Vitro Evaluation of Biocompatibility—MTT Assay
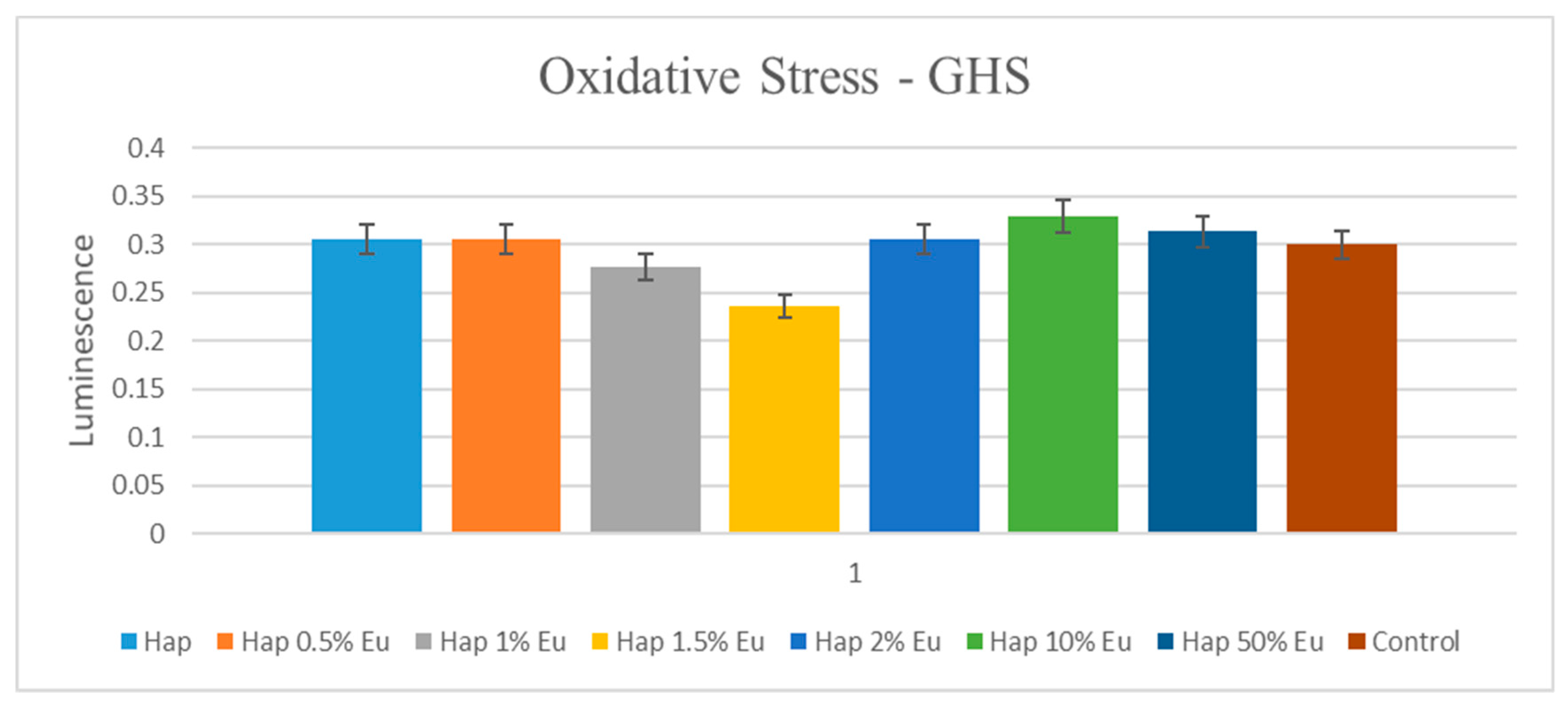
2.5.2. Oxidative Stress Assessment—GSH-Glo Glutathione Assay
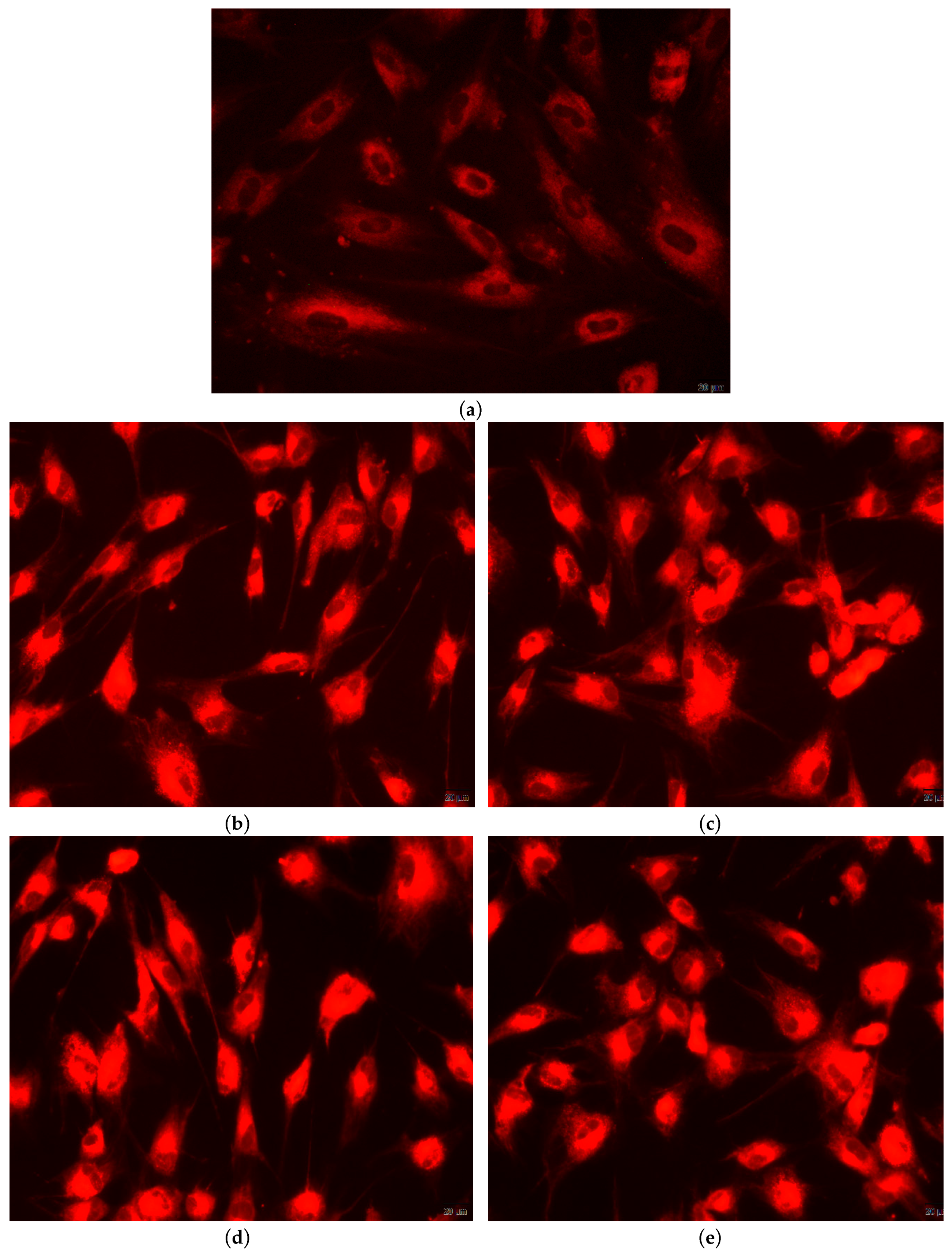
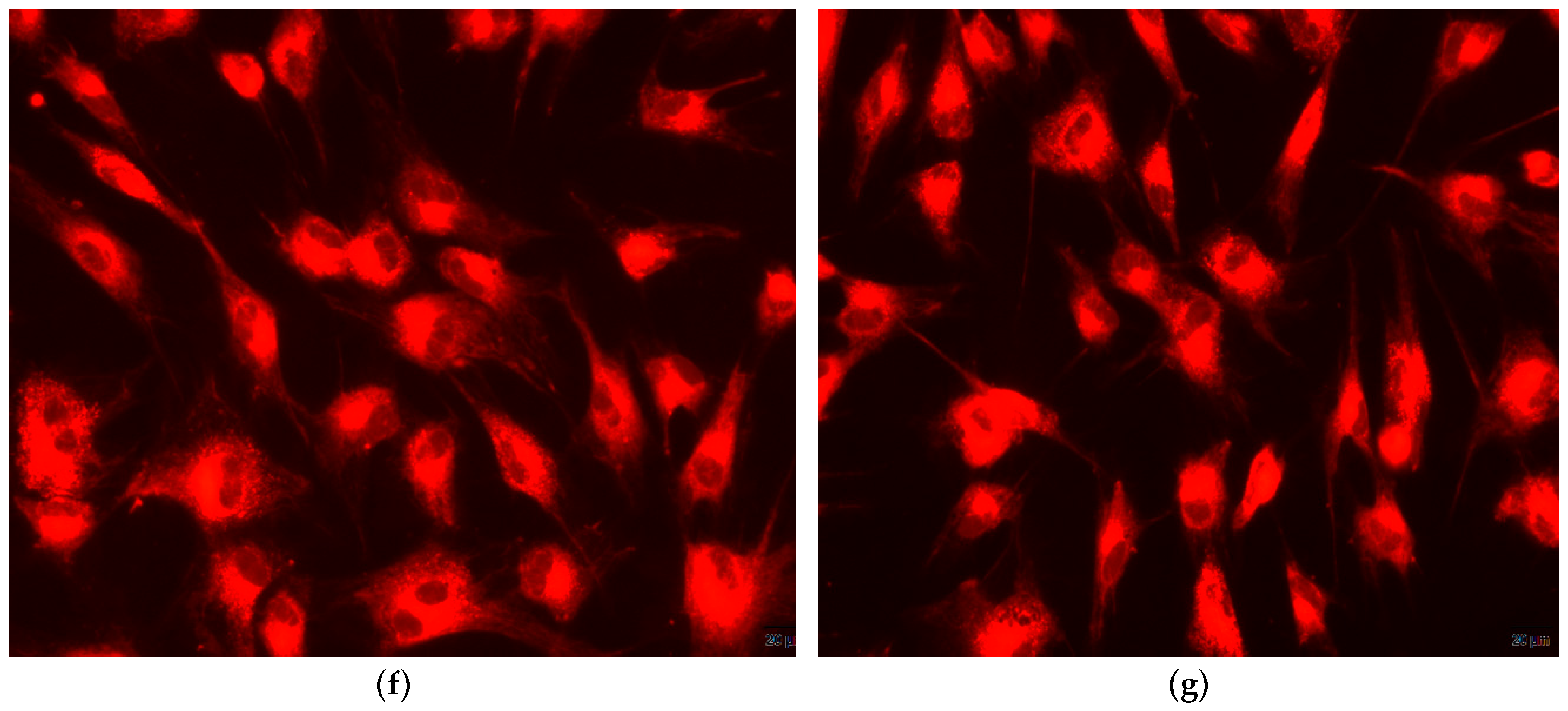
2.5.3. Qualitative In-Vitro Evaluation of Biocompatibility—Fluorescent Microscopy
3. Results and Discussions
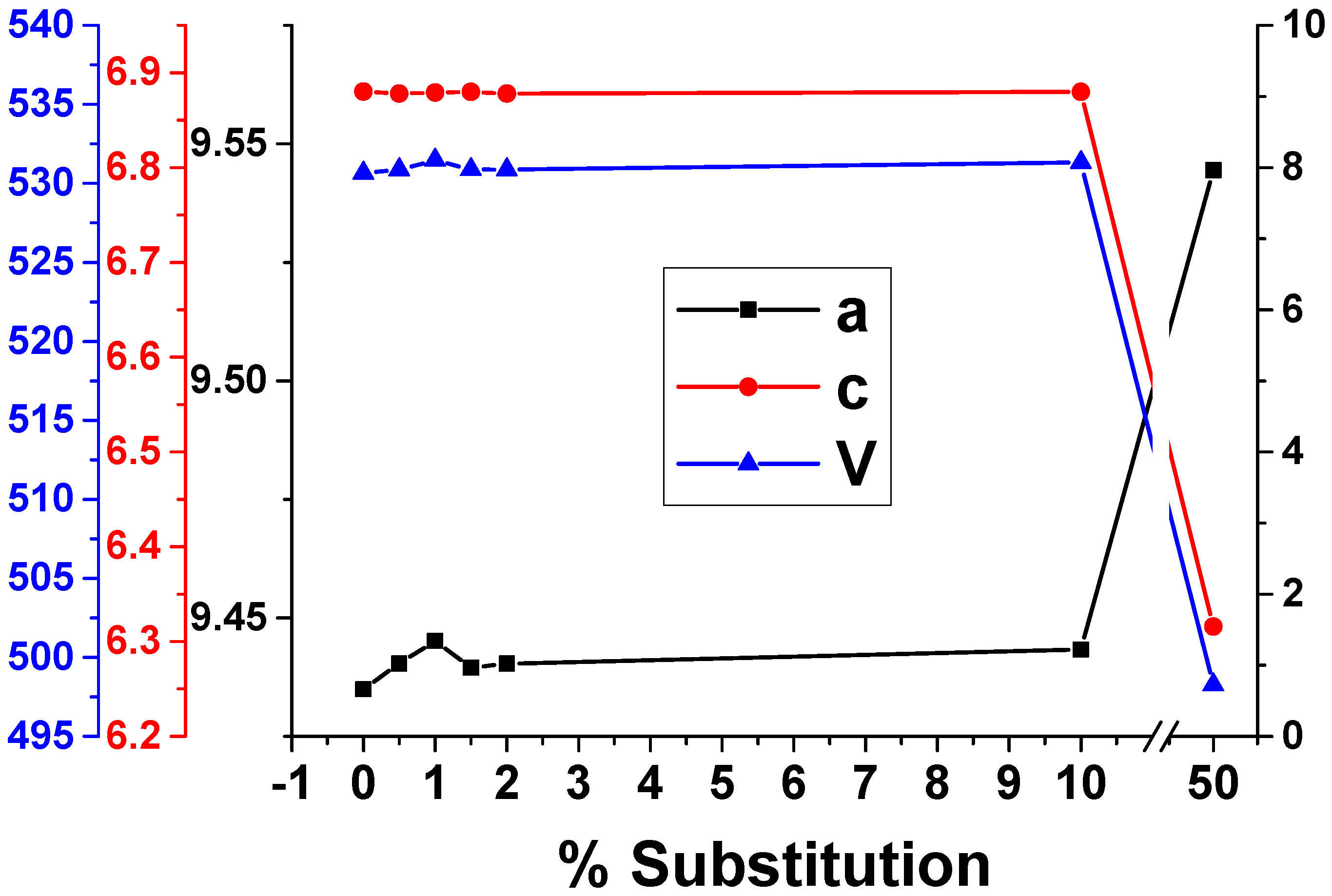
3.1. X-ray Diffraction
3.2. SEM Analysis
3.3. TEM Analysis
3.4. FTIR Spectra
3.5. Raman Spectroscopy
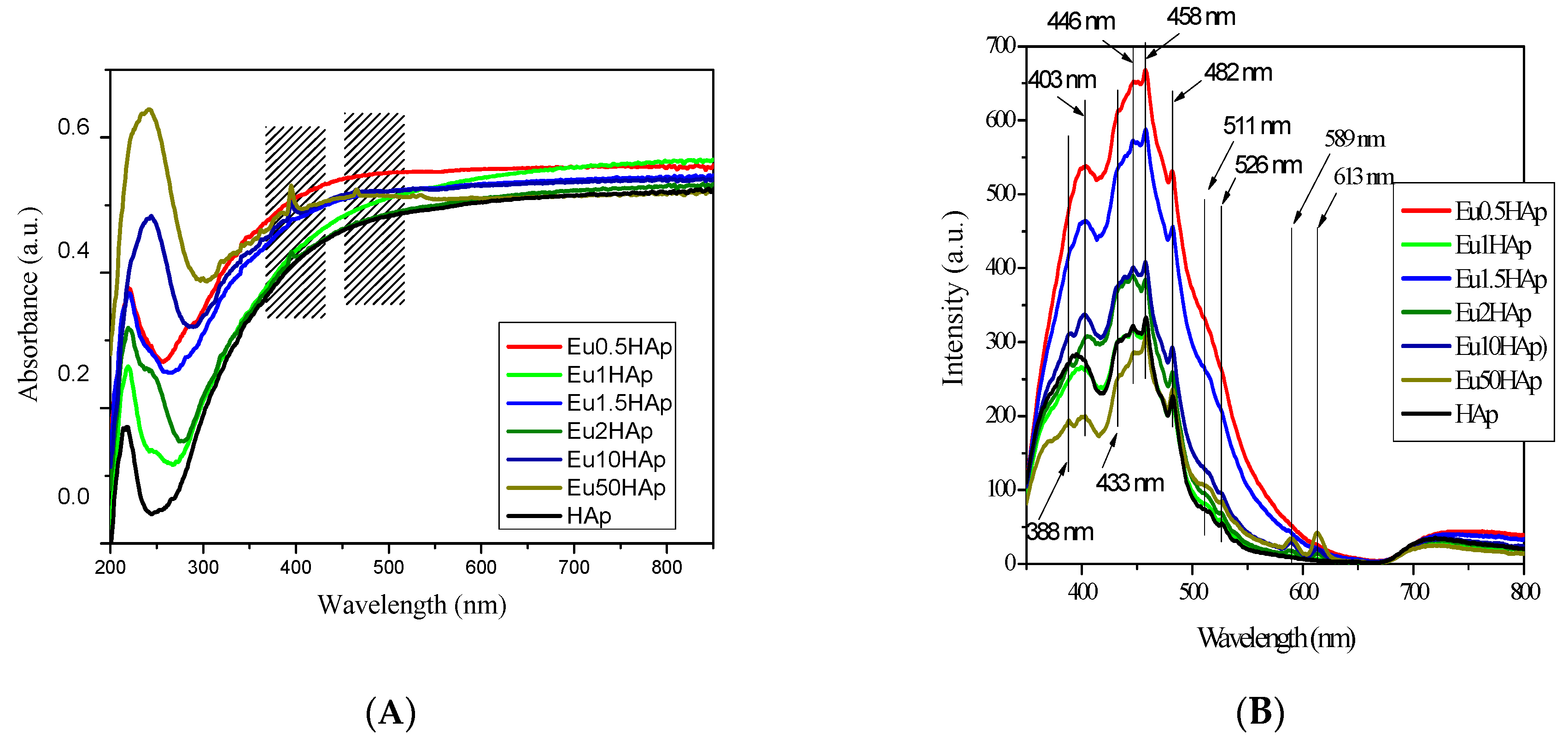
3.6. UV-Vis and PL Spectra
4. Biocompatibility and Cytotoxicity of EuXHAp Photoluminescent Ceramic Materials
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Y.; Wu, C.; Chang, J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater. Today 2019, 24, 41–56. [Google Scholar] [CrossRef]
- Ben-nissan, B.; Cazalbou, S.; Choi, A. Bioceramics. Biomater. Sci. Eng. 2019, 1, 16–33. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties (Les orthophosphates de calcium (CaPO4): Occurrence et Proprieties). Morphologie 2017, 101, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. A history of calcium orthophosphates (CaPO4) and their biomedical applications. Morphologie 2017, 101, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Pelin, I.M.; Maier, V.; Suflet, D.M.; Popescu, I.; Darie-Nita, R.N.; Aflori, M.; Butnaru, M. Formation and characterization of calcium orthophosphates in the presence of two different acidic macromolecules. J. Cryst. Growth 2017, 475, 266–273. [Google Scholar] [CrossRef]
- Sathiskumar, S.; Vanaraj, S.; Sabarinathan, D.; Bharath, S. Green synthesis of biocompatible nanostructured hydroxyapatite from Cirrhinus mrigala fish scale—A biowaste to biomaterial. Ceram. Int. 2019, 45, 7804–7810. [Google Scholar] [CrossRef]
- Beau, S.; Rouillon, T.; Millet, P.; Le, J.; Weiss, P.; Chopart, J.; Daltin, A. Synthesis of calcium-deficient hydroxyapatite nanowires and nanotubes performed by template-assisted electrodeposition. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 333–346. [Google Scholar]
- Xia, X.; Chen, J.; Shen, J.; Huang, D.; Duan, P.; Zou, G. Synthesis of hollow structural hydroxyapatite with different morphologies using calcium carbonate as hard template. Adv. Powder Technol. 2018, 29, 1562–1570. [Google Scholar] [CrossRef]
- Hashemi, S.; Javadpour, J.; Khavandi, A.; Erfan, M. Morphological evolution on the surface of hydrothermally synthesized hydroxyapatite microspheres in the presence of EDTMP. Ceram. Int. 2018, 44, 19743–19750. [Google Scholar]
- Turan, İ.; Kaygili, O.; Tatar, C.; Bulut, N.; Koytepe, S.; Ates, T. The effects of Ni-addition on the crystal structure, thermal properties and morphology of Mg-based hydroxyapatites synthesized by a wet chemical method. Ceram. Int. 2018, 44, 14036–14043. [Google Scholar]
- Wolff, J.; Hofmann, D.; Amelung, W.; Lewandowski, H.; Kaiser, K.; Bol, R. Applied Geochemistry Rapid wet chemical synthesis for 33 P-labelled hydroxyapatite—An approach for environmental research. Appl. Geochem. 2018, 97, 181–186. [Google Scholar] [CrossRef]
- Yelten-yilmaz, A.; Yilmaz, S. Wet chemical precipitation synthesis of hydroxyapatite (HA) powders. Ceram. Int. 2018, 44, 9703–9710. [Google Scholar] [CrossRef]
- Jiao, Y.; Lu, Y.; Xiao, G.; Xu, W.; Zhu, R. Preparation and characterization of hollow hydroxyapatite microspheres by the centrifugal spray drying method. Powder Technol. 2012, 217, 581–584. [Google Scholar] [CrossRef]
- Sabu, U.; Logesh, G.; Rashad, M.; Joy, A.; Balasubramanian, M. Microwave assisted synthesis of biomorphic hydroxyapatite. Ceram. Int. 2019, 45, 6718–6722. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Daliri-joupari, M. Bone-like hydroxyapatite mineralization on the bio-inspired PDA nanoparticles using microwave irradiation. Surf. Interfaces 2019, 15, 38–42. [Google Scholar] [CrossRef]
- Anwar, A.; Akbar, S. Novel continuous microwave assisted flow synthesis of nanosized manganese substituted hydroxyapatite. Ceram. Int. 2018, 44, 10878–10882. [Google Scholar] [CrossRef]
- Wu, S.; Hsu, H.; Hsu, S.; Chang, Y.; Ho, W. Synthesis of hydroxyapatite from eggshell powders through ball milling and heat treatment. J. Asian Ceram. Soc. 2016, 4, 85–90. [Google Scholar] [CrossRef]
- Hannora, A.E.; Ataya, S. Structure and compression strength of hydroxyapatite/titania nanocomposites formed by high energy ball milling. J. Alloy. Compd. 2016, 658, 222–233. [Google Scholar] [CrossRef]
- Ferro, A.C.; Guedes, M. Mechanochemical synthesis of hydroxyapatite using cuttle fish bone and chicken eggshell as calcium precursors. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 124–140. [Google Scholar] [CrossRef]
- Bouyarmane, H.; Gouza, A.; Masse, S.; Saoiabi, S.; Saoiabi, A.; Coradin, T.; Laghzizil, A. Nanoscale conversion of chlorapatite into hydroxyapatite using ultrasound irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 495, 187–192. [Google Scholar] [CrossRef]
- Nikolaev, A.L.; Gopin, A.V.; Severin, A.V.; Rudin, V.N.; Mironov, M.A.; Dezhkunov, N.V. Ultrasonic synthesis of hydroxyapatite in non-cavitation and cavitation modes. Ultrason. Sonochem. 2018, 44, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.J.; Katsarava, R. Other Miscellaneous Materials and Their Nanocomposites; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128146156. [Google Scholar]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Kubasiewicz-Ross, P.; Hadzik, J.; Seeliger, J.; Kozak, K.; Jurczyszyn, K.; Gerber, H.; Dominiak, M.; Kunert-Keil, C. New nano-hydroxyapatite in bone defect regeneration: A histological study in rats. Ann. Anat. 2017, 213, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, M.A.S.; Hirata, H.H.; Plepis, A.M.G.; Martins, V.C.A.; Cunha, M.R. Use of collagen/chitosan sponges mineralized with hydroxyapatite for the repair of cranial defects in rats. Injury 2018, 49, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Solonenko, A.P.; Blesman, A.I.; Polonyankin, D.A. Preparation and in vitro apatite-forming ability of hydroxyapatite and β-wollastonite composite materials. Ceram. Int. 2018, 44, 17824–17834. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, K.J.; Anand, V.; Islam, N.; Bhatia, G.; Kalia, N.; Singh, J. Lanthanide (=Ce, Pr, Nd and Tb) ions substitution at calcium sites of hydroxyl apatite nanoparticles as fluorescent bio probes: Experimental and density functional theory study. Ceram. Int. 2017, 43, 10097–10108. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Ramesh, S.; Tan, C.Y.; Chandran, H.; Fauzi, A.; Noor, M.; Krishnasamy, S.; Alengaram, U.J.; Ramesh, S. Effect of multi-ions doping on the properties of carbonated hydroxyapatite bioceramic. Ceram. Int. 2019, 45, 3473–3477. [Google Scholar] [CrossRef]
- Tesch, A.; Wenisch, C.; Herrmann, K.H.; Reichenbach, J.R.; Warncke, P.; Fischer, D.; Müller, F.A. Luminomagnetic Eu3+- and Dy3+-doped hydroxyapatite for multimodal imaging. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 422–431. [Google Scholar] [CrossRef]
- Zilm, M.E.; Yu, L.; Hines, W.A.; Wei, M. Magnetic properties and cytocompatibility of transition-metal-incorporated hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 112–119. [Google Scholar] [CrossRef]
- Li, H.; Sun, X.; Li, Y.; Li, B.; Liang, C.; Wang, H. Preparation and properties of carbon nanotube (Fe)/hydroxyapatite composite as magnetic targeted drug delivery carrier. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Zia, R.; Ijaz, A.; Hussain, T.; Mohsin, M.; Malik, A. Synthesis of monophasic Ag doped hydroxyapatite and evaluation of antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Mondal, S.; Jang, B.; Manivasagan, P. Biomimetic synthesis of metal—Hydroxyapatite (Au-HAp, Ag-HAp, Au-Ag- HAp): Structural analysis, spectroscopic characterization and biomedical application. Ceram. Int. 2018, 44, 20490–20500. [Google Scholar] [CrossRef]
- Gonzalez, G.; Costa-vera, C.; Borrero, L.J.; Soto, D.; Lozada, L.; Chango, J.I.; Diaz, J.C.; Lascano, L. Effect of carbonates on hydroxyapatite self-activated photoluminescence response. J. Lumin. 2018, 195, 385–395. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Stoica, A.E.; Vasile, B.S. Luminescent Hydroxyapatite Doped with Rare Earth Elements for Biomedical Applications. Nanomaterials 2019, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lécuyer, T.; Seguin, J.; Mignet, N.; Scherman, D.; Viana, B.; Richard, C. Imaging and therapeutic applications of persistent luminescence nanomaterials. Adv. Drug Deliv. Rev. 2019, 138, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Zhang, X.; Wu, D.; Han, Y.; Wickramaratne, M.N.; Dai, H.; Wang, X. Ultrasound-Assisted Synthesis and Characterization of Heparin-Coated Eu3+ Doped Hydroxyapatite Luminescent Nanoparticles. Colloid Interface Sci. Commun. 2019, 29, 17–25. [Google Scholar] [CrossRef]
- Kudryashova, V.A. Europium and terbium pyrrole-2-carboxylates: Structures, luminescence, and energy transfer. Inorg. Chim. Acta 2019, 492, 1–7. [Google Scholar]
- Corbin, B.A.; Hovey, J.L.; Thapa, B.; Schlegel, H.B.; Allen, M.J. Luminescence differences between two complexes of divalent europium. J. Organomet. Chem. 2018, 857, 88–93. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Koshelev, D.S.; Medvedko, A.V.; Kalyakina, A.S.; Bushmarinov, I.S.; Grishko, A.Y.; Schepers, U.; Bräse, S.; Vatsadze, S.Z. Europium 2-benzofuranoate: Synthesis and use for bioimaging. Opt. Mater. 2017, 74, 191–196. [Google Scholar] [CrossRef]
- Petrochenkova, N.V.; Mirochnik, A.G.; Emelina, T.B.; Sergeev, A.A.; Leonov, A.A.; Voznesenskii, S.S. Luminescent amine sensor based on europium(III) chelate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.A.R.M.; Valerio, M.E.G. X-ray absorption fine structure spectroscopy and photoluminescence study of multifunctional europium (III)-doped hydroxyapatite in the presence of cationic surfactant medium. J. Lumin. 2018, 201, 70–76. [Google Scholar] [CrossRef]
- Mugnaioli, E.; Reyes-gasga, J.; Kolb, U.; Hemmerlø, J. Evidence of Noncentrosymmetry of Human Tooth Hydroxyapatite Crystals. Chem. A Eur. J. 2014, 20, 6849–6852. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tan, J.; Lian, Y.; Liu, D. Preparation of Gelatin coated hydroxyapatite nanorods and the stability of its aqueous colloidal. Appl. Surf. Sci. 2008, 254, 2730–2735. [Google Scholar] [CrossRef]
- Mullica, D.F.; Milligan, W.O.; Beall, G.W. Crystal structures of Pr(OH)3, Eu(OH)3 and Tm(OH)3. J. Inorg. Nucl. Chem. 1979, 41, 525–532. [Google Scholar] [CrossRef]
- Terra, J.; Dourado, E.R.; Eon, J.G.; Ellis, D.E.; Gonzalez, G.; Rossi, A.M. The structure of strontium-doped hydroxyapatite: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2009, 11, 568–577. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Fahami, A.; Nasiri-Tabrizi, B.; Beall, G.W.; Basirun, W.J. Structural insights of mechanically induced aluminum-doped hydroxyapatite nanoparticles by Rietveld refinement. Chin. J. Chem. Eng. 2017, 25, 238–247. [Google Scholar] [CrossRef]
- Fowler, B. Infrared Studies of Apatites. I. Vibrational Assignments for Calcium, Strontium, and Barium Hydroxyapatites Utilizing Isotopic Substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Iconaru, S.; Motelica-Heino, M.; Predoi, D. Study on Europium-Doped Hydroxyapatite Nanoparticles by Fourier Transform Infrared Spectroscopy and Their Antimicrobial Properties. J. Spectrosc. 2013, 2013, 284285. [Google Scholar] [CrossRef]
- Sitarz, M.; Rokita, M.; Bułat, K. Infrared spectroscopy of different phosphates structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 722–727. [Google Scholar]
- Markovic, M.; Fowler, O.B. Preparation and Comprehensive Characterization of a Calcium Hydroxyapatite Reference Material Volume. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Cuscó, R.; Guitián, F.; de Aza, S.; Arttis, L. Differentiation between Hydroxyapatite and/I-Tricalcium Phosphate by Means of p-Raman Spectroscopy. J. Eur. Ceram. Soc. 1998, 2219, 1301–1305. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Massuyeau, F.; Andronescu, E.; Stan, M.S.; Predoi, D. Biocompatibility study of europium doped crystalline hydroxyapatite bioceramics. Dig. J. Nanomater. Biostructures 2011, 6, 1639–1647. [Google Scholar]
- Binnemans, K. Interpretation of europium (III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Al-kattan, A.; Dufour, P.; Dexpert-ghys, J.; Drouet, C. Preparation and Physicochemical Characteristics of Luminescent Apatite-Based Colloids. J. Phys. Chem. C 2010, 114, 2918–2924. [Google Scholar] [CrossRef]
- Yang, C.; Yang, P.; Wang, W.; Gai, S.; Wang, J.; Zhang, M.; Lin, J. Synthesis and characterization of Eu-doped hydroxyapatite through a microwave assisted microemulsion process. Solid State Sci. 2009, 11, 1923–1928. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Massuyeau, F.; Constantin, L.V.; Costescu, A.; Predoi, D. Synthesis, Structure, and Luminescent Properties of Europium-Doped Hydroxyapatite Nanocrystalline Powders. J. Nanomater. 2012, 2012, 942801. [Google Scholar] [CrossRef]
- Noushin Jalayer Naderi, R.Y.; Jamali, D.; Mohammad Rezvani, B. Cytotoxicity of nano-hydroxyapatite on gingiva-derived fibroblast cell line (HGF2): An in vitro study. Daneshvar Med. 2012, 19, 79–86. [Google Scholar]
- Paulina-Guadalupe, M.-M.; Gabriel-Alejandro, M.-C.; Nereyda, N.-M.; Nuria, P.-M.; Miguel-Ángel, C.-S.; Brenda-Erendida, C.-S.; Facundo, R. Facile Synthesis, Characterization, and Cytotoxic Activity of Europium-Doped Nanohydroxyapatite. Bioinorg. Chem. Appl. 2016, 2016, 1057260. [Google Scholar]
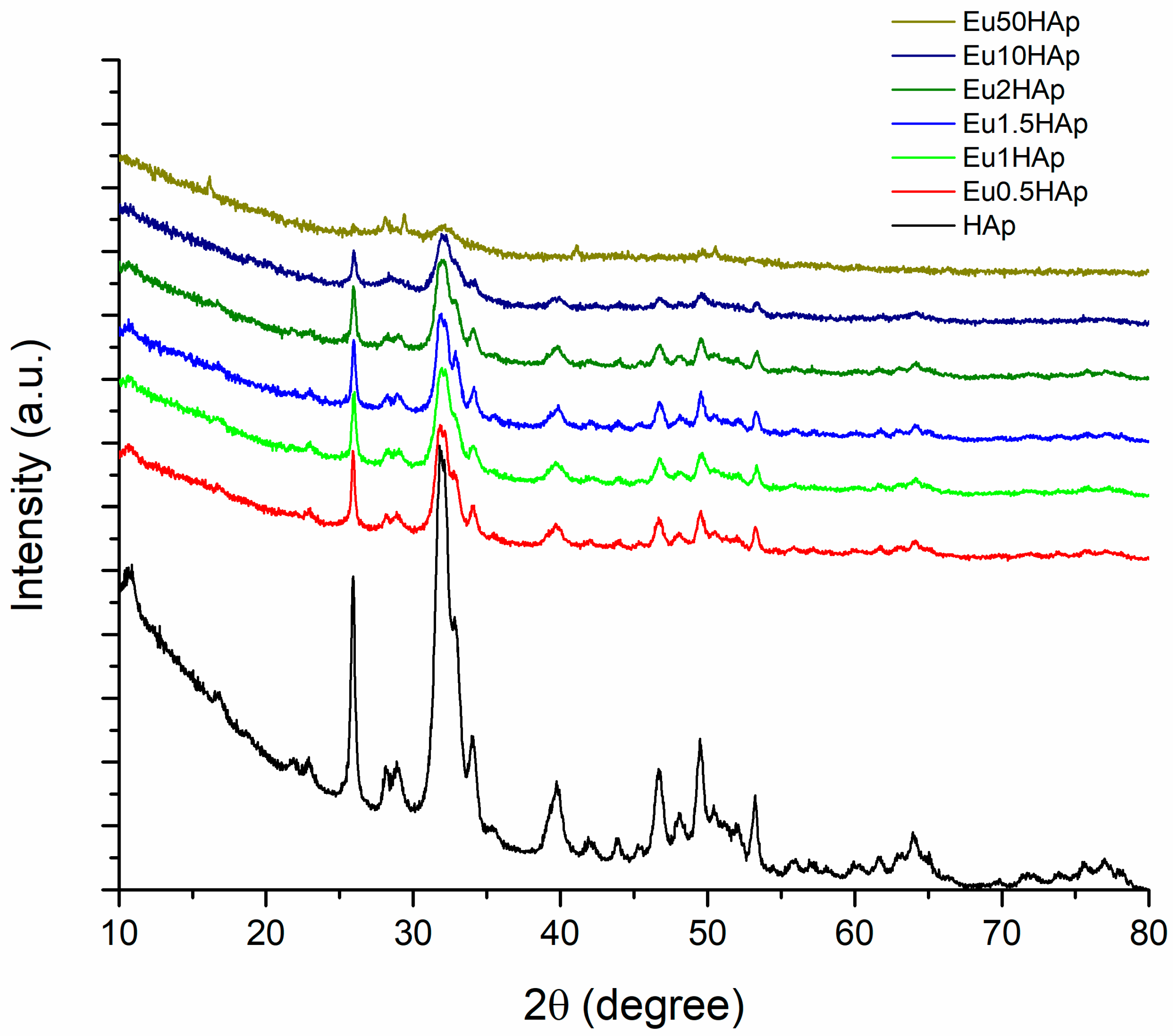
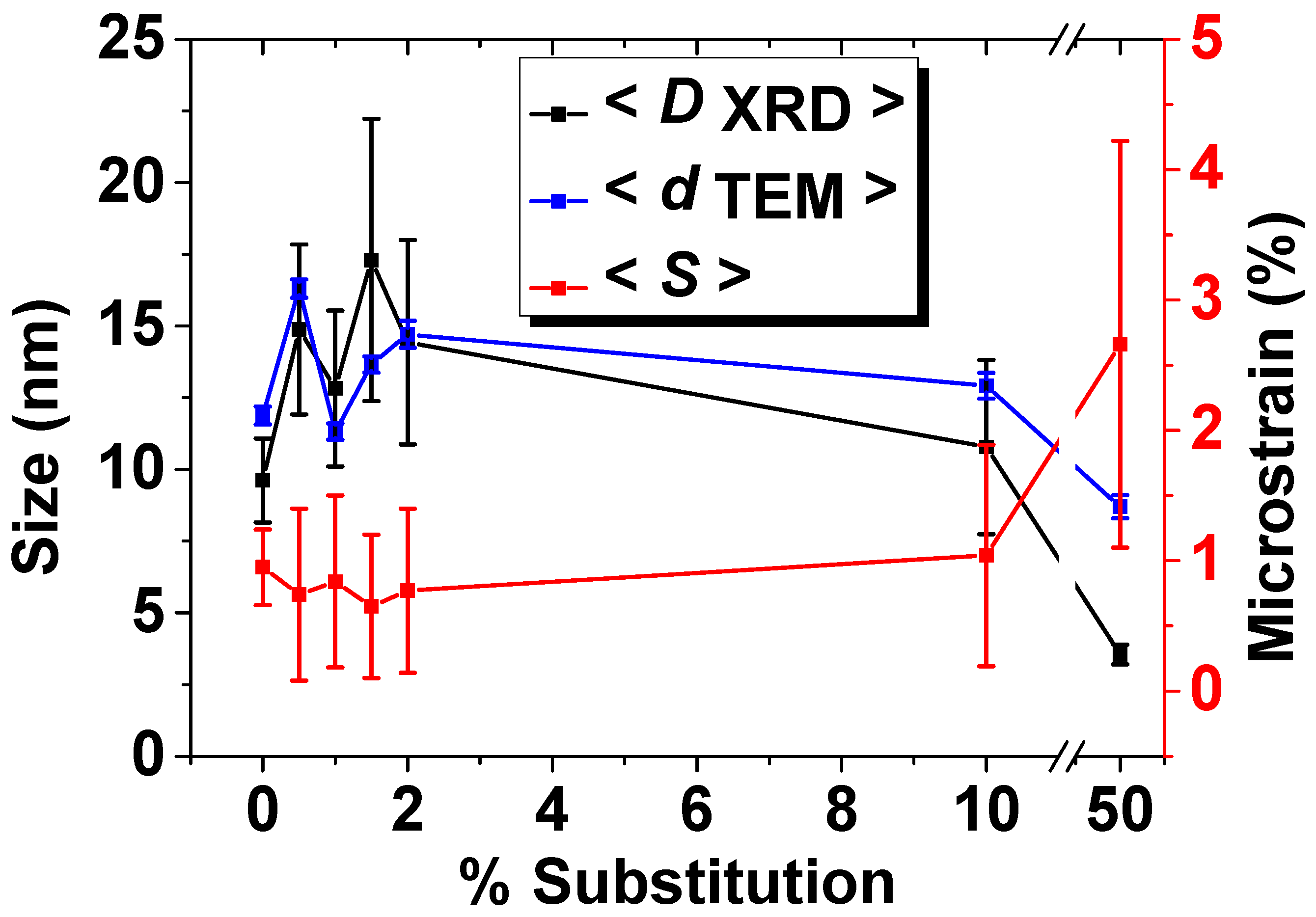

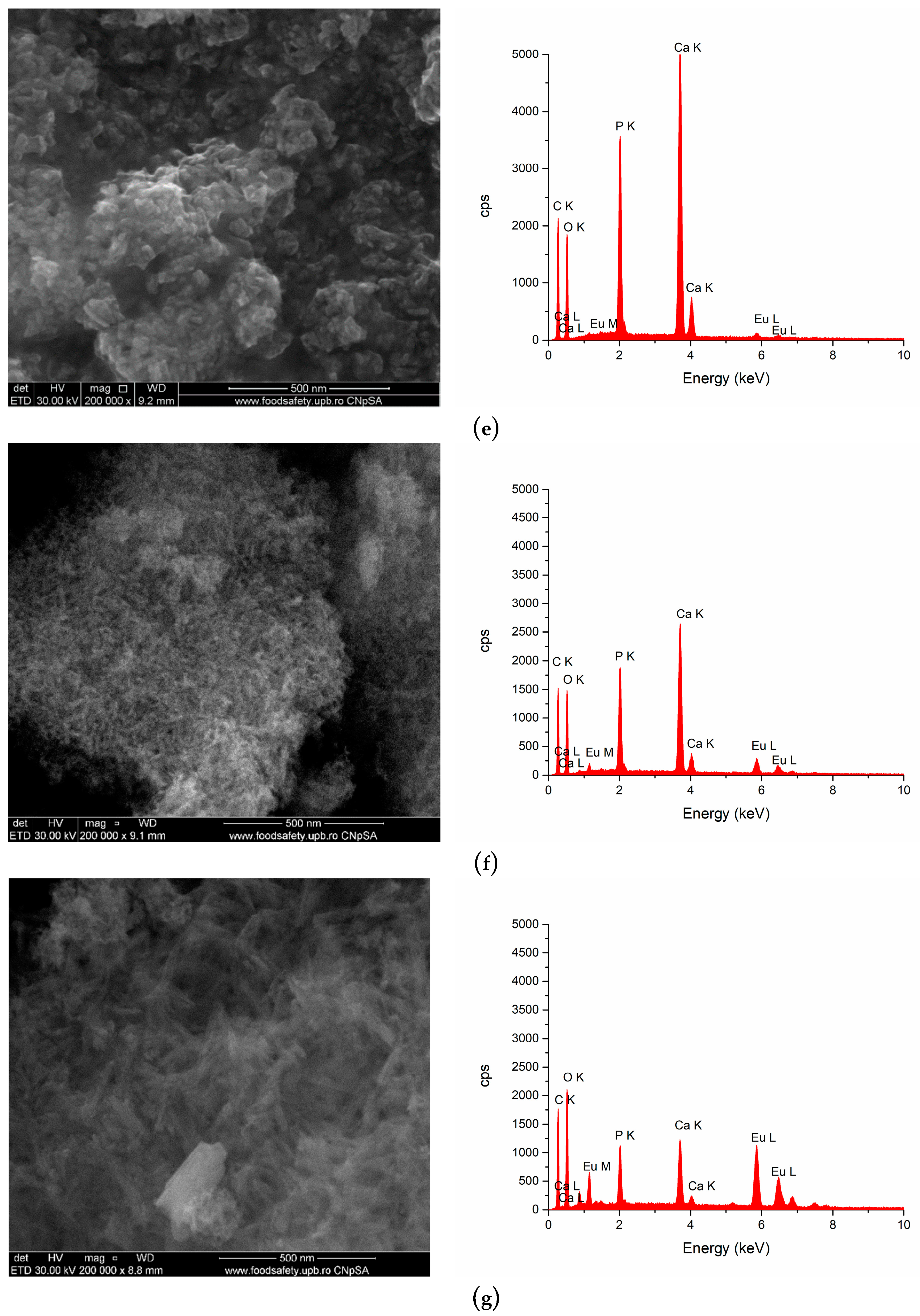
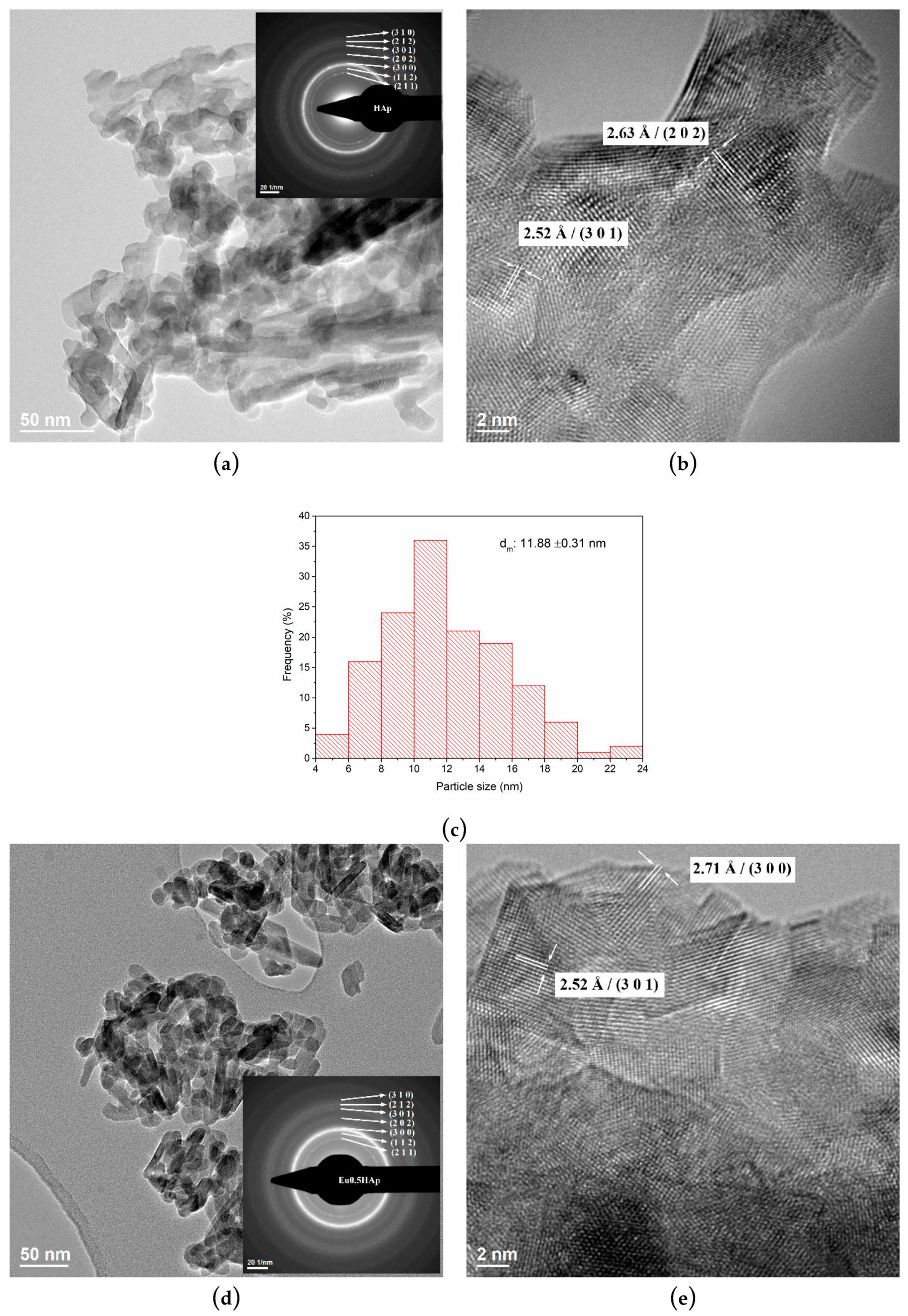
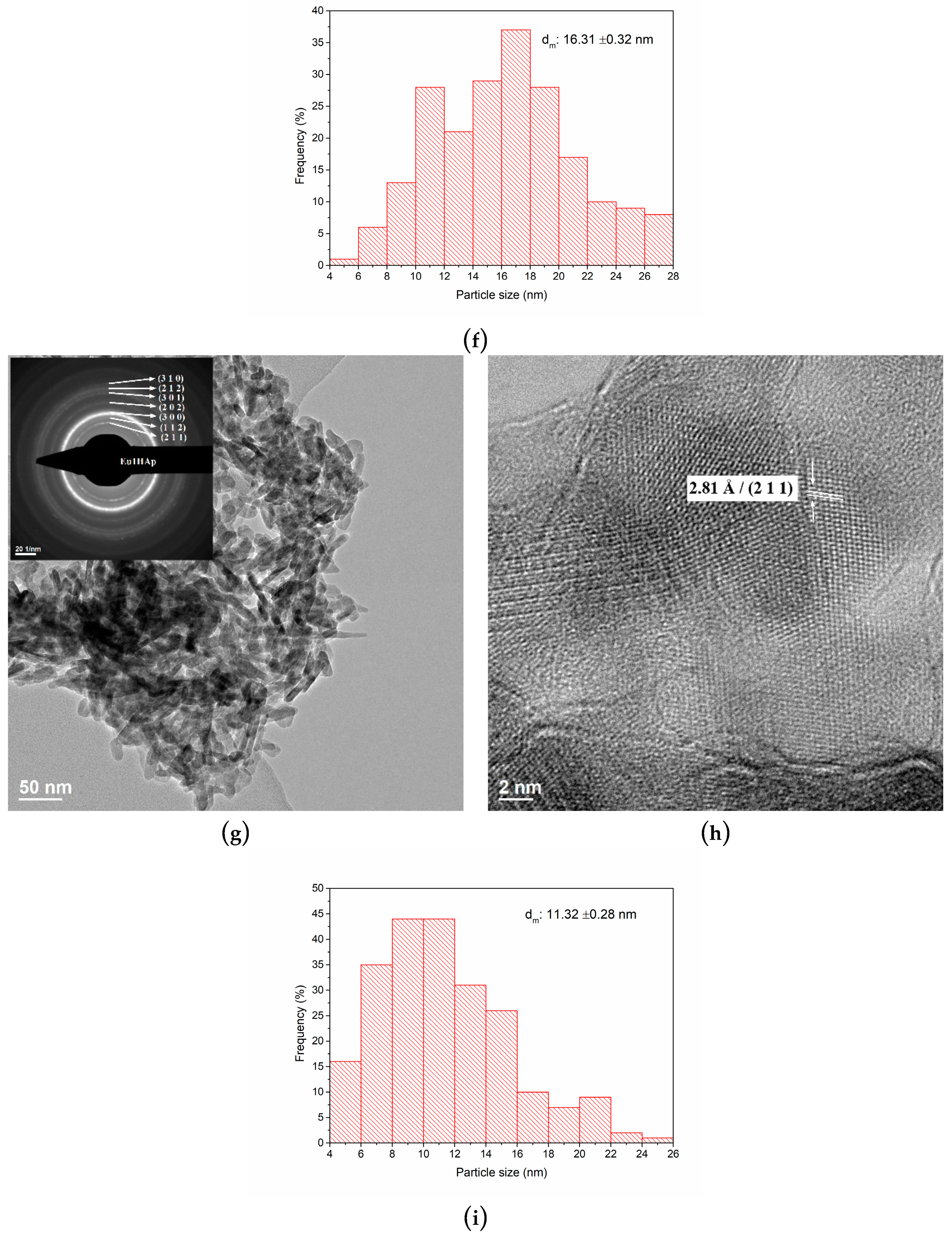
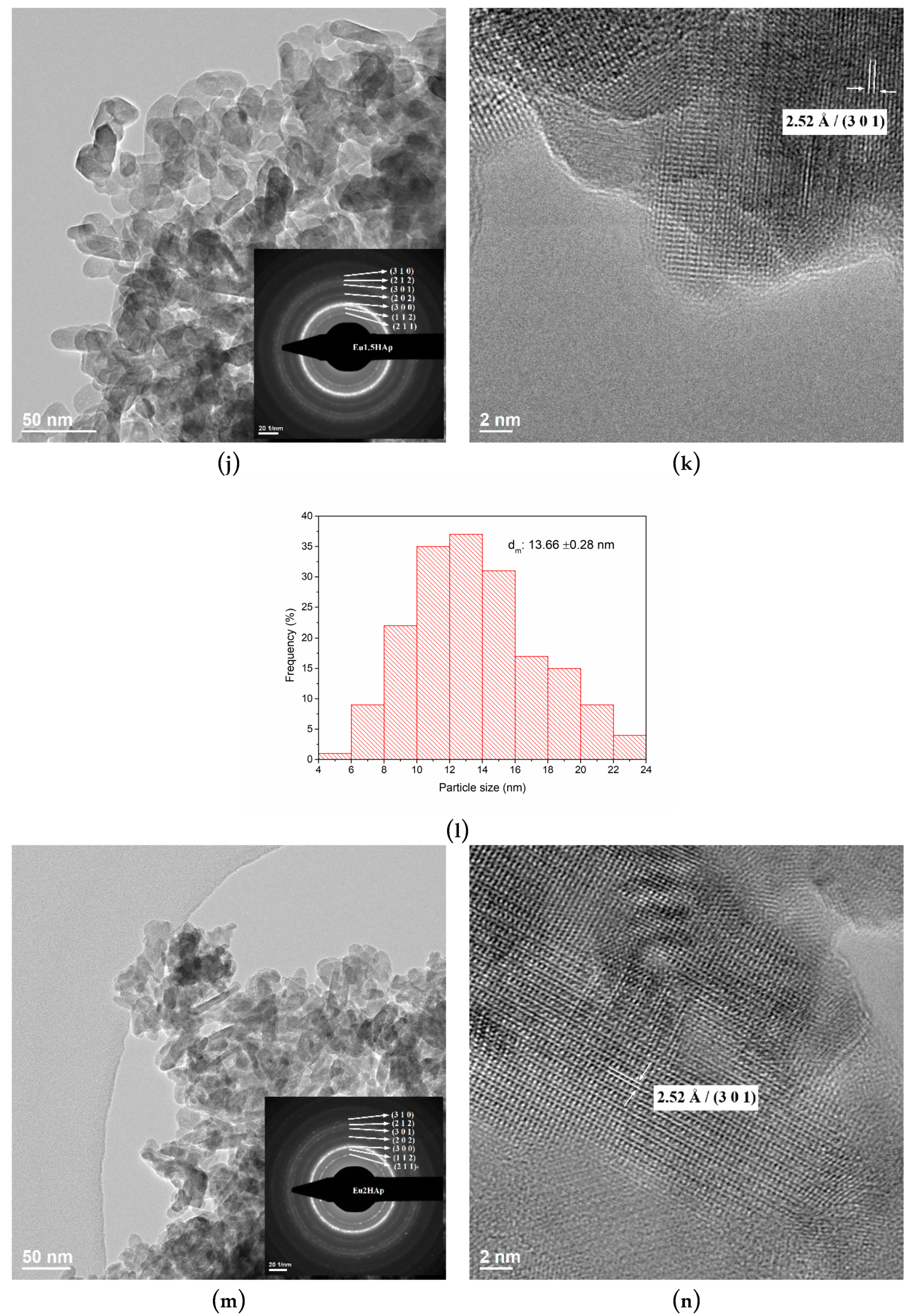
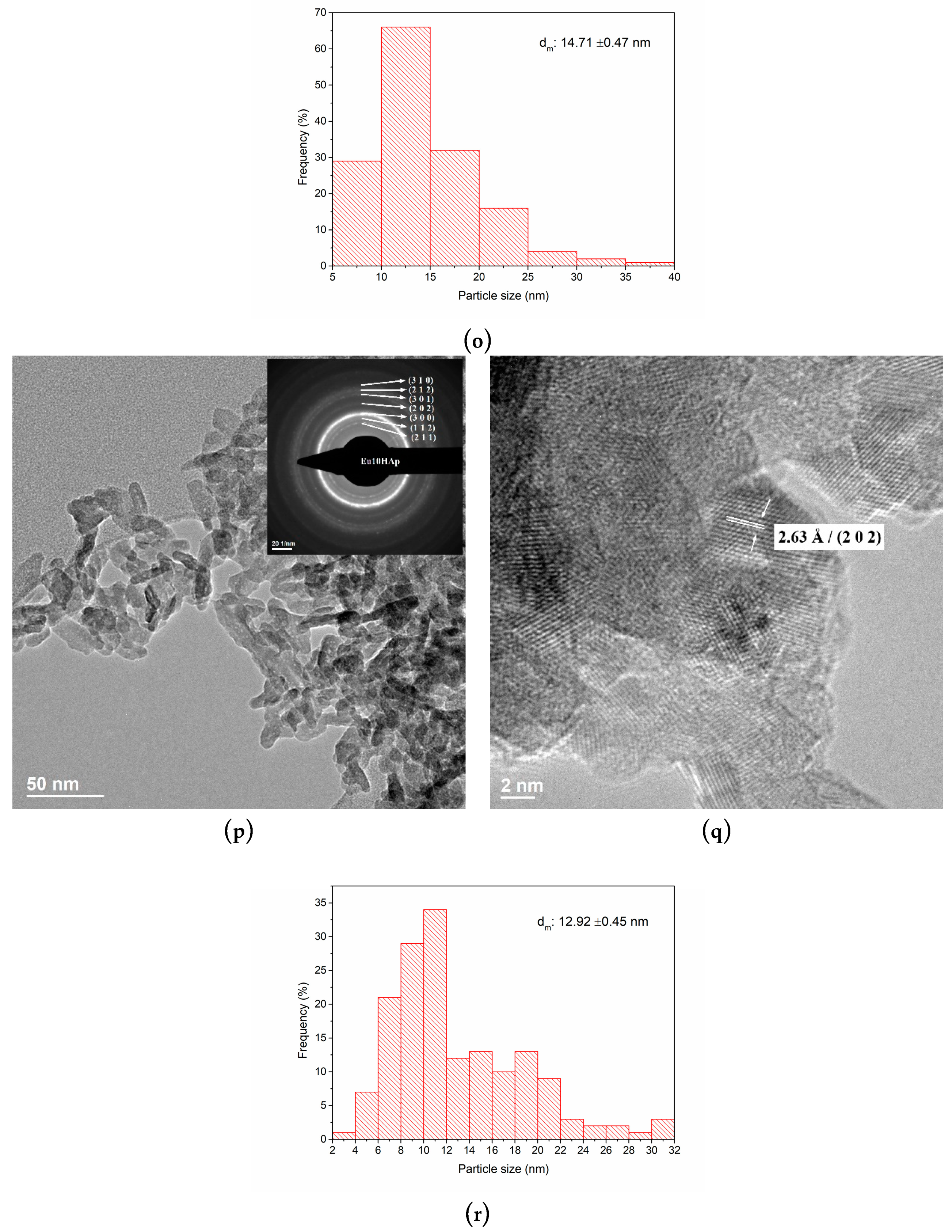
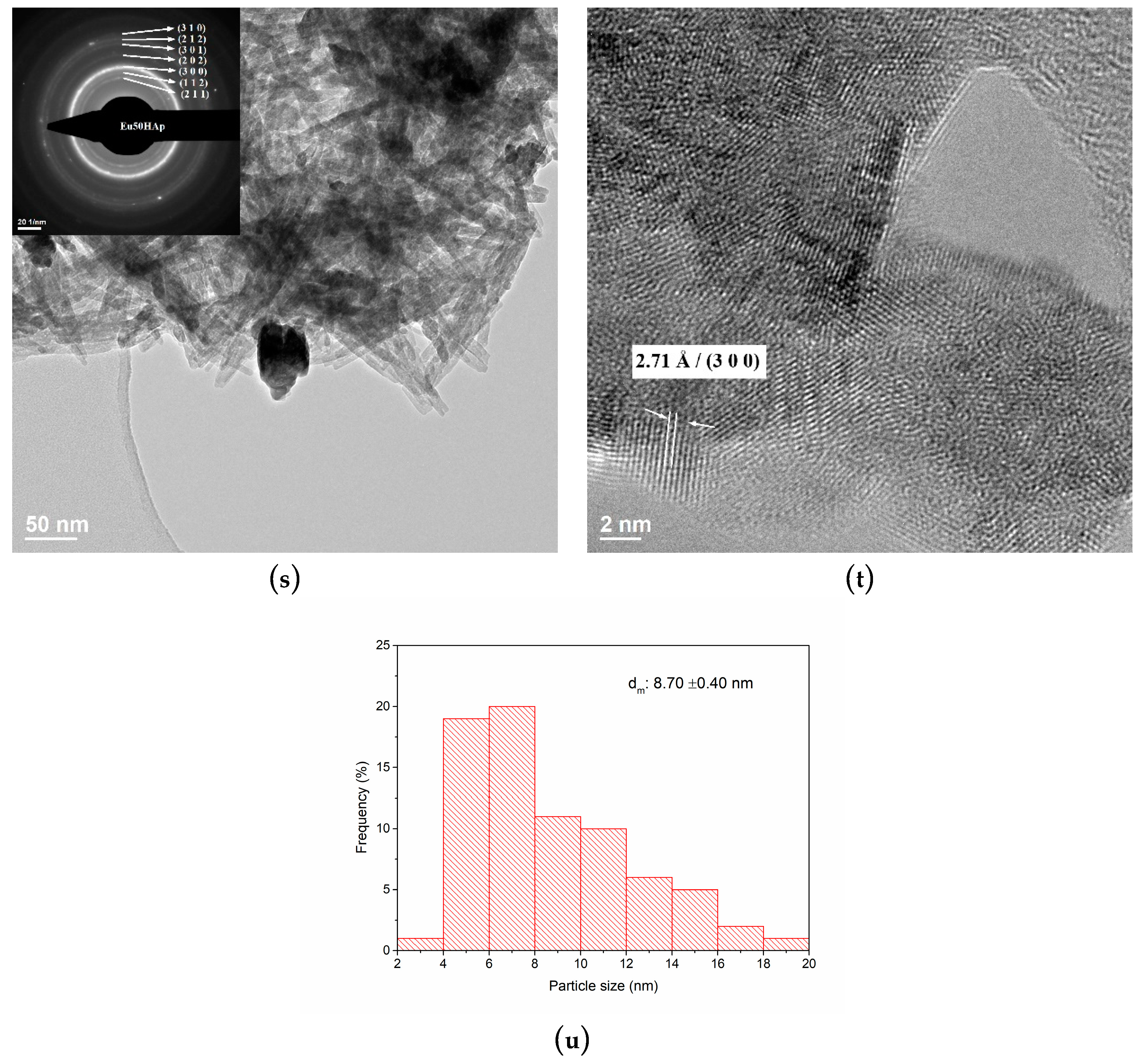
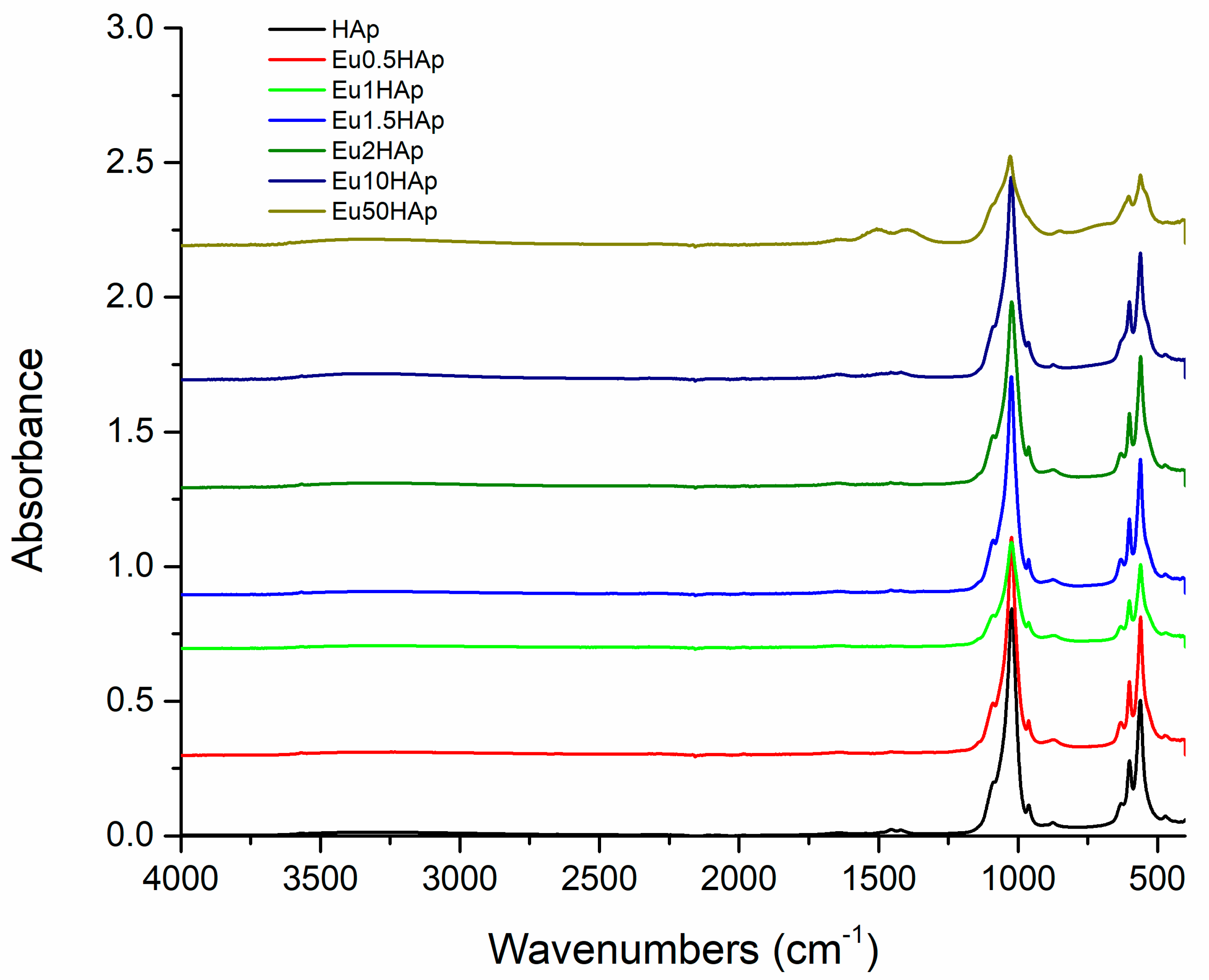
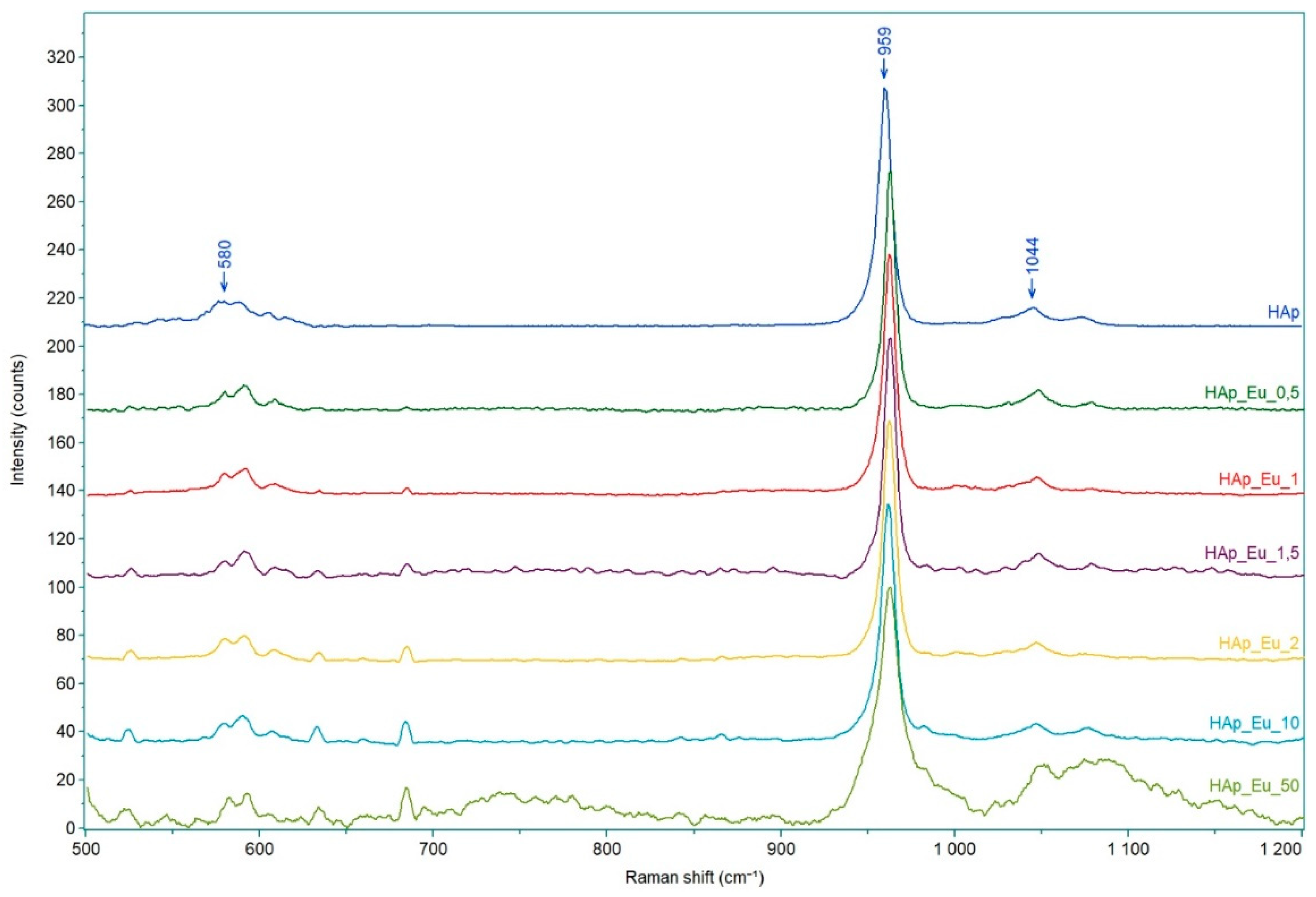
| No. | Samples | D/nm | S/% | Xc/% |
|---|---|---|---|---|
| 1 | HAp | 9.62 ± 1.46 | 0.95 ± 0.29 | 33.93 |
| 2 | Eu0.5HAp | 14.88 ± 2.96 | 0.74 ± 0.66 | 26.25 |
| 3 | Eu1HAp | 12.82 ± 2.72 | 0.84 ± 0.66 | 24.11 |
| 4 | Eu1.5HAp | 17.3 ± 4.92 | 0.65 ± 0.55 | 22.97 |
| 5 | Eu2HAp | 14.44 ± 3.56 | 0.77 ± 0.63 | 22.63 |
| 6 | Eu10HAp | 10.78 ± 3.04 | 1.04 ± 0.85 | 23.03 |
| 7 | Eu50HAp | 3.55 ± 0.34 | 2.66 ± 1.56 | 21.87 |
| Sample | a [Å] | c [Å] | V [Å3] | Rexp | Rp | Rwp | χ2 |
|---|---|---|---|---|---|---|---|
| HAp | 9.434979 ± 0.001401 | 6.8803397 ± 0.001042 | 530.6588 | 3.4323 | 3.99694 | 5.19277 | 2.28891 |
| Eu0.5HAp | 9.440372 ± 0.002067 | 6.878142 ± 0.001529 | 530.8601 | 3.96601 | 4.82349 | 6.554 | 2.7309 |
| Eu1HAp | 9.445147 ± 0.002444 | 6.879144 ± 0.001839 | 531.4745 | 3.92894 | 4.95602 | 6.61378 | 2.83367 |
| Eu1.5HAp | 9.439496 ± 0.001874 | 6.879717 ± 0.001413 | 530.883 | 3.7995 | 4.38638 | 5.76083 | 2.29889 |
| Eu2HAp | 9.440372 ± 0.002067 | 6.878142 ± 0.001529 | 530.8601 | 3.96601 | 4.82349 | 6.554 | 2.7309 |
| Eu10HAp | 9.443276 ± 0.002227 | 6.879882 ± 0.001665 | 531.321 | 3.88457 | 4.43662 | 5.82612 | 2.24943 |
| Eu50HAp | 9.544463 ± 0.021918 | 6.315845 ± 0.023992 | 498.2704 | 3.02385 | 3.11688 | 4.08783 | 1.82753 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andronescu, E.; Predoi, D.; Neacsu, I.A.; Paduraru, A.V.; Musuc, A.M.; Trusca, R.; Oprea, O.; Tanasa, E.; Vasile, O.R.; Nicoara, A.I.; et al. Photoluminescent Hydroxylapatite: Eu3+ Doping Effect on Biological Behaviour. Nanomaterials 2019, 9, 1187. https://doi.org/10.3390/nano9091187
Andronescu E, Predoi D, Neacsu IA, Paduraru AV, Musuc AM, Trusca R, Oprea O, Tanasa E, Vasile OR, Nicoara AI, et al. Photoluminescent Hydroxylapatite: Eu3+ Doping Effect on Biological Behaviour. Nanomaterials. 2019; 9(9):1187. https://doi.org/10.3390/nano9091187
Chicago/Turabian StyleAndronescu, Ecaterina, Daniela Predoi, Ionela Andreea Neacsu, Andrei Viorel Paduraru, Adina Magdalena Musuc, Roxana Trusca, Ovidiu Oprea, Eugenia Tanasa, Otilia Ruxandra Vasile, Adrian Ionut Nicoara, and et al. 2019. "Photoluminescent Hydroxylapatite: Eu3+ Doping Effect on Biological Behaviour" Nanomaterials 9, no. 9: 1187. https://doi.org/10.3390/nano9091187
APA StyleAndronescu, E., Predoi, D., Neacsu, I. A., Paduraru, A. V., Musuc, A. M., Trusca, R., Oprea, O., Tanasa, E., Vasile, O. R., Nicoara, A. I., Surdu, A. V., Iordache, F., Birca, A. C., Iconaru, S. L., & Vasile, B. S. (2019). Photoluminescent Hydroxylapatite: Eu3+ Doping Effect on Biological Behaviour. Nanomaterials, 9(9), 1187. https://doi.org/10.3390/nano9091187












