Optically Stimulated Nanodosimeters with High Storage Capacity
Abstract
1. Introduction
2. Materials and Methods
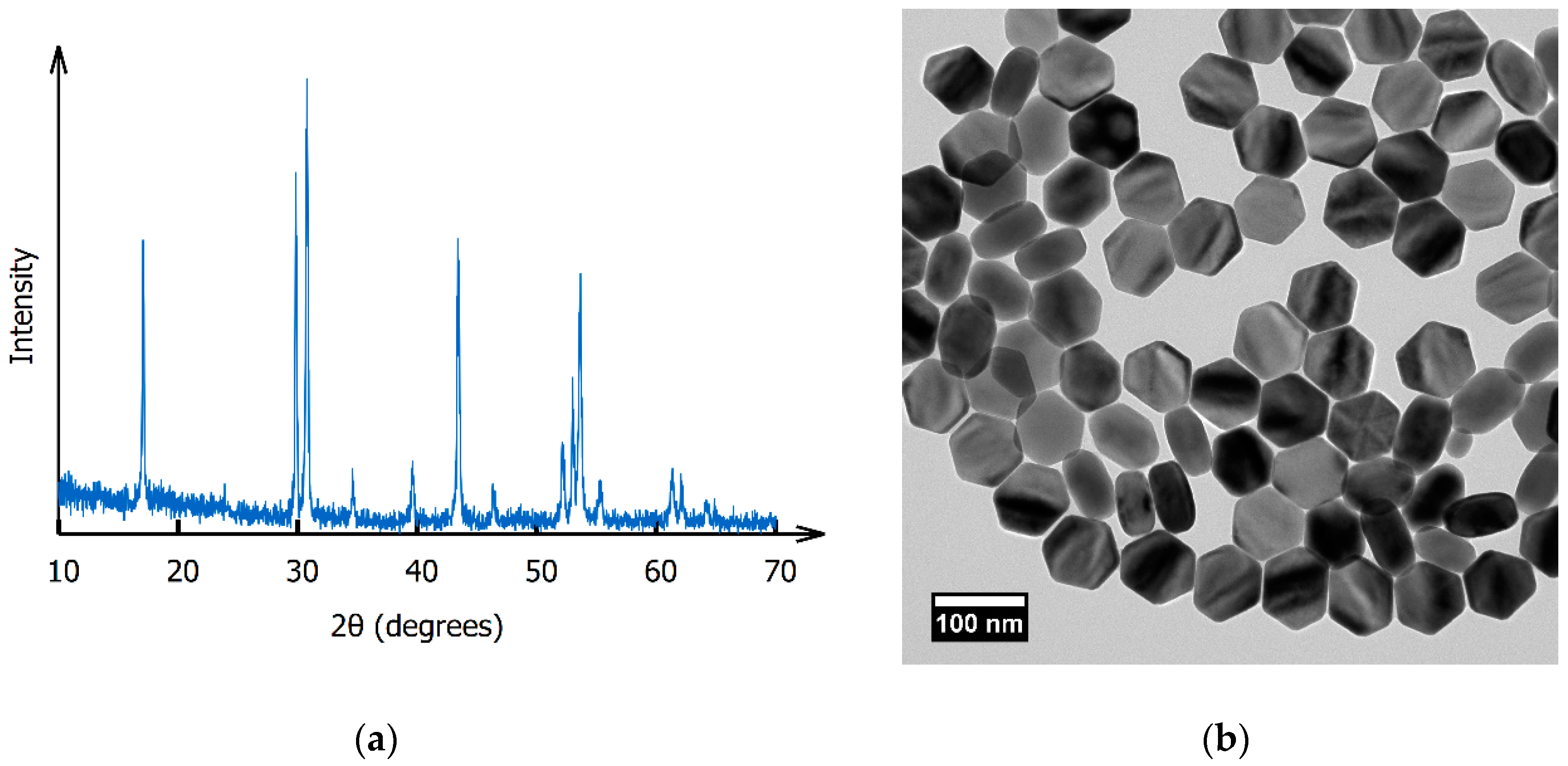
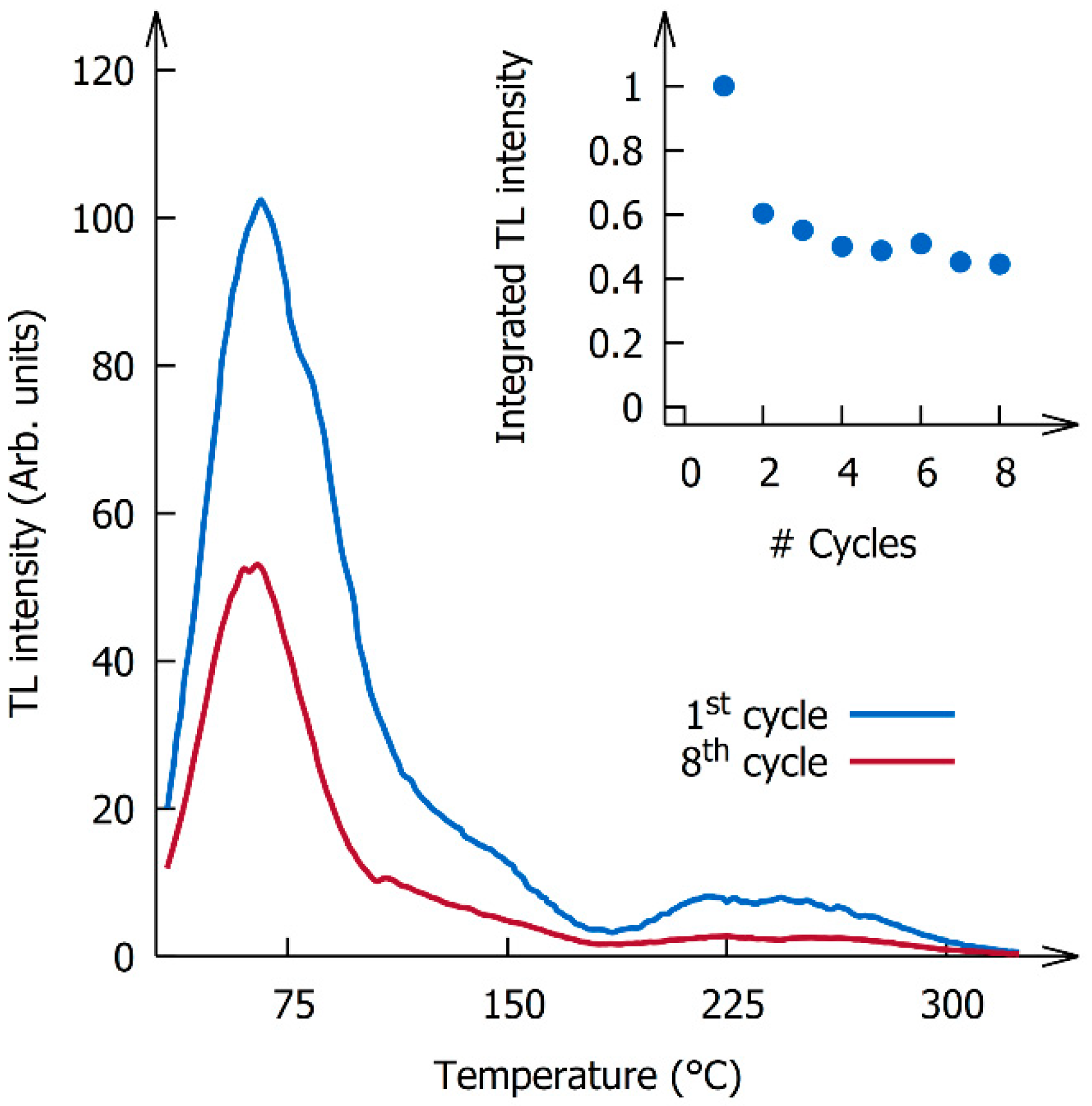
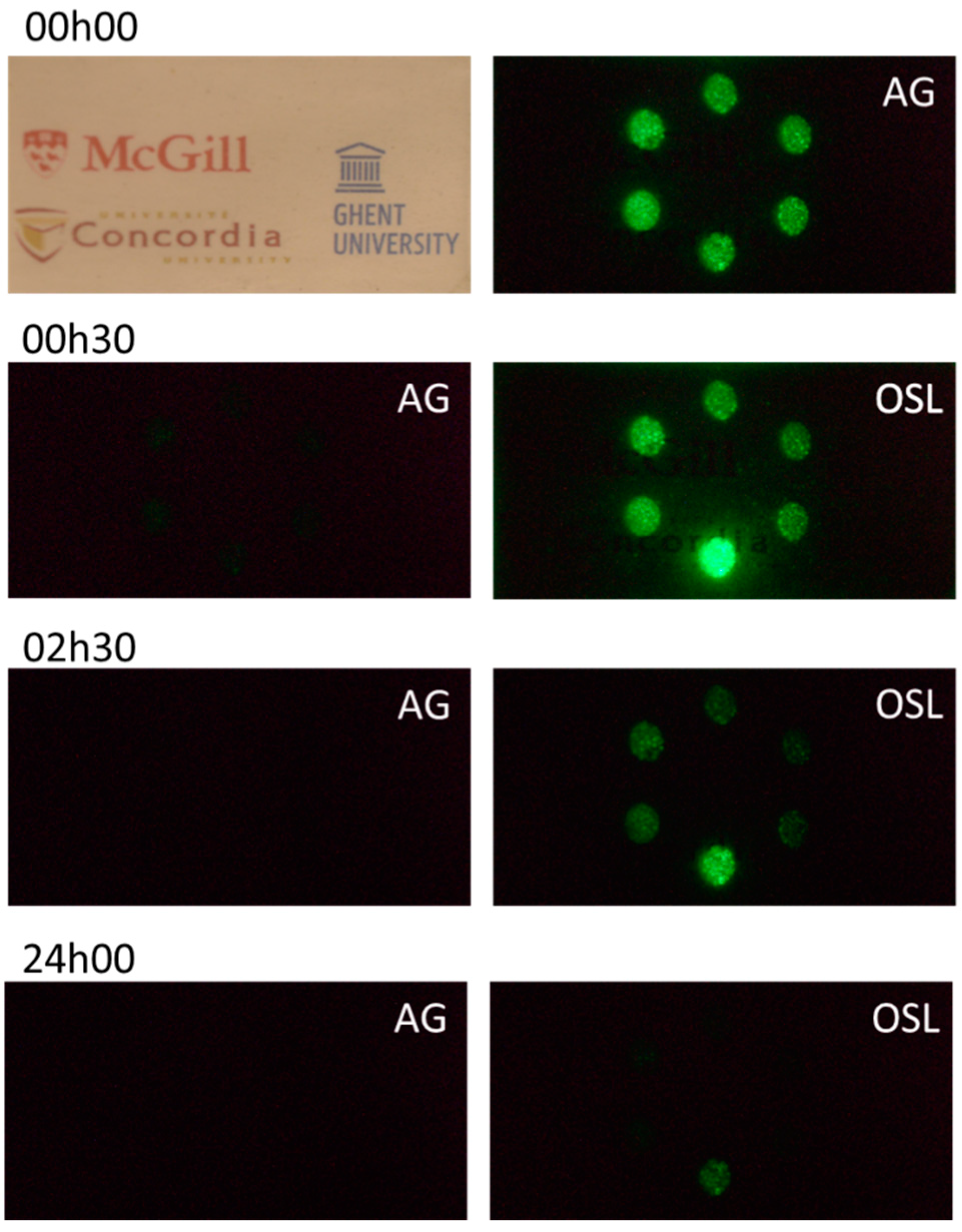
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smet, P.F.; Parmentier, A.B.; Poelman, D. Selecting Conversion Phosphors for White Light-Emitting Diodes. J. Electrochem. Soc. 2011, 158, R37–R54. [Google Scholar] [CrossRef]
- Chen, L.; Lin, C.-C.; Yeh, C.-W.; Liu, R.-S. Light Converting Inorganic Phosphors for White Light-Emitting Diodes. Materials 2010, 3, 2172–2195. [Google Scholar] [CrossRef]
- Xia, Z.; Xu, Z.; Chen, M.; Liu, Q. Recent Developments in the New Inorganic Solid-State LED Phosphors. Dalt. Trans. 2016, 45, 11214–11232. [Google Scholar] [CrossRef] [PubMed]
- Nikl, M. Scintillation Detectors for X-rays. Meas. Sci. Technol. 2006, 17, R37–R54. [Google Scholar] [CrossRef]
- Dorenbos, P. Light Output and Energy Resolution of Ce3+-Doped Scintillators. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2002, 486, 208–213. [Google Scholar] [CrossRef]
- Moretti, F.; Patton, G.; Belsky, A.; Fasoli, M.; Vedda, A.; Trevisani, M.; Bettinelli, M.; Dujardin, C. Radioluminescence Sensitization in Scintillators and Phosphors: Trap Engineering and Modeling. J. Phys. Chem. C 2014, 118, 9670–9676. [Google Scholar] [CrossRef]
- Andreo, P.; Burns, D.T.; Nahum, A.E.; Seuntjens, J.P.; Attix, F.H. Fundamentals of Ionizing Radiation Dosimetry; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Xu, J.; Tanabe, S. Persistent Luminescence Instead of Phosphorescence: History, Mechanism, and Perspective. J. Lumin. 2019, 205, 581–620. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Li, Z.-Y.; Zhang, J.-Y.; Lu, Y.; Guo, S.-Q.; Zhao, Q.; Wang, X.; Yong, Z.-J.; Li, H.; Ma, J.-P.; et al. X-Ray-Activated Long Persistent Phosphors Featuring Strong UVC Afterglow Emissions. Light Sci. Appl. 2018, 7, 88. [Google Scholar] [CrossRef]
- Sun, S.-K.; Wang, H.-F.; Yan, X.-P. Engineering Persistent Luminescence Nanoparticles for Biological Applications: From Biosensing/Bioimaging to Theranostics. Acc. Chem. Res. 2018, 51, 1131–1143. [Google Scholar] [CrossRef]
- Viana, B.; Sharma, S.K.; Gourier, D.; Maldiney, T.; Teston, E.; Scherman, D.; Richard, C. Long Term in Vivo Imaging with Cr3+ Doped Spinel Nanoparticles Exhibiting Persistent Luminescence. J. Lumin. 2016, 170, 879–887. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Zheng, S.; Li, J.; Fu, X.; Zhang, H. A New Near-Infrared Persistent Luminescence Nanoparticle as a Multifunctional Nanoplatform for Multimodal Imaging and Cancer Therapy. Biomaterials 2018, 152, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lv, Y.; Wang, L.; Chen, W.; Zhou, T.-L.; Takeda, T.; Hirosaki, N.; Xie, R.-J. Trap Depth Engineering of SrSi2O2N2:Ln2+,Ln3+ (Ln2+ = Yb, Eu; Ln3+ = Dy, Ho, Er) Persistent Luminescence Materials for Information Storage Applications. ACS Appl. Mater. Interfaces 2018, 10, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jin, Y.; Lv, Y.; Ju, G.; Liu, D.; Chen, L.; Li, Z.; Hu, Y. Trap Distribution Tailoring Guided Design of Super-Long-Persistent Phosphor Ba2SiO4:Eu2+,Ho3+ and Photostimulable Luminescence for Optical Information Storage. J. Mater. Chem. C 2018, 6, 6058–6067. [Google Scholar] [CrossRef]
- Li, W.; Zhuang, Y.; Zheng, P.; Zhou, T.-L.; Xu, J.; Ueda, J.; Tanabe, S.; Wang, L.; Xie, R.-J. Tailoring Trap Depth and Emission Wavelength in Y3Al5–xGaxO12:Ce3+,V3+ Phosphor-in-Glass Films for Optical Information Storage. ACS Appl. Mater. Interfaces 2018, 10, 27150–27159. [Google Scholar] [CrossRef] [PubMed]
- Bolek, P.; Kulesza, D.; Zych, E. On Site-Selective Optically and Thermally Induced Processes in Lu2O3:Tb,Ta Storage Phosphors. J. Lumin. 2019, 209, 274–282. [Google Scholar] [CrossRef]
- Yanagida, T.; Okada, G.; Kawaguchi, N. Ionizing-Radiation-Induced Storage-Luminescence for Dosimetric Applications. J. Lumin. 2019, 207, 14–21. [Google Scholar] [CrossRef]
- Leblans, P.; Vandenbroucke, D.; Willems, P. Storage Phosphors for Medical Imaging. Materials 2011, 4, 1034–1086. [Google Scholar] [CrossRef]
- Crawford, M.K.; Brixner, L.H. Photostimulable Phosphors for X-Ray Imaging: Applications and Mechanism. J. Lumin. 1991, 48–49, 37–42. [Google Scholar] [CrossRef]
- Von Seggern, H. Photostimulable X-Ray Storage Phosphors: A Review of Present Understanding. Brazilian J. Phys. 1999, 29, 254–268. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4:Eu2+, Dy3+. J. Electrochem. Soc. 1996, 143, 2670–2673. [Google Scholar] [CrossRef]
- Van den Eeckhout, K.; Smet, P.F.; Poelman, D. Persistent Luminescence in Eu2+-Doped Compounds: A Review. Materials 2010, 3, 2536–2566. [Google Scholar] [CrossRef]
- Maldiney, T.; Sraiki, G.; Viana, B.; Gourier, D.; Richard, C.; Scherman, D.; Bessodes, M.; Van den Eeckhout, K.; Poelman, D.; Smet, P.F. In Vivo Optical Imaging with Rare Earth Doped Ca2Si5N8 Persistent Luminescence Nanoparticles. Opt. Mater. Express 2012, 2, 261–268. [Google Scholar] [CrossRef]
- Le Masne de Chermont, Q.; Chaneac, C.; Seguin, J.; Pelle, F.; Maitrejean, S.; Jolivet, J.-P.; Gourier, D.; Bessodes, M.; Scherman, D. Nanoprobes with Near-Infrared Persistent Luminescence for in Vivo Imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 9266–9271. [Google Scholar] [CrossRef] [PubMed]
- Maldiney, T.; Lecointre, A.; Viana, B.; Bessière, A.; Gourier, D.; Bessodes, M.; Richard, C.; Scherman, D. Trap Depth Optimization to Improve Optical Properties of Diopside-Based Nanophosphors for Medical Imaging; Teherani, F.H., Look, D.C., Rogers, D.J., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2012; Volume 8263, p. 826318. [Google Scholar]
- Bessière, A.; Sharma, S.K.; Basavaraju, N.; Priolkar, K.R.; Binet, L.; Viana, B.; Bos, A.J.J.; Maldiney, T.; Richard, C.; Scherman, D.; et al. Storage of Visible Light for Long-Lasting Phosphorescence in Chromium-Doped Zinc Gallate. Chem. Mater. 2014, 26, 1365–1373. [Google Scholar] [CrossRef]
- Glais, E.; Pellerin, M.; Castaing, V.; Alloyeau, D.; Touati, N.; Viana, B.; Chanéac, C. Luminescence Properties of ZnGa2O4:Cr3+,Bi3+ Nanophosphors for Thermometry Applications. RSC Adv. 2018, 8, 41767–41774. [Google Scholar] [CrossRef]
- Cooper, D.R.; Capobianco, J.A.; Seuntjens, J. Radioluminescence Studies of Colloidal Oleate-Capped β-Na(Gd,Lu)F4:Ln3+ Nanoparticles (Ln = Ce, Eu, Tb). Nanoscale 2018, 10, 7821–7832. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, D.; Kuzmin, A.; Pliss, A.; Shao, W.; Xia, J.; Qu, J.; Prasad, P.N. ICG-Sensitized NaYF4:Er Nanostructure for Theranostics. Adv. Opt. Mater. 2018, 6, 1701142. [Google Scholar] [CrossRef]
- Hu, J.; Ortgies, D.H.; Martín Rodríguez, E.; Rivero, F.; Aguilar Torres, R.; Alfonso, F.; Fernández, N.; Carreño-Tarragona, G.; Monge, L.; Sanz-Rodriguez, F.; et al. Optical Nanoparticles for Cardiovascular Imaging. Adv. Opt. Mater. 2018, 6, 1800626. [Google Scholar] [CrossRef]
- Taylor, M.L.; Smith, R.L.; Dossing, F.; Franich, R.D. Robust Calculation of Effective Atomic Numbers: The Auto-ZEff Software. Med. Phys. 2012, 39, 1769–1778. [Google Scholar] [CrossRef]
- Van der Heggen, D.; Joos, J.J.; Rodríguez Burbano, D.; Capobianco, J.; Smet, P.F. Counting the Photons: Determining the Absolute Storage Capacity of Persistent Phosphors. Materials 2017, 10, 867. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Shen, Y.; Shi, F.; Song, C.; Liu, T.; Gao, P.; Lan, B.; Liu, M.; Wang, S.; et al. Ultra-high FRET efficiency NaGdF4:Tb3+-Rose Bengal biocompatible nanocomposite for X-ray excited photodynamic therapy application. Biomaterials 2018, 184, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Krumpel, A.H.; van der Kolk, E.; Zeelenberg, D.; Bos, A.J.J.; Krämer, K.W.; Dorenbos, P. Lanthanide 4f-Level Location in Lanthanide Doped and Cerium-Lanthanide Codoped NaLaF4 by Photo-and Thermoluminescence. J. Appl. Phys. 2008, 104, 073505. [Google Scholar] [CrossRef]
- Bettinelli, M.; Speghini, A.; Piccinelli, F.; Ueda, J.; Tanabe, S. Energy Transfer Processes in Sr3Tb0.90Eu 0.10(PO4)3. Opt. Mater. 2010, 33, 119–122. [Google Scholar] [CrossRef]
- Maldiney, T.; Richard, C.; Seguin, J.; Wattier, N.; Bessodes, M.; Scherman, D. Effect of Core Diameter, Surface Coating, and PEG Chain Length on the Biodistribution of Persistent Luminescence Nanoparticles in Mice. ACS Nano 2011, 5, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Huignard, A.; Buissette, V.; Laurent, G.; Gacoin, T.; Boilot, J.-P. Synthesis and Characterizations of YVO4:Eu Colloids. Chem. Mater. 2002, 14, 2264–2269. [Google Scholar] [CrossRef]
- Jursinic, P.A. Characterization of Optically Stimulated Luminescent Dosimeters, OSLDs, for Clinical Dosimetric Measurements. Med. Phys. 2007, 34, 4594–4604. [Google Scholar] [CrossRef] [PubMed]
- Yukihara, E.G.; McKeever, S.W.S. Optically Stimulated Luminescence (OSL) Dosimetry in Medicine. Phys. Med. Biol. 2008, 53, R351–R379. [Google Scholar] [CrossRef] [PubMed]
- Bekerat, H.; Devic, S.; DeBlois, F.; Singh, K.; Sarfehnia, A.; Seuntjens, J.; Shih, S.; Yu, X.; Lewis, D. Improving the Energy Response of External Beam Therapy (EBT) GafChromicTM Dosimetry Films at Low Energies (≤100 KeV). Med. Phys. 2014, 41, 022101. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van der Heggen, D.; Cooper, D.R.; Tesson, M.; Joos, J.J.; Seuntjens, J.; Capobianco, J.A.; Smet, P.F. Optically Stimulated Nanodosimeters with High Storage Capacity. Nanomaterials 2019, 9, 1127. https://doi.org/10.3390/nano9081127
Van der Heggen D, Cooper DR, Tesson M, Joos JJ, Seuntjens J, Capobianco JA, Smet PF. Optically Stimulated Nanodosimeters with High Storage Capacity. Nanomaterials. 2019; 9(8):1127. https://doi.org/10.3390/nano9081127
Chicago/Turabian StyleVan der Heggen, David, Daniel R. Cooper, Madeleine Tesson, Jonas J. Joos, Jan Seuntjens, John A. Capobianco, and Philippe F. Smet. 2019. "Optically Stimulated Nanodosimeters with High Storage Capacity" Nanomaterials 9, no. 8: 1127. https://doi.org/10.3390/nano9081127
APA StyleVan der Heggen, D., Cooper, D. R., Tesson, M., Joos, J. J., Seuntjens, J., Capobianco, J. A., & Smet, P. F. (2019). Optically Stimulated Nanodosimeters with High Storage Capacity. Nanomaterials, 9(8), 1127. https://doi.org/10.3390/nano9081127






