Conformal High-K Dielectric Coating of Suspended Single-Walled Carbon Nanotubes by Atomic Layer Deposition
Abstract
1. Introduction
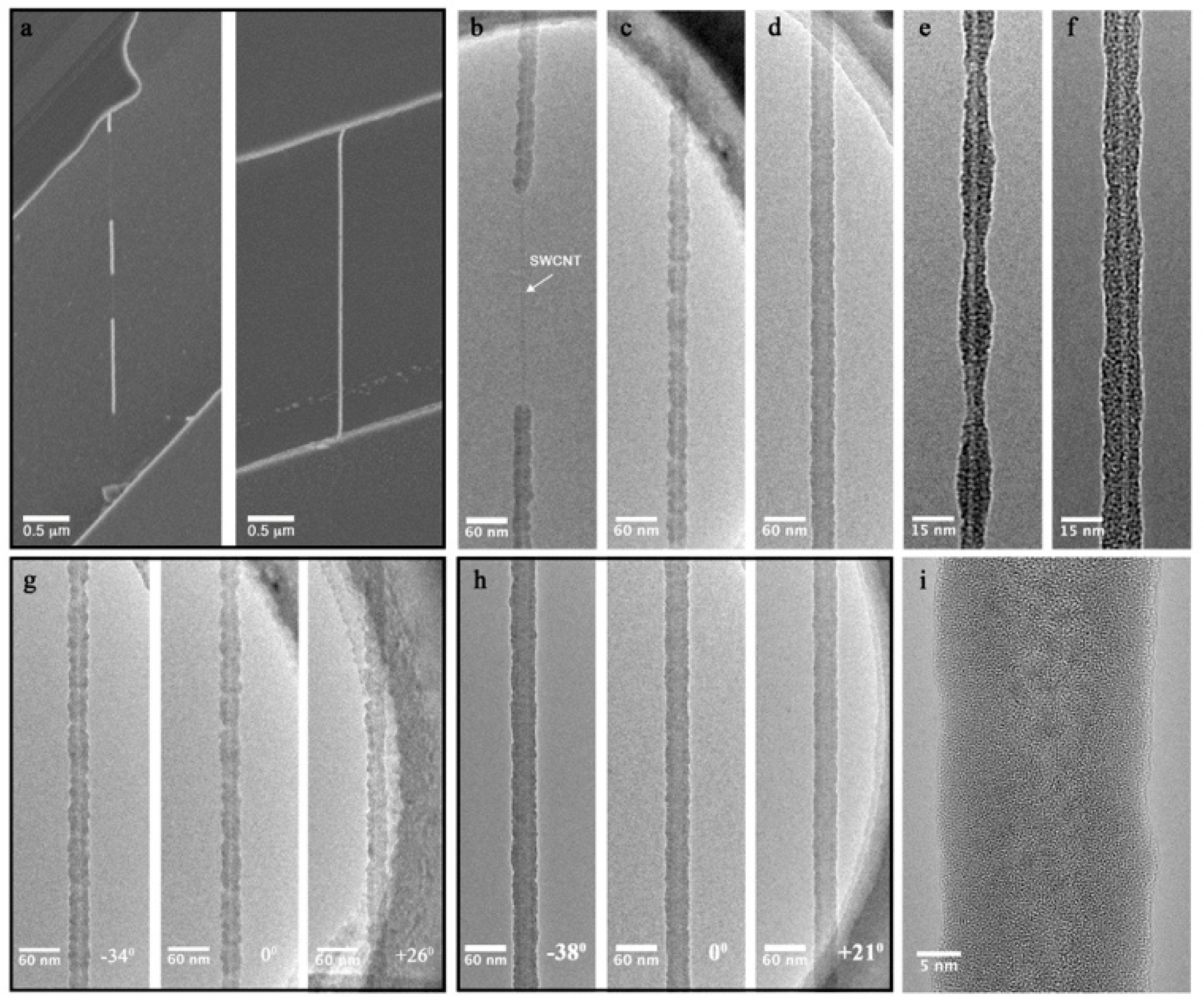
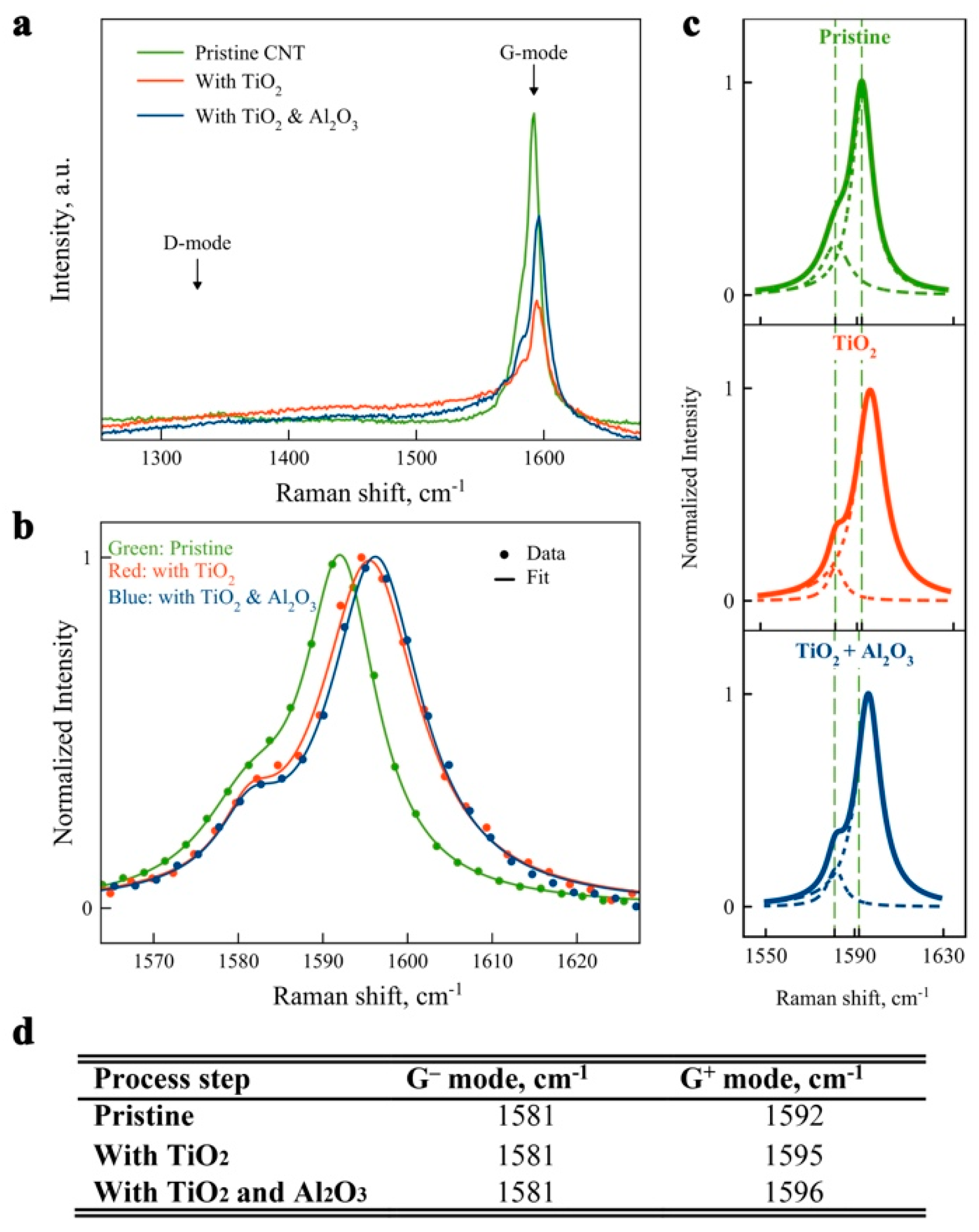
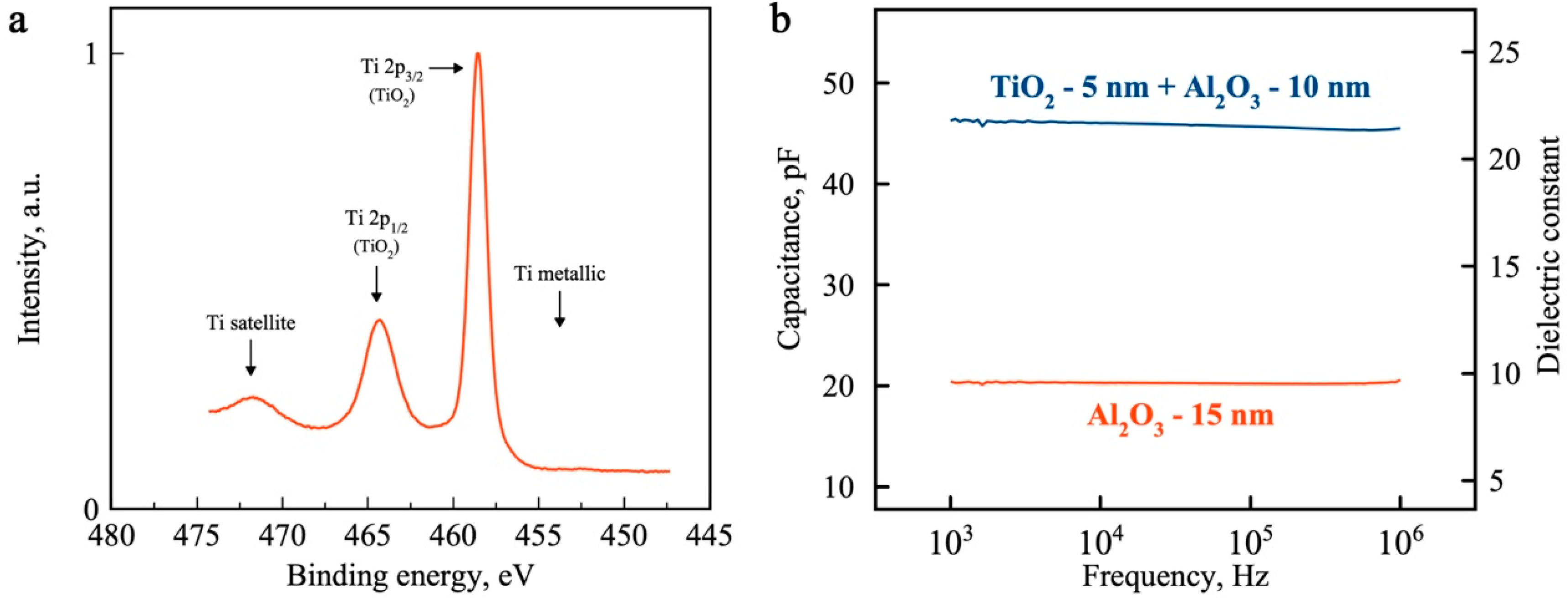
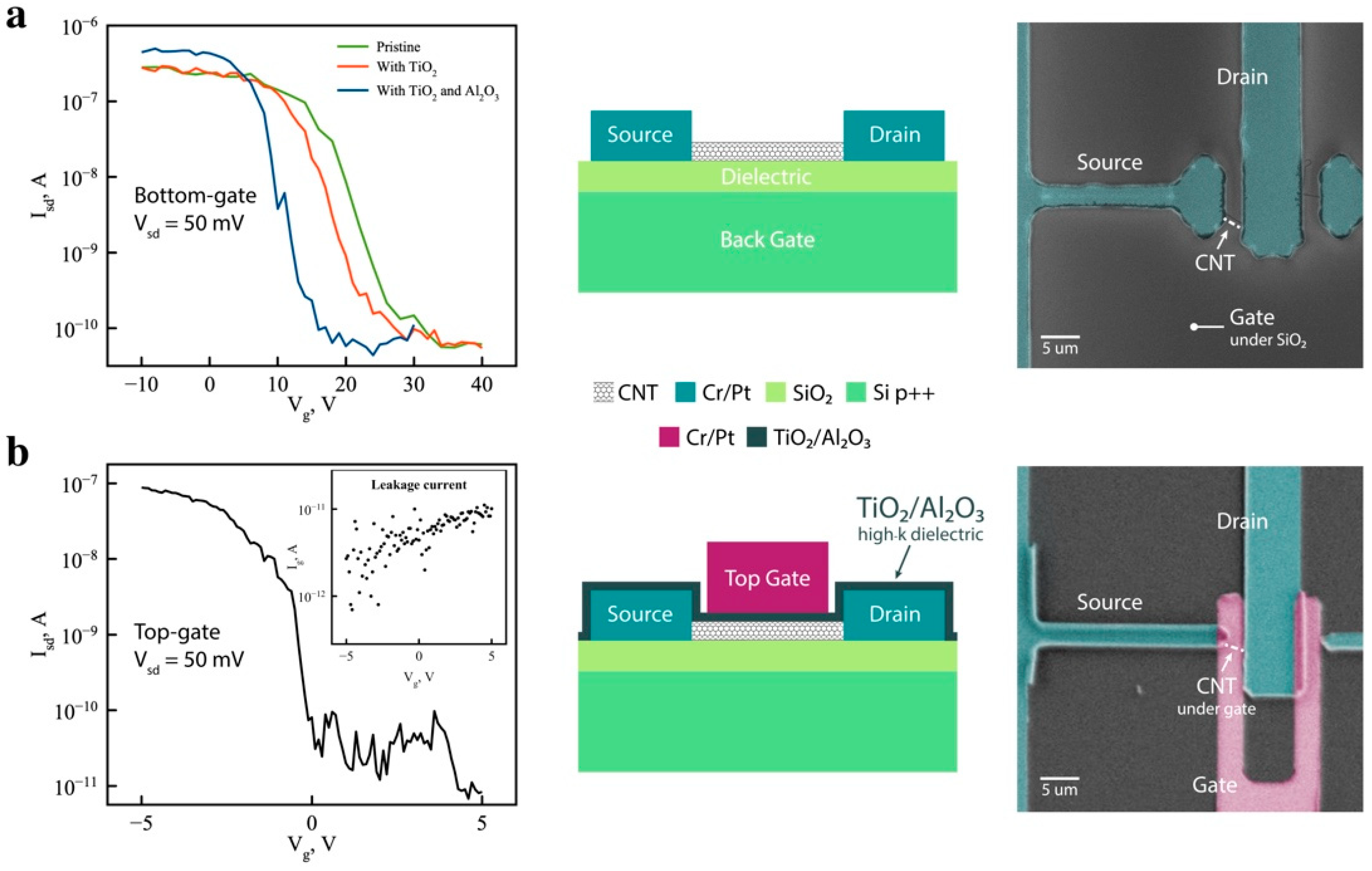
2. Results and Discussion
3. Methods
3.1. Synthesis
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coy, E.; Yate, L.; Kabacińska, Z.; Jancelewicz, M.; Jurga, S.; Iatsunskyi, I. Topographic reconstruction and mechanical analysis of atomic layer deposited Al2O3/TiO2 nanolaminates by nanoindentation. Mater. Des. 2016, 111, 584–591. [Google Scholar] [CrossRef]
- Grigoras, K.; Zavodchikova, M.Y.; Nasibulin, A.G.; Kauppinen, E.I.; Ernnolov, V.; Franssila, S. Atomic layer deposition of aluminum oxide films for carbon nanotube network transistor passivation. J. Nanosci. Nanotechnol. 2011, 11, 8818–8825. [Google Scholar] [CrossRef] [PubMed]
- Javey, A.; Guo, J.; Farmer, D.B.; Wang, Q.; Yenilmez, E.; Gordon, R.G.; Lundstrom, M.; Dai, H. Self-aligned ballistic molecular transistors and electrically parallel nanotube arrays. Nano Lett. 2004, 4, 1319–1322. [Google Scholar] [CrossRef]
- Hollander, M.J.; Labella, M.; Hughes, Z.R.; Zhu, M.; Trumbull, K.A.; Cavalero, R.; Snyder, D.W.; Wang, X.; Hwang, E.; Datta, S.; et al. Enhanced transport and transistor performance with oxide seeded high-κ gate dielectrics on wafer-scale epitaxial graphene. Nano Lett. 2011, 11, 3601–3607. [Google Scholar] [CrossRef] [PubMed]
- Dahal, A.; Addou, R.; Azcatl, A.; Coy-Diaz, H.; Lu, N.; Peng, X.; De Dios, F.; Kim, J.; Kim, M.J.; Wallace, R.M.; et al. Seeding atomic layer deposition of alumina on graphene with yttria. ACS Appl. Mater. Interfaces 2015, 7, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.; Brennan, B.; Azcatl, A.; Lu, N.; Dong, H.; Buie, C.; Kim, J.; Hinkle, C.L.; Kim, M.J.; Wallace, R.M. HfO2 on MoS2 by atomic layer deposition: Adsorption mechanisms and thickness scalability. ACS Nano 2013, 7, 10354–10361. [Google Scholar] [CrossRef]
- Kim, H.G.; Lee, H.B.R. Atomic Layer Deposition on 2D Materials. Chem. Mater. 2017, 29, 3809–3826. [Google Scholar] [CrossRef]
- Banerjee, S.; Hemraj-Benny, T.; Wong, S.S. Covalent surface chemistry of single-walled carbon nanotubes. Adv. Mater. 2005, 17, 17–29. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.J.; Lee, H.R.; Song, J.H.; Yi, S.N.; Ha, D.H. Oxygen plasma effects on the electrical conductance of single-walled carbon nanotube bundles. J. Phys. D Appl. Phys. 2010, 43, 305402. [Google Scholar] [CrossRef]
- Cognet, L.; Tsyboulski, D.A.; Rocha, J.D.R.; Doyle, C.D.; Tour, J.M.; Weisman, R.B. Stepwise quenching of exciton fluorescence in carbon nanotubes by single-molecule reactions. Science 2007, 316, 1465–1468. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Barnard, A.; McEuen, P.L. Bending and Twisting of Suspended Single-Walled Carbon Nanotubes in Solution. Nano Lett. 2009, 9, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; Mclean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Hill, D.E.; Lin, Y.; Rao, A.M.; Allard, L.F.; Sun, Y.P. Functionalization of carbon nanotubes with polystyrene. Macromolecules 2002, 35, 9466–9471. [Google Scholar] [CrossRef]
- Chen, R.J.; Bangsaruntip, S.; Drouvalakis, K.A.; Wong Shi Kam, N.; Shim, M.; Li, Y.; Kim, W.; Utz, P.J.; Dai, H. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. USA 2003, 100, 4984–4989. [Google Scholar] [CrossRef] [PubMed]
- Steuerman, D.W.; Star, A.; Narizzano, R.; Choi, H.; Ries, R.S.; Nicolini, C.; Stoddart, J.F.; Heath, J.R. Interactions between conjugated polymers and single-walled carbon nanotubes. J. Phys. Chem. B 2002, 106, 3124–3130. [Google Scholar] [CrossRef]
- Farmer, D.B.; Gordon, R.G. Atomic layer deposition on suspended single-walled carbon nanotubes via gas-phase noncovalent functionalization. Nano Lett. 2006, 6, 699–703. [Google Scholar] [CrossRef]
- Lin, Y.M.; Jenkins, K.A.; Valdes-Garcia, A.; Small, J.P.; Farmer, D.B.; Avouris, P. Operation of graphene transistors at gigahertz frequencies. Nano Lett. 2009, 9, 422–426. [Google Scholar] [CrossRef]
- Wang, L.; Travis, J.J.; Cavanagh, A.S.; Liu, X.; Koenig, S.P.; Huang, P.Y.; George, S.M.; Bunch, J.S. Ultrathin oxide films by atomic layer deposition on graphene. Nano Lett. 2012, 12, 3706–3710. [Google Scholar] [CrossRef]
- Chen, Z.; Farmer, D.; Xu, S.; Gordon, R.; Avouris, P.; Appenzeller, J. Externally assembled gate-all-around carbon nanotube field-effect transistor. IEEE Electron Device Lett. 2008, 29, 183–185. [Google Scholar] [CrossRef]
- Franklin, A.D.; Koswatta, S.O.; Farmer, D.B.; Smith, J.T.; Gignac, L.; Breslin, C.M.; Han, S.J.; Tulevski, G.S.; Miyazoe, H.; Haensch, W.; et al. Carbon nanotube complementary wrap-gate transistors. Nano Lett. 2013, 13, 2490–2495. [Google Scholar] [CrossRef] [PubMed]
- Suriyasena Liyanage, L.; Xu, X.; Pitner, G.; Bao, Z.; Wong, H.S.P. VLSI-compatible carbon nanotube doping technique with low work-function metal oxides. Nano Lett. 2014, 14, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, H.; Zhang, Z.; Wang, S.; Ding, L.; Zeng, Q.; Yang, L.; Pei, T.; Liang, X.; Gao, M.; et al. Growth and performance of yttrium oxide as an ideal high-κ gate dielectric for carbon-based electronics. Nano Lett. 2010, 6, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Franklin, N.W.; Chen, R.J.; Dai, H.J. Metal coating on suspended carbon nanotubes and its implication to metal-tube interaction. Chem. Phys. Lett. 2000, 331, 35–41. [Google Scholar] [CrossRef]
- Fallahazad, B.; Kim, S.; Colombo, L.; Tutuc, E. Dielectric thickness dependence of carrier mobility in graphene with HfO2 top dielectric. Appl. Phys. Lett. 2010, 97, 123105. [Google Scholar] [CrossRef]
- Kim, S.; Nah, J.; Jo, I.; Shahrjerdi, D.; Colombo, L.; Yao, Z.; Tutuc, E.; Banerjee, S.K. Realization of a high mobility dual-gated graphene field-effect transistor with Al2O3 dielectric. Appl. Phys. Lett. 2009, 94, 062107. [Google Scholar] [CrossRef]
- Robinson, J.A.; Labella, M.; Trumbull, K.A.; Weng, X.; Cavelero, R.; Daniels, T.; Hughes, Z.; Hollander, M.; Fanton, M.; Snyder, D. Epitaxial graphene materials integration: Effects of dielectric overlayers on structural and electronic properties. ACS Nano 2010, 97, 123105. [Google Scholar] [CrossRef]
- Zou, X.; Wang, J.; Chiu, C.-H.; Wu, Y.; Xiao, X.; Jiang, C.; Wu, W.-W.; Mai, L.; Chen, T.; Li, J.; et al. Interface Engineering for High-Performance Top-Gated MoS2 Field-Effect Transistors. Adv. Mater. 2014, 26, 6255–6261. [Google Scholar] [CrossRef]
- Son, S.; Yu, S.; Choi, M.; Kim, D.; Choi, C. Improved high temperature integration of Al2O3 on MoS2 by using a metal oxide buffer layer. Appl. Phys. Lett. 2015, 106, 021601. [Google Scholar] [CrossRef]
- Zhang, H.; Arutchelvan, G.; Meersschaut, J.; Gaur, A.; Conard, T.; Bender, H.; Lin, D.; Asselberghs, I.; Heyns, M.; Radu, I.; et al. MoS2 Functionalization with a Sub-nm Thin SiO2 Layer for Atomic Layer Deposition of High-κ Dielectrics. Chem. Mater. 2017, 29, 6772–6780. [Google Scholar] [CrossRef]
- Fallahazad, B.; Lee, K.; Lian, G.; Kim, S.; Corbet, C.M.; Ferrer, D.A.; Colombo, L.; Tutuc, E. Scaling of Al2O3 dielectric for graphene field-effect transistors. Appl. Phys. Lett. 2012, 100, 093112. [Google Scholar] [CrossRef]
- Robertson, J. High dielectric constant gate oxides for metal oxide Si transistors. Rep. Prog. Phys. 2006, 69, 327. [Google Scholar] [CrossRef]
- Maiti, A.; Ricca, A. Metal-nanotube interactions-Binding energies and wetting properties. Chem. Phys. Lett. 2004, 395, 7–11. [Google Scholar] [CrossRef]
- Burns, G.P. Titanium dioxide dielectric films formed by rapid thermal oxidation. J. Appl. Phys. 1989, 65, 2095–2097. [Google Scholar] [CrossRef]
- Gialampouki, M.A.; Lekka, C.E. TiN decoration of single-wall carbon nanotubes and graphene by density functional theory computations. J. Phys. Chem. C 2011, 115, 15172–15181. [Google Scholar] [CrossRef]
- Yang, C.K.; Zhao, J.; Lu, J.P. Binding energies and electronic structures of adsorbed titanium chains on carbon nanotubes. Phys. Rev. B Condens. Matter Mater. Phys. 2002, 66, 041403. [Google Scholar] [CrossRef]
- Khomyakov, P.A.; Giovannetti, G.; Rusu, P.C.; Brocks, G.; Van Den Brink, J.; Kelly, P.J. First-principles study of the interaction and charge transfer between graphene and metals. Phys. Rev. B Condens. Matter Mater. Phys. 2009, 79, 195425. [Google Scholar] [CrossRef]
- Rojas, M.I.; Leiva, E.P.M. Density functional theory study of a graphene sheet modified with titanium in contact with different adsorbates. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 155415. [Google Scholar] [CrossRef]
- Felten, A.; Suarez-Martinez, I.; Ke, X.; Van Tendeloo, G.; Ghijsen, J.; Pireaux, J.J.; Drube, W.; Bittencourt, C.; Ewels, C.P. The role of oxygen at the interface between titanium and carbon nanotubes. Chem. Phys. Chem. 2009, 10, 1799–1804. [Google Scholar] [CrossRef]
- Rao, A.M.; Eklund, P.C.; Bandow, S.; Thess, A.; Smalley, R.E. Evidence for charge transfer in doped carbon nanotube bundles from Raman scattering. Nature 1997, 388, 257–259. [Google Scholar] [CrossRef]
- Gao, B.; Jiang, L.; Ling, X.; Zhang, J.; Liu, Z. Chirality-dependent Raman frequency variation of single-walled carbon nanotubes under uniaxial strain. J. Phys. Chem. C 2008, 112, 20123–20125. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, J.; Dong, J. Raman modes of the deformed single-wall carbon nanotubes. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 72, 115411. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C.; Payne, B.P.; Grosvenor, A.P. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Nosho, Y.; Ohno, Y.; Kishimoto, S.; Mizutani, T. Relation between conduction property and work function of contact metal in carbon nanotube field-effect transistors. Nanotechnology 2006, 17, 3412–3415. [Google Scholar] [CrossRef]
- Khanal, D.R.; Levander, A.X.; Yu, K.M.; Liliental-Weber, Z.; Walukiewicz, W.; Grandal, J.; Snchez-Garca, M.A.; Calleja, E.; Wu, J. Decoupling single nanowire mobilities limited by surface scattering and bulk impurity scattering. J. Appl. Phys. 2011, 110, 033705. [Google Scholar] [CrossRef]
- Dürkop, T.; Getty, S.A.; Cobas, E.; Fuhrer, M.S. Extraordinary Mobility in Semiconducting Carbon Nanotubes. Nano Lett. 2004, 4, 35–39. [Google Scholar] [CrossRef]
- Kim, W.; Javey, A.; Vermesh, O.; Wang, Q.; Li, Y.; Dai, H. Hysteresis caused by water molecules in carbon nanotube field-effect transistors. Nano Lett. 2003, 3, 193–198. [Google Scholar] [CrossRef]
- Martin, M.; Mader, W.; Fromm, E. Oxidation of iron, aluminium and titanium films in the temperature range 50–200 °C. Thin Solid Films 1994, 250, 61–66. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemelbay, A.; Tikhonov, A.; Aloni, S.; Kuykendall, T.R. Conformal High-K Dielectric Coating of Suspended Single-Walled Carbon Nanotubes by Atomic Layer Deposition. Nanomaterials 2019, 9, 1085. https://doi.org/10.3390/nano9081085
Kemelbay A, Tikhonov A, Aloni S, Kuykendall TR. Conformal High-K Dielectric Coating of Suspended Single-Walled Carbon Nanotubes by Atomic Layer Deposition. Nanomaterials. 2019; 9(8):1085. https://doi.org/10.3390/nano9081085
Chicago/Turabian StyleKemelbay, Aidar, Alexander Tikhonov, Shaul Aloni, and Tevye R. Kuykendall. 2019. "Conformal High-K Dielectric Coating of Suspended Single-Walled Carbon Nanotubes by Atomic Layer Deposition" Nanomaterials 9, no. 8: 1085. https://doi.org/10.3390/nano9081085
APA StyleKemelbay, A., Tikhonov, A., Aloni, S., & Kuykendall, T. R. (2019). Conformal High-K Dielectric Coating of Suspended Single-Walled Carbon Nanotubes by Atomic Layer Deposition. Nanomaterials, 9(8), 1085. https://doi.org/10.3390/nano9081085




