Purification and Nitrogen Doping of Nanothin Exfoliated Graphite Through RF Thermal Plasma Treatment
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
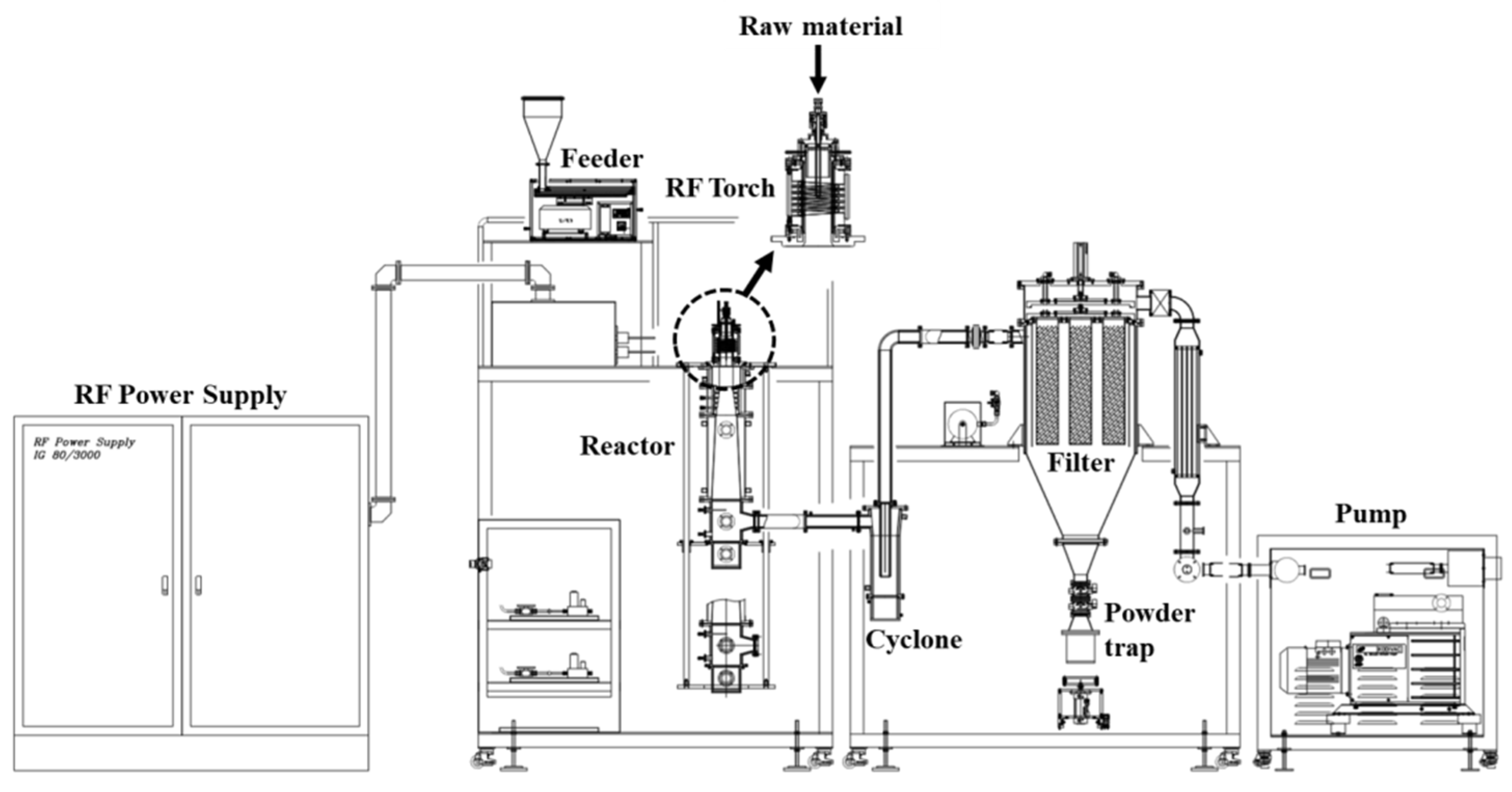
2.2. Configuration of the RF Plasma System
2.3. Synthesis of Nitrogen-Doped NTE Graphite
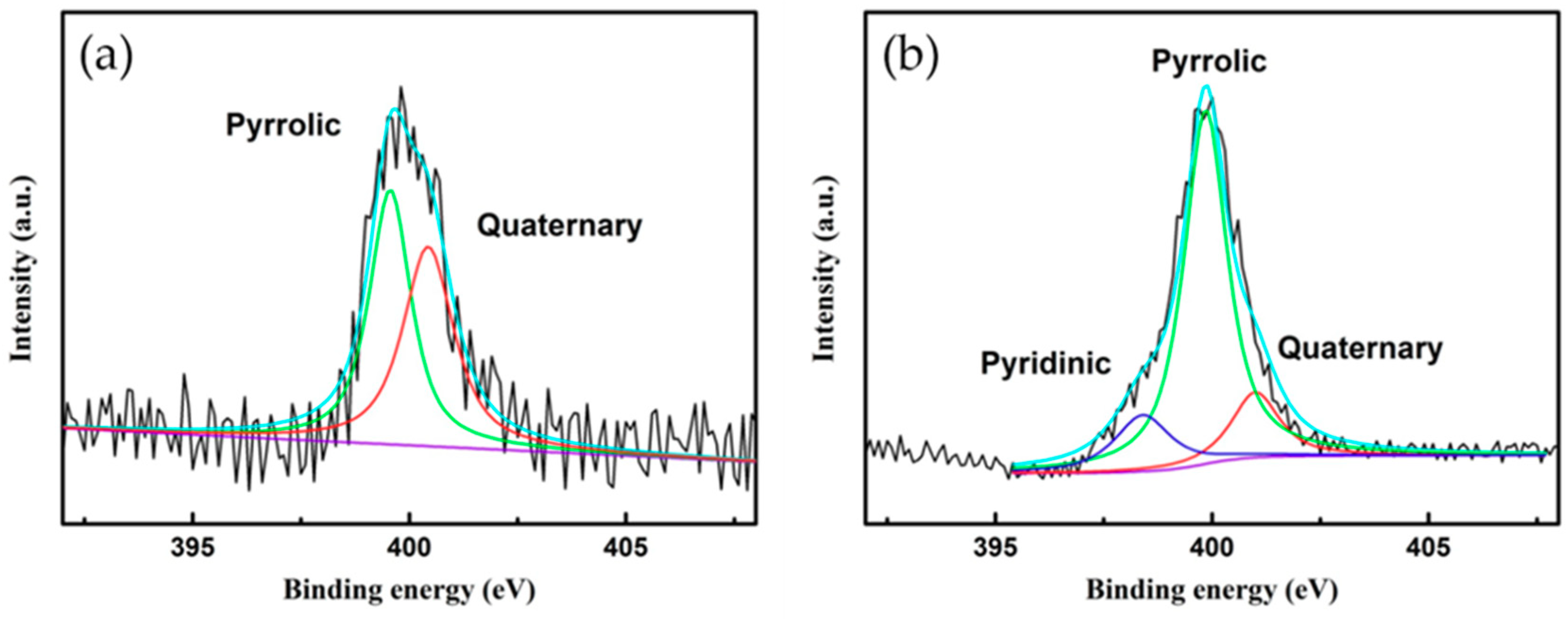
3. Results and Discussion
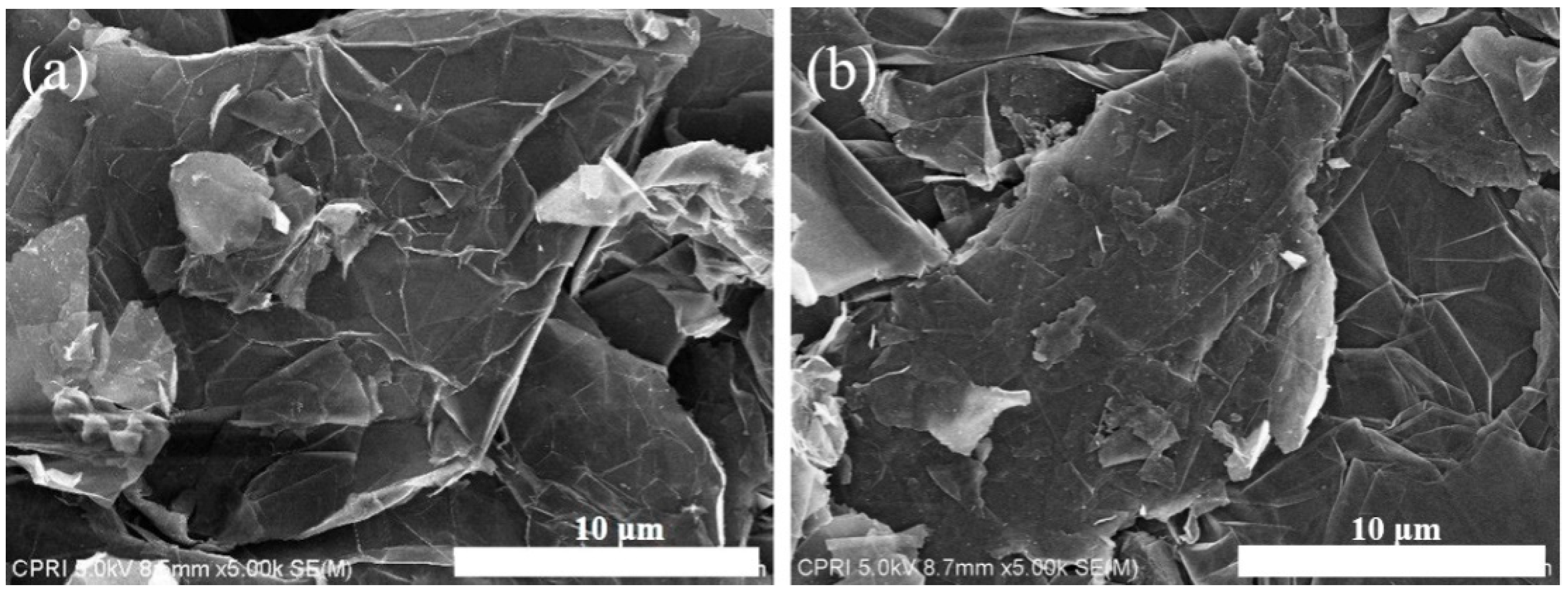
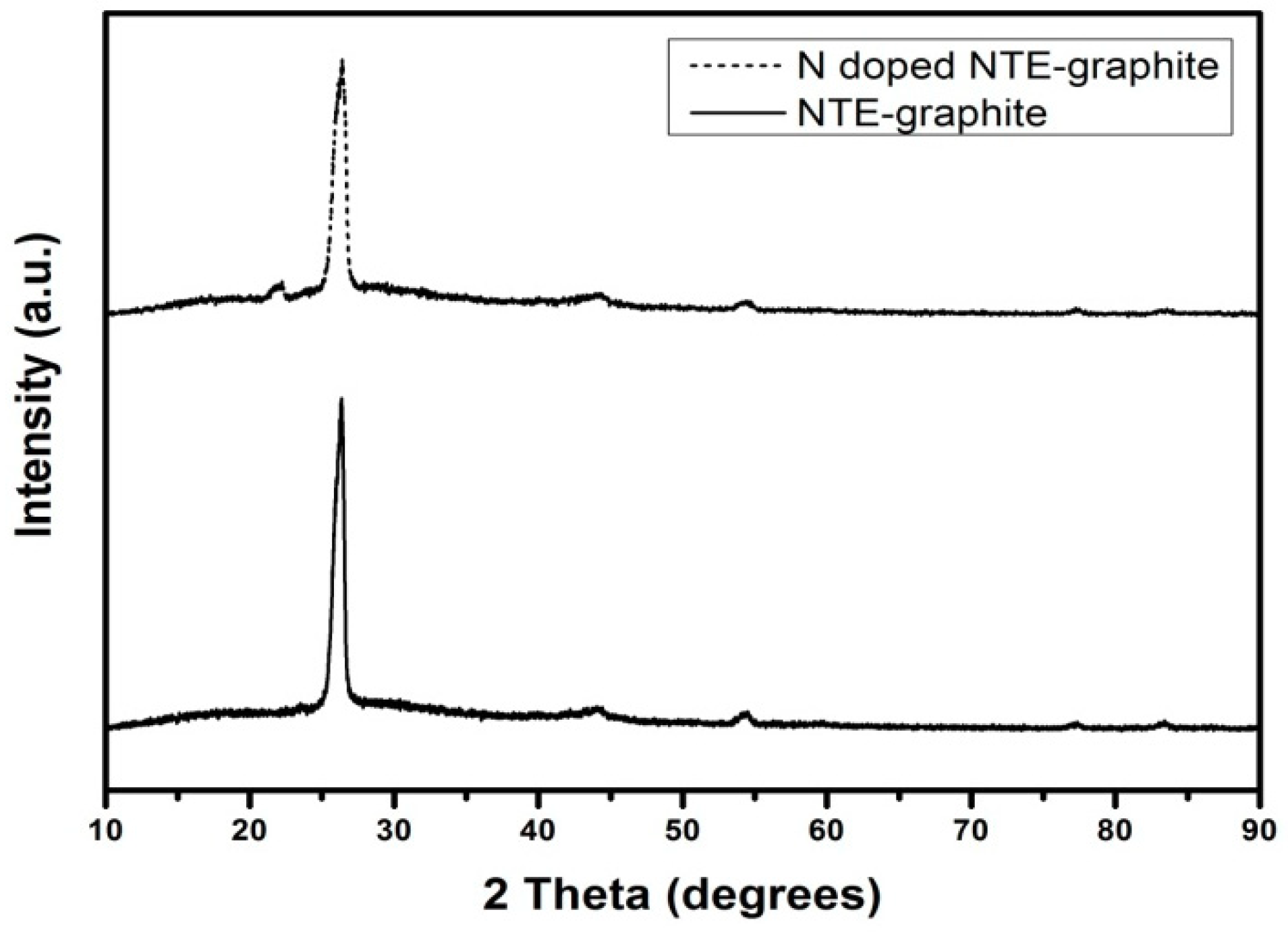
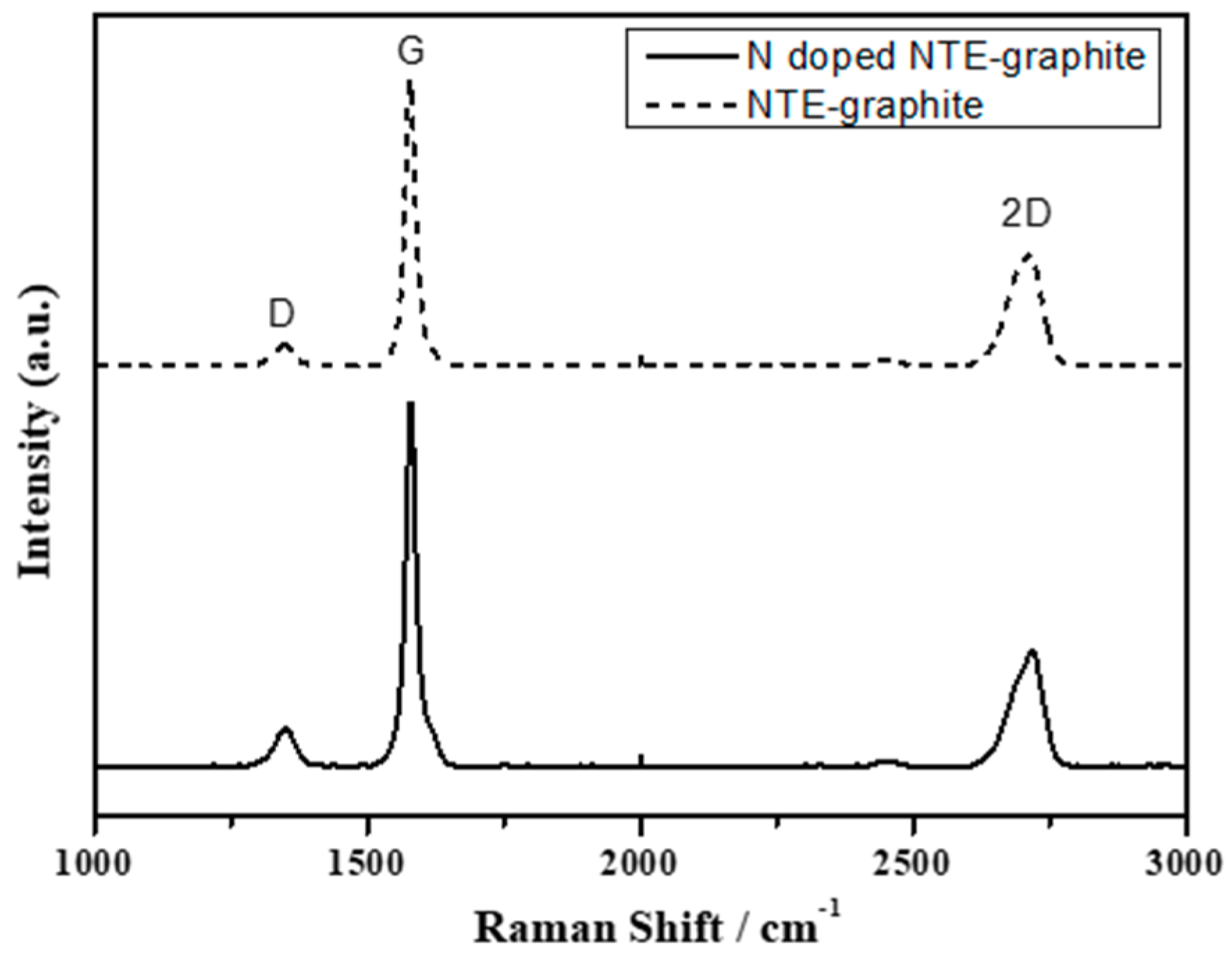
3.1. Analysis of N-Doped NTE Graphite and Untreated NTE Graphite
3.2. Electrical Performance of the N-Doped NTE Graphite Sheet
3.3. N Doping Process in the RF Thermal Plasma System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Obraztsov, A.N.; Obraztsova, E.A.; Tyurnina, A.V.; Zolotukhin, A.A. Chemical vapor deposition of thin graphite films of nanometer thickness. Carbon 2007, 45, 2017–2021. [Google Scholar] [CrossRef]
- Chae, S.J.; Güneş, F.; Kim, K.K.; Kim, E.S.; Han, G.H.; Kim, S.M.; Shin, H.; Yoon, S.-M.; Choi, J.-Y.; Park, M.H.; et al. Synthesis of large-area graphene layers on poly-nickel substrate by chemical vapor deposition: Wrinkle formation. Adv. Mater. 2009, 21, 2328–2333. [Google Scholar] [CrossRef]
- Zheng, G.; Wu, J.; Wang, W.; Pan, C. Characterizations of expanded graphite/polymer composites prepared by in situ polymerization. Carbon 2004, 42, 2839–2847. [Google Scholar] [CrossRef]
- Li, J.; Vaisman, L.; Marom, G.; Kim, J.-K. Br treated graphite nanoplatelets for improved electrical conductivity of polymer composites. Carbon 2007, 45, 744–750. [Google Scholar] [CrossRef] [Green Version]
- George, J.J.; Brandyopadhyay, A.; Bhowmick, A.K. New generation layered nanocomposites derived from ethylene-co-vinyl acetate and naturally occurring graphite. J. Appl. Polym. Sci. 2008, 108, 1603–1616. [Google Scholar] [CrossRef]
- Steurer, P.; Wissert, R.; Thomann, R.; Mülhaupt, R. Functionalized graphenes and thermoplastic nanocomposites based upon expanded graphite oxide. Macromol. Rapid Commun. 2009, 30, 316–327. [Google Scholar] [CrossRef]
- Boscá, A.; Pedrós, J.; Martínez, J.; Palacios, T.; Calle, F. Automatic graphene transfer system for improved material quality and efficiency. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Ong, B.K.; Poh, H.L.; Chua, C.K.; Pumera, M. Graphenes prepared by hummers, staudenmaier and hofmann methods for analysis of TNT-based nitroaromatic explosives in seawater. Electroanalysis 2012, 24, 2085–2093. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Lomeda, J.R.; Doyle, C.D.; Kosynkin, D.V.; Hwang, W.; Tour, J.M. Diazonium functionalization of surfactant-wrapped chemically converted graphene sheets. J. Am. Chem. Soc. 2008, 130, 16201–16206. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Shi, Y.; Huang, W.; Chen, P.; Li, L.J. Electrical detection of DNA hybridization with single-base specificity using transistors based on CVD-grown graphene sheets. Adv. Mater. 2010, 22, 1649–1653. [Google Scholar] [CrossRef]
- Suk, J.W.; Kitt, A.; Magnuson, C.W.; Hao, Y.; Ahmed, S.; An, J.; Swan, A.K.; Goldberg, B.B.; Ruoff, R.S. Transfer of CVD-grown monolayer graphene onto arbitrary substrates. ACS Nano 2011, 5, 6916–6924. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile synthesis and supercapacitor properties of graphene nanosheets. J. Phys. Chem. 2008, 112, 8192–8195. [Google Scholar]
- Seo, J.H.; Kim, J.S.; Lee, M.Y.; Ju, W.T.; Nam, I.T. Direct synthesis of nano-sized glass powders with spherical shape by RF (radio frequency) thermal plasma. Thin Solid Film. 2011, 519, 7111–7115. [Google Scholar] [CrossRef]
- Oh, S.-M.; Ishigaki, T. Preparation of pure rutile and anatase TiO2 nanopowders using RF thermal plasma. Thin Solid Film. 2004, 457, 186–191. [Google Scholar] [CrossRef]
- Ko, S.-M.; Koo, S.-M.; Cho, W.-S.; Hwnag, K.-T.; Kim, J.-H. Synthesis of SiC nano-powder from organic precursors using RF inductively coupled thermal plasma. Ceram. Int. 2012, 38, 1959–1963. [Google Scholar] [CrossRef]
- Song, S.-K.; Son, B.; Kim, B.; Lee, M.; Sin, M.; Choi, S.; Lee, K.-H.; Kim, S.-I. Development of 80 kW RF thermal plasma torch system for mass production and research of Si nano-powder manufacturing process. J. Korean Vac. Soc. 2013, 22, 66–78. [Google Scholar] [CrossRef]
- Liu, J.; Takeshi, D.; Orejon, D.; Sasaki, K.; Lyth, S.M. Defective nitrogen-doped graphene foam: A metal-free, non-precious electrocatalyst for the oxygen reduction reaction in acid. J. Electrochem. Soc. 2014, 161, F544–F550. [Google Scholar] [CrossRef]
- Cai, M.; Thorpe, D.; Adamson, D.H.; Schniepp, H.C. Methods of graphite exfoliation. J. Mater. Chem. 2012, 22, 24992–25002. [Google Scholar] [CrossRef]
- Mou, Z.; Chen, X.; Du, Y.; Wang, X.; Yang, P.; Wang, S. Forming mechanism of nitrogen doped graphene prepared by thermal solid-state reaction of graphite oxide and urea. Appl. Surf. Sci. 2011, 258, 1704–1710. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Granzier-Nakajima, T.; Fujisawa, K.; Anil, V.; Terrones, M.; Yeh, Y.-T. Controlling nitrogen doping in graphene with atomic precision: Synthesis and characterization. Nanomaterials 2019, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Meyer, J.; Roth, S.; Skákalová, V. Growth and properties of few-layer graphene prepared by chemical vapor deposition. Carbon 2010, 48, 1088–1094. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.K.; Reina, A.; Shi, Y.; Park, H.; Li, L.-J.; Lee, Y.H.; Kong, J. Enhancing the conductivity of transparent graphene films via doping. Nanotechnology 2010, 21, 285205. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Tian, C.; Tan, T.; Xie, Y.; Shi, K.; Li, M.; Fu, H. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012, 2, 4498–4506. [Google Scholar] [CrossRef]
- Bernhard, A.M.; Peitz, D.; Elsener, M.; Schildhauer, T.; Kröcher, O. Catalytic urea hydrolysis in the selective catalytic reduction of NOx: Catalyst screening and kinetics on anatase TiO2 and ZrO2. Catal. Sci. Technol. 2013, 3, 942–951. [Google Scholar] [CrossRef]

| Plate Power | Plasma Forming Gas | Raw Material | Feeding Rate of Raw Material | Carrier Gas | |
|---|---|---|---|---|---|
| Central Gas | Sheath Gas | ||||
| 30 kW | 30 L/min Ar | 50 L/min Ar | Mixed powder 1 of NTE graphite and CO(NH2)2 | 1 kg/h | 10 L/min Ar |
| Materials | Elements (wt%) | |||
|---|---|---|---|---|
| C | N | H | S | |
| Untreated NTE graphite | 91.73 | 0.83 | 0.29 | 0.59 |
| N-doped NTE graphite | 90.85 | 3.99 | 0.48 | - |
| Materials | Bonding Structure | Total | ||
|---|---|---|---|---|
| Pyrrolic | Pyridinic | Quaternary | ||
| Untreated NTE graphite | 0.18 | - | 0.18 | 0.36 |
| N-doped NTE graphite | 0.26 | 1.28 | 0.26 | 1.79 |
| Materials | Sheet Resistance (Rs, mΩ/sq) | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Untreated NTE Graphite + Hot PRESS | 80.34 | 80.7 | 75.37 | 74.88 | 77.36 | 79.51 | 71.17 | 72.33 |
| N-doped NTE Graphite + Hot PRESS | 58.63 | 62.56 | 65 | 74.48 | 67.34 | 62.23 | 71.68 | 64.48 |
| Untreated NTE Graphite + Roll Process | 78.77 | 75.41 | 74.32 | 76.09 | 79.55 | 80.92 | 72.33 | 82.93 |
| N-doped NTE Graphite + Roll Process | 58.21 | 62.97 | 59.88 | 63.73 | 63.73 | 64.98 | 68.36 | 62.43 |
| Materials | Average Rs (mΩ/sq) | Thickness (μm) | Calibrated Rs (mΩ/sq at 100 μm) | Resistivity (Ω m) | ||||
| Untreated NTE Graphite + Hot PRESS | 76.46 | 113 | 86.40 | 8.63 × 10−6 | ||||
| N-doped NTE Graphite + Hot PRESS | 65.80 | 115.8 | 76.20 | 7.61 × 10−6 | ||||
| Untreated NTE Graphite + Roll Process | 77.54 | 111 | 86.07 | 8.60 × 10−6 | ||||
| N-doped NTE Graphite + Roll Process | 63.04 | 107.5 | 67.76 | 6.77 × 10−6 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, B.-K.; Lee, K.-H.; Kim, T.-H.; Shin, M.-S.; Choi, S.-Y.; Cho, G. Purification and Nitrogen Doping of Nanothin Exfoliated Graphite Through RF Thermal Plasma Treatment. Nanomaterials 2019, 9, 995. https://doi.org/10.3390/nano9070995
Son B-K, Lee K-H, Kim T-H, Shin M-S, Choi S-Y, Cho G. Purification and Nitrogen Doping of Nanothin Exfoliated Graphite Through RF Thermal Plasma Treatment. Nanomaterials. 2019; 9(7):995. https://doi.org/10.3390/nano9070995
Chicago/Turabian StyleSon, Byung-Koo, Kyu-Hang Lee, Tae-Hee Kim, Myung-Sun Shin, Sun-Yong Choi, and Guangsup Cho. 2019. "Purification and Nitrogen Doping of Nanothin Exfoliated Graphite Through RF Thermal Plasma Treatment" Nanomaterials 9, no. 7: 995. https://doi.org/10.3390/nano9070995
APA StyleSon, B.-K., Lee, K.-H., Kim, T.-H., Shin, M.-S., Choi, S.-Y., & Cho, G. (2019). Purification and Nitrogen Doping of Nanothin Exfoliated Graphite Through RF Thermal Plasma Treatment. Nanomaterials, 9(7), 995. https://doi.org/10.3390/nano9070995





