A Facile Method to Synthesize CdSe-Reduced Graphene Oxide Composite with Good Dispersion and High Nonlinear Optical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Oxide (GO)
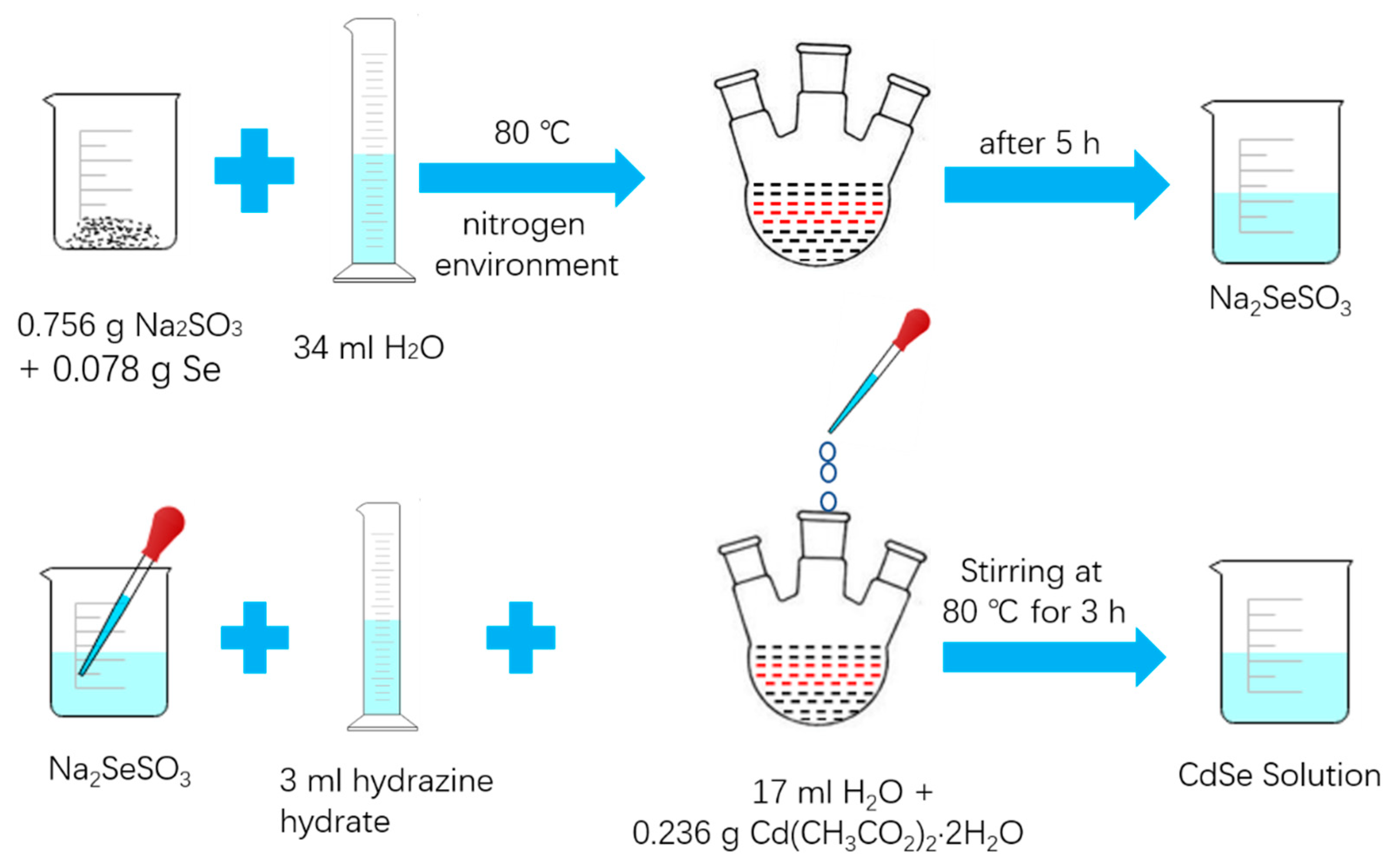
2.3. Synthesis of CdSe Nanoparticle
2.3.1. Preparation of Sodium Selenosulfate (Na2SeSO3) Solution
2.3.2. Synthesis of CdSe Nanoparticle
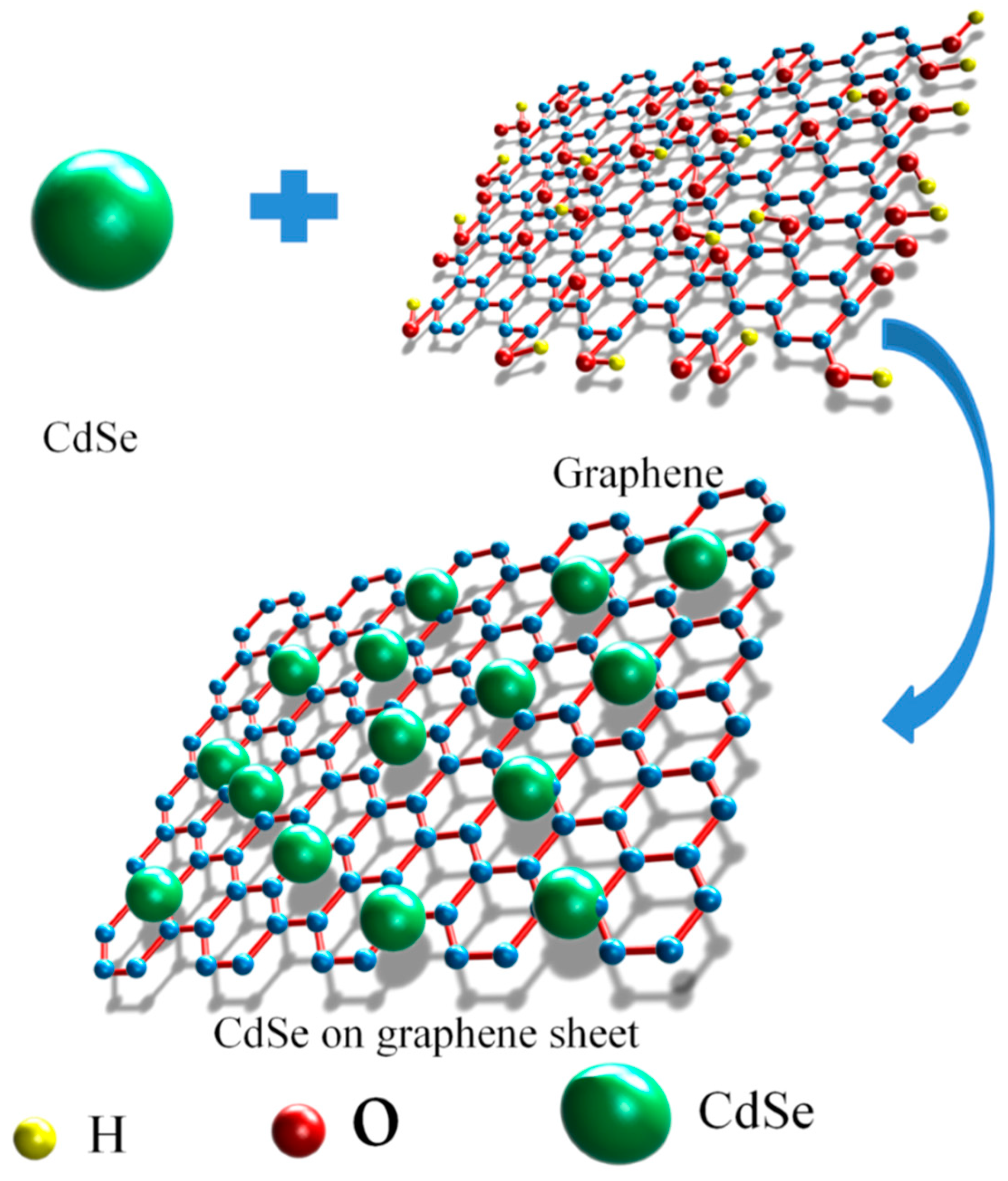
2.4. Synthesis of CdSe/RGO Nanocomposite
2.5. Sample Characterization
3. Results and Discussion
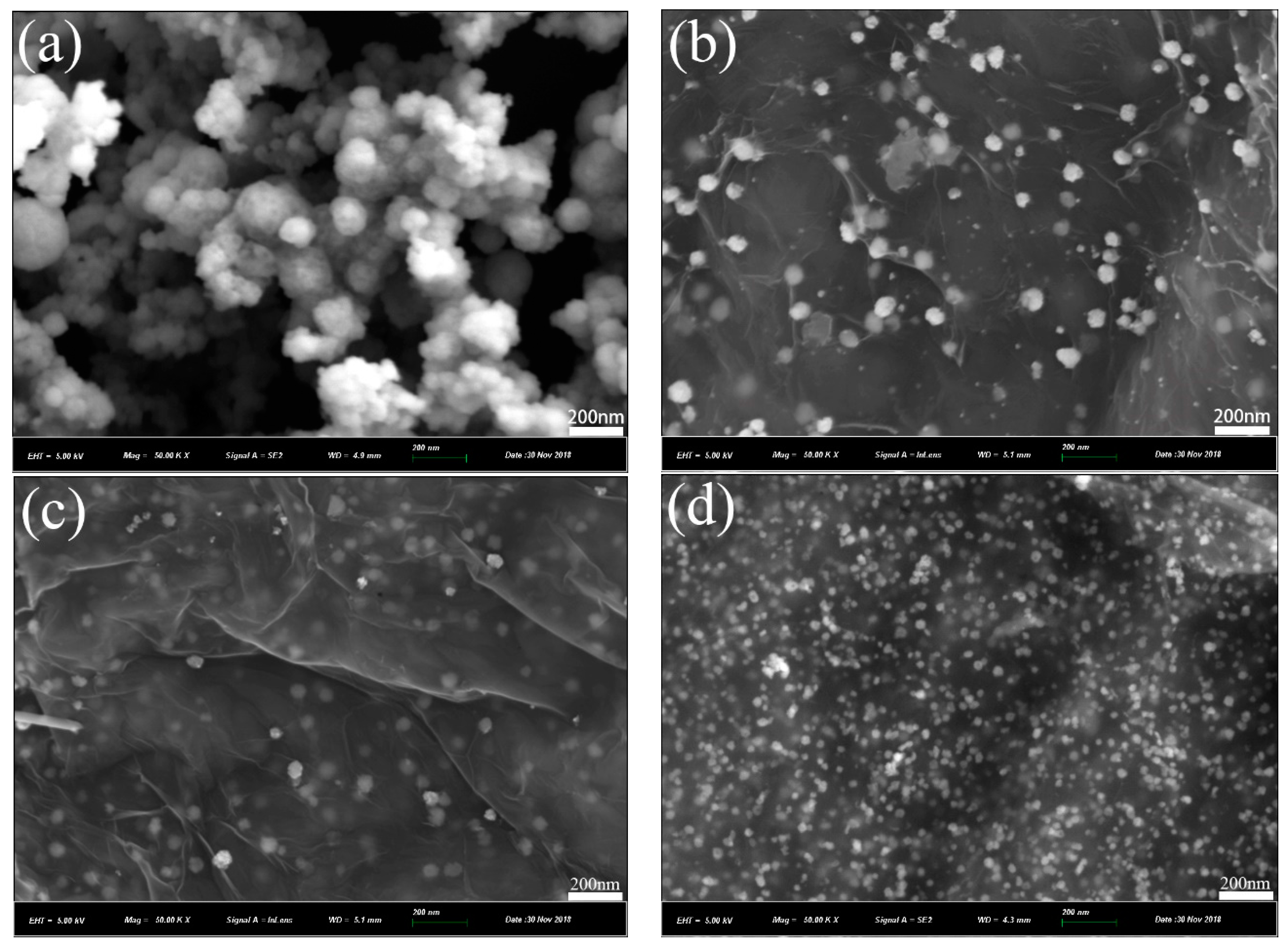
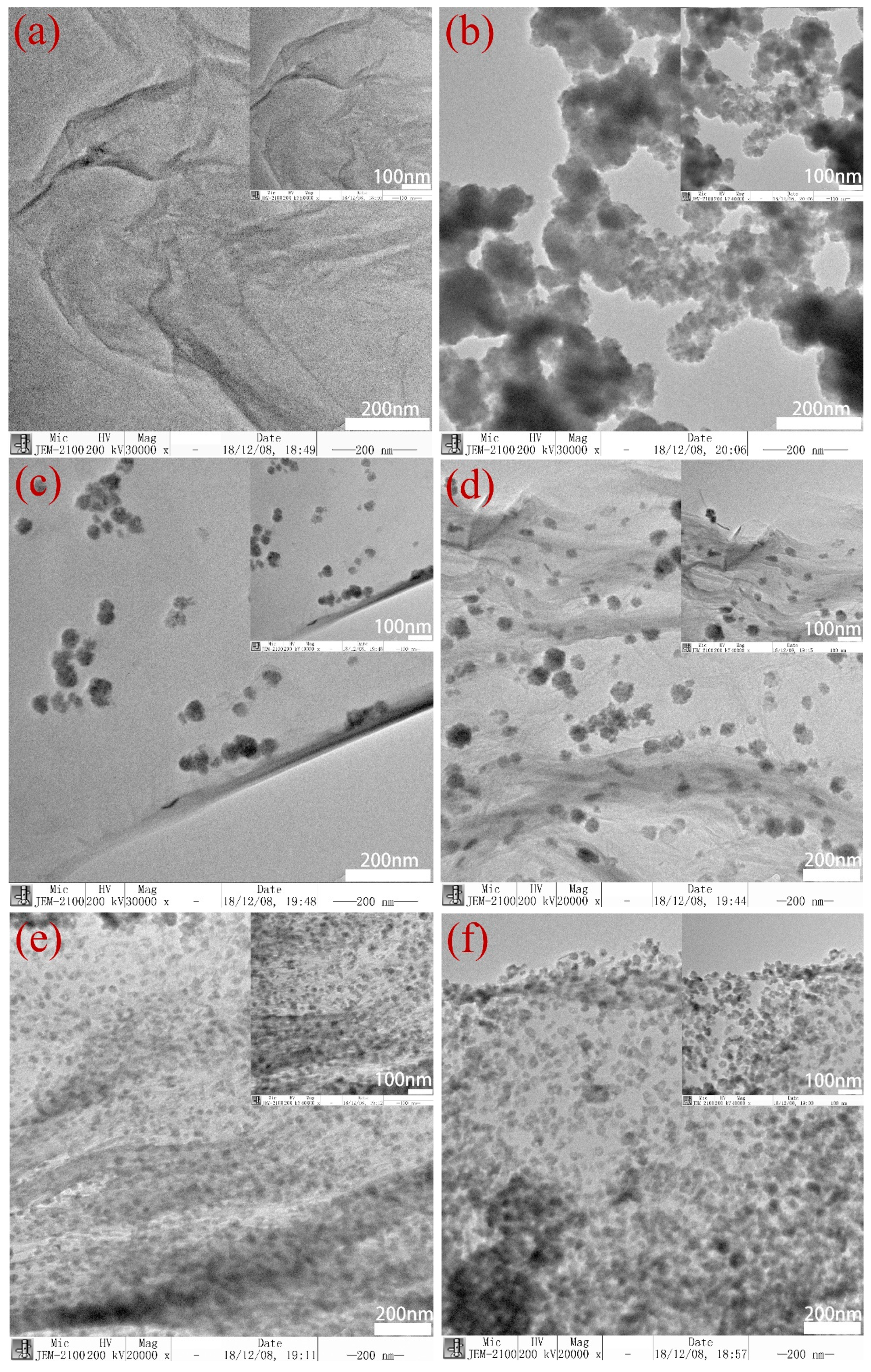
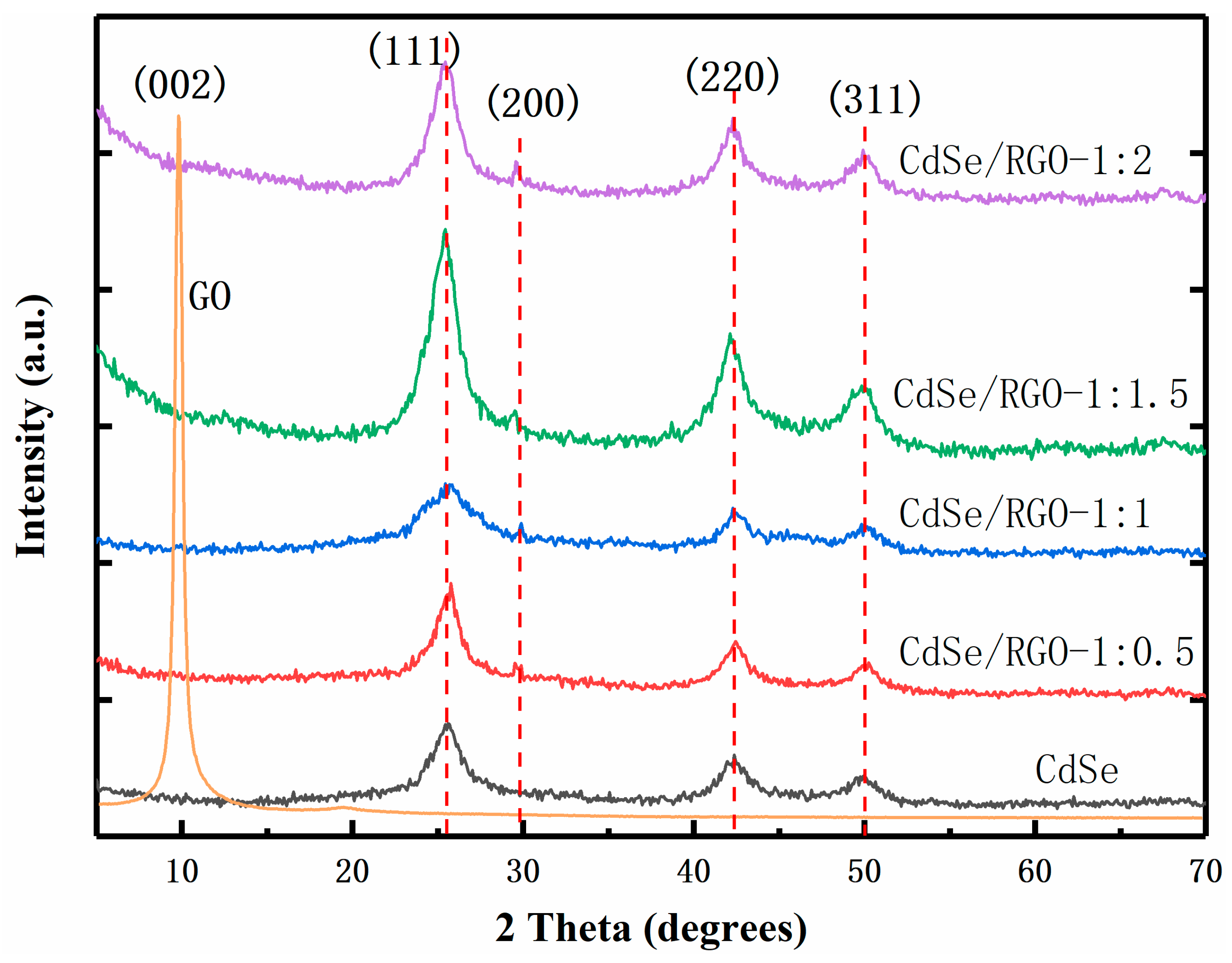
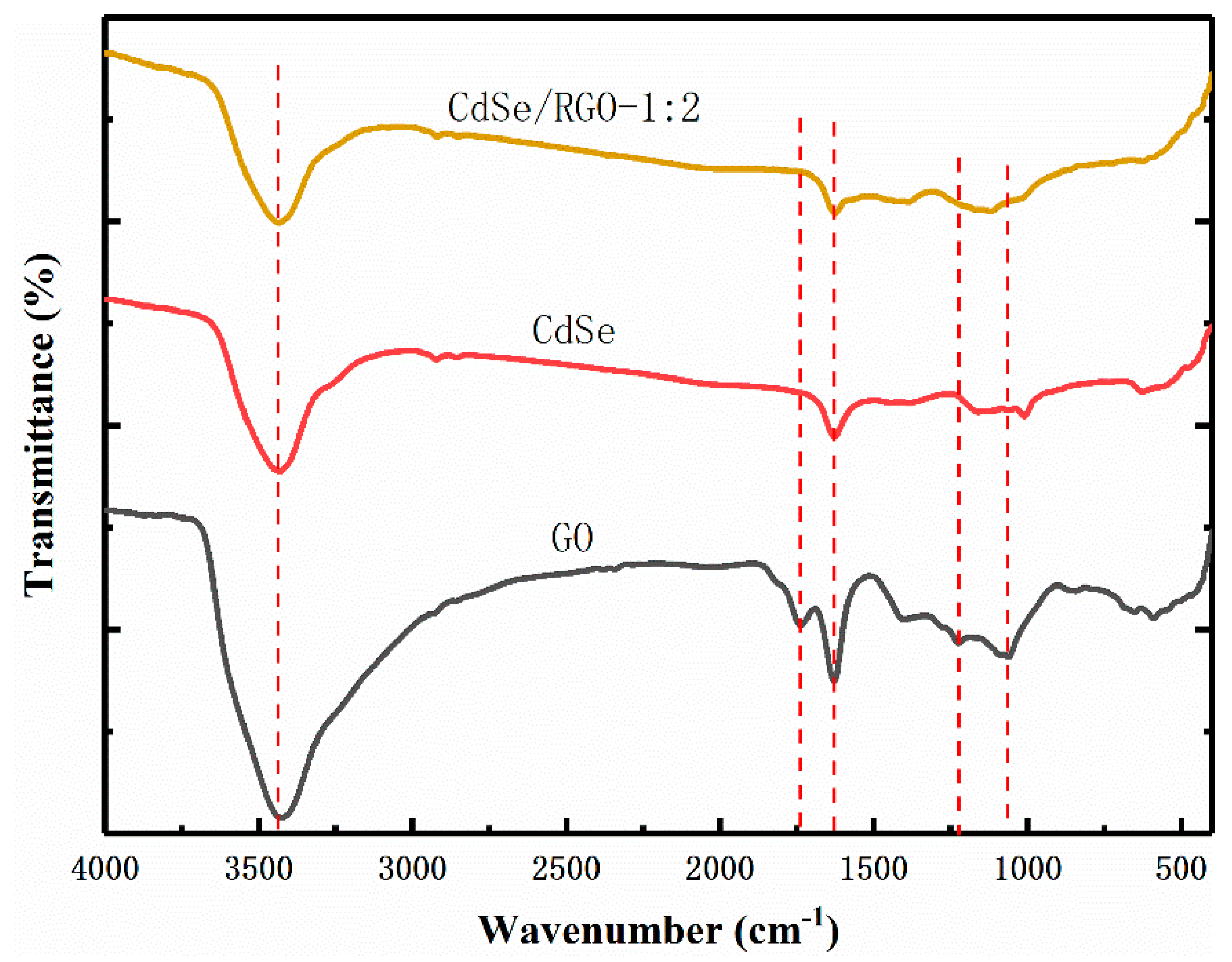
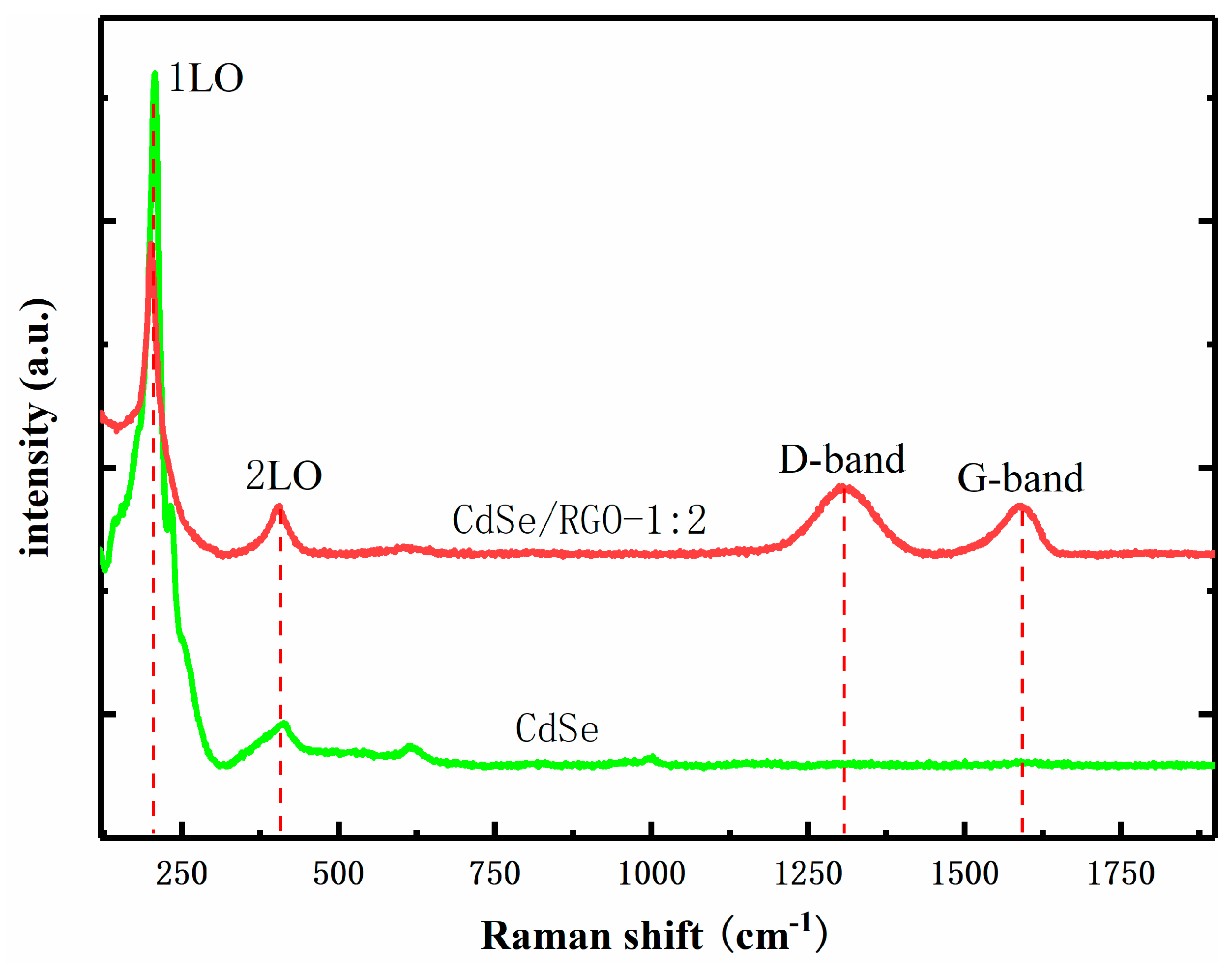
3.1. Structural and Morphology Characterization
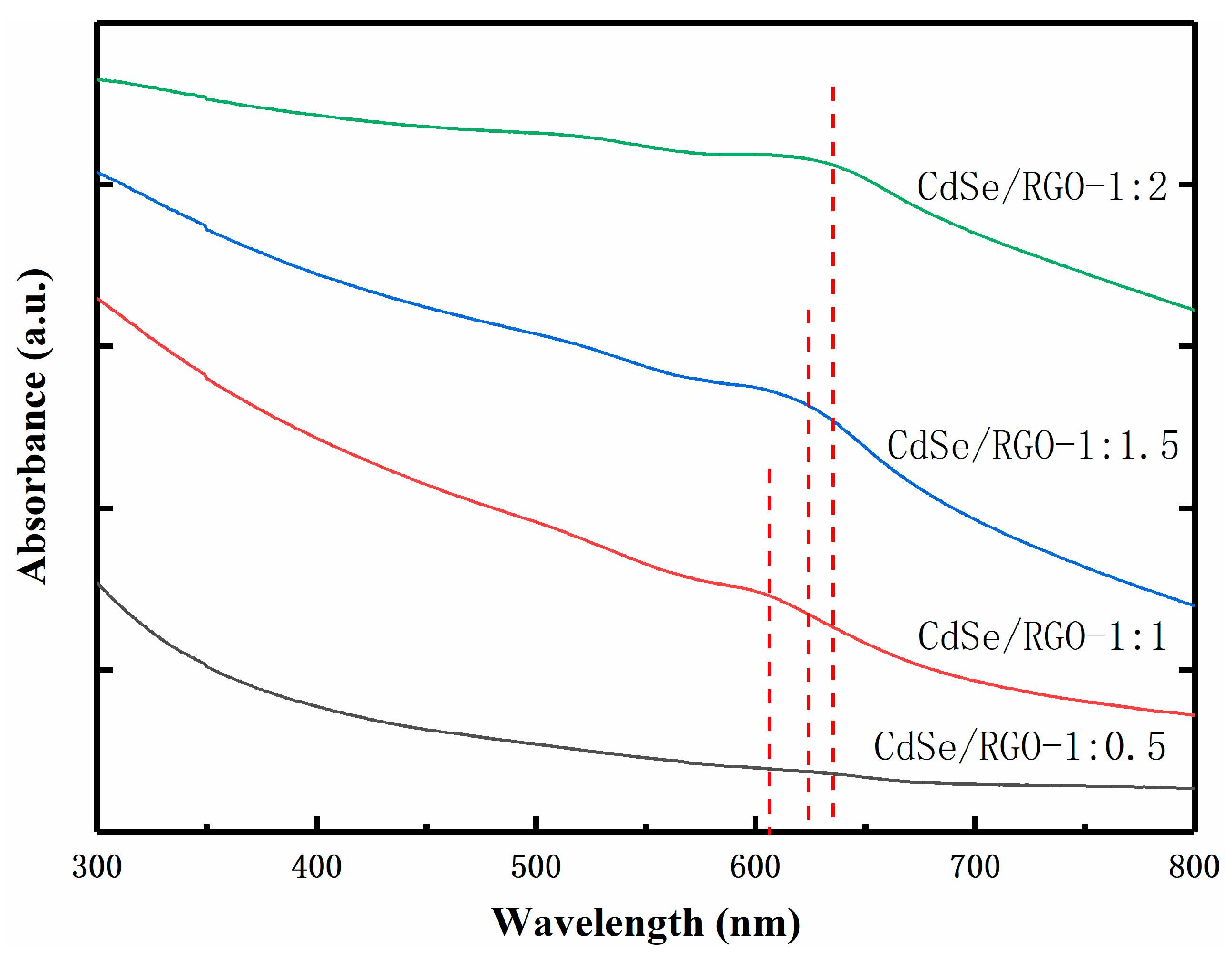
3.2. Linear Optical Properties
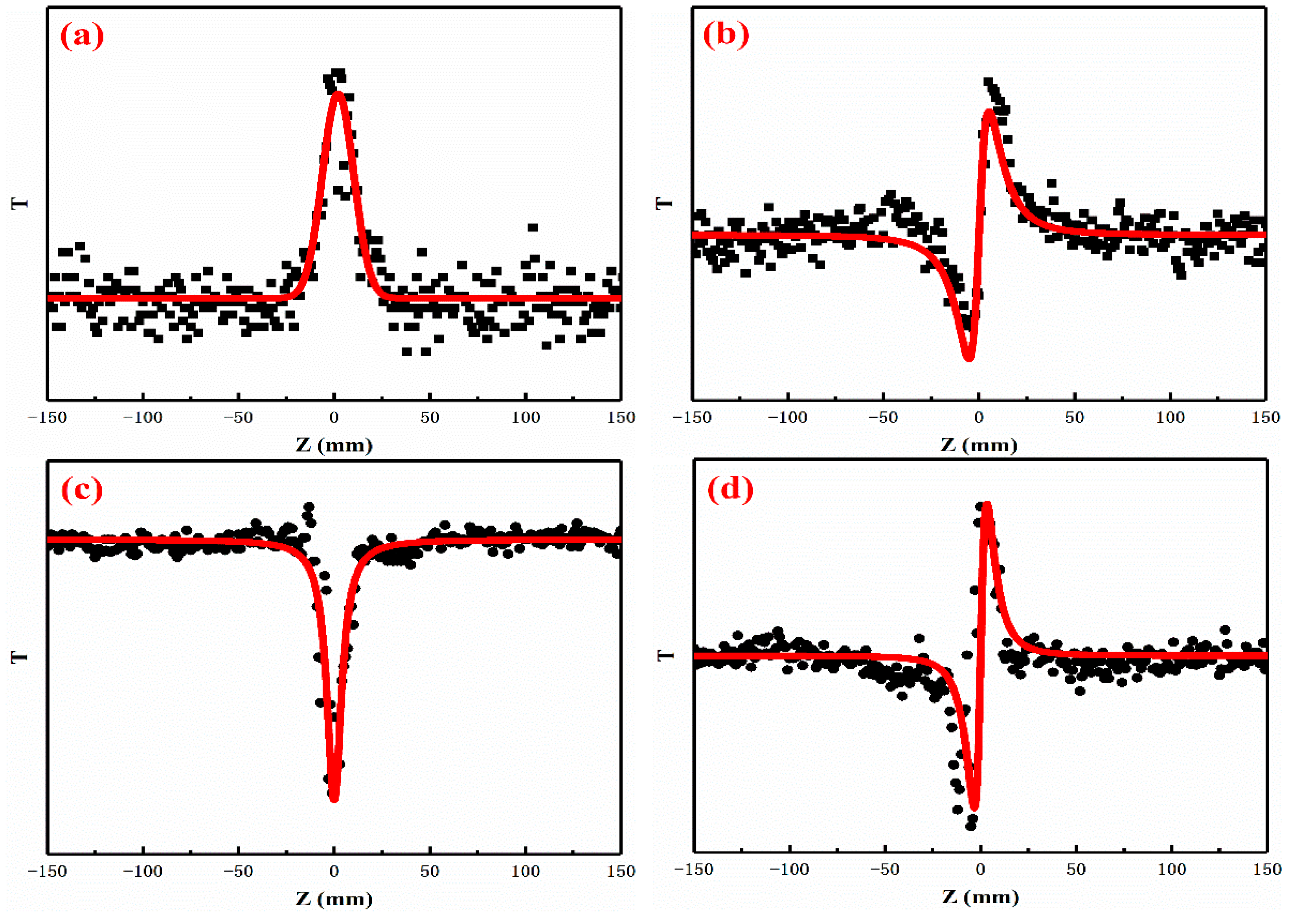
3.3. Nonlinear Optical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geim, K.; MacDonald, A.H. Graphene: Exploring carbon flatland. Phys. Today 2007, 60, 35. [Google Scholar]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Liu, K.P.; Yang, G.H.; Wang, C.M.; Zhang, J.R.; Zhu, J.J. Fabrication of Graphene–Quantum Dots Composites for Sensitive Electrogenerated Chemiluminescence Immunosensing. Adv. Funct. Mater. 2011, 21, 869–878. [Google Scholar] [CrossRef]
- Kanodarwalaa, F.K.; Wang, F.; Reeceb, P.J.; Strideac, J.A. Deposition of CdSe quantum dots on graphene sheets. J. Lumin. 2014, 146, 46–52. [Google Scholar] [CrossRef]
- Li, H.N.; Li, Y.; Aljarb, A.; Shi, Y.M.; Li, L.J. Epitaxial Growth of Two-Dimensional Layered Transition-Metal Dichalcogenides: Growth Mechanism, Controllability, and Scalability. Chem. Rev. 2018, 118, 6134–6150. [Google Scholar] [CrossRef] [PubMed]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zheng, Z.; Gao, H.L.; Ma, J.; Qin, X. SnO2/graphene composite as highly reversible anode materials for lithium ion batteries. J. Power Sources 2013, 240, 149–154. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Z.; Wang, H.; Cheng, W.; Chen, M.X.; Shangguan, W.F.; Ding, G.F. TiO2-graphene nanocomposites for photocatalytic hydrogen production from splitting water. Int. J. Hydrogen Energy 2012, 37, 2224–2230. [Google Scholar] [CrossRef]
- Whetten, R.L.; Shafigullin, M.N.; Khoury, J.T.; SSchaaff, T.; Vezmar, I.; Alvarez, M.M.; Wilkinson, A. Crystal Structures of Molecular Gold Nanocrystal Arrays. Acc. Chem. Res. 1999, 32, 397–406. [Google Scholar] [CrossRef]
- Tavakolian, E.; Tashkhourian, J. Sonication-assisted preparation of a nanocomposite consisting of reduced graphene oxide and CdSe quantum dots, and its application to simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim. Acta 2018, 185, 456. [Google Scholar] [CrossRef]
- Cao, Y.W.; Wang, C.; Zhu, B.H.; Gu, Y.Z. A facile method to synthesis high-quality CdSe quantum dots for large and tunable nonlinear absorption. Opt. Mater. 2017, 66, 59–64. [Google Scholar] [CrossRef]
- Zhu, B.H.; Wang, F.F.; Liao, C.; Zhang, H.C.; Zhang, J.Y.; Cui, Y.P.; Ye, Y.H.; Gu, Y.Z. Size confinement and origins of two-photon absorption and refraction in CdSe quantum dots. Opt. Express 2019, 27, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lim, J.; Song, J.; Heo, H.; An, K.; Kim, J.; Lee, S.; Char, K.; Song, H.J.; Lee, C. CdSe tetrapod interfacial layer for improving electron extraction in planar heterojunction perovskite solar cells. Nanotechnology 2018, 30, 065401. [Google Scholar] [CrossRef] [PubMed]
- Klimov, V.I.; Mikhailovsky, A.A.; Xu, S.; Malko, A.; Hollingsworth, J.A.; Leatherdale, C.A.; Eisler, H.J.; Bawendi, M.G. Optical gain and stimulated emission in nanocrystal quantum dots. Science 2000, 290, 314–317. [Google Scholar] [CrossRef]
- Oh, W.C.; Chen, M.L.; CHO, K.W.Y.; Kim, C.; Meng, Z.D.; Zhu, L. Synthesis of Graphene-CdSe Composite by a Simple Hydrothermal Method and Its Photocatalytic Degradation of Organic Dyes. Chin. J. Catal. 2011, 32, 1577–1583. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Kamat, P.V. CdSe–Graphene Oxide Light-Harvesting Assembly: SizeDependent Electron Transfer and Light Energy Conversion Aspects. ChemPhysChem 2014, 15, 2129–2135. [Google Scholar] [CrossRef]
- Nyoni, S.; Nyokong, T. Development of Graphene/CdSe Quantum Dots-Co Phthalocyanine Nanocomposite for Oxygen Reduction Reaction. Electroanalysis 2014, 26, 2261–2272. [Google Scholar] [CrossRef]
- Zhu, B.H.; Wang, F.F.; Cao, Y.W.; Wang, C.; Wang, J.; Gu, Y.Z. Nonlinear optical enhancement induced by synergistic effect of graphene nanosheets and CdS nanocrystals. Appl. Phys. Lett. 2016, 108, 252106. [Google Scholar] [CrossRef]
- Zhu, B.H.; Wang, F.F.; Wang, G.X.; Gu, Y.Z. Oxygen-containing-defect-induced synergistic nonlinear optical enhancement of graphene/CdS nanohybrids under single pulse laser irradiation. Photonics Res. 2018, 6, 1158–1169. [Google Scholar] [CrossRef]
- Zhu, B.H.; Wang, F.F.; Li, P.; Wang, C.; Gu, Y.Z. Surface oxygen-containing defects of graphene nanosheets with tunable nonlinear optical absorption and refraction. Phys. Chem. Chem. Phys. 2018, 20, 27105–27114. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.B.; Ye, Z.L.; Chenet, D.A.; Ye, Y.; O’Brien, K.; Hone, J.C.; Zhang, X. Edge Nonlinear Optics on a MoS2 Atomic Monolayer. Science 2014, 344, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Davoody, A.H.; Knezevic, I. Nonlinear optical response in graphene nanoribbons: The critical role of electron scattering. Phys. Rev. B 2018, 97, 245403. [Google Scholar] [CrossRef]
- Ghosh, T.; Lee, J.H.; Meng, Z.D.; Ullah, K.; Park, C.Y.; Nikam, V.; Oh, W.C. Graphene oxide based CdSe photocatalysts: Synthesis, characterization and comparative photocatalytic efficiency of rhodamine B and industrial dye. Mater. Res. Bull. 2013, 48, 1268–1274. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Dong, Y.J.; Deng, Z.X.; Kou, H.Z.; Gao, S.; Li, Y.D. Selective Synthesis and Magnetic Properties of α-MnSe and MnSe2 Uniform Microcrystals. J. Phys. Chem. B 2002, 106, 9261–9265. [Google Scholar] [CrossRef]
- Peng, Q.; Dong, Y.J.; Deng, Z.X.; Li, Y.D. Selective Synthesis and Characterization of CdSe Nanorods and Fractal Nanocrystals. Inorg. Chem. 2002, 41, 5249–5254. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, N.N.; Zhang, G.Y.; Du, G.X. One-pot hydrothermal synthesis of ZnS–reduced graphene oxide composites with enhanced photocatalytic properties. CrystEngComm 2014, 16, 214–222. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Z.; Li, Y.; Li, Y.Y.; Chen, Y.M.; Wang, W.G.; Ye, Y.P.; Deng, P. Microwave-assisted in situ synthesis of reduced graphene oxide/Mn3O4 composites for supercapacitor applications. RSC Adv. 2015, 5, 45061–45067. [Google Scholar] [CrossRef]
- Olusola, O.I.; Echendu, O.K.; Dharmadasa, I.M. Development of CdSe thin films for application in electronic devices. Mater. Electron. 2015, 26, 1066–1076. [Google Scholar] [CrossRef]
- Sheik-bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Stryland, E.W.V. Sensitive measurement of optical nonlinearities using a single beam. IEEE Photonics Technol. Lett. 1990, 26, 760–769. [Google Scholar] [CrossRef]
| Sample | Reχ(3)/10−12 esu | Imχ(3)/10−12 esu | β/cm•GW−1 | n2/10−11 esu | χ(3)/10−12 esu |
|---|---|---|---|---|---|
| CdSe | 3.79 | 7.63 | 11.47 | 2.68 | 8.52 |
| RGO | 1.12 | 1.42 | 0.75 | 0.21 | 1.81 |
| CdSe/RGO-1:2 | 69.23 | 149.21 | 224.42 | 48.90 | 164.49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhu, B.; Li, P.; Zhang, Z.; Li, L.; Gu, Y. A Facile Method to Synthesize CdSe-Reduced Graphene Oxide Composite with Good Dispersion and High Nonlinear Optical Properties. Nanomaterials 2019, 9, 957. https://doi.org/10.3390/nano9070957
Li P, Zhu B, Li P, Zhang Z, Li L, Gu Y. A Facile Method to Synthesize CdSe-Reduced Graphene Oxide Composite with Good Dispersion and High Nonlinear Optical Properties. Nanomaterials. 2019; 9(7):957. https://doi.org/10.3390/nano9070957
Chicago/Turabian StyleLi, Pengchao, Baohua Zhu, Peng Li, Zhihao Zhang, Luyao Li, and Yuzong Gu. 2019. "A Facile Method to Synthesize CdSe-Reduced Graphene Oxide Composite with Good Dispersion and High Nonlinear Optical Properties" Nanomaterials 9, no. 7: 957. https://doi.org/10.3390/nano9070957
APA StyleLi, P., Zhu, B., Li, P., Zhang, Z., Li, L., & Gu, Y. (2019). A Facile Method to Synthesize CdSe-Reduced Graphene Oxide Composite with Good Dispersion and High Nonlinear Optical Properties. Nanomaterials, 9(7), 957. https://doi.org/10.3390/nano9070957




