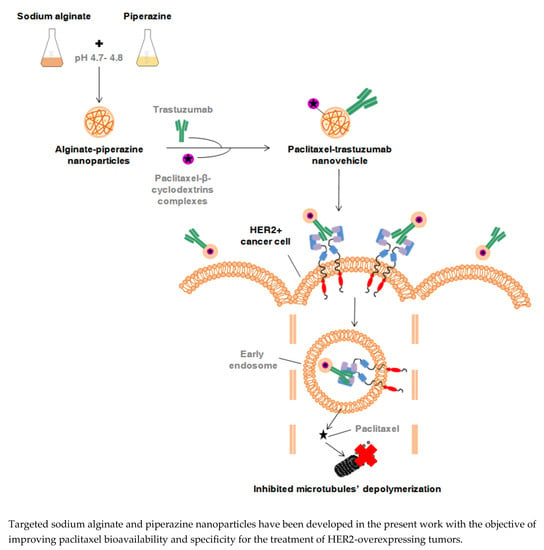
Paclitaxel-Trastuzumab Mixed Nanovehicle to Target HER2-Overexpressing Tumors
Abstract
:1. Introduction
2. Materials and Methods
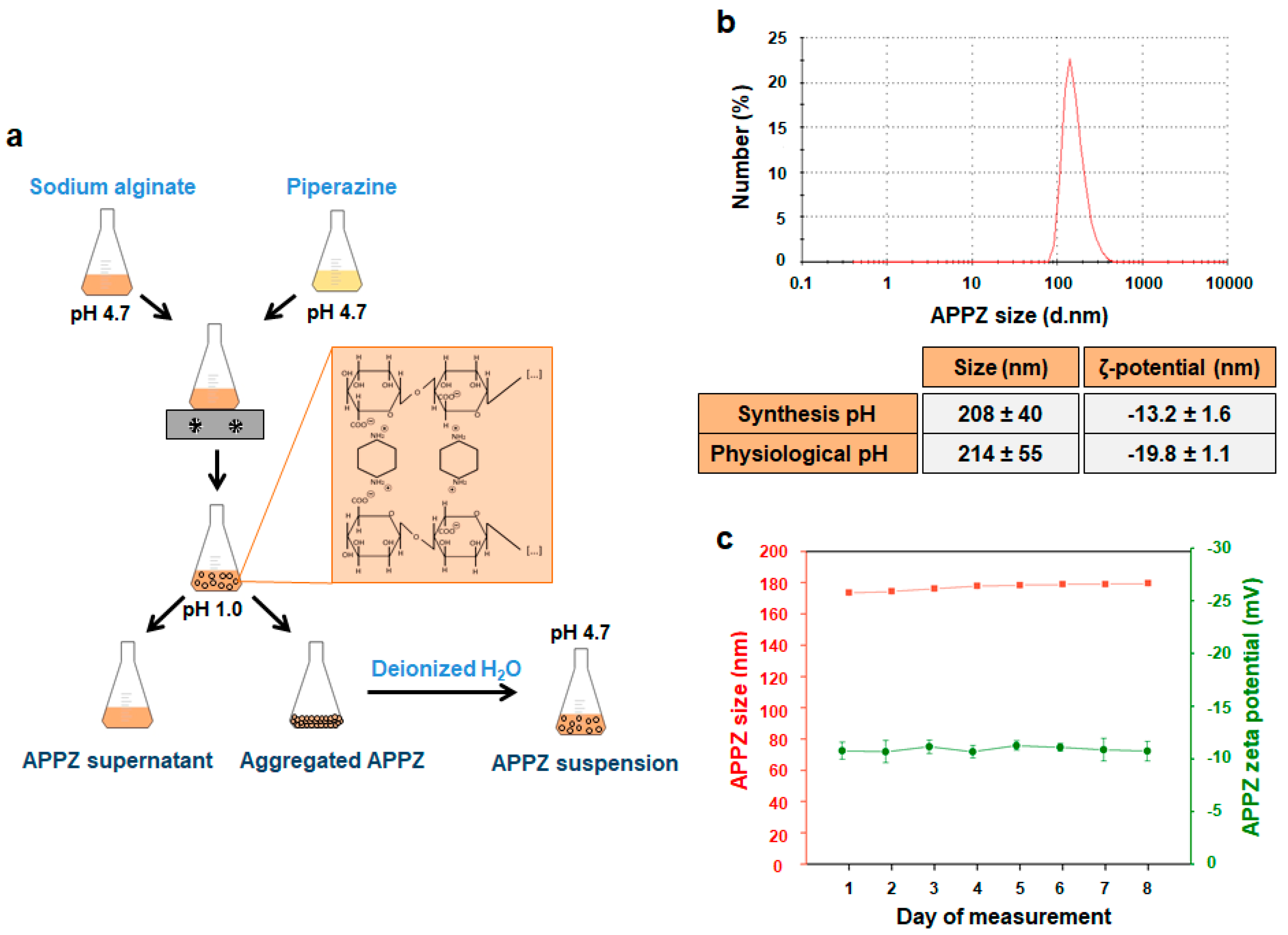
2.1. APPZ Synthesis and Characterization
2.2. Preparation of PTX:βCD Inclusion Complexes
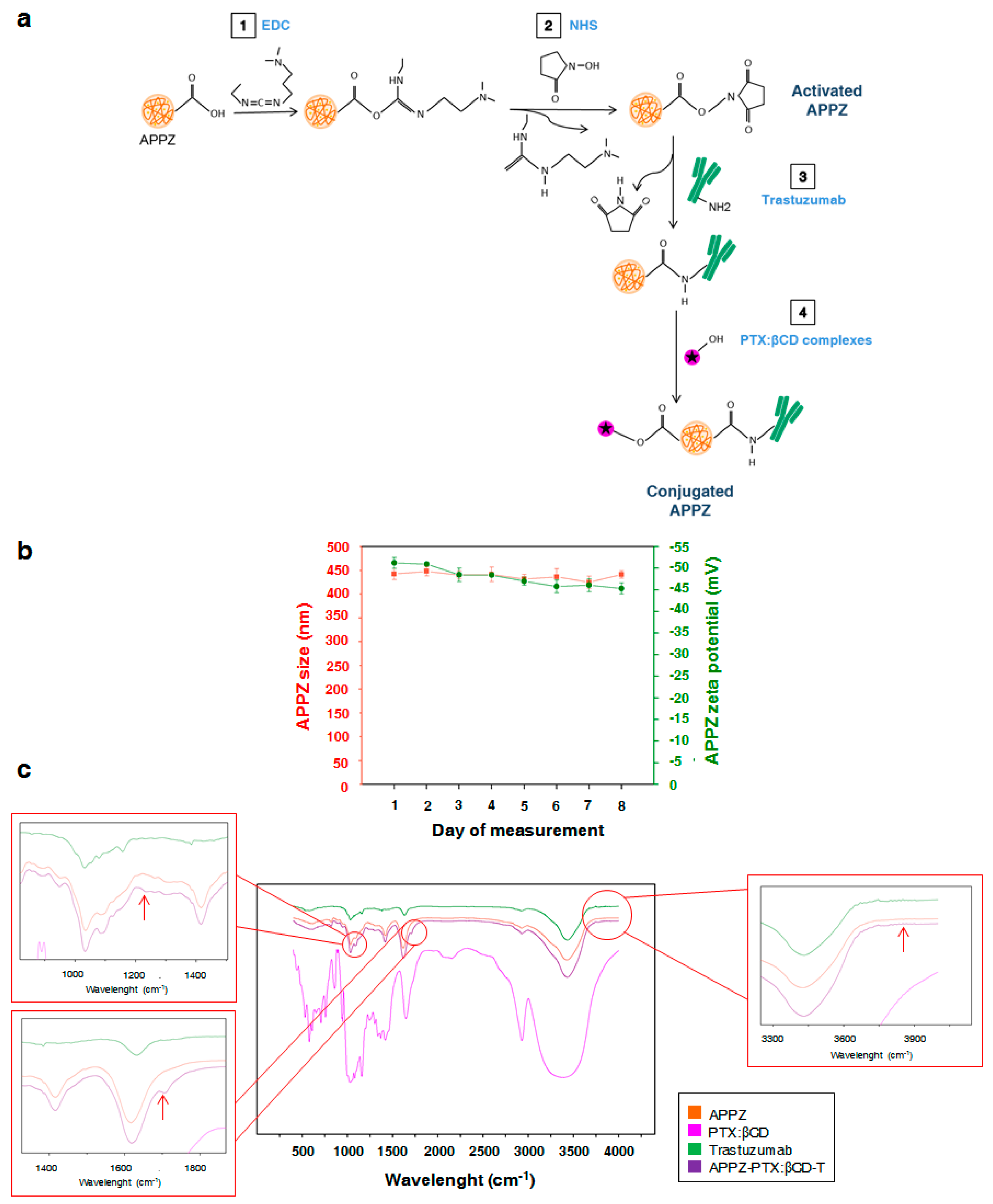
2.3. APPZ Conjugation with Trastuzumab and PTX:βCD Complexes and Conjugated APPZ Characterization
2.4. Determination of PTX and Trastuzumab Conjugation Efficiencies on APPZ
2.5. Cell Culture
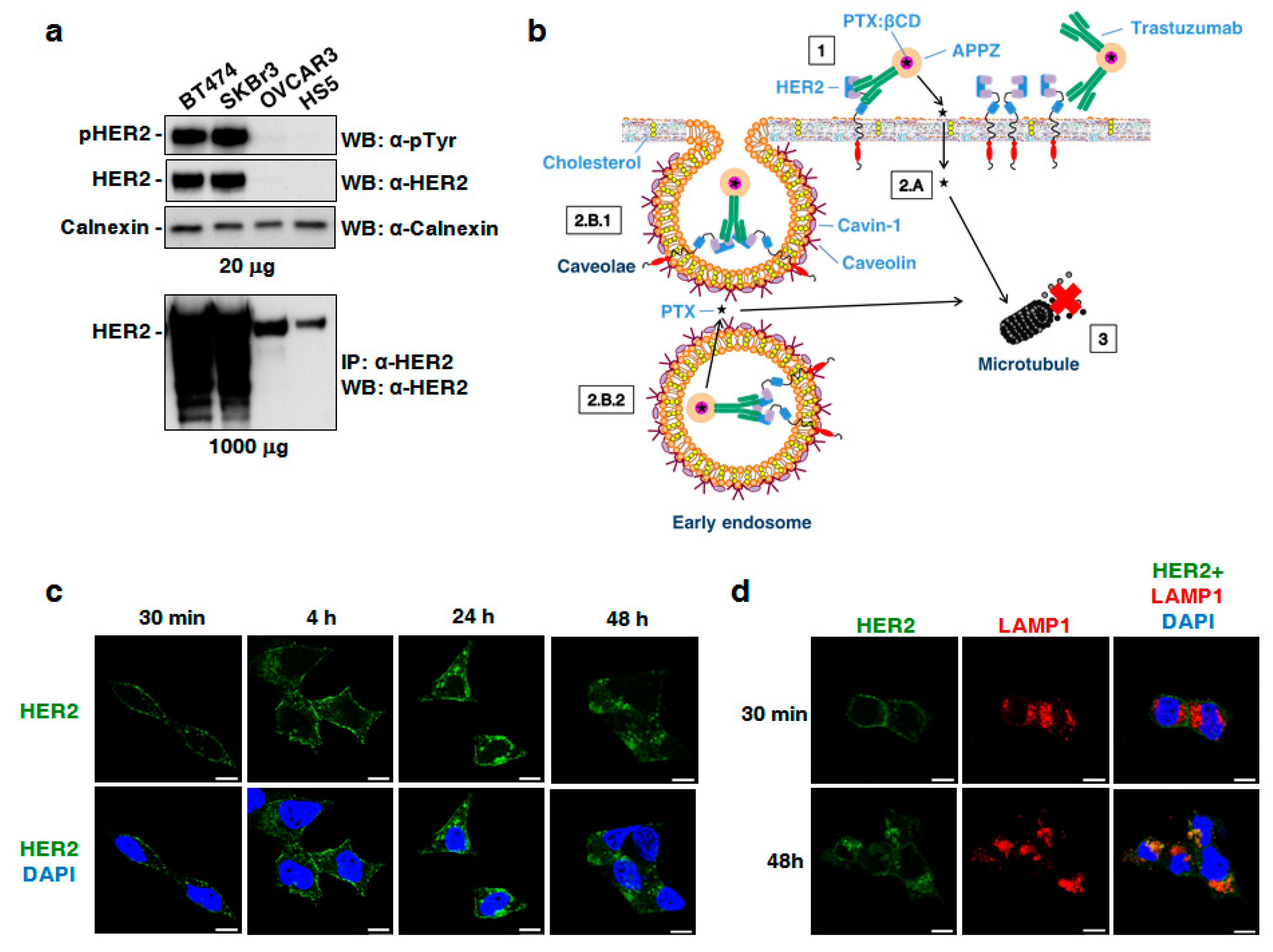
2.6. Determination of BT474, SKBR3, OVCAR3, and HS5 Cellular Levels of HER2-Expression
2.7. Conjugated APPZ Internalization in HER2-Overexpressing Cancer Cells
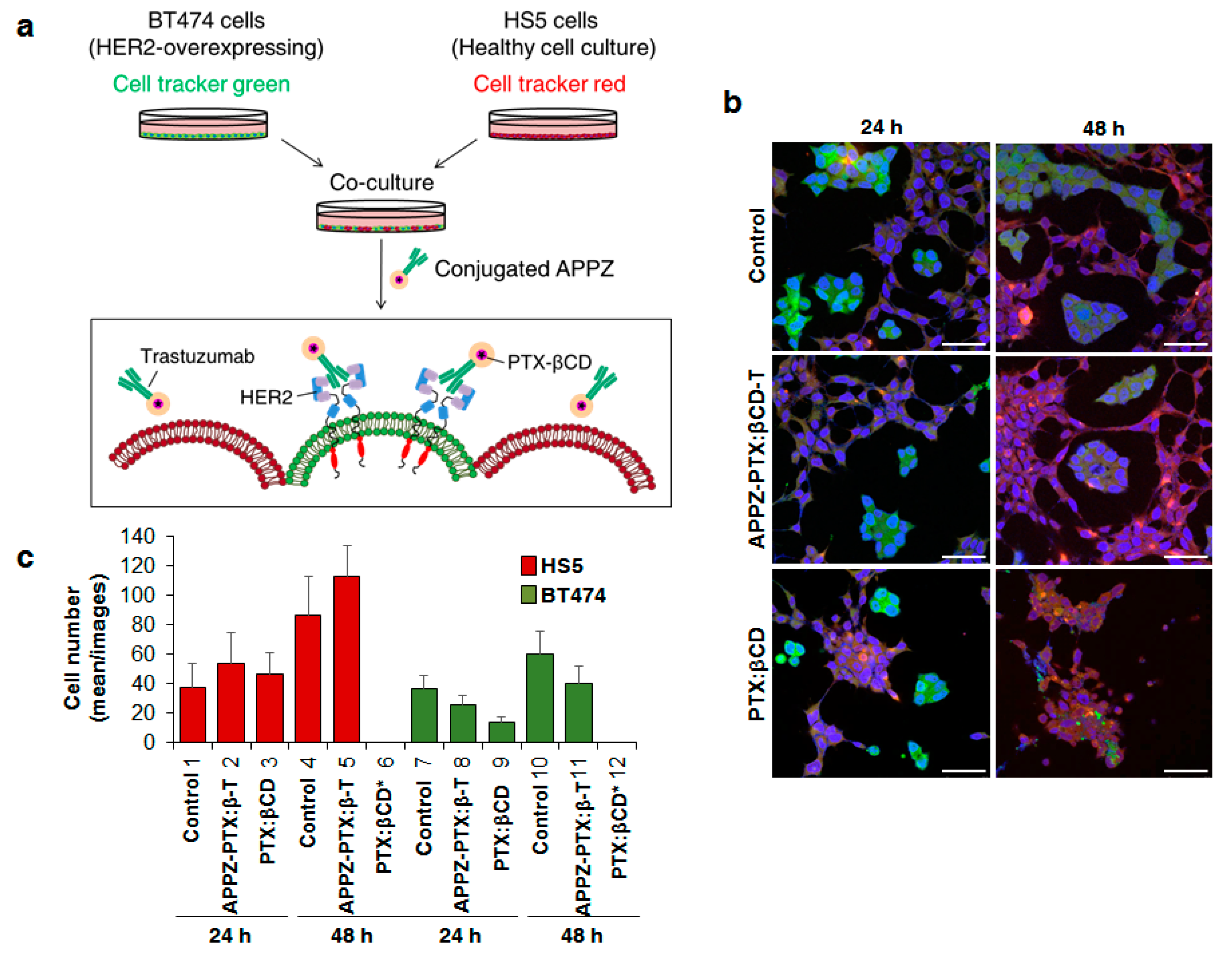
2.8. Conjugated APPZ Specificity: Targeting HER2-Positive Cancer Cells
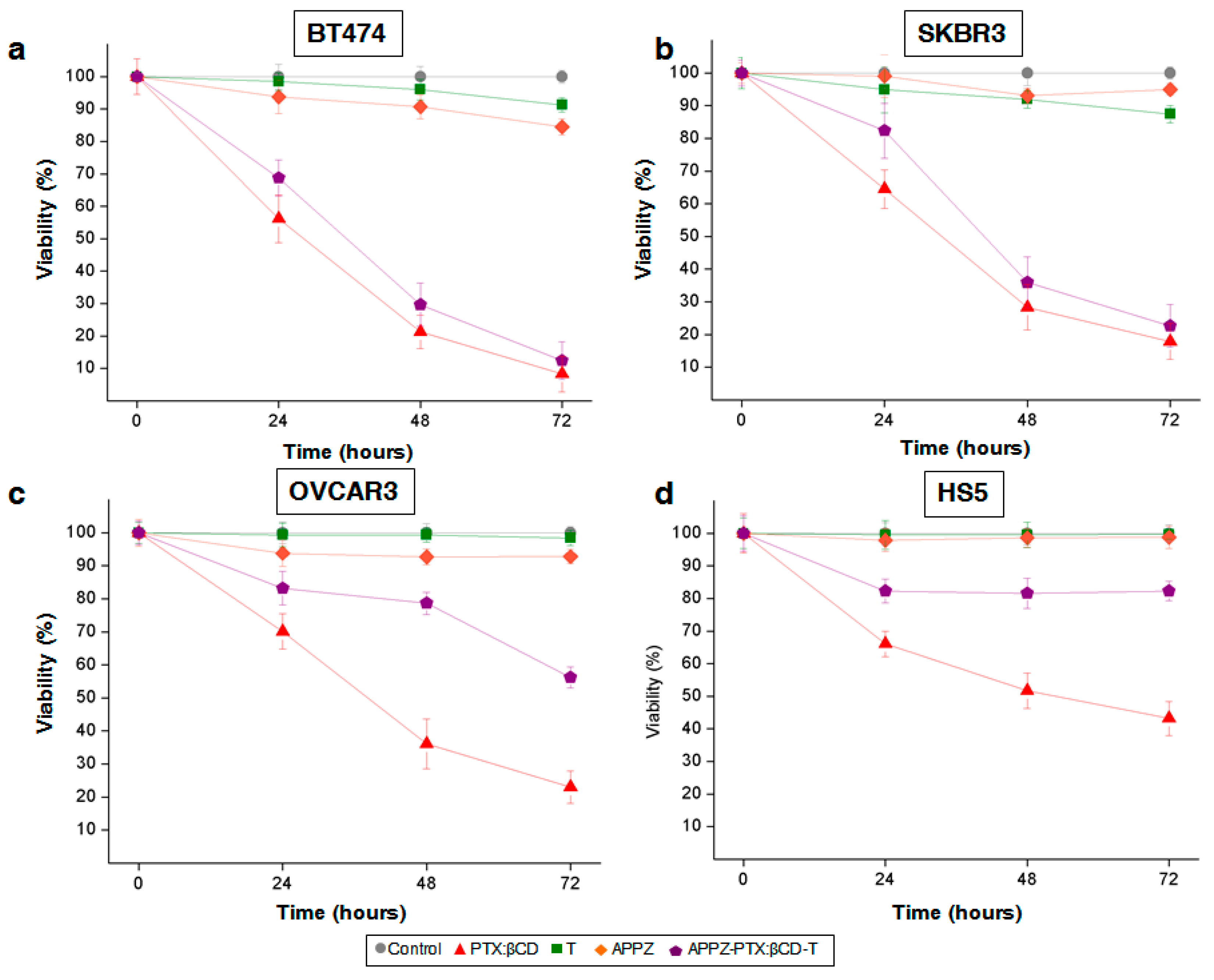
2.9. Conjugated APPZ Cytotoxic Effect According to Cellular Levels of HER2-Expression
2.10. Statistical Analyses
3. Results
3.1. Stable APPZ Synthesis and Characterization
3.2. APPZ Conjugation with Trastuzumab and PTX:βCD Complexes and Later Characterization
3.3. Conjugated APPZ are Internalized in HER2-Overexpressing Cancer Cells by Endocytosis
3.4. Conjugated APPZ Present HER2-Overexpressing Specificity
3.5. Conjugated APPZ Have Different Cytotoxic Effect According to Cellular Levels of HER2-Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luzzati, T.; Parenti, A.; Rughi, T. Economic growth and cancer incidence. Ecol. Econ. 2018, 146, 381–396. [Google Scholar] [CrossRef]
- Dickens, E.; Ahmed, S. Principles of cancer treatment chemotherapy. Surgery 2017, 36, 134–138. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Zak, J.K. Biomaterial-based regional chemotherapy: Local anticancer drug delivery to enhance chemotherapy and minimize its size-effects. Mater. Sci. Eng. C 2016, 62, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Abandansari, H.S.; Abuali, M.; Nabid, M.R.; Niknejad, H. Enhance chemotherapy efficacy and minimize anticancer drug side effects by using reversible pH- and redox- responsive cross-linked unimolecular micelles. Polymer 2017, 116, 16–26. [Google Scholar] [CrossRef]
- Wang, F.; Porter, M.; Konstantopoulos, A.; Zhang, P.; Cui, H. Preclinical development of drug delivery systems for paclitaxel-based cancer chemotherapy. J. Control. Release 2017, 267, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef] [PubMed]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of nano-paclitaxel. Adv. Drug. Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Khan, A.R.; Fu, M.; Ji, J.; Yu, A.; Zhai, G. Current development in the formulations of non-injection administration of paclitaxel. Int. J. Pharm. 2018, 542, 242–252. [Google Scholar] [CrossRef]
- Khuroo, T.; Verma, D.; Khuroo, A.; Ali, A.; Iqbal, Z. Simultaneous delivery of paclitaxel and erlotinib from dual drug loaded PLGA nanoparticles: Formulation development, thorough optimization and in vitro release. J. Mol. Liq. 2018, 257, 52–68. [Google Scholar] [CrossRef]
- Gupta, D.; Kumar, M.; Tyagi, P.; Kapoor, S.; Tyagi, A.; Barman, T.K.; Kharbanda, S.; Kufe, D.; Singh, H. Concomitant delivery of paclitaxel and NuBCP-9 peptide for synergistic enhancement of cancer therapy. Nanomedicine 2018, 14, 1301–1313. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagesh, P.K.B.; Hatami, E.; Wagh, S.; Dan, N.; Tripathi, M.K.; Khan, S.; Hafrez, B.B.; Meibohm, B.; Chauhan, S.C.; et al. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J. Colloid Interface Sci. 2019, 535, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, Q.; Zhang, X.; Zhu, J.; Sun, B. Trastuzumab-cisplatin conjugates for targeted delivery of cisplatin to HER2-overexpressing cancer cells. Biomed. Pharmacother. 2015, 72, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Tran, T.H.; Thapa, R.K.; Phung, C.D.; Shin, B.S.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; Kim, J.O. Targeted co-delivery of polypyrrole and rapamycin by trastuzumab-conjugated liposomes for combined chemo-photothermal therapy. Int. J. Pharm. 2017, 527, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Sun, Y.; Zhang, X.Y.; Sun, B.W.; Wang, Q.C.; Zhu, Z. Biological evaluation of a novel Herceptin-platinum (II) conjugate for efficient and cancer cell specific delivery. Biomed. Pharmacother. 2015, 73, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Morimura, O.; Minami, T.; Kijima, T.; Koyama, S.; Otsuka, T.; Kinehara, Y.; Osa, A.; Higashiguchi, M.; Miyake, K.; Nagatomo, I.; et al. Trastuzumab emtansine suppresses the growth of HER2-positive small-cell lung cancer in preclinical models. Biochem. Biophys. Res. Commun. 2017, 488, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Daniels, B.; Kiely, B.E.; Lord, S.J.; Houssami, N.; Lu, C.Y.; Ward, R.L.; Pearson, S.A. Trastuzumab for metastatic breast cancer: real world outcomes from an Australian whole-of-population cohort (2001-2006). Breast 2018, 38, 7–13. [Google Scholar] [CrossRef]
- Baselga, J.; Manikhas, A.; Cortés, J.; Llombart, A.; Roman, L.; Semiglazov, V.F.; Byakhov, M.; Lokanatha, D.; Forenza, S.; Goldfarb, R.H.; et al. Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer. Ann. Oncol. 2014, 25(3), 592–598. [Google Scholar] [CrossRef]
- Román, J.V.; Rodríguez-Rodríguez, J.A.; Martín del Valle, E.M.; Galán, M.A. Synthesis of a new nanoparticle system based on the electrostatic alginate-piperazine interactions. Polym. Adv. Technol. 2016, 27, 623–629. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Feng, Y.; Li, K.; Yan, H.; Gao, P.; Xiao, T.; Wang, C. Aggregation behavior of derivatives of sodium alginate and N-octyl-D-glucopyranoside in aqueous solutions. Colloids Surf. A 2015, 479, 11–17. [Google Scholar] [CrossRef]
- Shah, A.; Shah, A.M.; Parveen, N.; Rehman, Z.; Khan, S.Z.; Rana, U.A.; Khan, S.U.; Nisar, J.; Lashin, A.; Qureshi, R. Synthesis and electrochemical investigations of piperazines. Electrochim. Acta 2016, 220, 705–711. [Google Scholar] [CrossRef]
- Fytas, C.; Zoidis, G.; Tsotinis, A.; Fytas, G.; Khan, M.A.; Khtar, S.A. Novel1-(2-aryl-2-adamantyl) piperazine derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 93, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Martín del Valle, E.M. Cyclodextrins and their uses: a review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Shelley, H.; Babu, R.J. Role of cyclodextrins in nanoparticle-based drug delivery systems. J. Pharm. Sci. 2018, 107, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Oroujeni, M.; Kaboudin, B.; Xia, W.; Jöhsson, P.; Ossipov, D.A. Conjugation of cyclodextrin to magnetic Fe3O4 nanoparticles via polydopamine coating for drug delivery. Prog. Org. Coat. 2018, 114, 154–161. [Google Scholar] [CrossRef]
- Basha, R.Y.; Kumar, S.; Doble, M. Dual delivery of tuberculosis drugs via cyclodextrin conjugated curdlan nanoparticles to infect macrophages. Carbohydr. Polym. 2019, 218, 53–62. [Google Scholar] [CrossRef]
- Shah, M.; Shah, V.; Ghosh, A.; Zhang, Z.; Minko, T. Molecular inclusion complexes of β-cyclodextrins derivatives enhance aqueous solubility and cellular internalization of paclitaxel: Preformulation and in vitro assessments. J. Pharm. Pharmacol. 2015, 2(2), 8. [Google Scholar]
- Bhatt, P.; Lalani, R.; Vhora, I.; Patil, S.; Amrutiya, J.; Misra, A.; Mashru, R. Liposomes encapsulating native and cyclodextrin enclosed paclitaxel: Enhanced loading efficiency and its pharmacokinetic evaluation. Int. J. Pharm. 2018, 536(1), 95–107. [Google Scholar] [CrossRef]
- Alcaro, S.; Ventura, C.A.; Paolino, D.; Battaglia, D.; Ortuso, F.; Cattel, L.; Puglisi, G.; Fresta, M. Preparation, characterization, molecular modelling and in vitro activity of paclitaxel-cyclodextrins complexes. Bioorg. Med. Chem. Lett. 2002, 12, 1637–1641. [Google Scholar] [CrossRef]
- Hamada, H.; Ishihara, K.; Masuoka, N.; Mikuni, K.; Nakajima, N. Enhancement of water-solubility and bioactivity of paclitaxel using modified cyclodextrins. J. Biosci. Bioeng. 2006, 102, 369–371. [Google Scholar] [CrossRef]
- Badea, I.; Ciutaru, D.; Lazar, L.; Nicolescu, D.; Tudose, A. Rapid HPLC method for the determination of paclitaxel in pharmaceutical forms without separation. J. Pharm. Biomed. 2004, 34, 501–507. [Google Scholar] [CrossRef]
- Chung, W.J.; Lee, D.Y.; Yoo, S.Y. Chemical modulation of M13 bacteriophage and its functional opportunities for nanomedicine. Int. J. Nanomed. 2014, 9, 5825–5836. [Google Scholar]
- Campuzano, S.; Yánez-Sedeño, P.; Pingarrón, J.M. Electrochemical affinity biosensors in food safety. Chemosensors 2017, 5, 8. [Google Scholar] [CrossRef]
- Kelestemur, S.; Altunbek, M.; Culha, M. Influence of EDC/NHS coupling chemistry on stability and cytotoxicity of ZnO nanoparticles modified with proteins. Appl. Surf. Sci. 2017, 403, 455–463. [Google Scholar] [CrossRef]
- Hua, J.; Li, Z.; Xia, W.; Yang, N.; Gong, J.; Zhang, J.; Qiao, C. Preparation and properties of EDC/NHS mediated crosslinking poly (gamma-glutamic acid)/ epsilon-polylysine hydrogels. Mater. Sci. Eng. C 2016, 61, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Montero, J.C.; Seoane, S.; Ocaña, A.; Pandiella, A. P-Rex1 participates in neuregulin- ErbB signal trasduction and its expression correlates with patient outcome in breast cancer. Oncogene 2011, 30, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Esparís-Ogando, A.; Díaz-Rodríguez, E.; Montero, J.C.; Yuste, L.; Crespo, P.; Pandiella, A. Erk5 participates in neuregulin signal trasduction and is constitutively active in breast cancer cells overexpressing Erb2. Mol. Cell. Biol. 2002, 22, 270–285. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Inmmunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Haug, A.; Larsen, B. Separation of uronic acids by paper electrophoresis. Acta Chem. Scand. 1961, 15, 1395–1396. [Google Scholar] [CrossRef]
- Khalili, F.; Henri, A.; East, A.L.L. pKa values of some piperazines at (198, 302, 313 and 323) K. J. Chem. Eng. Data 2009, 54, 2914–2917. [Google Scholar] [CrossRef]
- Kaufman, P.A.; Wildiers, H.; Freyer, G.; Kemeny, M.; Gonçalves, A.; Jerusalem, G.; Stopeck, A.; Vrindavanam, N.; Dalenc, F.; Nanayakkara, N.; et al. Phase 1b study of trebananib plus paclitaxel and trastuzumab in patients with HER2-positive locally recurrent or metastatic breast cancer. Clin. Breast Cancer 2019, 19(1), 47–57. [Google Scholar] [CrossRef] [PubMed]
- Van Ramshorst, M.S.; van Werkhoven, E.; Mandjes, I.A.M.; Schot, M.; Wesseling, J.; Vrancken Peeters, M.T.F.D.; Meerum Terwogt, J.M.; Bos, M.E.M.; Oosterkamp, H.M.; Rodenhuis, S.; et al. Trastuzumab in combination with weekly paclitaxel and carboplatin as neo-adjuvant treatment for HER2-positive breast cancer: the TRAIN study. Eur. J. Cancer 2017, 74, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Subik, K.; Lee, J.F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.C.; Bonfiglio, T.; Hicks, D.G.; et al. The expression patterns of ER, PR, HER2, CK516, EGFR, Ki-67 and AR by inmmunohistochemical analysis in breast cancer cell lines. Breast Cancer 2010, 4, 35–41. [Google Scholar] [PubMed]
- He, J.; Jing, Y.; Li, W.; Qian, X.; Xu, Q.; Li, F.S.; Liu, L.Z.; Jiang, B.H.; Jiang, Y. Roles and mechanism of miR-199a and miR-125b in tumor angiogenesis. Plos ONE 2013, 8, e56647. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Park, S.W. An evolving role of Piperazine moieties in drug design and discovery. Mini-Rev. Med. Chem. 2013, 13, 1579–1601. [Google Scholar] [CrossRef] [PubMed]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev 2019. In press. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, V.J.; Chen, Y. Nanoparticles – A review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Alessandrini, F.; Pezzè, L.; Ciribilli, Y. LAMPs: Shedding light on cancer biology. Semin. Oncol. 2017, 44, 239–253. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto, C.; Centa, A.; Rodríguez-Rodríguez, J.A.; Pandiella, A.; Martín del Valle, E.M. Paclitaxel-Trastuzumab Mixed Nanovehicle to Target HER2-Overexpressing Tumors. Nanomaterials 2019, 9, 948. https://doi.org/10.3390/nano9070948
Nieto C, Centa A, Rodríguez-Rodríguez JA, Pandiella A, Martín del Valle EM. Paclitaxel-Trastuzumab Mixed Nanovehicle to Target HER2-Overexpressing Tumors. Nanomaterials. 2019; 9(7):948. https://doi.org/10.3390/nano9070948
Chicago/Turabian StyleNieto, Celia, Ariana Centa, Jesús A. Rodríguez-Rodríguez, Atanasio Pandiella, and Eva M. Martín del Valle. 2019. "Paclitaxel-Trastuzumab Mixed Nanovehicle to Target HER2-Overexpressing Tumors" Nanomaterials 9, no. 7: 948. https://doi.org/10.3390/nano9070948
APA StyleNieto, C., Centa, A., Rodríguez-Rodríguez, J. A., Pandiella, A., & Martín del Valle, E. M. (2019). Paclitaxel-Trastuzumab Mixed Nanovehicle to Target HER2-Overexpressing Tumors. Nanomaterials, 9(7), 948. https://doi.org/10.3390/nano9070948