A Journey Through the Landscapes of Small Particles in Binary Colloidal Assemblies: Unveiling Structural Transitions from Isolated Particles to Clusters upon Variation in Composition
Abstract
1. Introduction
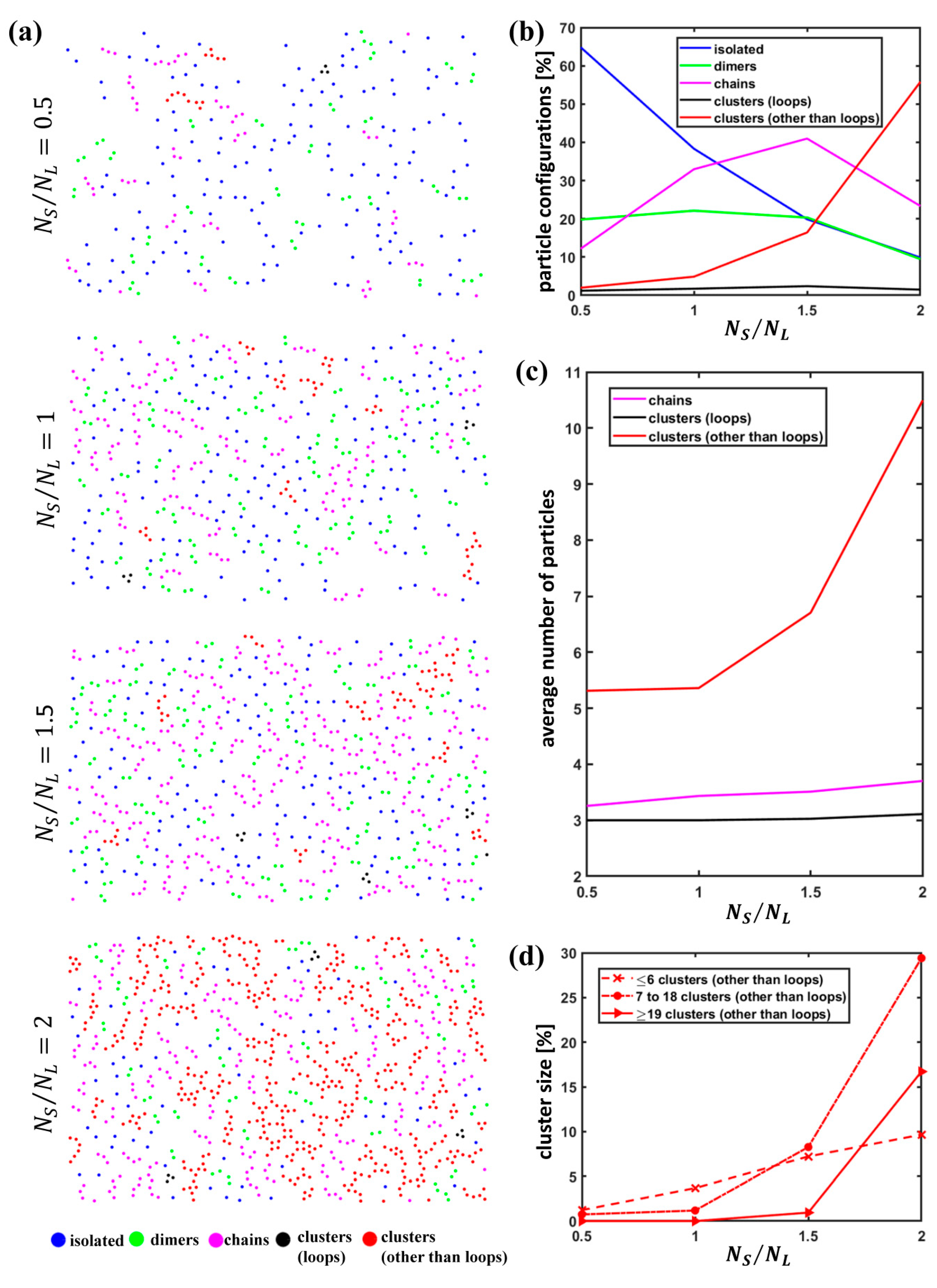
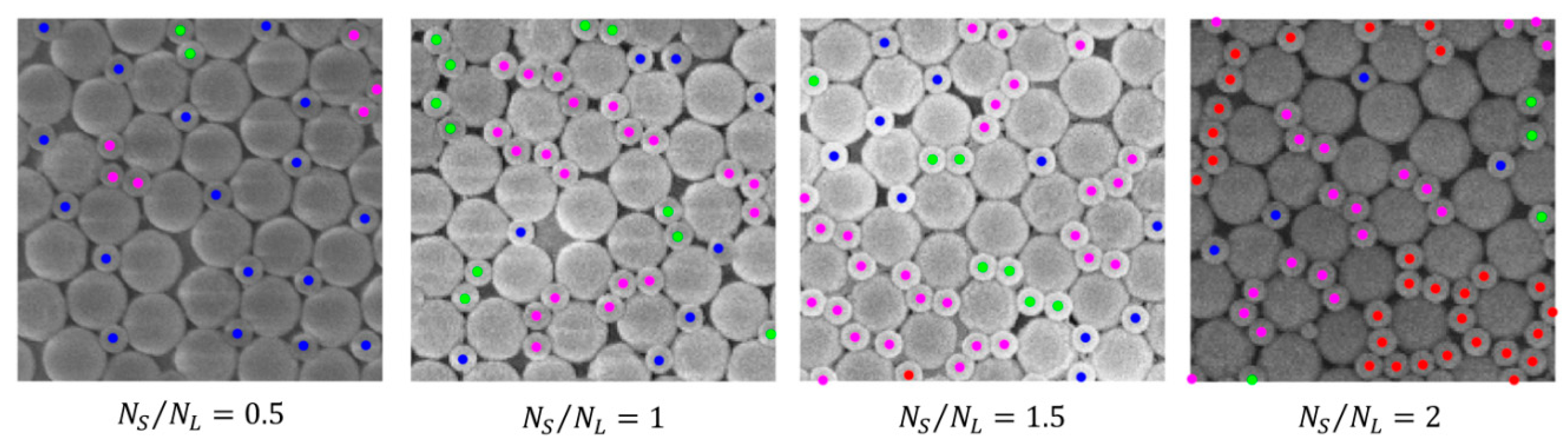
- Examination of the configurations assumed by S particles, i.e., identification of isolated particles, dimers, chains, rings and clusters;
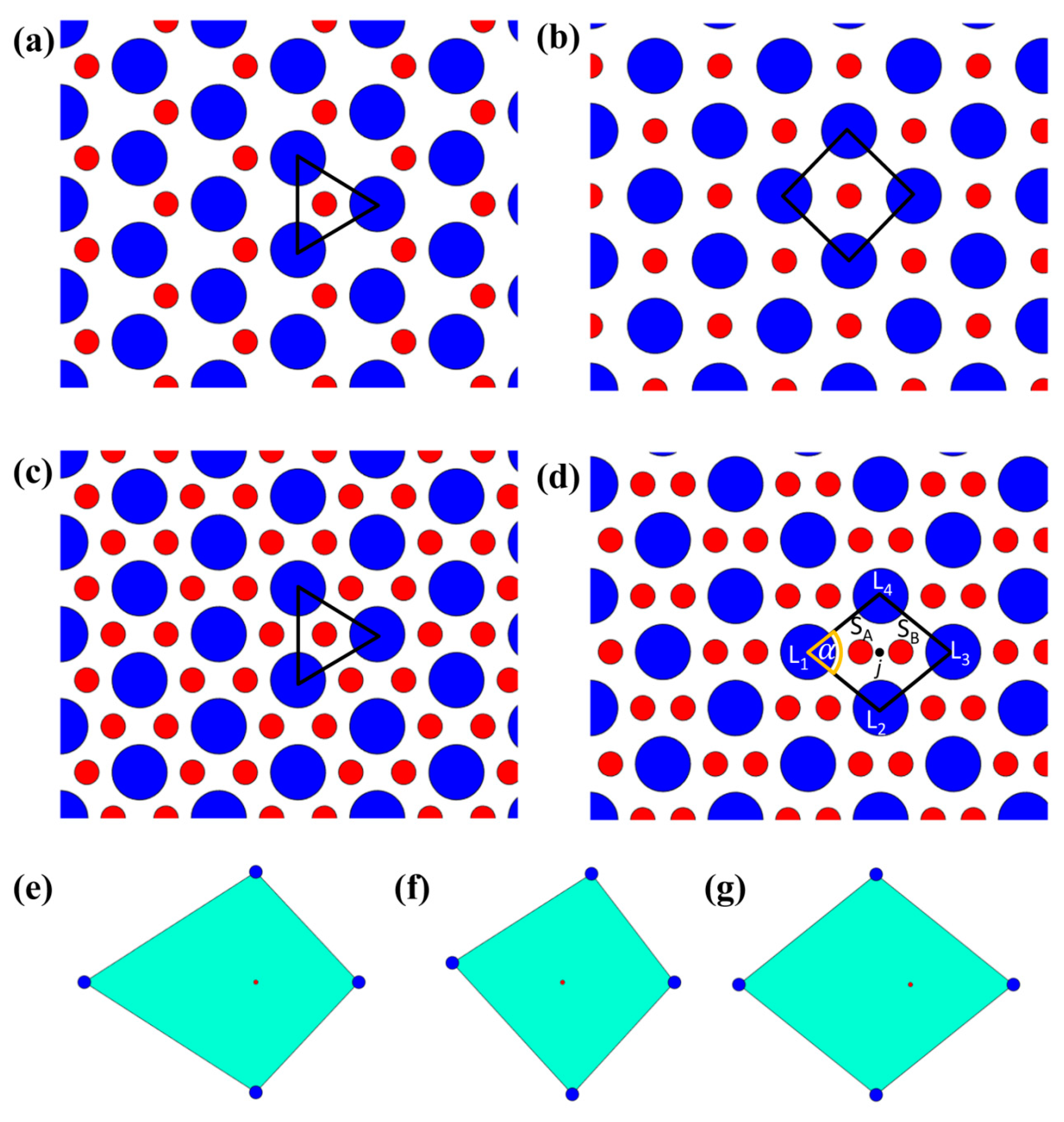
- Examination of the configurations assumed by L particles relative to S particles in order to identify specific recurrent patterns characterized by angular and radial uniformity of L particles with respect to S particles (for instance, isolated S particles in the interstices between three L particles arranged in an equilateral triangle and between four L particles arranged in a square or S particle dimers in the interstices between four L particles arranged in a rhombus);
- Examination of the configurations assumed by S particles relative to L particles, e.g., determination of L particles “caged” by S particles and analysis of the angular and radial uniformity of S particles around L particles.
2. Materials and Methods
2.1. Colloidal Self-Assembly
2.2. Characterization and Analysis of Particle Configurations
- Isolated particles: a particle belongs to this group if , i.e., if it does not have any neighbor within a distance from its centroid;
- Dimers: two particles and belong to this group if has only the neighbor within a distance from its centroid and if has only the neighbor within a distance from its centroid;
- Chains: particles belong to a chain if they are such that each of the particles belonging to the chain has only two neighbors within a distance from its centroid (neighborhood cardinality 2) except for two particles that have just one neighbor (neighborhood cardinality 1) and such a neighbor has a neighborhood cardinality equal to 2; roughly speaking, in a chain, all the particles have two neighbors except for the particles at the two extremes of the chain that have just one neighbor;
- Clusters: particles belong to a cluster if they belong to a connected component that does not fall into any of the previous categories; within this set, we can distinguish loops; particles belong to a loop if they all have a neighborhood cardinality equal to 2, i.e., if they all have two neighbors within a distance from their centroid; a loop can be interpreted as a sort of “closed” chain or ring.
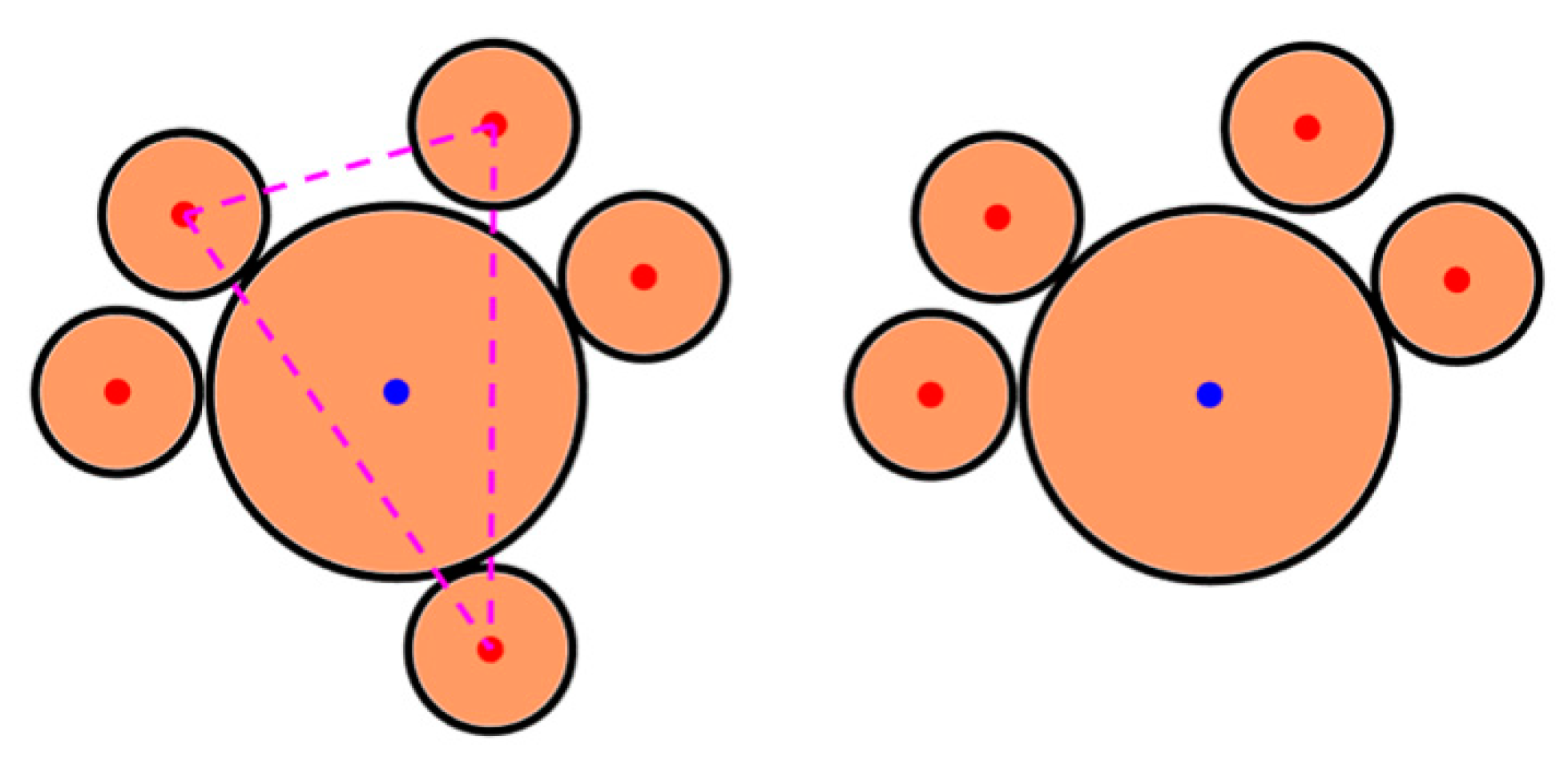
- The center of the L particle lies within the triangle;
- All sides of the triangle (i.e., the center-to-center distances of the three S nearest neighbors) are smaller than .
3. Results and Discussion
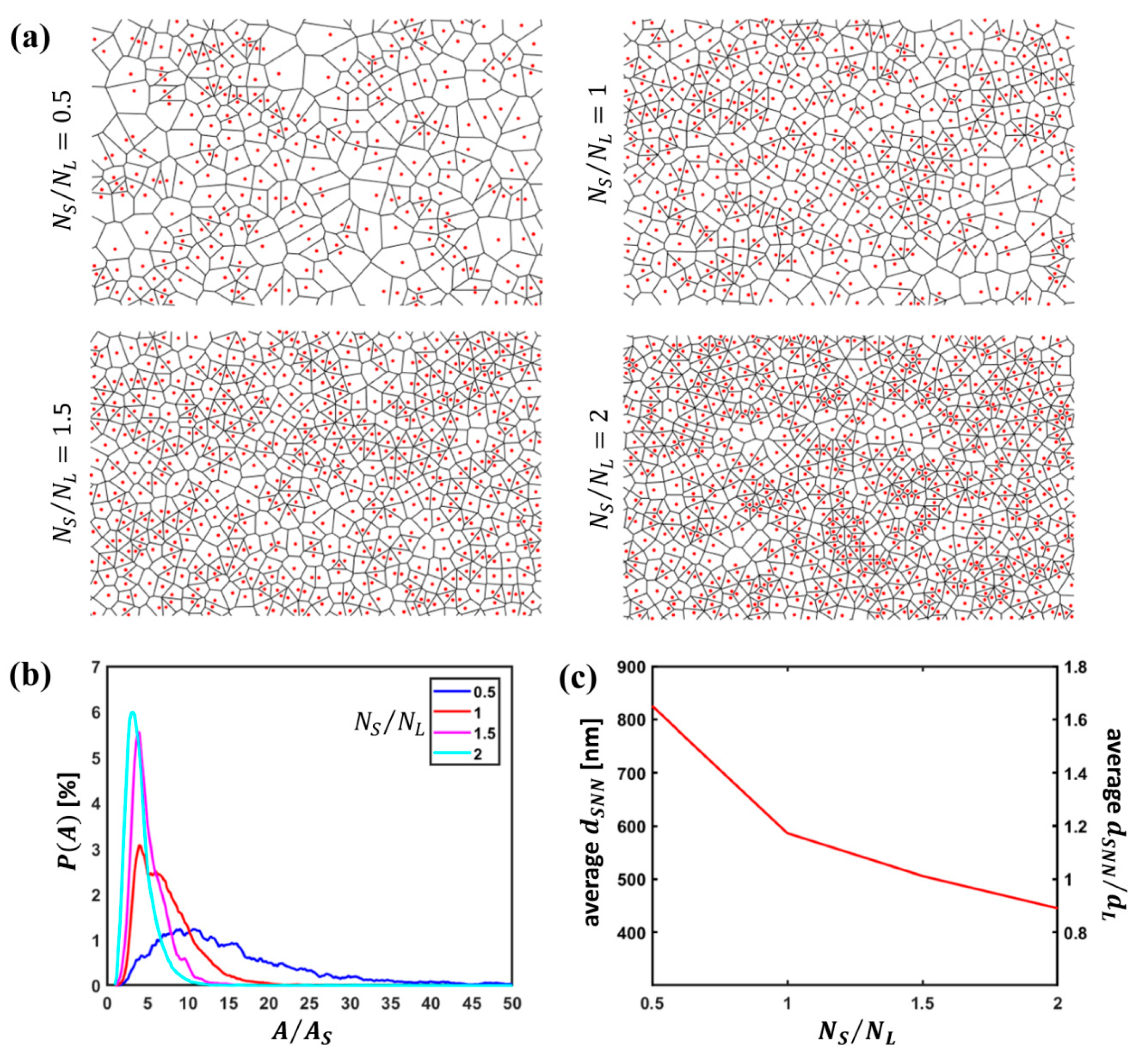
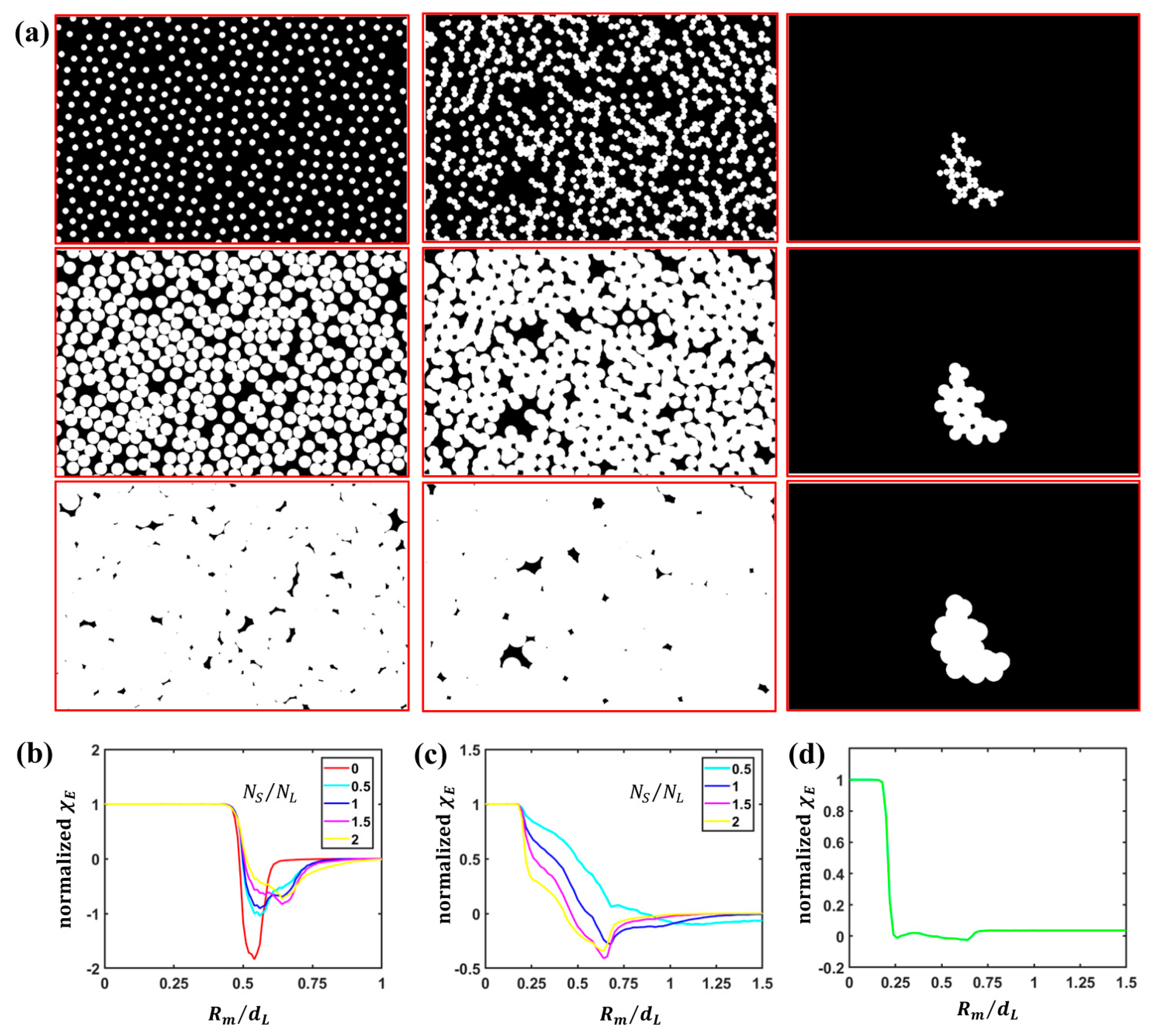
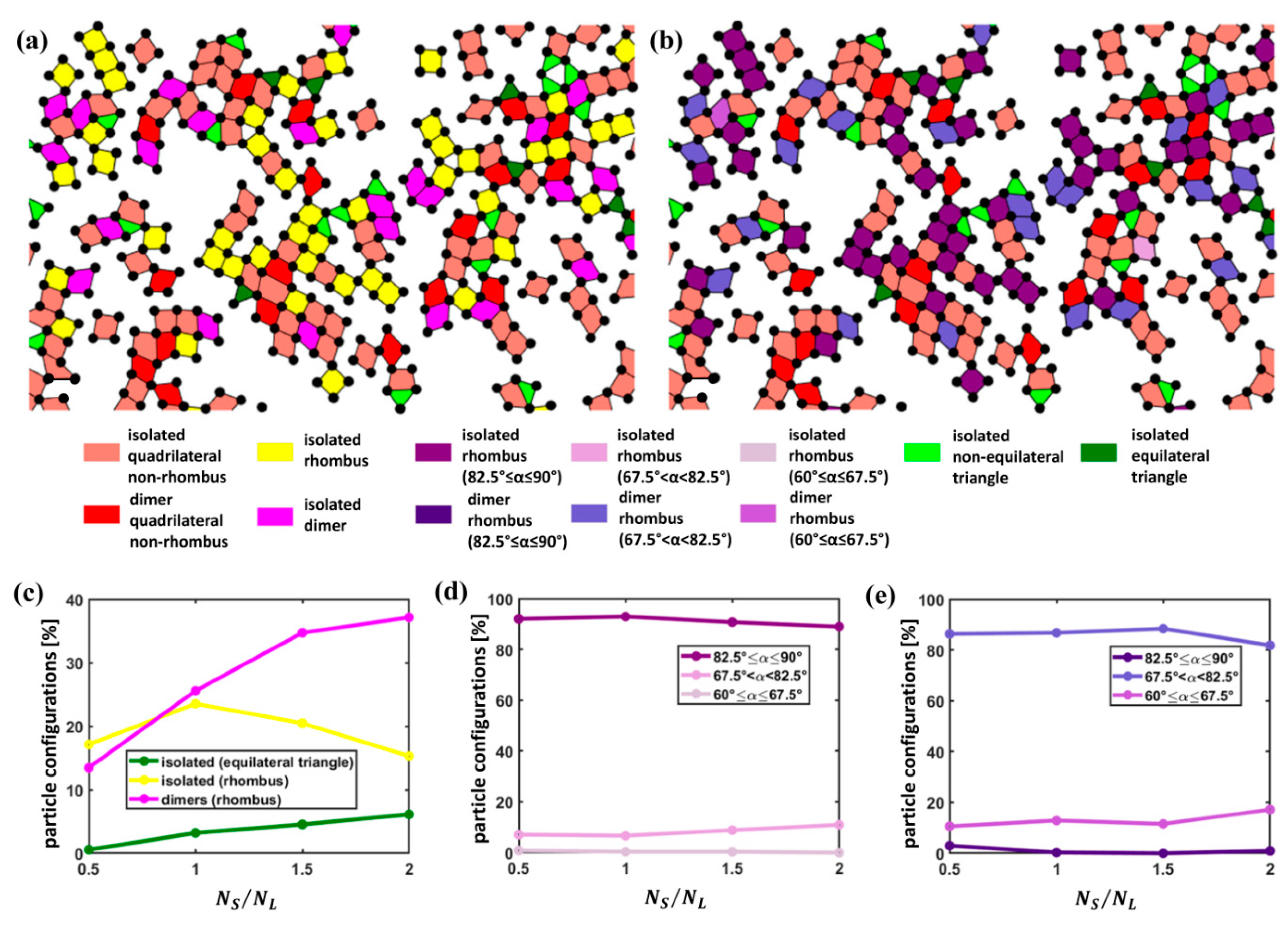
3.1. Quantitative Analysis of S Particle Configurations
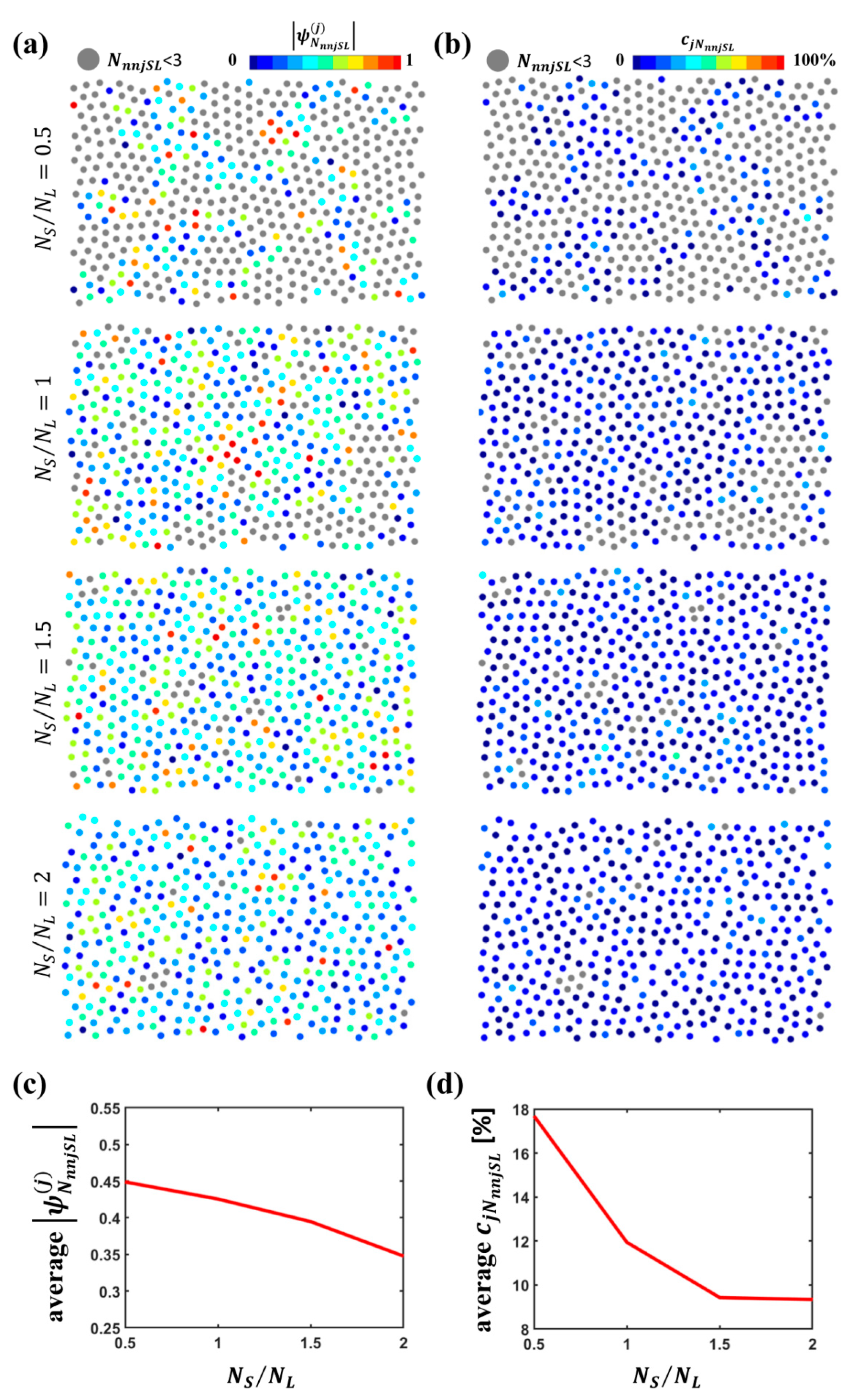
3.2. Quantitative Analysis of L Particle Configurations Relative to S Particles
- Isolated S particles present alternatively in the interstices between L particles arranged in a hexagonal pattern;
- Isolated S particles present in the interstices between L particles arranged in a square pattern;
- Isolated S particles present in all the interstices between L particles arranged in a hexagonal pattern.
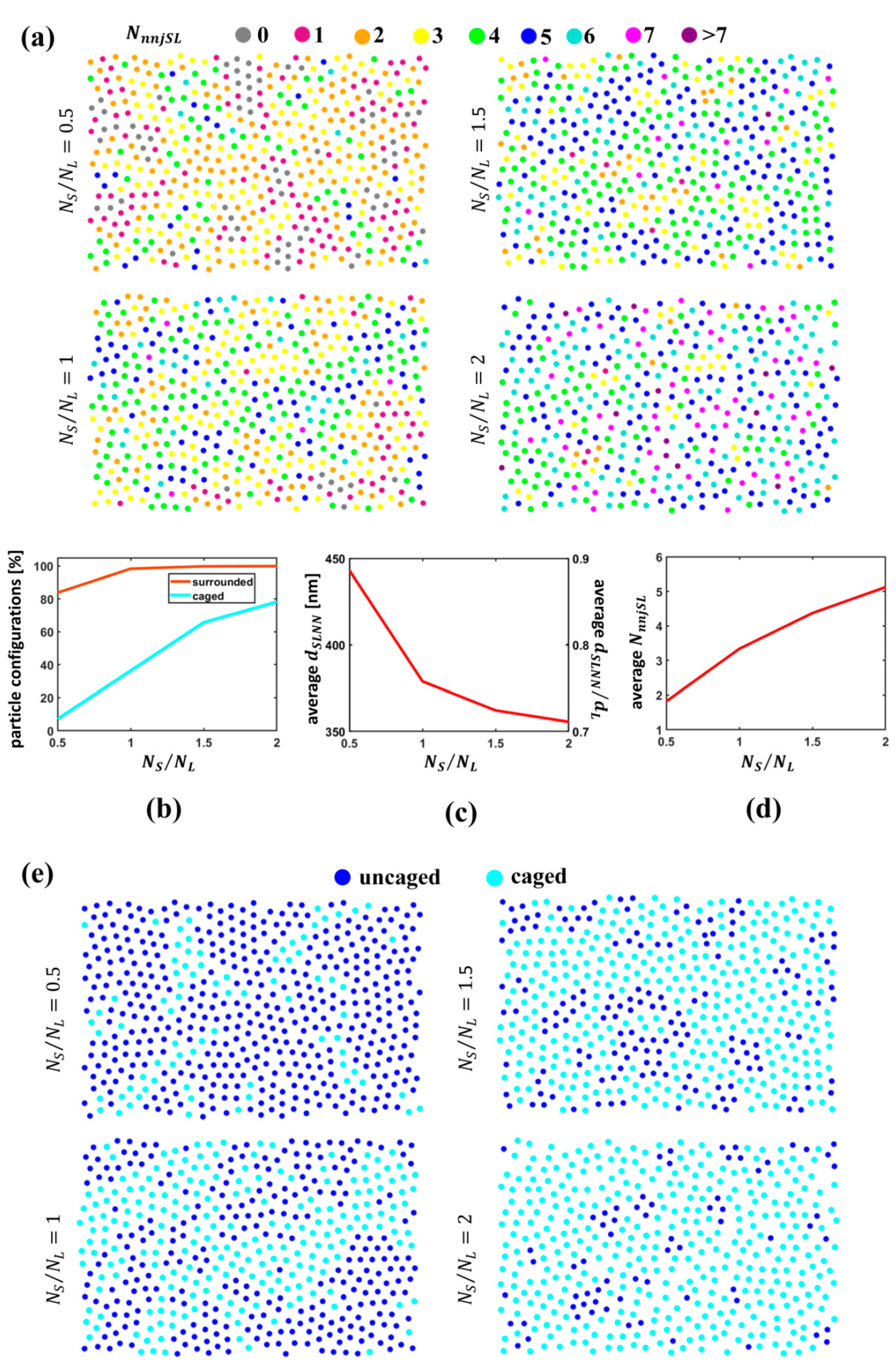
3.3. Quantitative Analysis of S Particle Configurations Relative to L Particles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lotito, V.; Zambelli, T. Approaches to self-assembly of colloidal monolayers: A guide for nanotechnologists. Adv. Colloid Interface Sci. 2017, 246, 217–274. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, Y.; Duan, G.; Jia, L.; Cai, W. Phase diagram, design of monolayer binary colloidal crystals, and their fabrication based on ethanol-assisted self-assembly at the air/water interface. ACS Nano 2012, 6, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; de Viguerie, L.; Jonas, U.; Weiss, C.K.; Landfester, K. Wafer-scale fabrication of ordered binary colloidal monolayers with adjustable stoichiometries. Adv. Funct. Mater. 2011, 21, 3064–3073. [Google Scholar] [CrossRef]
- Lotito, V.; Zambelli, T. Self-assembly of single-sized and binary colloidal particles at air/water interface by surface confinement and water discharge. Langmuir 2016, 2016 32, 9582–9590. [Google Scholar] [CrossRef]
- Likos, C.N.; Henley, C.L. Complex alloy phases for binary hard-disc mixtures. Philos. Mag. B 1993, 68, 85–113. [Google Scholar] [CrossRef]
- Pham, A.T.; Seto, R.; Schönke, J.; Joh, D.Y.; Chilkoti, A.; Fried, E.; Yellen, B.B. Crystallization kinetics of binary colloidal monolayers. Soft Matter 2016, 12, 7735–7746. [Google Scholar] [CrossRef] [PubMed]
- Ristenpart, W.D.; Aksay, I.A.; Saville, D.A. Electrically guided assembly of planar superlattices in binary colloidal suspensions. Phys. Rev. Lett. 2003, 90, 128303. [Google Scholar] [CrossRef] [PubMed]
- Assoud, L.; Ebert, F.; Keim, P.; Messina, R.; Maret, G.; Löwen, H. Ultrafast quenching of binary colloidal suspensions in an external magnetic field. Phys. Rev. Lett. 2009, 102, 23801. [Google Scholar] [CrossRef]
- Löwen, H.; Horn, T.; Neuhaus, T.; ten Hagen, B. Two-dimensional colloidal mixtures in magnetic and gravitational fields. Eur. Phys. J. Spec. Top. 2013, 222, 2961–2972. [Google Scholar] [CrossRef][Green Version]
- Hamanaka, T.; Onuki, A. Transitions among crystal, glass, and liquid in a binary mixture with changing particle-size ratio and temperature. Phys. Rev. E 2006, 74, 011506. [Google Scholar] [CrossRef]
- Romanov, S.G.; Orlov, S.; Ploss, D.; Weiss, C.K.; Vogel, N.; Peschel, U. Engineered disorder and light propagation in a planar photonic glass. Sci. Rep. 2016, 6, 27264. [Google Scholar] [CrossRef]
- Lotito, V.; Zambelli, T. Pattern formation in binary colloidal assemblies: Hidden symmetries in a kaleidoscope of structures. Langmuir 2018, 34, 7827–7843. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Duraia, E.-S.M.; Velev, O.D.; Amiri, M.D.; Beall, G.W. Formation of periodic size-segregated stripe pattern via directed self-assembly of binary colloids and its mechanism. Appl. Surf. Sci. 2018, 435, 512–520. [Google Scholar] [CrossRef]
- Tkachenko, A.V. Generic phase diagram of binary superlattices. Proc. Natl. Acad. Sci. USA 2016, 113, 10269–10274. [Google Scholar] [CrossRef]
- Law, A.D.; Buzza, D.M.A.; Horozov, T.S. Two-dimensional colloidal alloys. Phys. Rev. Lett. 2011, 106, 128302. [Google Scholar] [CrossRef]
- Fornleitner, J.; Kahl, G.; Likos, C.N. Tailoring the phonon band structure in binary colloidal mixtures. Phys. Rev. E 2010, 81, 060401(R). [Google Scholar] [CrossRef]
- Still, T. High Frequency Acoustics in Colloid-Based Meso and Nanostructures by Spontaneous Brillouin Light Scattering; Springer Theses; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Vogel, N. Surface Patterning with Colloidal Monolayers; Springer Theses; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Lohr, M.A.; Still, T.; Ganti, R.; Gratale, M.D.; Davidson, Z.S.; Aptowicz, K.B.; Goodrich, C.P.; Sussman, D.M.; Yodh, A.G. Vibrational and structural signatures of the crossover between dense glassy and sparse gel-like attractive colloidal packings. Phys. Rev. E 2014, 90, 062305. [Google Scholar] [CrossRef] [PubMed]
- Gratale, M.D.; Yunker, P.J.; Chen, K.; Still, T.; Aptowicz, K.B.; Yodh, A.G. Phonons in two-dimensional colloidal crystals with bond-strength disorder. Phys. Rev. E 2013, 87, 052301. [Google Scholar] [CrossRef] [PubMed]
- Gratale, M.D.; Ma, X.; Davidson, Z.S.; Still, T.; Habdas, P.; Yodh, A.G. Vibrational properties of quasi-two-dimensional colloidal glasses with varying interparticle attraction. Phys. Rev. E 2016, 94, 042606. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Still, T.; Schoenholz, S.; Aptowicz, K.B.; Schindler, M.; Maggs, A.C.; Liu, A.J.; Yodh, A.G. Phonons in two-dimensional soft colloidal crystals. Phys. Rev. E 2013, 88, 022315. [Google Scholar] [CrossRef] [PubMed]
- Still, T.; Chen, W.; Retch, M.; Jonas, U.; Fytas, G. Colloidal systems: A promising material class for tailoring sound propagation at high frequencies. J. Phys. Condens. Matter 2008, 20, 404203. [Google Scholar] [CrossRef]
- Lotito, V.; Zambelli, T. Self-assembly and nanosphere lithography for large-area plasmonic patterns on graphene. J. Colloid Interface Sci. 2015, 447, 202–210. [Google Scholar] [CrossRef]
- Smith, N.L.; Coukouma, A.; Dubnik, S.; Asher, S.A. Debye ring diffraction elucidation of 2D photonic crystal self-assembly and ordering at the air-water interface. Phys. Chem. Chem. Phys. 2017, 19, 31813–31822. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Ge, L.; Liu, X.; Zhou, Y.; Chang, J.; Ali, H.; Pan, C.; Wang, T.; Wang, M. Large area highly ordered monolayer composite microsphere arrays—Fabrication and tunable surface plasmon linewidth. RSC Adv. 2018, 8, 39735–39741. [Google Scholar] [CrossRef]
- Saadatfar, M.; Takeuchi, H.; Robins, V.; Francois, N.; Hiraoka, Y. Pore configuration landscape of granular crystallization. Nat. Commun. 2017, 8, 15082. [Google Scholar] [CrossRef]
- Ardanza-Trevijano, S.; Zuriguel, I.; Arévalo, R.; Maza, D. Topological analysis of tapped granular media using persistent homology. Phys. Rev. E 2014, 89, 052212. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.; Ardanza-Trevijano, S.; Poduska, K.M.; Yethiraj, A.; Gonzalez-Viñas, W. Quantifying disorder in colloidal films spin-coated onto patterned substrates. Phys. Rev. E 2017, 95, 032607. [Google Scholar] [CrossRef]
- Robins, V.; Turner, K. Principal component analysis of persistent homology rank functions with case studies of spatial point patterns, sphere packing and colloids. Phys. D 2016, 334, 99–117. [Google Scholar] [CrossRef]
- Hegelsen, G.; Skjeltorp, A.T. An experimental system for studying dynamic behavior of magnetic particles. J. Appl. Phys. 1991, 69, 8277–8284. [Google Scholar]
- Lumsdon, S.O.; Kaler, E.W.; Williams, J.P.; Velev, O.D. Dielectrophoretic assembly of oriented and switchable two-dimensional photonic crystals. Appl. Phys. Lett. 2003, 82, 949–951. [Google Scholar] [CrossRef]
- Lumsdon, S.O.; Kaler, E.W.; Velev, O.D. Two-dimensional crystallization of microspheres by a coplanar AC electric field. Langmuir 2004, 20, 2108–2116. [Google Scholar] [CrossRef]
- Atsumi, C.; Araoka, S.; Landenberger, K.B.; Kanazawa, A.; Nakamura, J.; Ohtsuki, C.; Aoshima, S.; Sugawara-Narutaki, A. Ring-like assembly of silica nanospheres in the presence of amphiphilic block copolymer: Effects of particle size. Langmuir 2018, 34, 7751–7758. [Google Scholar] [CrossRef]
- Rey, M.; Yu, T.; Bley, K.; Landfester, K.; Buzza, D.M.; Vogel, N. Amphiphile-induced anisotropic colloidal self-assembly. Langmuir 2018, 34, 9990–10000. [Google Scholar] [CrossRef]
- Semmler, J.; Bley, K.; Klupp Taylor, R.N.; Stingl, M.; Vogel, N. Particulate coatings with optimized haze properties. Adv. Funct. Mater. 2019, 29, 1806025. [Google Scholar] [CrossRef]
- Rey, M.; Yu, T.; Guenther, R.; Bley, K.; Vogel, N. A dirty story: Improving colloidal monolayer formation by understanding the effect of impurities at the air/water interface. Langmuir 2019, 35, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, S.; Moldenaers, P.; Vermant, J. Control over colloidal aggregation in monolayers of latex particles at the oil-water interface. Langmuir 2006, 22, 4936–4945. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.J.; Earnshaw, J.C. Experimental study of colloidal aggregation in two dimensions. I. Structural aspects. Phys. Rev. A 1992, 46, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Bergström, L. Structure and formation of particle monolayers at liquid interface. In Colloidal Particles at Liquid Interfaces; Binks, B.P., Horozov, T.S., Eds.; Cambridge University Press: New York, NY, USA, 2006; pp. 77–107. [Google Scholar]
- Ovcharov, P.V.; Kryuchkov, N.P.; Zaytsev, K.I.; Yurchenko, S.O. Particle-resolved phase identification in two-dimensional condensable systems. J. Phys. Chem. C 2017, 121, 26860–26868. [Google Scholar] [CrossRef]
- Zhan, H.; Chem, Y.; Liu, Y.; Lau, W.; Bao, C.; Li, M.; Lu, Y.; Mei, J.; Hui, D. Precision-trimming 2D inverse-opal lattice on elastomer to ordered nanostructures with variable size and morphology. Langmuir 2017, 33, 4881–4889. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Sander, M.; Koopman, W.; Schuetz, R.; Bargheer, M.; Koetz, J. Deposition of gold nanotriangles in large scale close-packed monolayers for X-ray-based temperature calibration and SERS monitoring of plasmon-driven catalytic reactions. ACS Appl. Mater. Interfaces 2017, 9, 20247–20253. [Google Scholar] [CrossRef]
- Maiti, S.; André, A.; Banerjee, R.; Hagenlocher, J.; Konovalov, O.; Schreiber, F.; Scheele, M. Monitoring self-assembly and ligand exchange of PbS nanocrystal superlattices at the liquid/air interface in real time. J. Phys. Chem. Lett. 2018, 9, 739–744. [Google Scholar] [CrossRef]
- Blair, V.E.; Celebi, K.; Müllen, K.; Vermant, J. Electrically conductive thin films derived from bulk graphite and liquid–liquid interface assembly. Adv. Mater. Interfaces 2019, 6, 1801570. [Google Scholar] [CrossRef]
- Tang, J.S.J.; Bader, R.S.; Goerlitzer, E.S.A.; Wendisch, J.F.; Bourret, G.R.; Rey, M.; Vogel, N. Surface patterning with SiO2@PNiPAm core−shell particles. ACS Omega 2018, 3, 12089–12098. [Google Scholar] [CrossRef]
- Maestro, A.; Santini, E.; Guzman, E. Physico-chemical foundations of particle-laden fluid interfaces. Eur. Phys. J. E 2018, 41, 97. [Google Scholar] [CrossRef]
- Song, Q.; Schönherr, H. Control of orientation, formation of ordered structures, and self-sorting of surface-functionalized microcubes at the air-water interface. Langmuir 2019, 35, 6742–6751. [Google Scholar] [CrossRef]
- Mayarani, M.; Basavaraj, M.G.; Satapathy, D.K. Loosely packed monolayer coffee stains in dried drops of soft colloids. Nanoscale 2017, 9, 18798–18803. [Google Scholar] [CrossRef]
- Moučka, F.; Nezbeda, I. Detection and characterization of structural changes in the hard-disk fluid under freezing and melting conditions. Phys. Rev. Lett. 2005, 94, 040601. [Google Scholar] [CrossRef]
- Reis, P.M.; Ingale, R.A.; Shattuck, M.D. Crystallization of a quasi-two-dimensional granular fluid. Phys. Rev. Lett. 2006, 96, 258001. [Google Scholar] [CrossRef]
- Wang, Z.; Alsayed, A.M.; Yodh, A.G.; Han, Y. Two-dimensional freezing criteria for crystallizing colloidal monolayers. J. Chem. Phys. 2010, 132, 154501. [Google Scholar]
- Dillmann, P.; Maret, G.; Keim, P. Two-dimensional colloidal systems in time-dependent magnetic fields. Eur. Phys. J. Spec. Top. 2013, 222, 2941–2959. [Google Scholar] [CrossRef]
- Pham, A.T.; Zhuang, Y.; Detwiler, P.; Socolar, J.E.S.; Charbonneau, P.; Yellen, B.B. Phase diagram and aggregation dynamics of a monolayer of paramagnetic colloids. Phys. Rev. E 2017, 95, 052607. [Google Scholar] [CrossRef]
- Lavergne, F.A.; Diana, S.; Aarts, D.G.A.L.; Dullens, R.P.A. Anomalous grain growth in a polycrystalline monolayer of colloidal hard spheres. Phys. Rev. X 2017, 7, 041064. [Google Scholar] [CrossRef]
- Ebert, F.; Maret, G.; Keim, P. Partial clustering prevents global crystallization in a binary 2D colloidal glass former. Eur. Phys. J. E 2009, 29, 311–318. [Google Scholar] [CrossRef][Green Version]
- Dillmann, P.; Maret, G.; Keim, P. Comparison of 2D melting criteria in a colloidal system. J. Phys. Condens. Matter 2012, 24, 464118. [Google Scholar] [CrossRef]
- Hoffmann, N.; Likos, C.N.; Löwen, H. Microphase structuring in two-dimensional magnetic colloid mixtures. J. Phys. Condens. Matter 2006, 18, 10193–10211. [Google Scholar] [CrossRef]
- Ebert, F.; Maret, G.; Keim, P. Local crystalline order in a 2D colloidal glass former. Eur. Phys. J. E 2008, 26, 161–168. [Google Scholar] [CrossRef]
- Kim, J.; Sung, B.J. Dynamics and spatial correlation of voids in dense two dimensional colloids. J. Chem. Phys. 2014, 141, 014502. [Google Scholar] [CrossRef]
- Royall, C.P.; Vermolen, E.C.M.; van Blaaderen, A.; Tanaka, H. Controlling competition between crystallization and glass formation in binary colloids with an external field. J. Phys. Condens. Matter 2008, 20, 404225. [Google Scholar] [CrossRef]
- Williams, I.; Oguz, E.C.; Bartlett, P.; Löwen, H.; Royall, C.P. Flexible confinement leads to multiple relaxation regimes in glassy colloidal liquids. J. Chem. Phys. 2015, 142, 024505. [Google Scholar] [CrossRef]
- Tanaka, H. Bond orientational ordering in liquids: Towards a unified description of water-like anomalies, liquid-liquid transitions, glass transition, and crystallization. Eur. Phys. J. E 2012, 35, 113. [Google Scholar] [CrossRef]
- Van Blaaderen, A.; Wiltzius, P. Real-space structure of colloidal hard-sphere glasses. Science 1995, 270, 1177–1179. [Google Scholar] [CrossRef]
- Kuleshova, V.L.; Panfilova, E.V.; Prohorov, E.P. Automated device for vertical deposition of colloidal opal films. In Proceedings of the 2018 International Russian Automation Conference, Sochi, Russia, 9–16 September 2018. [Google Scholar]
- Lotito, V.; Sennhauser, U.; Hafner, C.; Bona, G.-L. Fully metal-coated scanning near-field optical microscopy probes with spiral corrugations for superfocusing under arbitrarily oriented linearly polarised excitation. Plasmonics 2011, 6, 327–336. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Rodriguez, M.A.; Elnathan, R.; Ditcovski, R.; Grillo, F.; Conley, G.M.; Timpu, F.; Rauh, A.; Geisel, K.; Ellenbogen, T.; Grange, R.; et al. Tunable 2D binary colloidal alloys for soft nanotemplating. Nanoscale 2018, 10, 22189–22195. [Google Scholar] [CrossRef]
- Lotito, V.; Sennhauser, U.; Hafner, C.; Bona, G.-L. Interaction of an asymmetric scanning near field optical microscopy probe with fluorescent molecules. Prog. Electromagn. Res. 2011, 121, 281–299. [Google Scholar] [CrossRef]
- Lotito, V.; Sennhauser, U.; Hafner, C. Finite element analysis of asymmetric scanning near field optical microscopy probes. J. Comput. Theor. Nanosci. 2010, 7, 1596–1609. [Google Scholar] [CrossRef]
- Chibani, H.; Dukenbayev, K.; Mensi, M.; Sekatskii, S.K.; Dietler, G. Near-field scanning optical microscopy using polymethylmethacrylate optical fiber probes. Ultramicroscopy 2010, 110, 211–215. [Google Scholar] [CrossRef]
- Kawasaki, T.; Tanaka, H. Structural signature of slow dynamics and dynamic heterogeneity in two-dimensional colloidal liquids: Glassy structural order. J. Phys. Condens. Matter 2011, 23, 194121. [Google Scholar] [CrossRef]
- Van der Hoeven, J.E.S.; van der Wee, E.B.; de Winter, D.A.; Hermes, M.; Liu, Y.; Fokkema, J.; Bransen, M.; van Huis, M.A.; Gerritsen, H.C.; de Jongh, P.E.; et al. Bridging the gap: 3D real-space characterization of colloidal assemblies via FIB-SEM tomography. Nanoscale 2019, 11, 5304–5316. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotito, V.; Zambelli, T. A Journey Through the Landscapes of Small Particles in Binary Colloidal Assemblies: Unveiling Structural Transitions from Isolated Particles to Clusters upon Variation in Composition. Nanomaterials 2019, 9, 921. https://doi.org/10.3390/nano9070921
Lotito V, Zambelli T. A Journey Through the Landscapes of Small Particles in Binary Colloidal Assemblies: Unveiling Structural Transitions from Isolated Particles to Clusters upon Variation in Composition. Nanomaterials. 2019; 9(7):921. https://doi.org/10.3390/nano9070921
Chicago/Turabian StyleLotito, Valeria, and Tomaso Zambelli. 2019. "A Journey Through the Landscapes of Small Particles in Binary Colloidal Assemblies: Unveiling Structural Transitions from Isolated Particles to Clusters upon Variation in Composition" Nanomaterials 9, no. 7: 921. https://doi.org/10.3390/nano9070921
APA StyleLotito, V., & Zambelli, T. (2019). A Journey Through the Landscapes of Small Particles in Binary Colloidal Assemblies: Unveiling Structural Transitions from Isolated Particles to Clusters upon Variation in Composition. Nanomaterials, 9(7), 921. https://doi.org/10.3390/nano9070921





