Effect of Ce Doping on the Structure and Chemical Stability of Nano-α-Fe2O3
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Raw Materials
2.2. Preparation of Nano-Iron Oxide
2.3. Characterization
- (1)
- XRD analysis. The crystal structure was measured by using X-ray diffraction (XRD) (PANalytical, X’pert PRO, Almelo, Netherlands) with Cu Kα (λ = 1.5418 Å). The scan rate and 2θ range of the samples were 2 deg/min and 10–70°, respectively. Moreover, the crystal structure was analyzed with jade6 software (Materials Data Inc., California, CA, USA) and in accordance with the databases of Powder Diffraction Flile (PDF) provided by the International Centre for Diffraction Data (ICDD). The crystalline size of nano-iron oxide can be estimated from the full width at half-maximum diffraction peak by the Scherrer equation.where D is the crystalline size (nm), K is a geometric factor (use K = 0.9), λ is the X-ray wavelength (1.5418 Å), β is the peak full width at half maximum (FWHM) in radians and θ is the Bragg’s angle of the peaks.
- (2)
- SEM-EDS analysis. The samples were first sprayed gold and then investigated for their morphology by scanning electron microscopy (SEM) (Zeiss, ULTRA PLU, Oberkochen, Germany).
- (3)
- TEM analysis. Transmission electron microscopy (TEM) (JEOL, JEM 2100, Tokyo, Japan) was used to study the Ce-doped α-Fe2O3’s internal phase at 200 keV.
- (4)
- FTIR analysis. The bonding styles of samples were studied using Fourier transform infrared (FTIR) (Thermo Nicolet, Nicolet-380, Minneapolis, MN, USA) in the 4000–400 cm−1 range using the KBr dilution technique.
- (5)
- XPS analysis. The element type and chemical valence state of the sample surface was measured by using the American Thermo Scientific ESCALAB 250Xi electron spectrometer (Waltham, MA, USA).
- (6)
- Electrochemistry analysis. Potentiodynamic polarization measurements were performed with an electrochemical workstation (Metrohm, Autolab, Utrecht, Switzerland). The scan rate was 3 mV s−1, with the scanning potential ranging from −0.5 to +1 V (vs. OCP). Samples, liquid paraffin and carbon powder were mixed in a 1:4:5 mass ratio as working electrode [20], a platinum plate (Pt) was used as the counter electrode and a saturated calomel electrode (SCE) was the reference electrode. The measurement was performed at room temperature in a 3.5% NaCl solution. The analysis was carried out using the NOVA 1.10 software (Metrohm, Beijing, China) provided by the Autolab electrochemical workstation.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aslani, F.; Bagheri, S.; Julkapli, N.M. Effects of Engineered Nanomaterials on Plants Growth: An Overview. Sci. World J. 2014, 2014, 641759. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, D.; Tang, G. Hydrothermal synthesis and visible-light photocatalytic activity of α-Fe2O3/TiO2 composite hollow microspheres. Ceram. Int. 2013, 39, 8633–8640. [Google Scholar] [CrossRef]
- Hu, C.Y.; Kou, S.W.; Guo, J.J. The Application Research of Ship Metal Corrosion in Marine Environment. Adv. Mater. Res. 2014, 936, 1091–1096. [Google Scholar] [CrossRef]
- Alexiadis, V.I.; Boukos, N.; Verykios, X.E. Influence of the composition of Fe2O3/Al2O3 catalysts on the rate of production and quality of carbon nanotubes. Mater. Chem. Phys. 2011, 128, 96–108. [Google Scholar] [CrossRef]
- He, S.P.; Gao, Q.; Yu, X.T. Preparation of Red Iron Oxide and the Use in Coatings. Chem. Prod. Technol. 2011, 18, 29–32. (In Chinese) [Google Scholar]
- Qin, W. Talking about the Application of Iron Oxide Red Pigment in Ceramic Industry. Foshan Ceram. 2011, 21, 9–12. (In Chinese) [Google Scholar]
- Mohammadikish, M. Hydrothermal synthesis, characterization and optical properties of ellipsoid shape α-Fe2O3. Nanocryst. Ceram. Int. 2014, 40, 1351–1358. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N.; Venkateswarlu, K. Effect of various additives on morphology and corrosion behavior of ceramic coatings developed on AZ31 magnesium alloy by plasma electrolytic oxidation. Ceram. Int. 2012, 38, 4607–4615. [Google Scholar] [CrossRef]
- Hayes, S.A.; Yu, P.; O’Keefe, T.J. The phase stability of cerium species in aqueous systems - I. E-pH diagram for the Ce-HClO4-H2O system. J. Electrochem. Soc. 2002, 149, C623–C630. [Google Scholar] [CrossRef]
- Liu, F.; Song, H.Q.; Huang, Z.R. Anti-corrosion Properties of Protective Coatings for Transmission Line Iron Tower in Coastal Area. Equip. Environ. Eng. 2015, 12, 76–82. (In Chinese) [Google Scholar]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic-inorganic hybrid sol-gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, D.Z. Effect of CeO2 doping on catalytic activity of Fe2O3/γ-Al2O3 catalyst for catalytic wet peroxide oxidation of azo dyes. J. Hazard. Mater. 2007, 143, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.C. Upgrading of vacuum residue with chemical looping partial oxidation over Ce doped Fe2O3. Energy 2018, 162, 542–553. [Google Scholar] [CrossRef]
- Tambe, S.P.; Singh, S.K.; Patri, M. Effect of pigmentation on mechanical and anticorrosive properties of thermally sprayable EVA and EVAl coatings. Prog. Org. Coat. 2011, 72, 315–320. [Google Scholar] [CrossRef]
- Singh, S.K.; Tambe, S.P.; Raja, V.S. Thermally sprayable polyethylene coatings for marine environment. Prog. Org. Coat. 2007, 60, 186–193. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. Preparation of polyaniline-Fe2O3, composite and its anticorrosion performance. Synth. Met. 2007, 157, 751–757. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Sinha, T.J.M.; Khanna, A.S. Effect of nano-Al2O3, particles on the corrosion behavior of alkyd based waterborne coatings. J. Coat. Technol. Res. 2009, 6, 353–368. [Google Scholar] [CrossRef]
- Huo, J.Z. Preparation of Composite Iron Oxides via Hydrothermal Process. Adv. Mater. Res. 2012, 583, 162–166. [Google Scholar] [CrossRef]
- Liu, H.D.; Zhang, J.L.; Xu, D.D. Easy one-step hydrothermal synthesis of nitrogen-doped reduced graphene oxide/iron oxide hybrid as efficient supercapacitor material. J. Solid State Electrochem. 2015, 19, 135–144. [Google Scholar] [CrossRef]
- Zou, Y.D.; Wang, J.; Mo, J.H. Voltammetric Determination of Amino Acids by Modified Carbon Paste Electrodes I. Study on the Electrochemical Behaviour of Tryptophan and Tyrosine on Polyamide-modified Carbon Paste Electrode. J. Instrum. Anal. 1999, 18, 25–28. (In Chinese) [Google Scholar]
- Dijken, A.V.; Meulenkamp, E.A.; Vanmaekelbergh, D. The luminescence of nanocrystalline ZnO particles: The mechanism of the ultraviolet and visible emission. J. Lumin. 2000, 87, 454–456. [Google Scholar] [CrossRef]
- Blanca-Romero, A.; Flores-Riveros, A.; Rivas-Silva, J.F. Structure Study of ZnO: Eu with the Supercell Method. J. Nano Res. 2010, 9, 25–30. [Google Scholar] [CrossRef]
- Perez-Alonso, F.J. Synergy of FexCe1−xO2 mixed oxides for N2O decomposition. J. Catal. 2006, 239, 340–346. [Google Scholar] [CrossRef]
- Tyson, T.A.; Leon, J.M.D.; Conradson, S.D.; Bishop, A.R.; Neumeier, J.J.; Röder, H.; Zang, J. Evidence for a local lattice distortion in Ca-doped LaMnO3. Phys. Rev. B 1996, 53, 13985. [Google Scholar] [CrossRef] [PubMed]
- Kools, F. Reaction-induced grain growth impediment during sintering of strontium hexaferrite with silica addition. Solid State Ion. 1985, 16, 251–259. [Google Scholar] [CrossRef]
- Fu, Y.P.; Lin, C.H.; Hsu, C.S. Preparation of ultrafine CeO2, powders by microwave-induced combustion and precipitation. J. Alloys Compd. 2005, 391, 110–114. [Google Scholar] [CrossRef]
- Baneshi, J.; Haghighi, M.; Jodeiri, N. Homogeneous precipitation synthesis of CuO-ZrO2-CeO2-Al2O3, nanocatalyst used in hydrogen production via methanol steam reforming for fuel cell applications. Energy Convers. Manag. 2014, 87, 928–937. [Google Scholar] [CrossRef]
- Khan, S.B.; Faisal, M.; Rahman, M.M. Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for environmental applications. Sci. Total Environ. 2011, 409, 2987–2992. [Google Scholar] [CrossRef]
- Saberi, A.; Golestani-Fard, F.; Sarpoolaky, H. Chemical synthesis of nanocrystalline magnesium aluminate spinel via nitrate-citrate combustion route. J. Alloys Compd. 2008, 462, 142–146. [Google Scholar] [CrossRef]
- Khan, I.A.; Belkhedkar, M.R.; Salodkar, R.V.; Ubale, A.U. Physical properties of nanostructured CeO2 thin films grown by SILAR method. AIP Conf. Proc. 2018, 1953, 030102. [Google Scholar]
- Mazloom, J. Relation between physical properties, enhanced photodegradation of organic dyes and antibacterial potential of Sn1−xSbxO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 2183–2192. [Google Scholar] [CrossRef]
- Chen, J.Y.; Jiang, G.M.; Teng, Y. Research on infrared spectra of (Fe,N) doped TiO2 by the electronegativity principle. Spectrosc. Spectr. Anal. 2017, 37, 2305–2310. [Google Scholar]
- Chen, J.Y.; Yan, J.K.; Gan, G.Y. The Effect of Cu Doping on the Transformation from Rutile to Anatase and Cu Occupation Tendency in TiO2 Solid Solution. J. Spectrosc. 2019, 2019, 6470601. [Google Scholar] [CrossRef]
- Meng, F.; Wang, L.; Cui, J. Controllable synthesis and optical properties of nano-CeO2, via a facile hydrothermal route. J. Alloys Compd. 2013, 556, 102–108. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Ramezanzadeh, B. A Detailed Molecular Dynamics Simulation and Experimental Investigation on the Interfacial Bonding Mechanism of an Epoxy Adhesive on Carbon Steel Sheets Decorated with a Novel Cerium-Lanthanum Nanofilm. ACS Appl. Mater. Interfaces 2017, 9, 17536. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, X.; Zhang, J. Surfactant-free CuO mesocrystals with controllable dimensions: Green ordered-aggregation-driven synthesis, formation mechanism and their photochemical performances. CrystEngComm 2013, 15, 867–877. [Google Scholar] [CrossRef]
- Yang, X.; Lian, X.; Liu, S. Investigation of enhanced photoelectrochemical property of cerium doped hematite film prepared by sol-gel route. Int. J. Electrochem. Sci. 2013, 8, 3721–3730. [Google Scholar]
- Hellgren, N.; Johansson, M.P.; Broitman, E. Role of nitrogen in the formation of hard and elastic CN x thin films by reactive magnetron sputtering. Phys. Rev. B 1999, 59, 5162. [Google Scholar] [CrossRef]
- Tauc, J. Electronic Properties of Amorphous Materials: Changes are considered which occur when the long-range order typical for crystals disappears. Science 1967, 158, 1543–1548. [Google Scholar] [CrossRef]
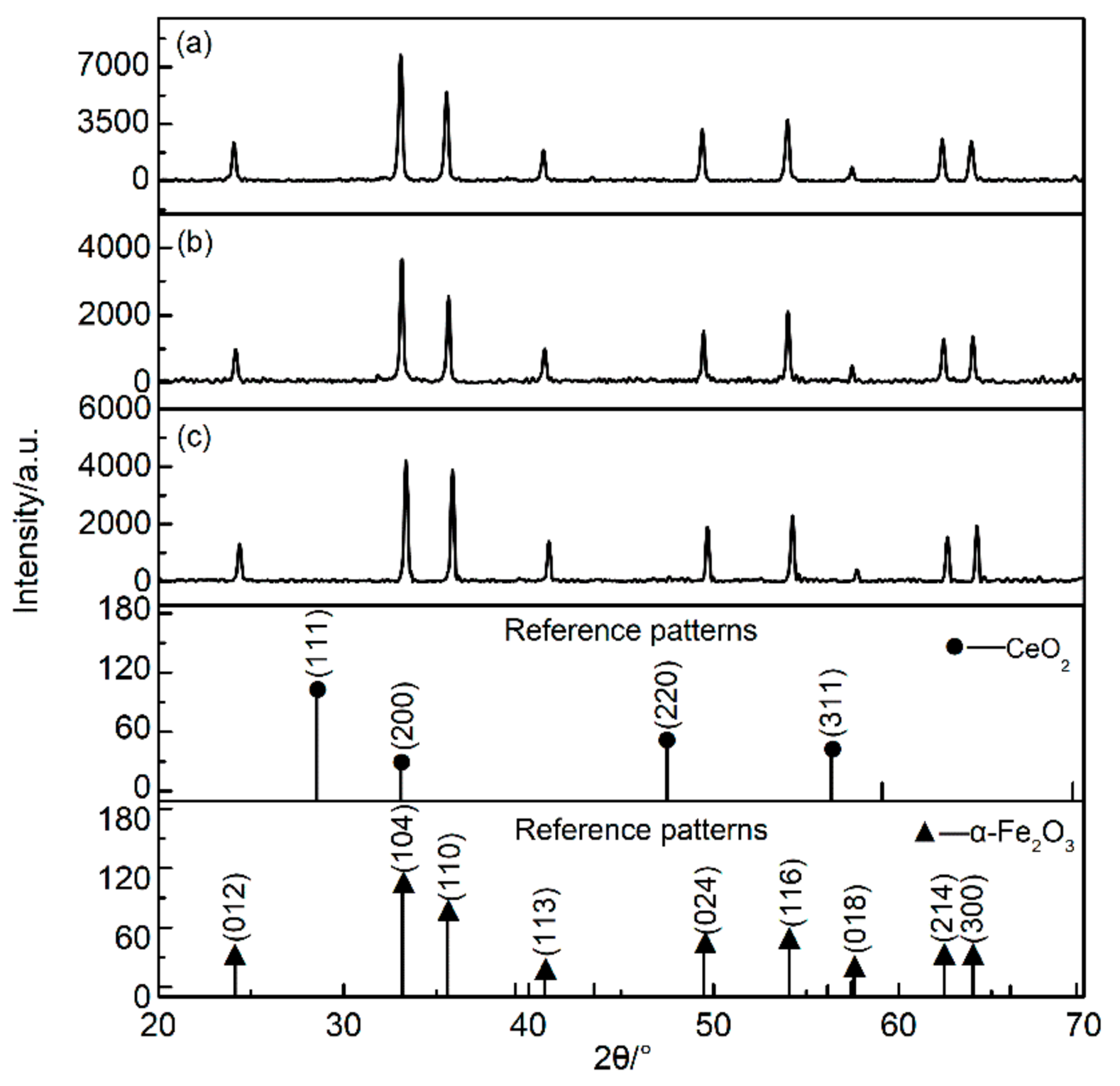
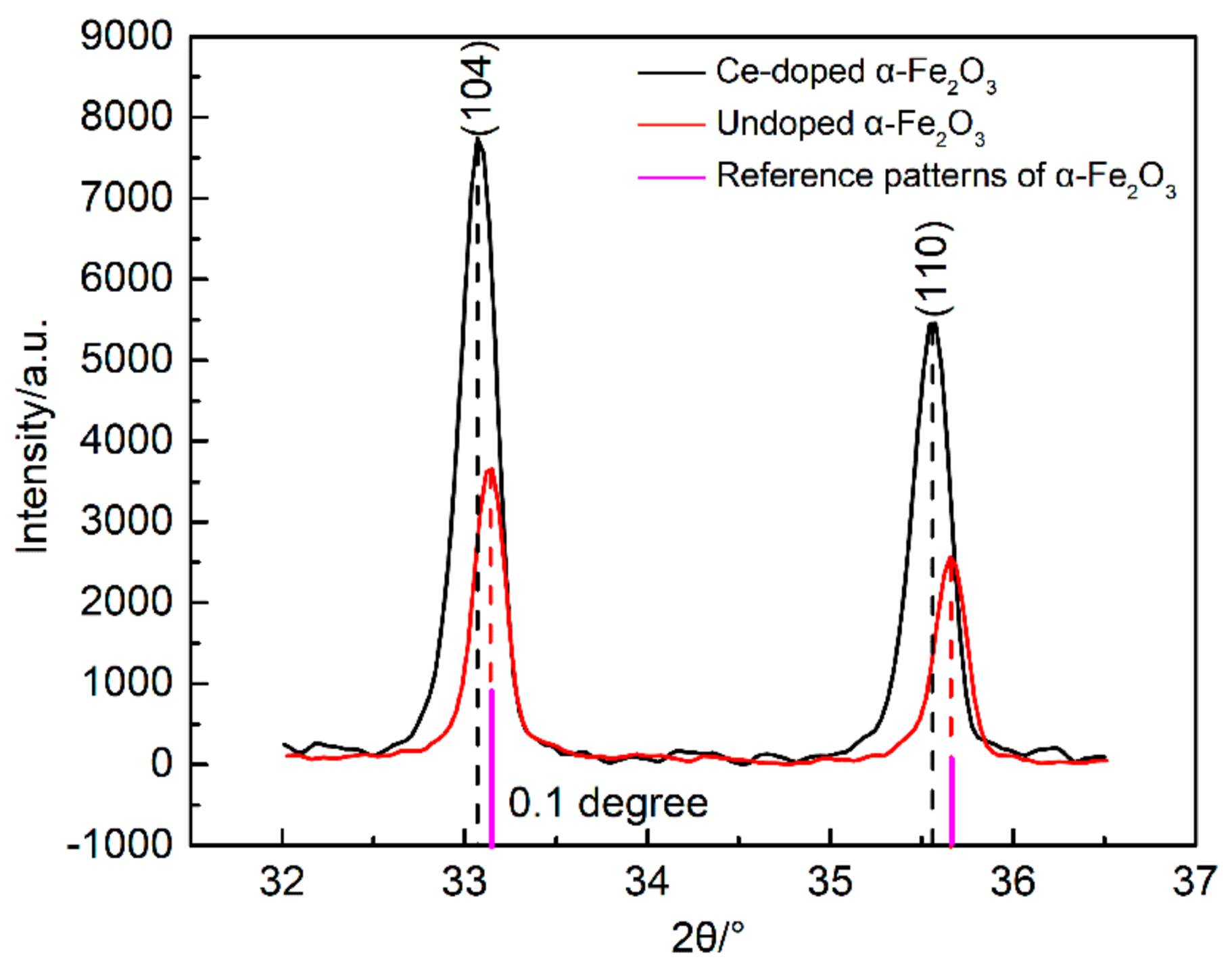

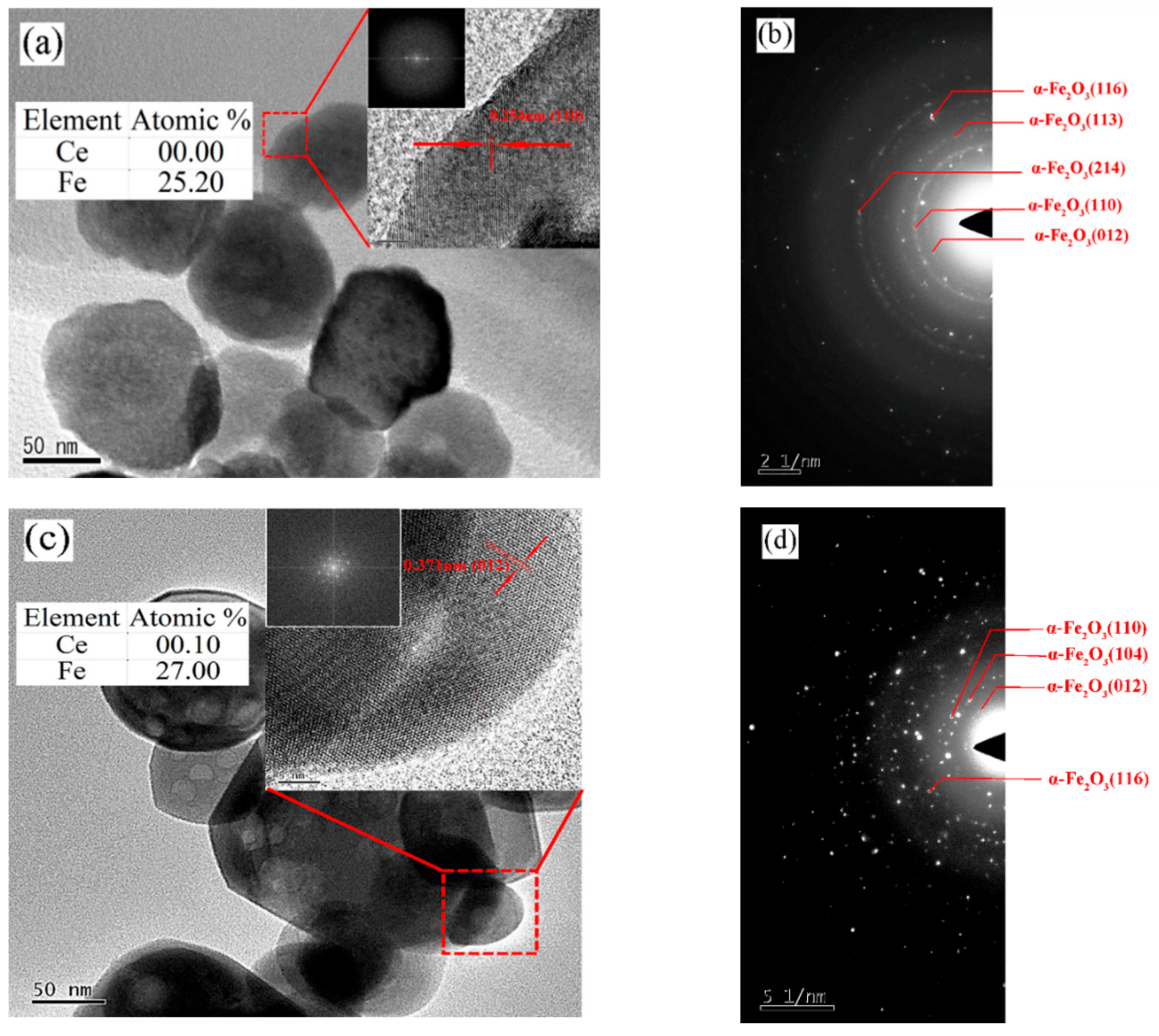
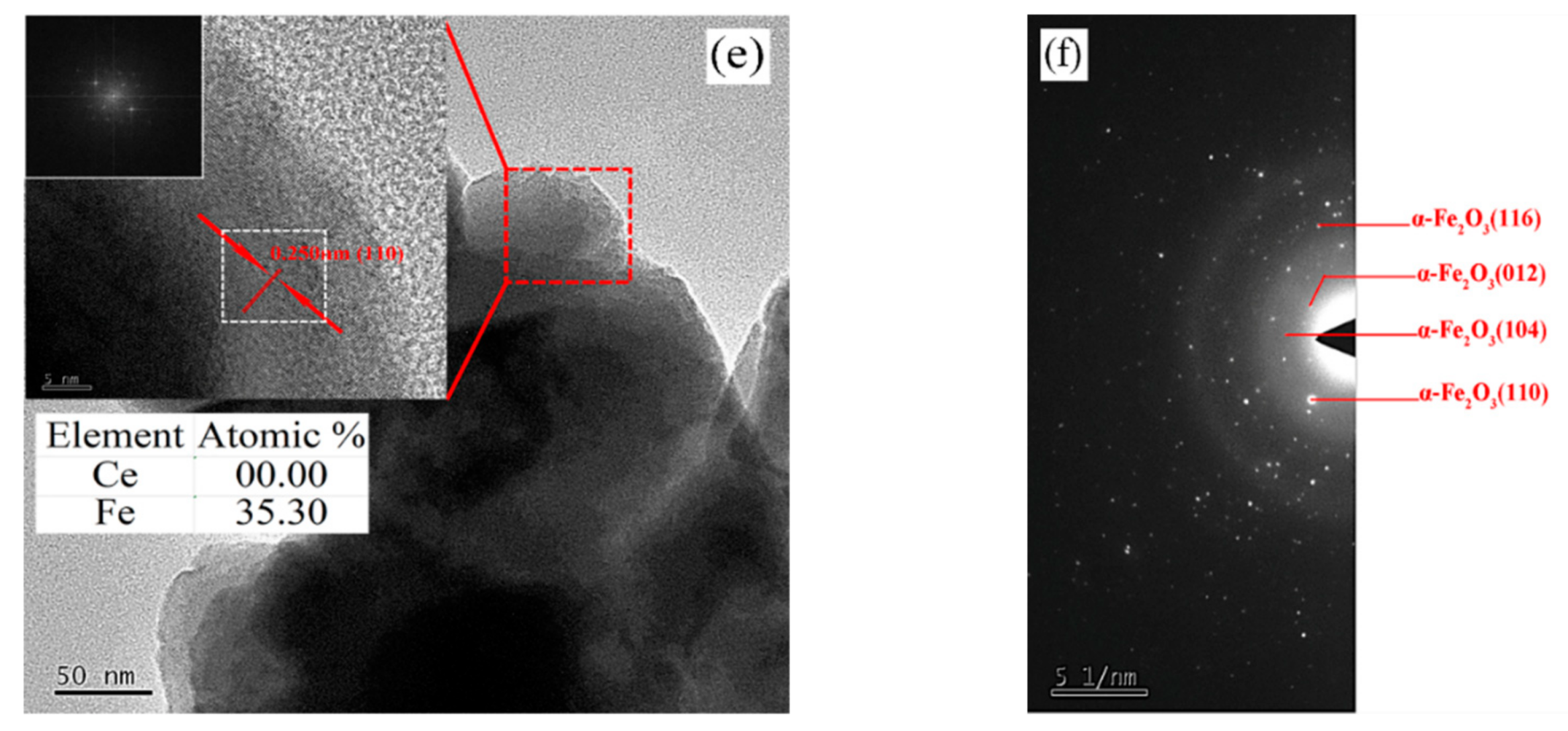
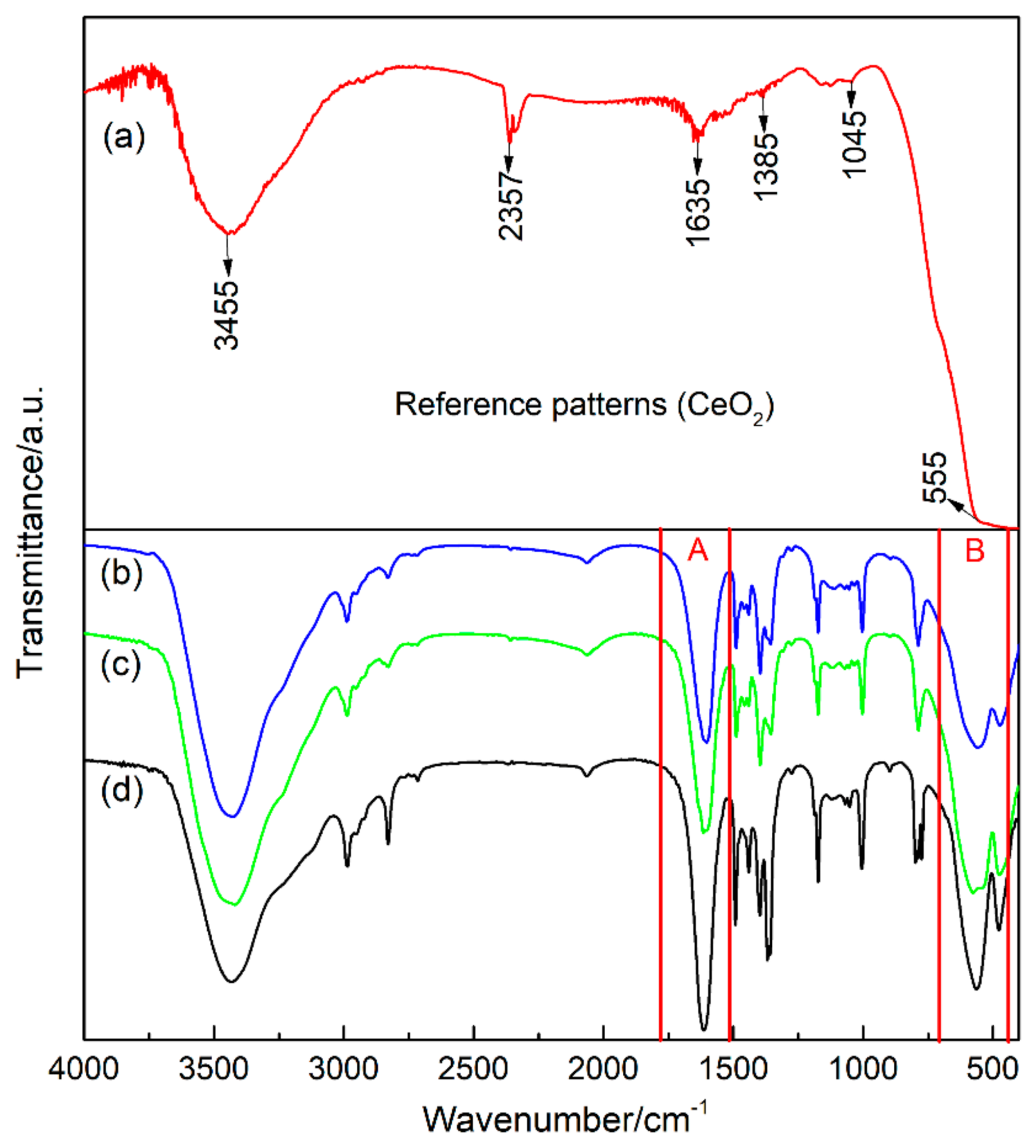
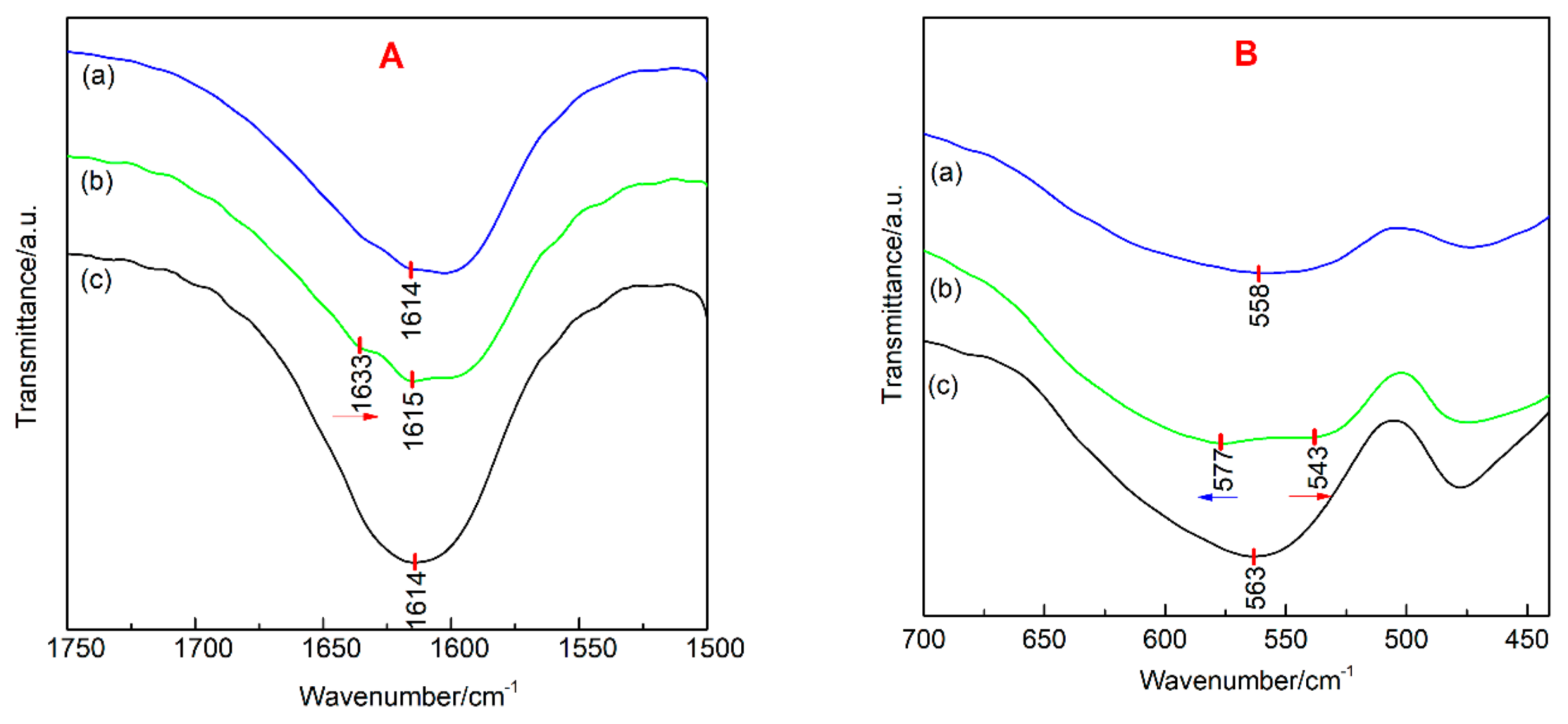
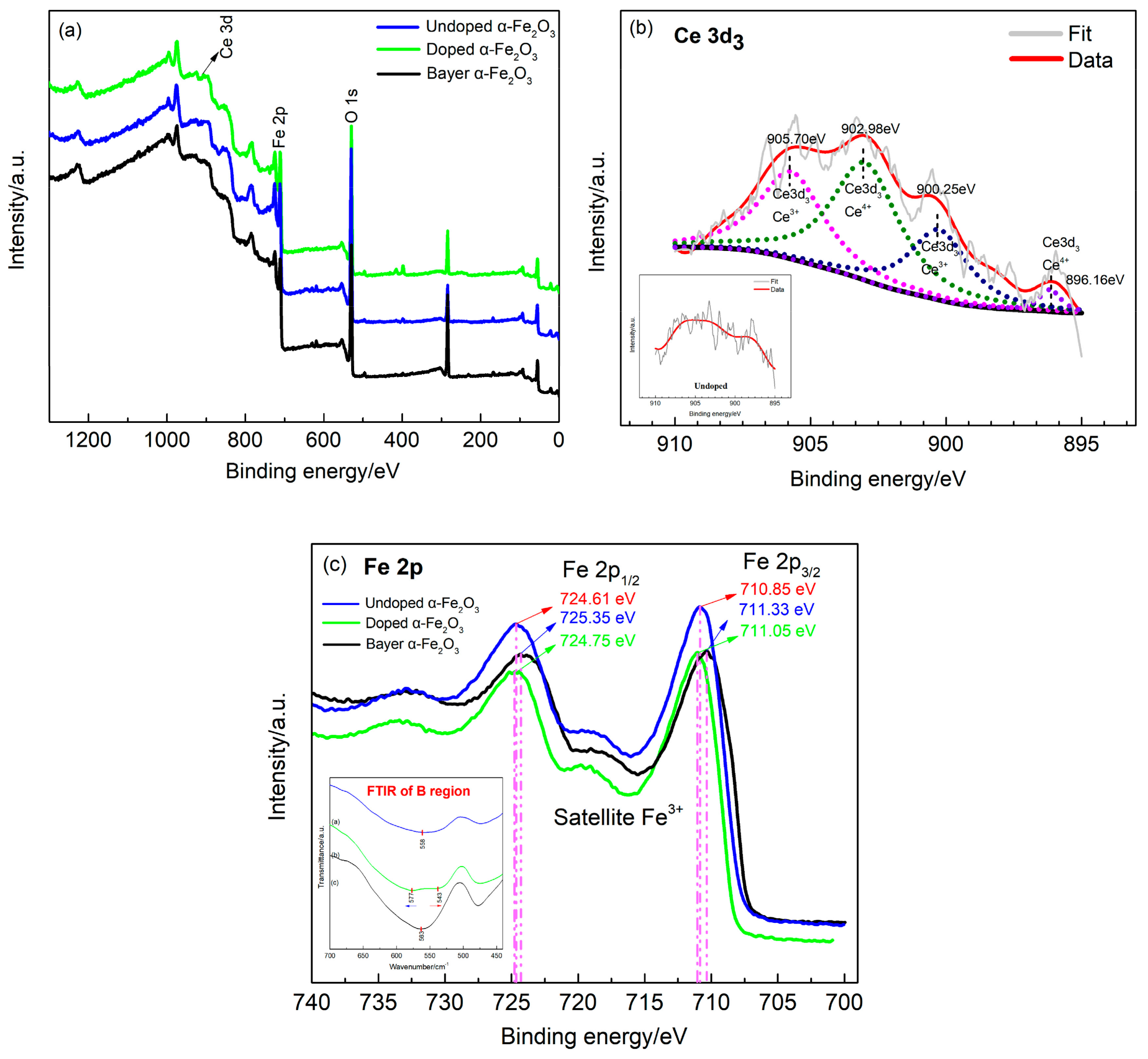
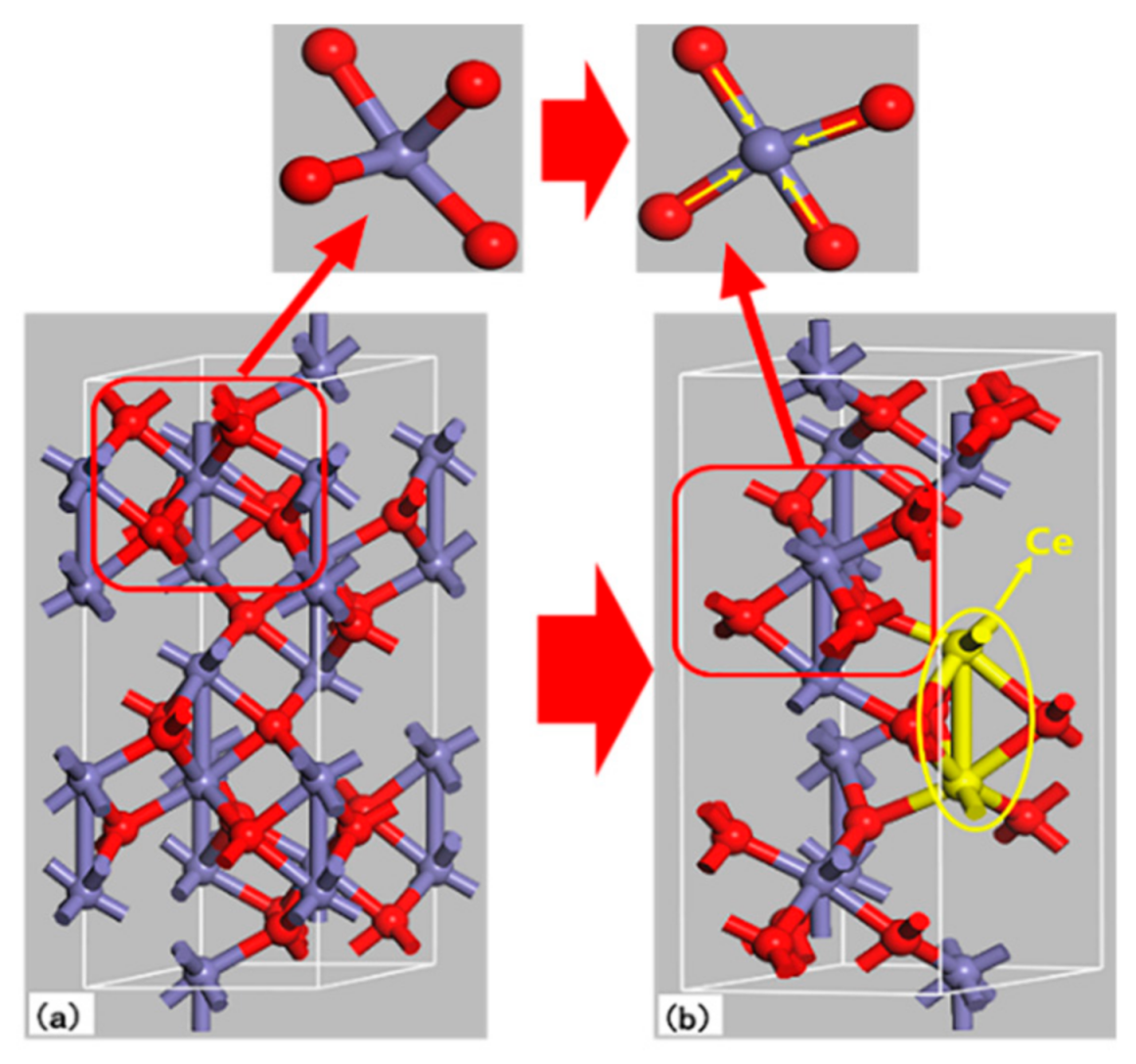

| Sample | Average Particle Size/nm | Lattice Constant/a | Lattice Constant/b | Lattice Constant/c | FWHM (104)/deg |
|---|---|---|---|---|---|
| Ce-doped α-Fe2O3 | 63.9 | 0.5042 | 0.5042 | 1.3771 | 0.235 |
| Undoped α-Fe2O3 | 82.9 | 0.5034 | 0.5034 | 1.3772 | 0.188 |
| Bayer α-Fe2O3 | 77.0 | 0.5014 | 0.5014 | 1.3713 | 0.231 |
| Sample | Ecorr/mV | Icorr/μA·cm−2 | Ebrea/mV |
|---|---|---|---|
| Ce-doped α-Fe2O3 | −44.08 | 0.01 | 252.23 |
| Undoped α-Fe2O3 | −155.12 | 0.12 | 29.37 |
| Bayer α-Fe2O3 | −46.46 | 0.03 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, J.; Shi, P.; Jiang, M.; Liu, C.; Li, X. Effect of Ce Doping on the Structure and Chemical Stability of Nano-α-Fe2O3. Nanomaterials 2019, 9, 1039. https://doi.org/10.3390/nano9071039
Ning J, Shi P, Jiang M, Liu C, Li X. Effect of Ce Doping on the Structure and Chemical Stability of Nano-α-Fe2O3. Nanomaterials. 2019; 9(7):1039. https://doi.org/10.3390/nano9071039
Chicago/Turabian StyleNing, Junxiang, Peiyang Shi, Maofa Jiang, Chengjun Liu, and Xiaoliang Li. 2019. "Effect of Ce Doping on the Structure and Chemical Stability of Nano-α-Fe2O3" Nanomaterials 9, no. 7: 1039. https://doi.org/10.3390/nano9071039
APA StyleNing, J., Shi, P., Jiang, M., Liu, C., & Li, X. (2019). Effect of Ce Doping on the Structure and Chemical Stability of Nano-α-Fe2O3. Nanomaterials, 9(7), 1039. https://doi.org/10.3390/nano9071039






