Polyethylenimine Assisted Bio-Inspired Surface Functionalization of Hexagonal Boron Nitride for Enhancing the Crystallization and the Properties of Poly(Arylene Ether Nitrile)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PEN
2.3. PEI Assisted Bio-Inspired Surface Functionalization of h-BN
2.4. Preparation of h-BN@(PDA+PEI)/PEN Nanocomposite Films
2.5. Characterization
3. Results and Discussion
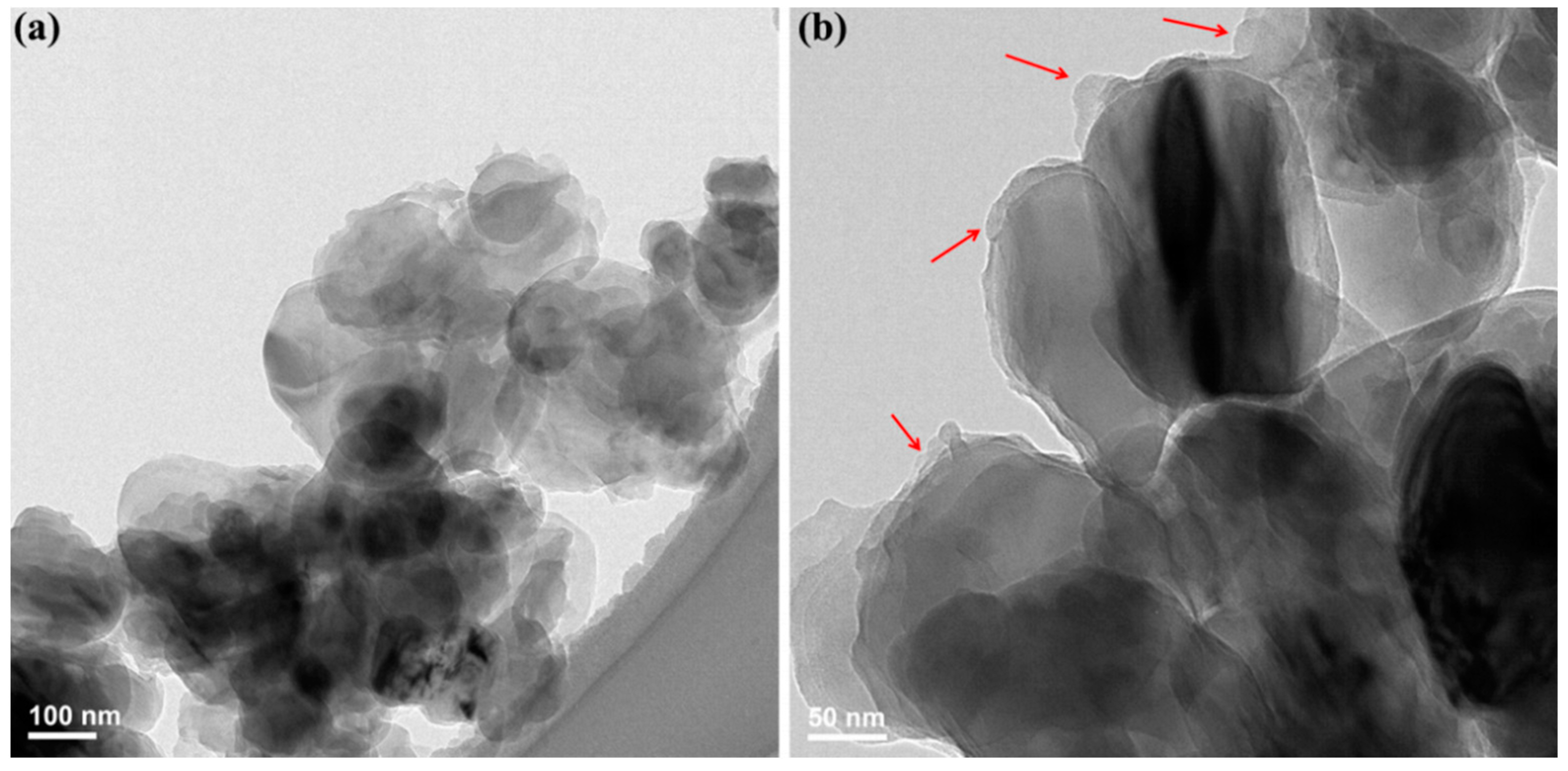
3.1. Structure and Morphology of Pure h-BN and h-BN@(PDA+PEI)
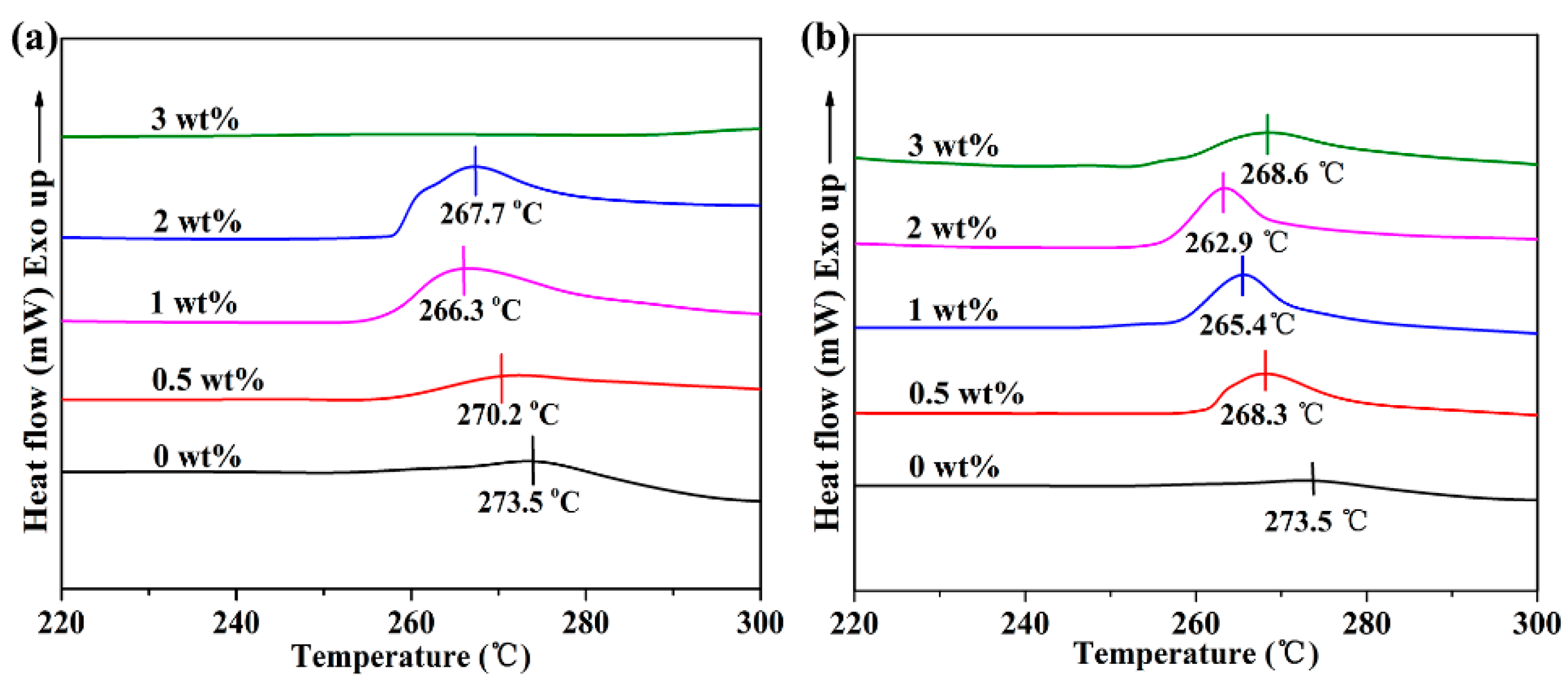
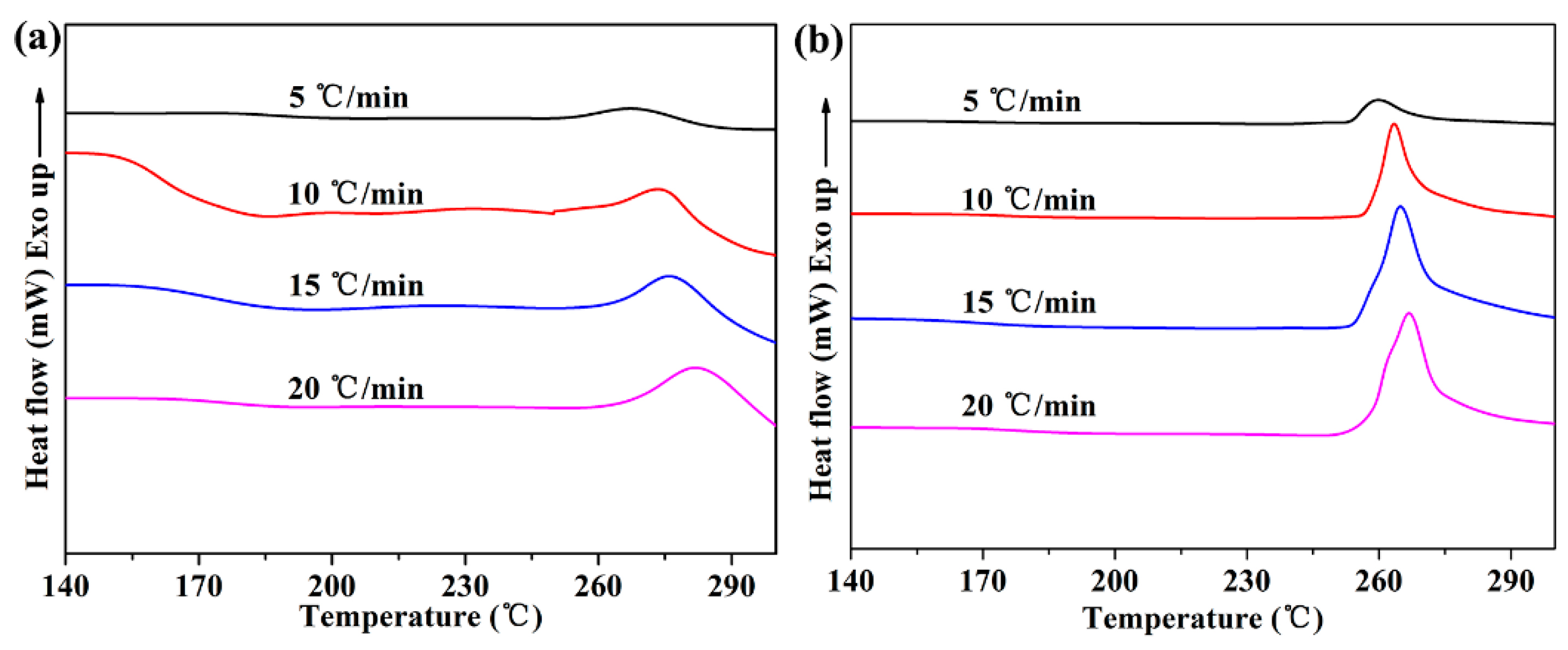
3.2. The Effect of h-BN@(PDA+PEI) on the Non-Isothermal Crystallization of PEN
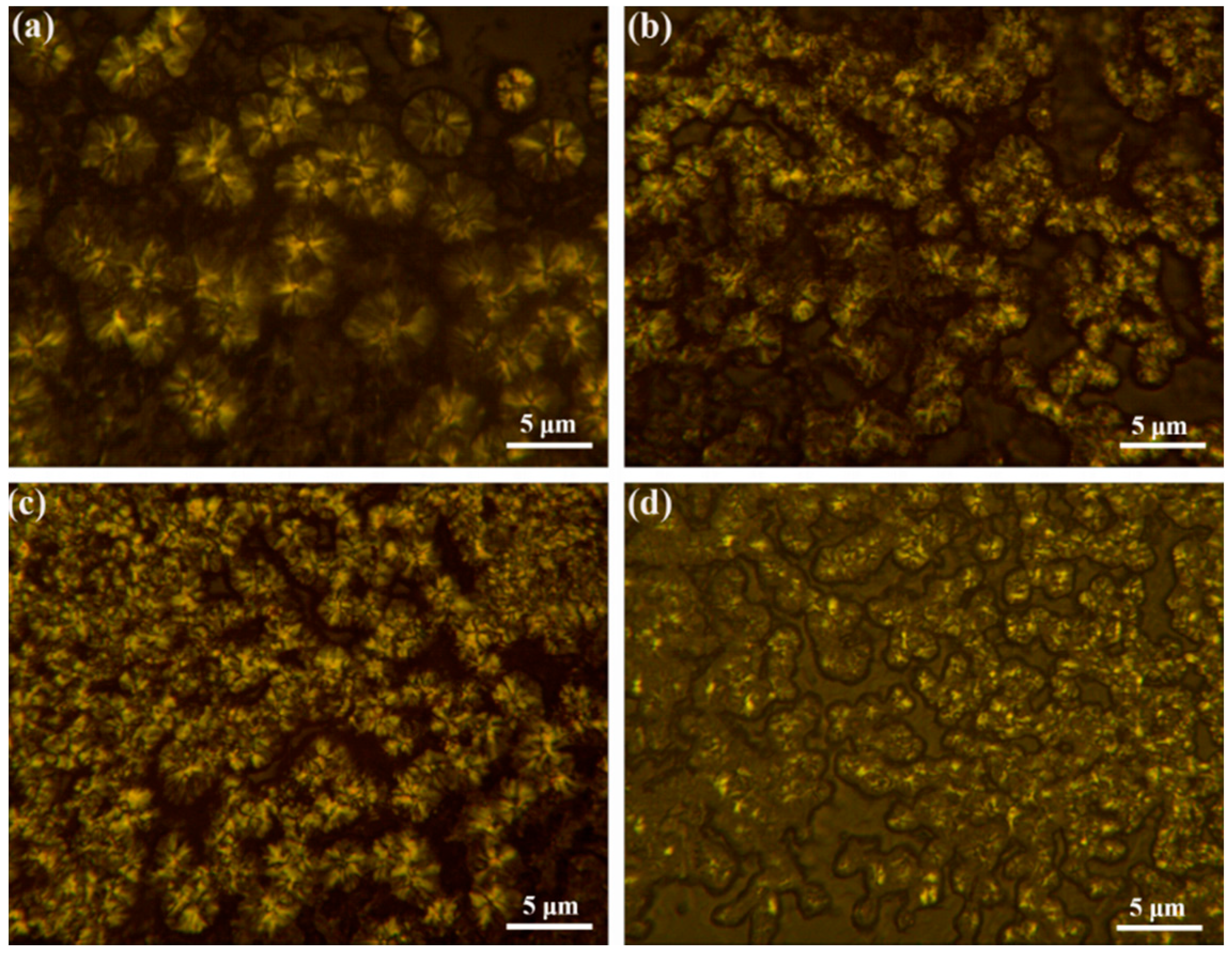
3.3. The Effect of h-BN@(PDA+PEI) on the Crystal Structure and Spherulite Morphology of PEN
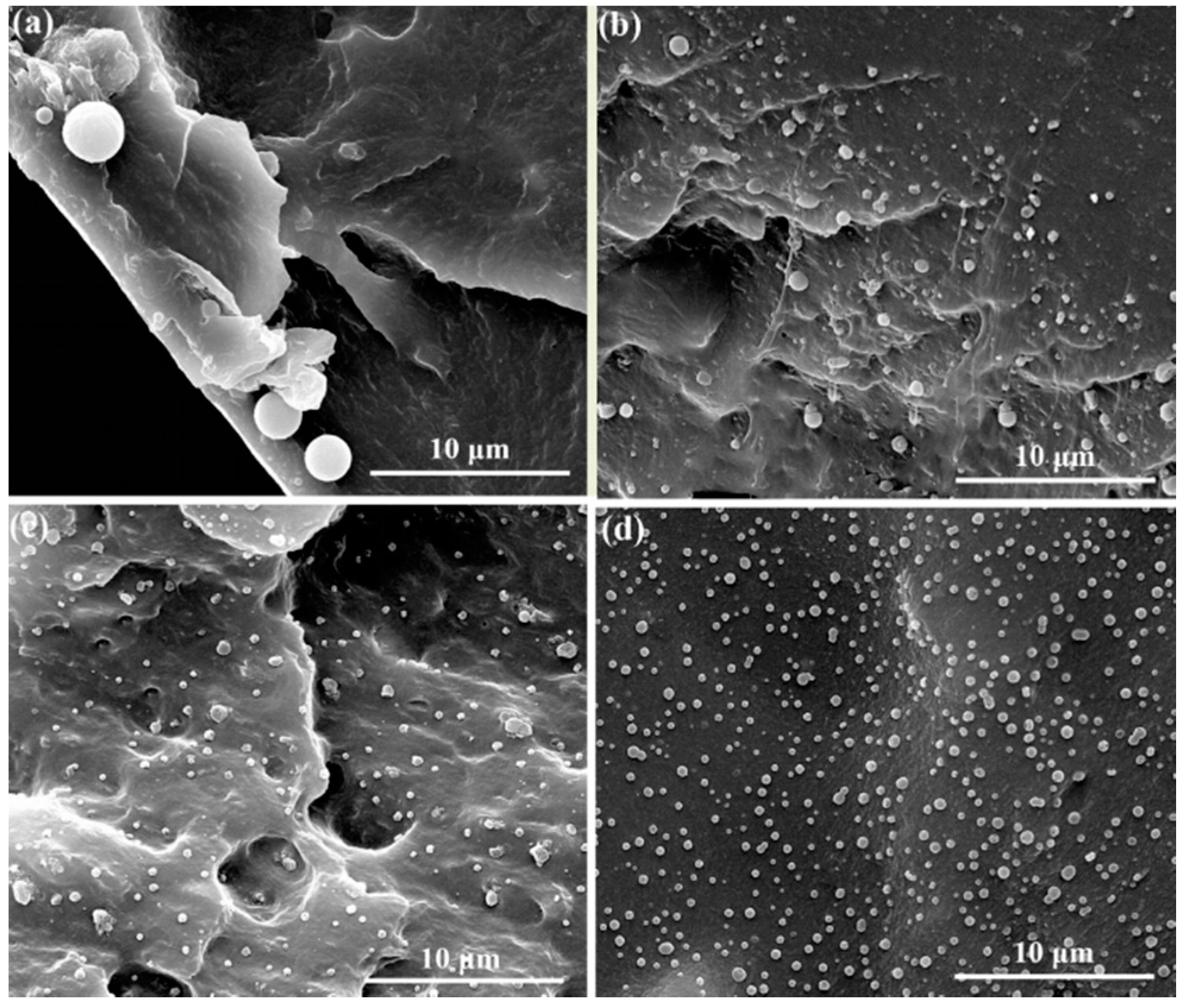
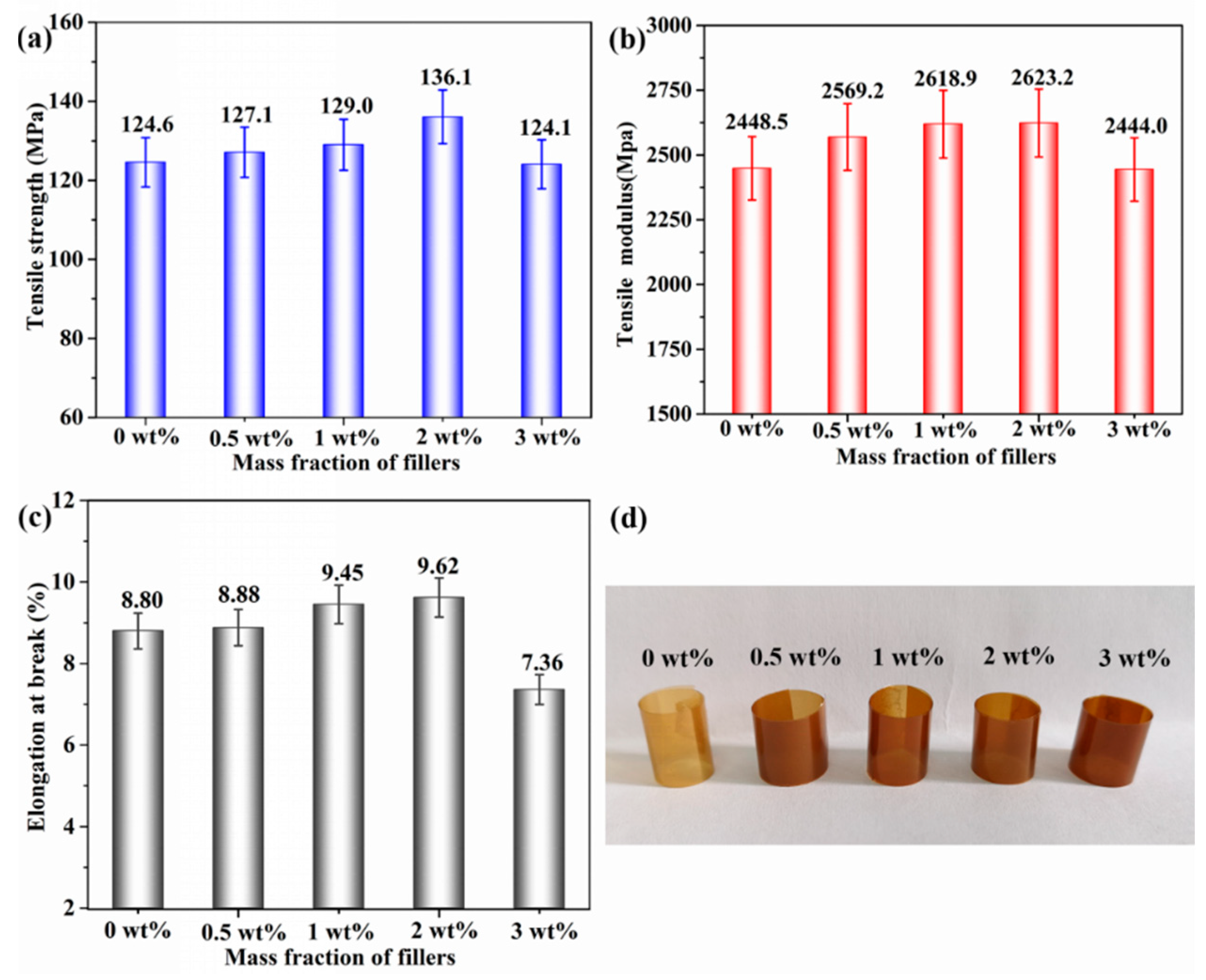
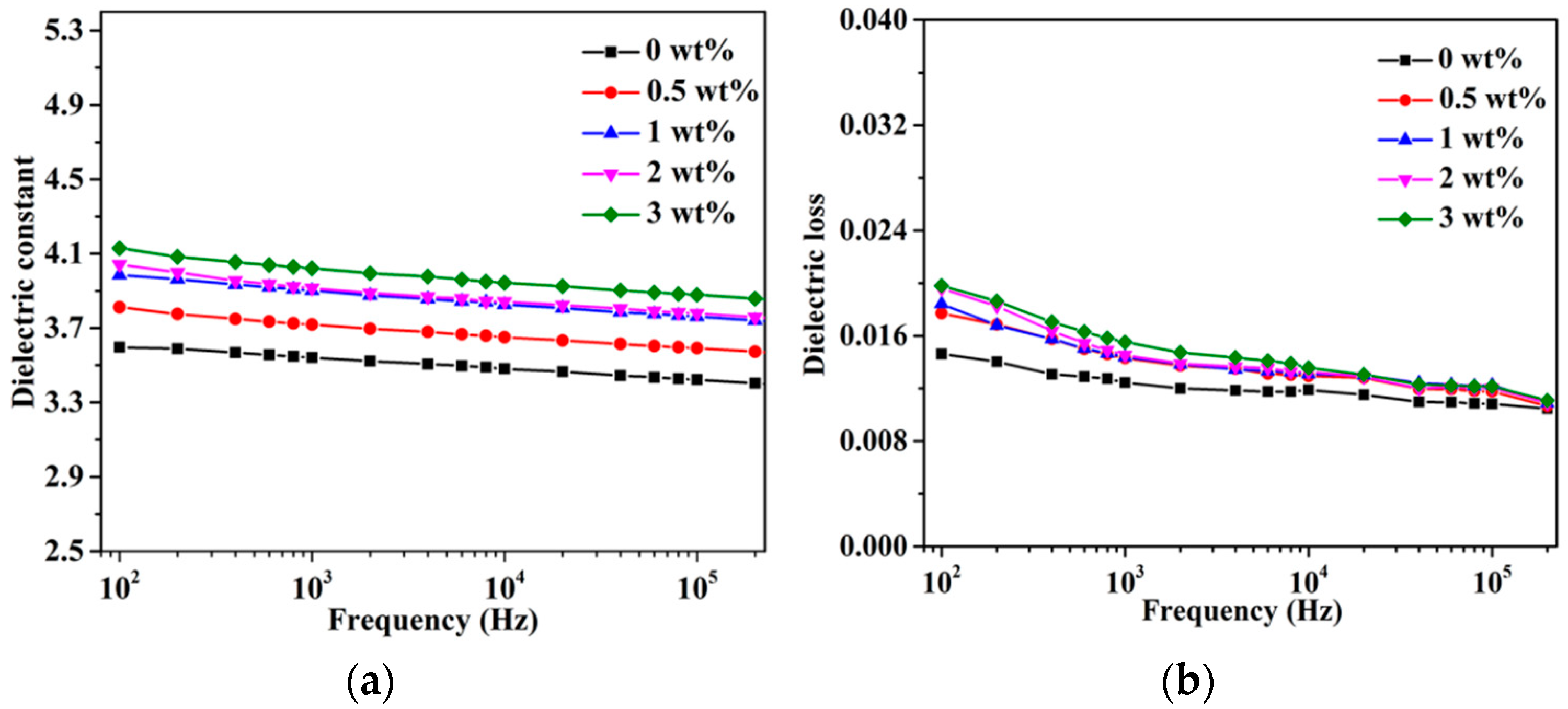
3.4. Mechanical and Dielectric Properties of h-BN@(PDA+PEI)/PEN Nanocomposite Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhan, Y.; He, S.; Wan, X.; Zhao, S.; Bai, Y. Thermally and chemically stable poly(arylene ether nitrile)/halloysite nanotubes intercalated graphene oxide nanofibrous composite membranes for highly efficient oil/water emulsion separation in harsh environment. J. Membr. Sci. 2018, 567, 76–88. [Google Scholar] [CrossRef]
- Wan, X.; Zhan, Y.; Zeng, G.; He, Y. Nitrile functionalized halloysite nanotubes/poly(arylene ether nitrile) nanocomposites: Interface control, characterization, and improved properties. Appl. Surf. Sci. 2017, 393, 1–10. [Google Scholar] [CrossRef]
- Wei, R.; Tu, L.; You, Y.; Zhan, C.; Wang, Y.; Liu, X. Fabrication of crosslinked single-component polyarylene ether nitrile composite with enhanced dielectric properties. Polymer 2019, 161, 162–169. [Google Scholar] [CrossRef]
- You, Y.; Wang, Y.; Tu, L.; Tong, L.; Wei, R.; Liu, X. Interface modulation of core-Shell structured BaTiO3@polyaniline for novel dielectric materials from its nanocomposite with polyarylene ether nitrile. Polymers 2018, 10, 1378. [Google Scholar] [CrossRef]
- Tong, L.; Wei, R.; You, Y.; Liu, X. Post Self-crosslinking of phthalonitrile-terminated polyarylene ether nitrile crystals. Polymers 2018, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Huang, X.; Pu, Z.; Jia, K.; Liu, X. Enhanced crystallinity, mechanical and dielectric properties of biphenyl polyarylene ether nitriles by unidirectional hot-stretching. J. Polym. Res. 2015, 22, 221. [Google Scholar] [CrossRef]
- Zhu, S.E.; Wang, L.L.; Chen, H.; Yang, W.; Yuen, A.C.; Chen, T.B.; Luo, C.; Bi, W.M.; Hu, E.Z.; Zhang, J.; et al. Comparative Studies on Thermal, Mechanical, and Flame Retardant Properties of PBT Nanocomposites via Different Oxidation State Phosphorus-Containing Agents Modified Amino-CNTs. Nanomaterials 2018, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Jia, Y.; Xu, X.; Luo, J.; Han, J.; Sun, X. Crystallization and properties of poly(ethylene terephthalate)/layered double hydroxide nanocomposites. J. Colloid. Interface Sci. 2019, 539, 54–64. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Zheng, W.-G.; Yan, Q.; Yang, Y.; Wang, J.-W.; Lu, Z.-H.; Ji, G.-Y.; Yu, Z.-Z. Electrically conductive polyethylene terephthalate/graphene nanocomposites prepared by melt compounding. Polymer 2010, 51, 1191–1196. [Google Scholar] [CrossRef]
- Cheng, F.O.; Mong, T.H.; Jia, R.L. The nucleating effect of montmorillonite on crystallization of PET/montmorillonite nanocomposite. J. Polym. Res. 2003, 10, 127–132. [Google Scholar]
- Avella, M.; Cosco, S.; Lorenzo, M.L.; Pace, E.D.; Errico, M.E. Influence of CaCO3 na-noparticles shape on thermal and crystallization behavior of isotactic polypropylene based nanocomposites. J. Therm. Anal. Calorim. 2005, 80, 131–136. [Google Scholar] [CrossRef]
- Cai, L.; Dou, Q. Investigation on the melting and crystallization behaviors, mechanical properties and morphologies of polypropylene/sericite composites. J. Mater. Sci. 2018, 54, 3600–3618. [Google Scholar] [CrossRef]
- Zhou, R.-J.; Burkhart, T. Polypropylene/SiO2 nanocomposites filled with different nanosilicas: Thermal and mechanical properties, morphology and interphase characterization. J. Membr. Sci. 2010, 46, 1228–1238. [Google Scholar] [CrossRef]
- Li, M.; Hu, D.; Wang, Y.; Shen, C. Nonisothermal crystallization kinetics of poly(lactic acid) formulations comprising talc with poly(ethylene glycol). Polym. Eng. Sci. 2010, 50, 2298–2305. [Google Scholar] [CrossRef]
- Tong, Z.; Zhuo, W.; Zhou, J.; Huang, R.; Jiang, G. Crystallization behavior and enhanced toughness of poly(ethylene terephthalate) composite with noncovalent modified graphene functionalized by pyrene-terminated molecules: A comparative study. J. Membr. Sci. 2017, 52, 10567–10580. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Deng, L.; Fang, H.; Zhang, Y.; Wang, Z. More dominant shear flow effect assisted by added carbon nanotubes on crystallization kinetics of isotactic polypropylene in nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, K.; Xu, M.; Liu, X. Crystallization behaviors and properties of poly (arylene ether nitrile) nanocomposites induced by aluminum oxide and multi-walled carbon nanotubes. J. Mater. Sci. 2018, 53, 14361–14374. [Google Scholar] [CrossRef]
- Jedrzejczak-Silicka, M.; Trukawka, M.; Dudziak, M.; Piotrowska, K.; Mijowska, E. Hexagonal Boron Nitride Functionalized with Au Nanoparticles-Properties and Potential Biological Applications. Nanomaterials 2018, 8, 605. [Google Scholar] [CrossRef]
- Shen, H.; Guo, J.; Wang, H.; Zhao, N.; Xu, J. Bioinspired modification of h-BN for high thermal conductive composite films with aligned structure. ACS Appl. Mater. Interfaces 2015, 7, 5701–5708. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-R.; Lin, D.-W.; Gao, Y.; Xu, J.; Guo, B.-H. Prominent nucleating effect of finely dispersed hydroxyl-functional hexagonal boron nitride on biodegradable poly(butylene succinate). Ind. Eng. Chem. Res. 2014, 53, 4689–4696. [Google Scholar] [CrossRef]
- Rosely, C.V.S.; Nagendra, B.; Sivaprasad, V.P.; Gowd, E.B. Influence of boron nitride nanosheets on the crystallization and polymorphism of poly(l-lactide). J. Phys. Chem. B 2018, 122, 6442–6451. [Google Scholar] [CrossRef]
- Wang, C.; Yin, J.; Wang, R.; Jiao, T.; Huang, H.; Zhou, J.; Zhang, L.; Peng, Q. Facile Preparation of Self-Assembled Polydopamine-Modified Electrospun Fibers for Highly Effective Removal of Organic Dyes. Nanomaterials 2019, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, D.A.; Song, K.; Lee, G.H.; Park, M.; Kang, Y.M. Carbon-coated si nanoparticles anchored between reduced graphene oxides as an extremely reversible anode material for high energy-density li-ion battery. Adv. Energy Mater. 2016, 6, 1600904. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, B.; Xu, H.; Chen, J.; Liu, Q.; Deng, Y.; Zeng, H. Highly regenerable mussel-inspired Fe3O4@polydopamine-Ag core-shell microspheres as catalyst and adsorbent for methylene blue removal. ACS Appl. Mater. Interfaces 2014, 6, 8845–8852. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, H.-C.; Liang, H.-Q.; Wan, L.-S.; Xu, Z.-K. Nanofiltration membranes via co-deposition of polydopamine/polyethylenimine followed by cross-linking. J. Membr. Sci. 2015, 476, 50–58. [Google Scholar] [CrossRef]
- Wang, Y.; Kai, Y.; Tong, L.; You, Y.; Huang, Y.; Liu, X. The frequency independent functionalized MoS2 nanosheet/poly(arylene ether nitrile) composites with improved dielectric and thermal properties via bio-inspired surface chemistry. Appl. Surf. Sci. 2019, 481, 1239–1248. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.U.; Jung, B.M.; Byun, J.-H.; Yi, J.-W.; Lee, S.-B.; Kim, B.-S. Simultaneous enhancement of mechanical, electrical and thermal properties of graphene oxide paper by embedding dopamine. Carbon 2013, 65, 296–304. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Exfoliation of Hexagonal Boron Nitride (h-BN) in Liquide Phase by Ion Intercalation. Nanomaterials 2018, 8, 716. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, J.; Liu, M.; Li, Y.; Ruan, J.; Li, Q.; Tian, J.; Zhu, X.; Zhang, X.; Wei, Y. Synthesis of polyacrylamide immobilized molybdenum disulfide (MoS2 @PDA@PAM) composites via mussel-inspired chemistry and surface-initiated atom transfer radical polymerization for removal of copper (II) ions. J. Taiwan Inst. Chem. Eng. 2018, 86, 174–184. [Google Scholar] [CrossRef]
- Sharma, R.; Maiti, S.N. Effects of SEBS-g-MA copolymer on non-isothermal crystallization kinetics of polypropylene. J. Mater. Sci. 2015, 50, 447–456. [Google Scholar] [CrossRef]
- Márquez, Y.; Franco, L.; Turon, P.; Martínez, J.; Puiggalí, J. Study of non-isothermal crystallization of polydioxanone and analysis of morphological changes occurring during heating and cooling processes. Polymers 2016, 8, 351. [Google Scholar] [CrossRef]
- Nagendra, B.; Mohan, K.; Gowd, E.B. Polypropylene/layered double hydroxide (LDH) nanocomposites: Influence of LDH particle size on the crystallization behavior of polypropylene. ACS Appl. Mater. Interfaces 2015, 7, 12399–12410. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly (ethylene terephthalate) determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Kalkar, A.K.; Deshpande, V.D.; Kulkarni, M.J. Nonisothermal crystallization kinetics of poly (phenylene sulphide) in composites with a liquid crystalline polymer. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1070–1100. [Google Scholar] [CrossRef]
- Chen, K.; Yu, J.; Qiu, Z. Effect of Low octavinyl-polyhedral oligomeric silsesquioxanes loading on the crystallization kinetics and morphology of biodegradable poly(ethylene succinate-co-5.1 mol % ethylene adipate) as an efficient nucleating agent. Ind. Eng. Chem. Res. 2013, 52, 1769–1774. [Google Scholar] [CrossRef]
- Snyder, C.R.; Marand, H. Effect of chain transport in the secondary surface nucleation based flux theory and in the Lauritzen-Hoffman crystal growth rate formalism. Macromolecules 1997, 30, 2759–2766. [Google Scholar] [CrossRef]
- Ou, B.; Zhou, Z.; Liu, Q.; Liao, B.; Xiao, Y.; Liu, J.; Zhang, X.; Li, D.; Xiao, Q.; Shen, S. Mechanical properties and nonisothermal crystallization kinetics of polyamide 6/functionalized TiO2 nanocomposites. Polym. Compos. 2014, 35, 294–300. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 2002, 29, 1702–1706. [Google Scholar] [CrossRef]


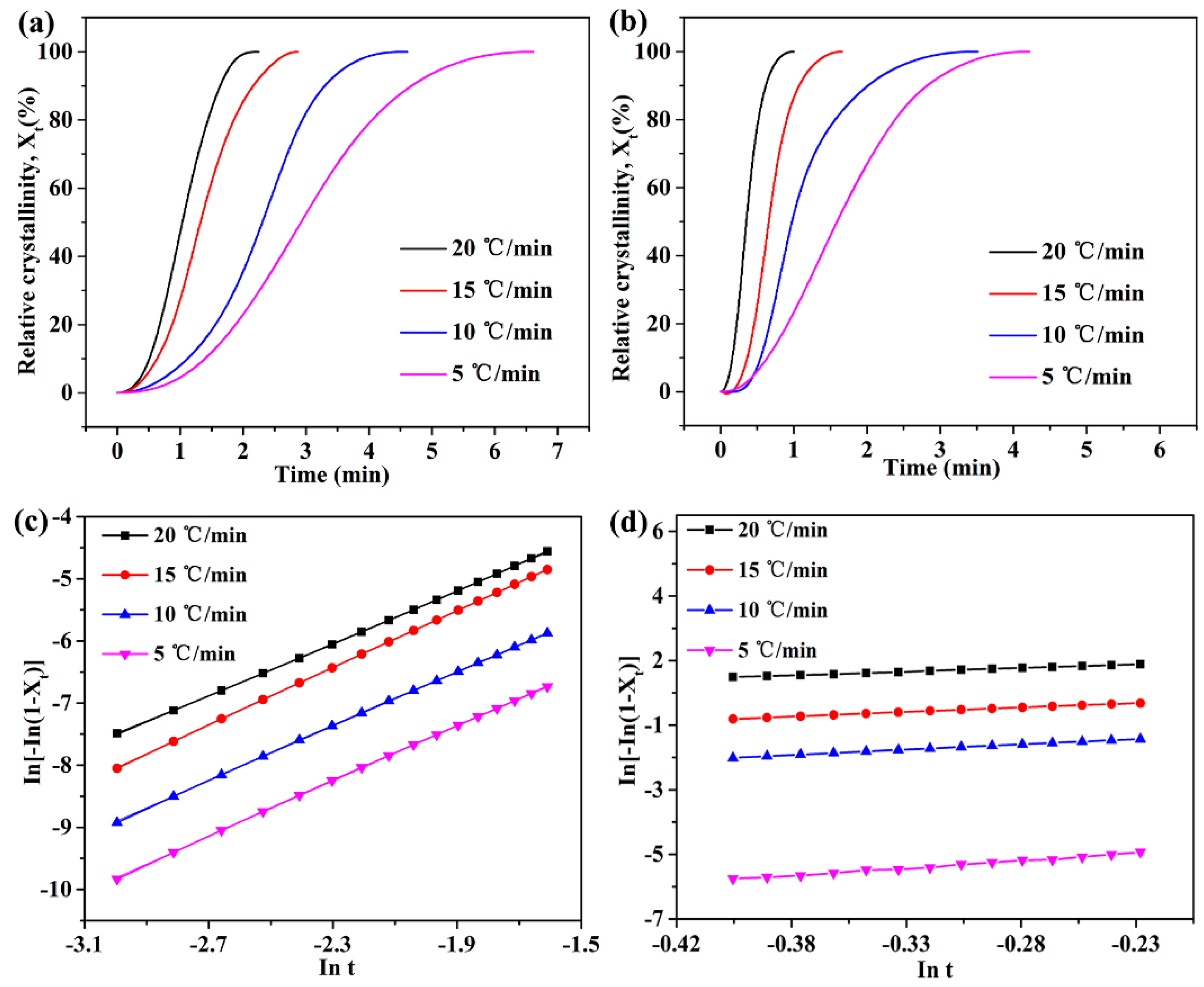
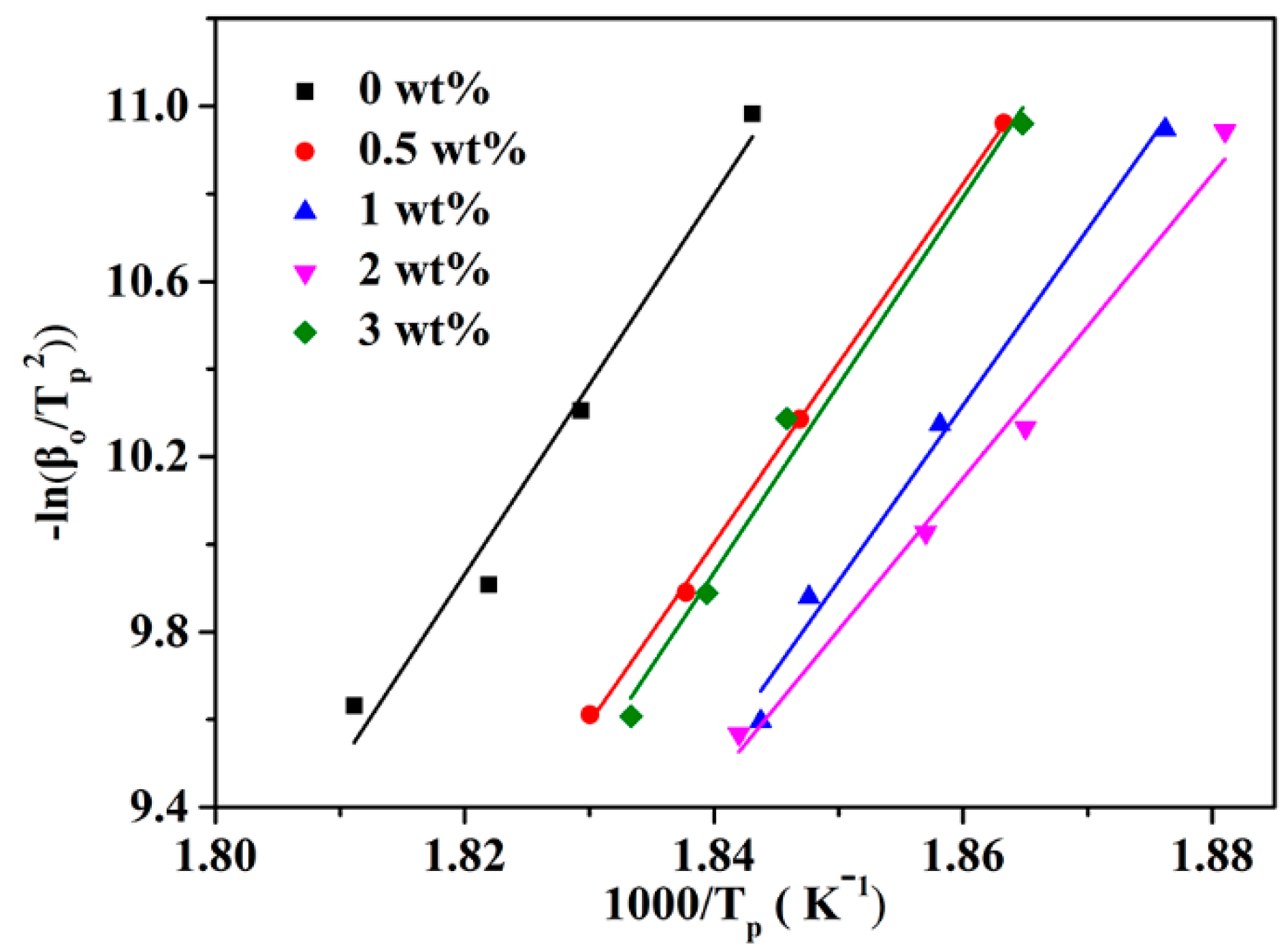

| Samples | β | n | InKt | InKc | Kc | Tp (°C) | Eα (KJ/mol) |
|---|---|---|---|---|---|---|---|
| 0 wt% | 5 | 2.23 | −3.140 | −0.628 | 0.534 | 269.4 | 359.66 |
| 10 | 2.18 | −2.346 | −0.235 | 0.791 | 273.5 | ||
| 15 | 2.29 | −2.295 | −0.153 | 0.858 | 275.7 | ||
| 20 | 2.22 | −1.560 | −0.078 | 0.925 | 278.9 | ||
| 0.5 wt% | 5 | 2.38 | −2.582 | −0.516 | 0.597 | 263.5 | 340.81 |
| 10 | 2.27 | −1.155 | −0.116 | 0.890 | 268.3 | ||
| 15 | 2.42 | −0.616 | −0.0410 | 0.960 | 270.9 | ||
| 20 | 2.23 | 0.015 | 0.001 | 1.000 | 273.3 | ||
| 1 wt% | 5 | 2.61 | −2.008 | −0.402 | 0.669 | 259.8 | 333.93 |
| 10 | 2.57 | −1.156 | −0.116 | 0.891 | 265.0 | ||
| 15 | 2.56 | −0.183 | −0.012 | 0.988 | 268.1 | ||
| 20 | 2.25 | 1.499 | 0.075 | 1.078 | 269.2 | ||
| 2 wt% | 5 | 3.31 | −1.363 | −0.273 | 0.761 | 258.5 | 292.84 |
| 10 | 3.15 | −0.518 | −0.052 | 0.949 | 262.9 | ||
| 15 | 2.62 | 1.019 | 0.068 | 1.070 | 264.3 | ||
| 20 | 2.48 | 2.073 | 0.124 | 1.132 | 266.2 | ||
| 3 wt% | 5 | 2.15 | −1.829 | −0.366 | 0.694 | 263.1 | 355.98 |
| 10 | 2.34 | −0.917 | −0.092 | 0.912 | 268.6 | ||
| 15 | 2.21 | −0.136 | −0.009 | 0.990 | 270.5 | ||
| 20 | 2.06 | 0.336 | 0.017 | 1.017 | 272.3 |
| Sample | Neat | 0.5 wt% | 1 wt% | 2 wt% | 3 wt% |
|---|---|---|---|---|---|
| Crystallinity (%) | 6.56 | 8.22 | 11.32 | 14.90 | 7.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tong, L.; You, Y.; Tu, L.; Zhou, M.; Liu, X. Polyethylenimine Assisted Bio-Inspired Surface Functionalization of Hexagonal Boron Nitride for Enhancing the Crystallization and the Properties of Poly(Arylene Ether Nitrile). Nanomaterials 2019, 9, 760. https://doi.org/10.3390/nano9050760
Wang Y, Tong L, You Y, Tu L, Zhou M, Liu X. Polyethylenimine Assisted Bio-Inspired Surface Functionalization of Hexagonal Boron Nitride for Enhancing the Crystallization and the Properties of Poly(Arylene Ether Nitrile). Nanomaterials. 2019; 9(5):760. https://doi.org/10.3390/nano9050760
Chicago/Turabian StyleWang, Yajie, Lifen Tong, Yong You, Ling Tu, Meirong Zhou, and Xiaobo Liu. 2019. "Polyethylenimine Assisted Bio-Inspired Surface Functionalization of Hexagonal Boron Nitride for Enhancing the Crystallization and the Properties of Poly(Arylene Ether Nitrile)" Nanomaterials 9, no. 5: 760. https://doi.org/10.3390/nano9050760
APA StyleWang, Y., Tong, L., You, Y., Tu, L., Zhou, M., & Liu, X. (2019). Polyethylenimine Assisted Bio-Inspired Surface Functionalization of Hexagonal Boron Nitride for Enhancing the Crystallization and the Properties of Poly(Arylene Ether Nitrile). Nanomaterials, 9(5), 760. https://doi.org/10.3390/nano9050760





