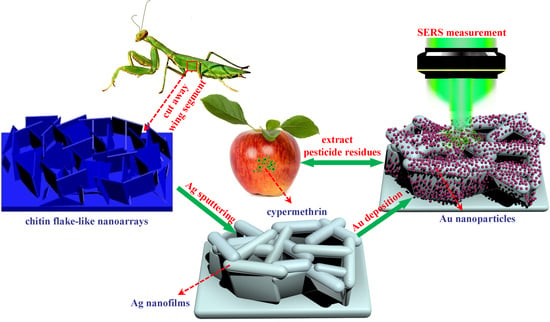
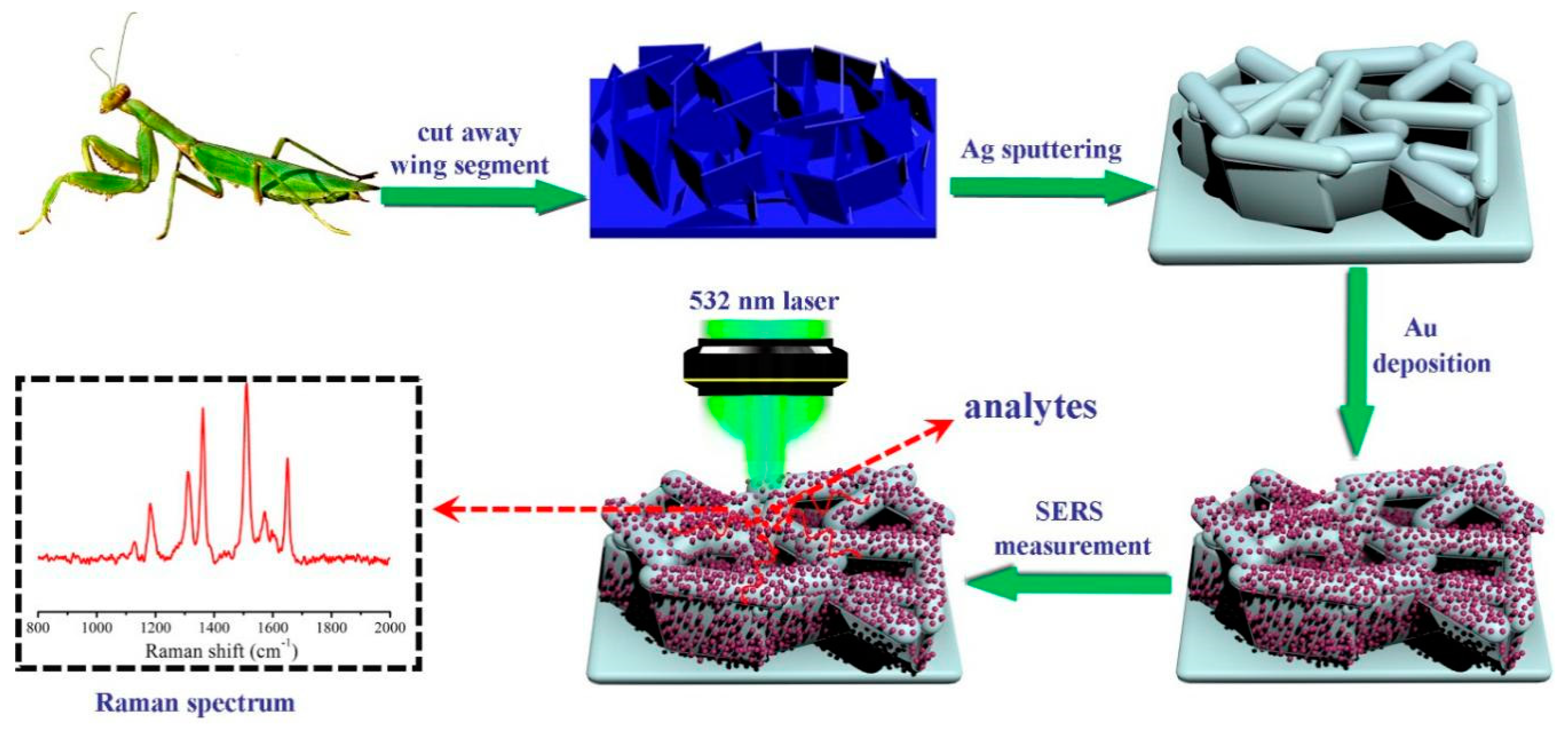
Preparation of a Novel SERS Platform Based on Mantis Wing with High-Density and Multi-Level “Hot Spots”
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Sample Preparation
2.3. SERS Measurements
2.4. Detection of Pesticide Residues on Apple Peels
3. Results and Discussion
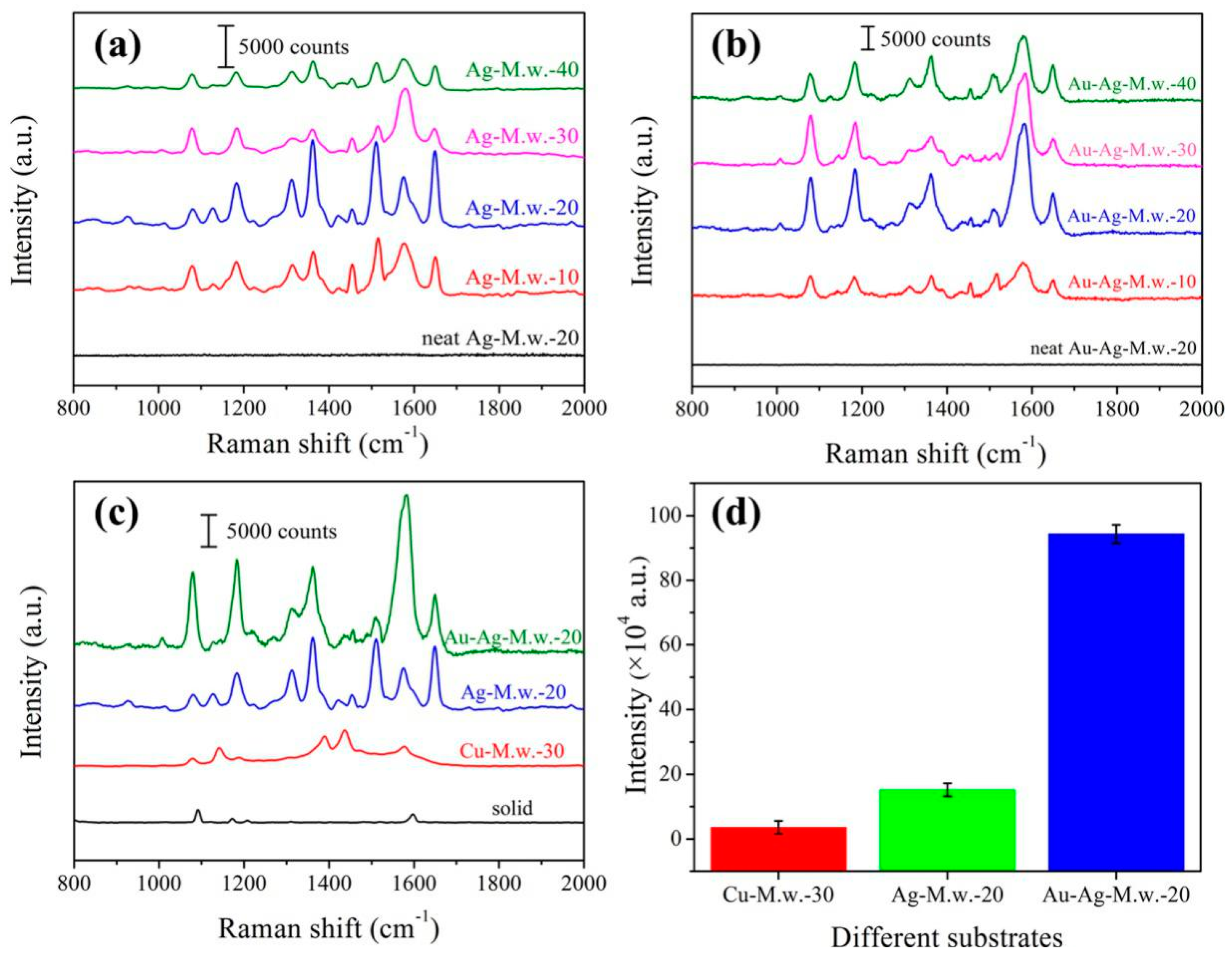
3.1. Comparing the SERS Substrates
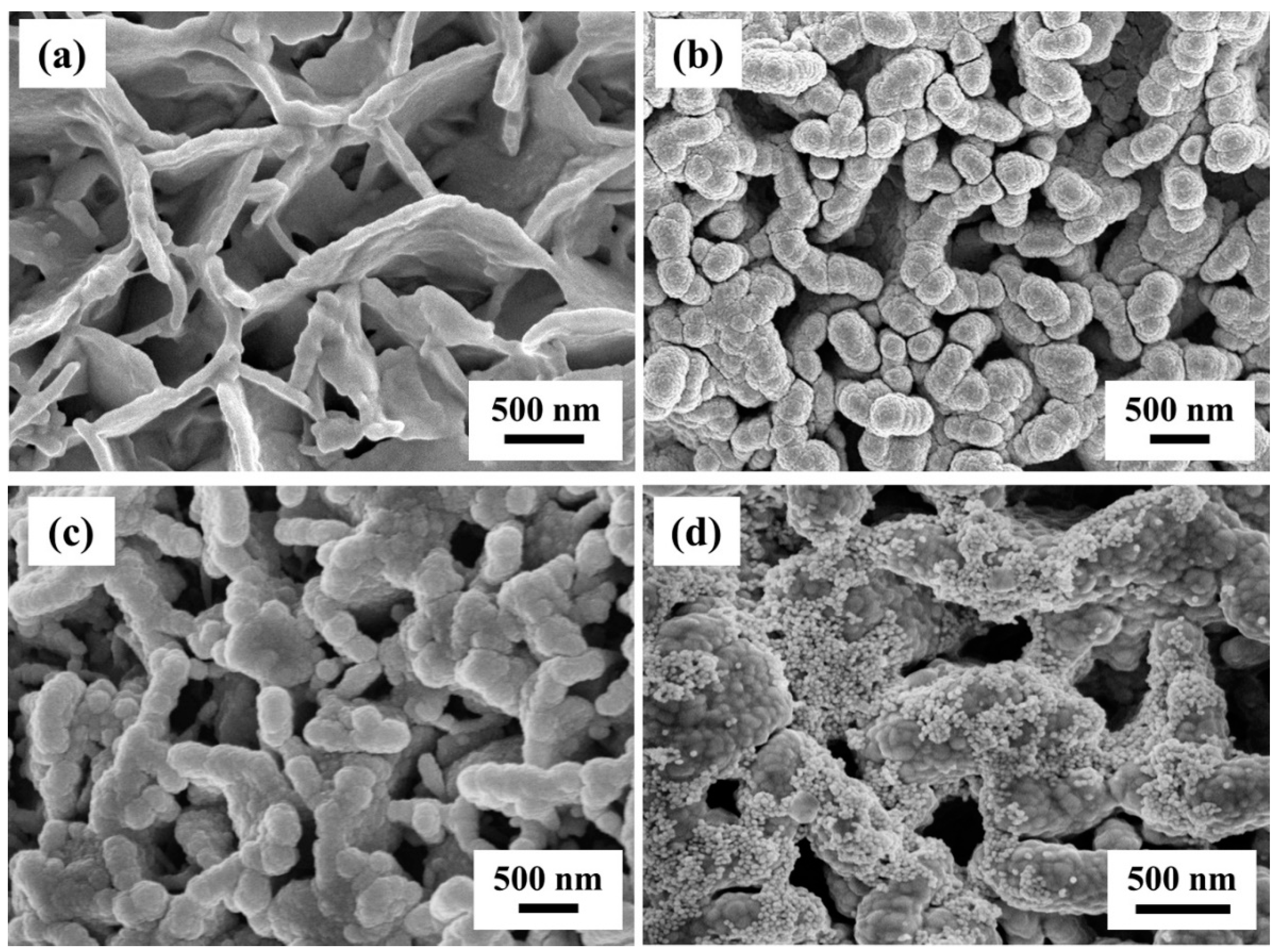
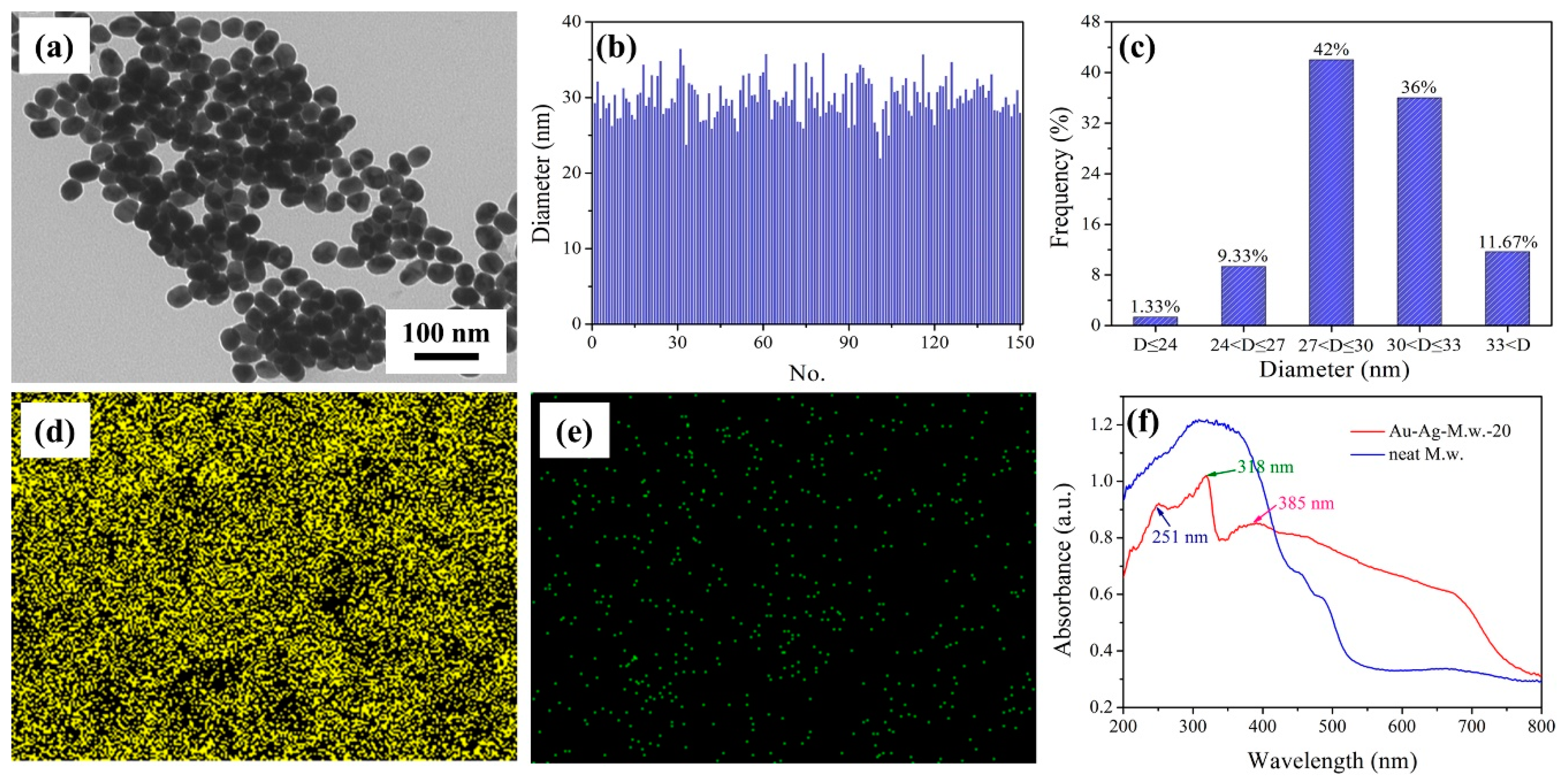
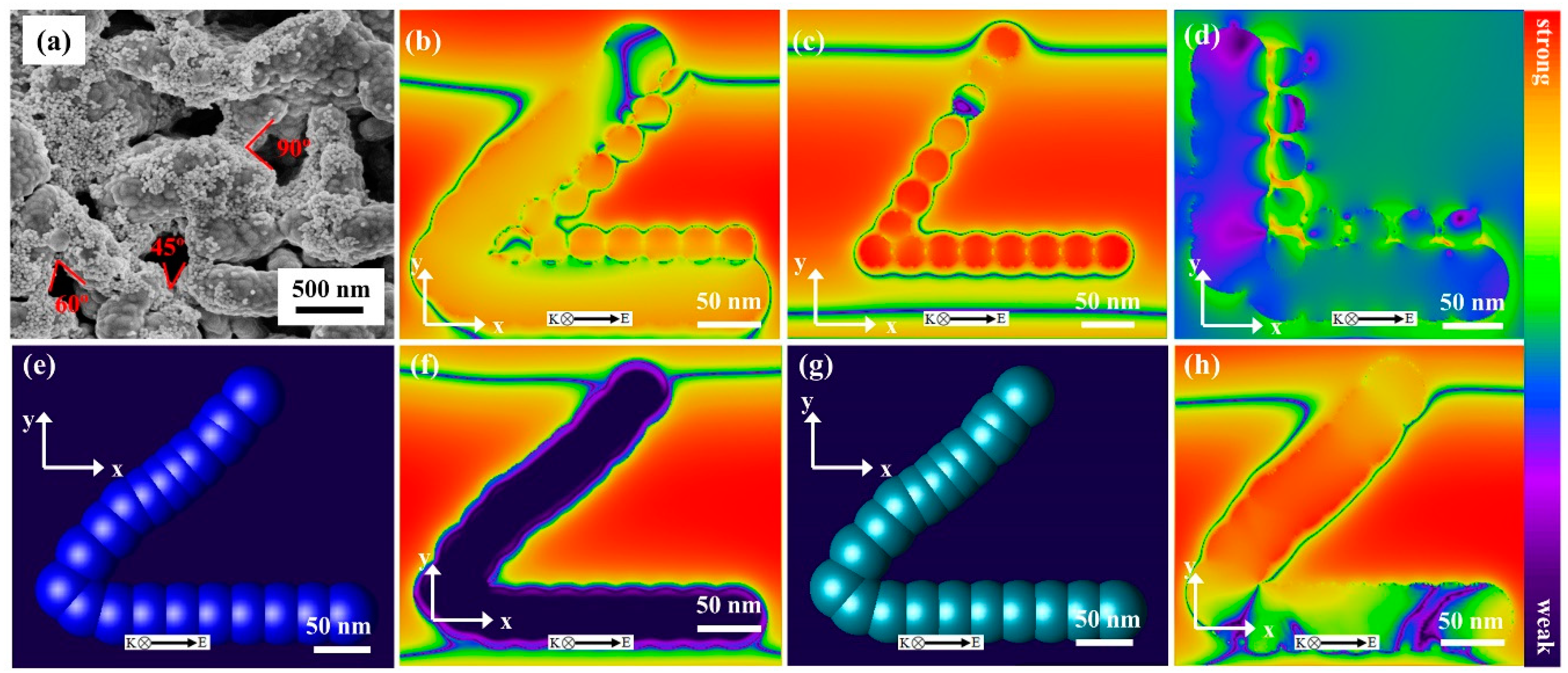
3.2. Morphology Characterization
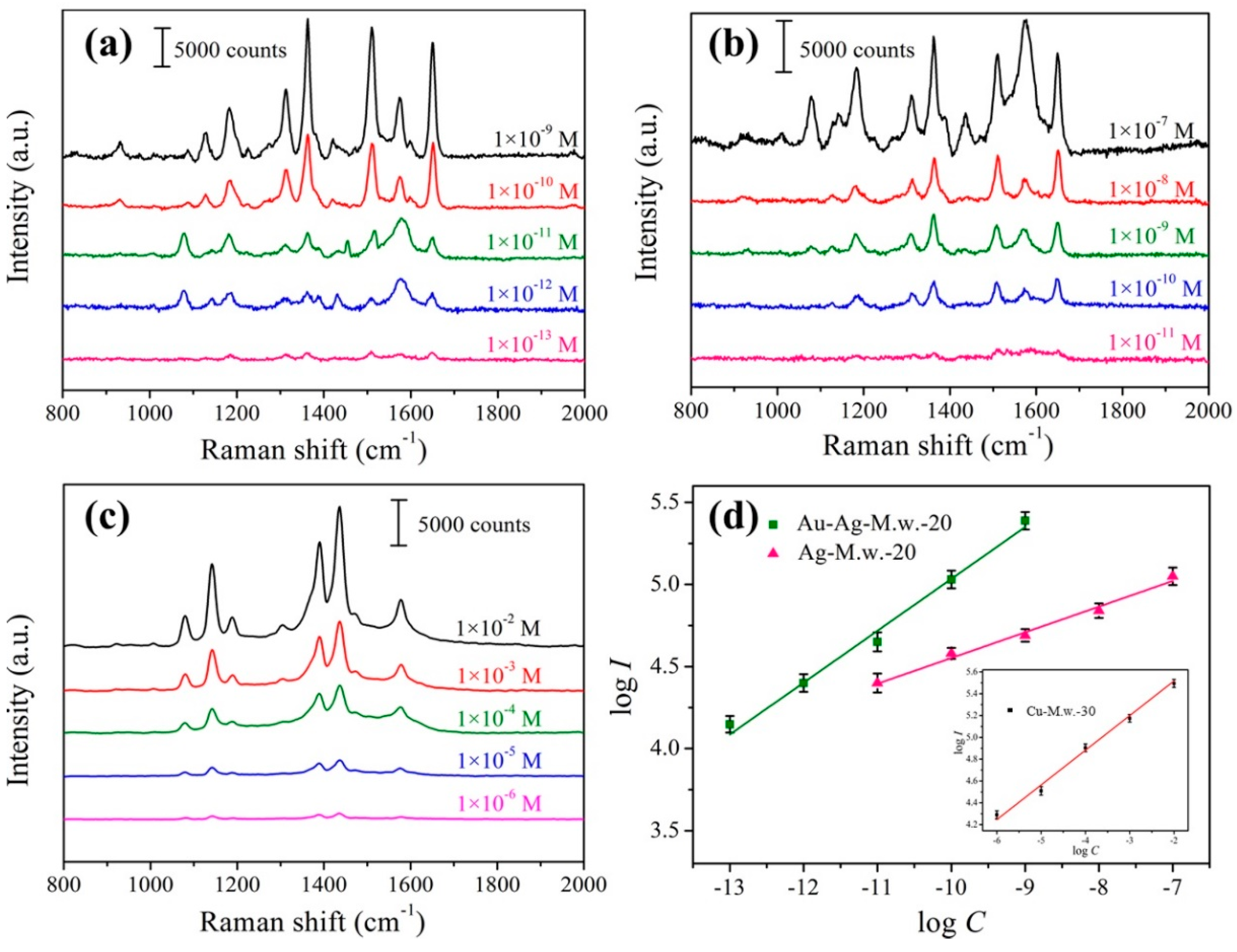
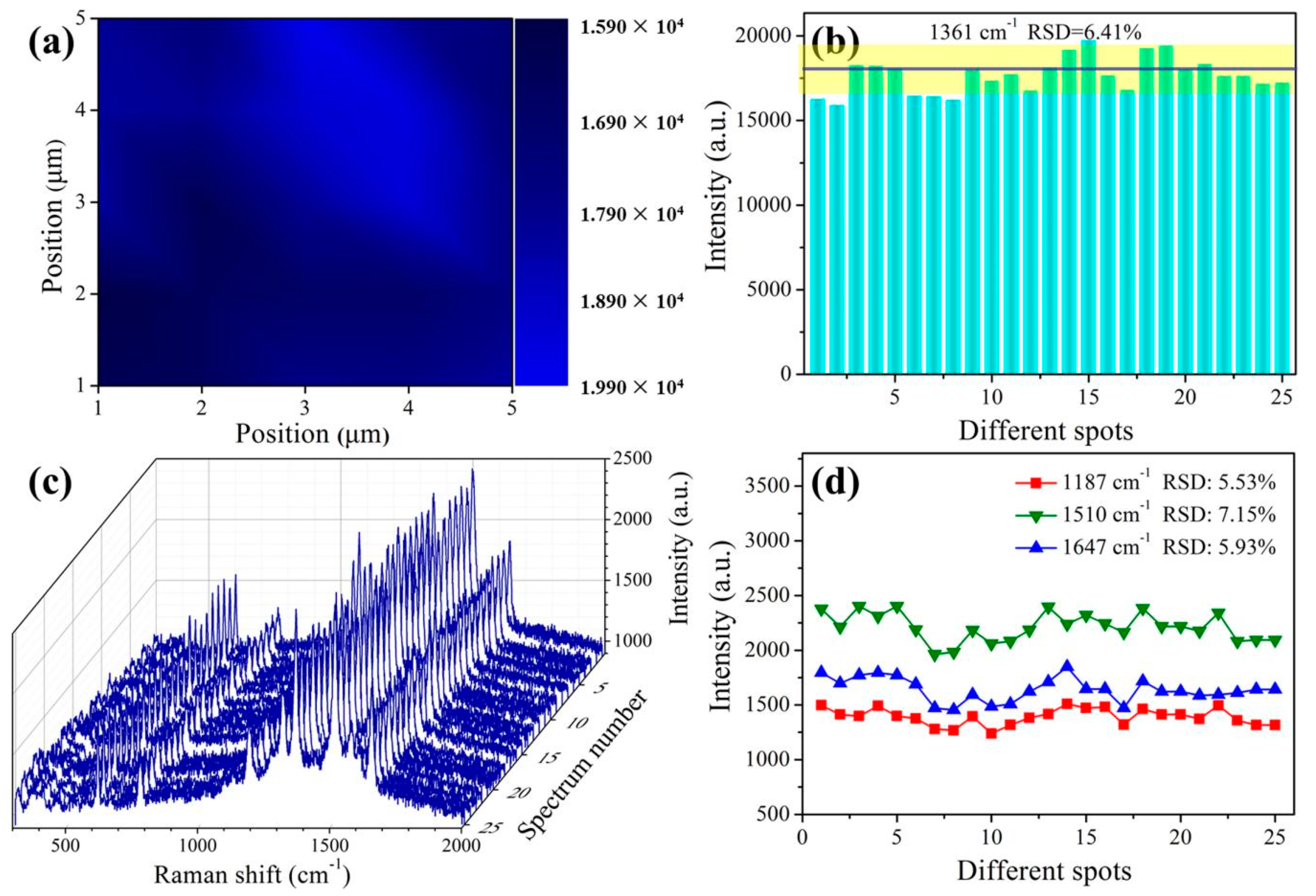
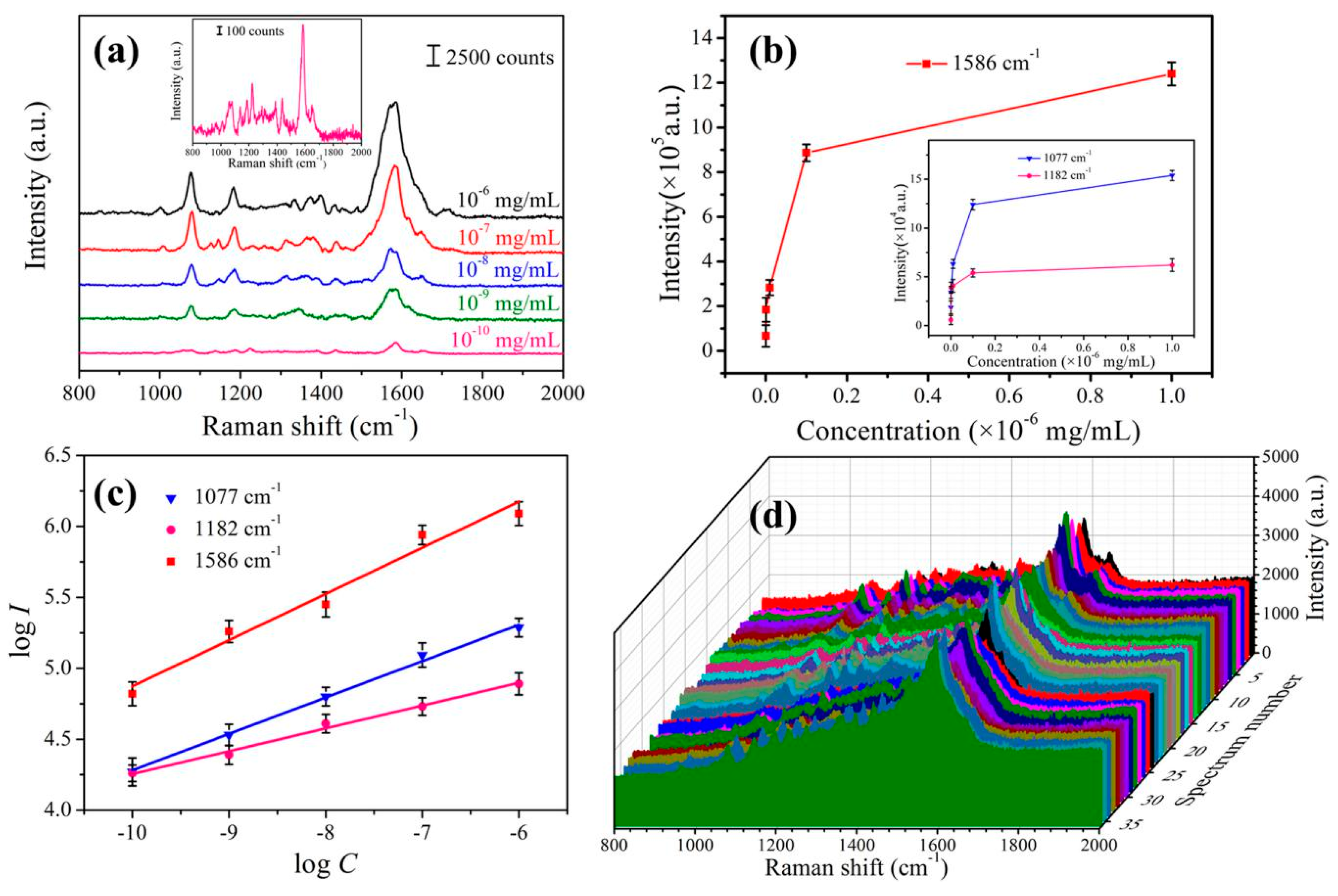
3.3. SERS Performances of the Prepared Substrate
3.4. EF Calculation
3.5. Collection of Pesticide Residues by Au-Ag-M.w.-20 Substrate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Jiang, L.; Hu, J.; Wang, A.D.; Li, X.W.; Lu, Y.F. Optical field enhancement in Au nanoparticle-decorated nanorod arrays prepared by femtosecond laser and their tunable SERS applications. ACS Appl. Mater. Interfaces 2017, 10, 297–1305. [Google Scholar]
- Wang, C.; Liu, B.X.; Dou, X.C. Silver nanotriangles-loaded filter paper for ultrasensitive SERS detection application benefited by interspacing of sharp edges. Sens. Actuat. B Chem. 2016, 231, 357–364. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, M.J.; Li, J.J.; Li, X.; Zhao, J.W. Multi-branched gold nanostars with fractal structure for SERS detection of the pesticide thiram. Spectrochim. Acta Part A 2018, 189, 586–593. [Google Scholar] [CrossRef]
- Yan, X.Y.; Wang, M.L.; Sun, X.; Wang, Y.H.; Shi, G.C.; Ma, W.L.; Hou, P. Sandwich-like Ag@Cu@CW SERS substrate with tunable nanogaps and component based on the Plasmonic nanonodule structures for sensitive detection crystal violet and 4-aminothiophenol. Appl. Surf. Sci. 2019, 479, 879–886. [Google Scholar] [CrossRef]
- Li, Y.; Lu, R.; Shen, J.Y.; Han, W.Q.; Sun, X.Y.; Li, J.S.; Wang, L.J. Electrospun flexible poly (bisphenol A carbonate) nanofibers decorated with Ag nanoparticles as effective 3D SERS substrates for trace TNT detection. Analyst 2017, 142, 4756–4764. [Google Scholar] [CrossRef]
- Ilkhani, H.; Hughes, T.; Zhong, C.J.; Hepel, M. Nanostructured SERS-electrochemical biosensors for testing of anticancer drug interactions with DNA. Biosens. Bioelectron. 2016, 80, 257–264. [Google Scholar] [CrossRef]
- Guerrini, L.; Graham, D. Molecularly-mediated assemblies of plasmonic nanoparticles for surface-enhanced Raman spectroscopy applications. Chem. Soc. Rev. 2012, 41, 7085–7107. [Google Scholar] [CrossRef]
- Baia, M.; Baia, L.; Astilean, S.; Popp, J. Surface-enhanced Raman scattering efficiency of truncated tetrahedral Ag nanoparticle arrays mediated by electromagnetic couplings. Appl. Phys. Lett. 2006, 88, 143121–143123. [Google Scholar] [CrossRef]
- Ru, E.C.L.; Etchegoin, P.G. Rigorous justification of the |E|4, enhancement factor in surface enhanced Raman spectroscopy. Chem. Phys. Lett. 2006, 423, 63–66. [Google Scholar]
- Wan, G.P.; Peng, X.G.; Zeng, M.; Yu, L.; Wang, K.; Li, X.Y.; Wang, G.Z. The preparation of Au@TiO2 yolk-shell nanostructure and its applications for degradation and detection of methylene blue. Nanoscale Res. Lett. 2017, 12, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Yan, X.Y.; Li, J.K.; Jiao, T.F.; Cai, C.; Zou, G.D.; Wang, R.; Wang, M.L.; Zhang, L.X.; Peng, Q.M. Preparation of self-assembled composite films constructed by chemically-modified MXene and dyes with surface-enhanced Raman scattering characterization. Nanomaterials 2019, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Velleman, L.; Scarabelli, L.; Sikdar, D.; Kornyshev, A.A.; Liz-Marzán, L.M.; Edel, J.B. Monitoring plasmon coupling and SERS enhancement through in situ nanoparticle spacing modulation. Faraday Discuss. 2017, 205, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.C.; Wang, M.L.; Zhu, Y.Y.; Wang, Y.H.; Yan, X.Y.; Sun, X.; Xu, H.J.; Ma, W.L. Biomimetic synthesis of Ag-coated glasswing butterfly arrays as ultra-sensitive SERS substrates for efficient trace detection of pesticides. Beilstein J. Nanotechnol. 2019, 10, 578–588. [Google Scholar] [CrossRef]
- Huang, W.S.; Sun, I.W.; Huang, C.C. Promotion of SERS and catalytic activities with bimetallic and ternary concave nanolayers. J. Mater. Chem. A 2018, 6, 13041–13049. [Google Scholar] [CrossRef]
- Hong, W.E.; Hsu, I.L.; Huang, S.Y.; Lee, C.W.; Ko, H.; Tsai, P.J.; Shiehcd, D.B.; Huang, C.C. Assembled growth of 3D Fe3O4@Au nanoparticles for efficient photothermal ablation and SERS detection of microorganisms. J. Mater. Chem. B 2018, 6, 5689–5697. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Si, T.T.; Liu, J.; Zhou, G.W. In-situ grown silver nanoparticles on nonwoven fabrics based on mussel-inspired polydopamine for highly sensitive SERS Carbaryl pesticides detection. Nanomaterials 2019, 9, 384. [Google Scholar] [CrossRef]
- Tseng, M.L.; Huang, Y.W.; Hsiao, M.K.; Huang, H.W.; Chen, H.M.; Chen, Y.L.; Chu, C.H.; Chu, N.N.; He, Y.J.; Chang, C.M.; et al. Fast fabrication of a Ag nanostructure substrate using the femtosecond laser for broad-band and tunable plasmonic enhancement. ACS Nano 2012, 6, 5190–5197. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering(SERS). Phys. Rev. Lett. 1997, 78, 667–1670. [Google Scholar] [CrossRef]
- Xu, B.B.; Zhang, Y.L.; Zhang, W.Y.; Liu, X.Q.; Wang, J.N.; Zhang, X.L.; Zhang, D.D.; Jiang, H.B.; Zhang, R.; Sun, H.B. Silver-coated rose petal: Green, facile, low-cost and sustainable fabrication of a SERS substrate with unique superhydrophobicity and high efficiency. Adv. Opt. Mater. 2013, 1, 56–60. [Google Scholar] [CrossRef]
- Huang, J.A.; Zhao, Y.Q.; Zhang, X.J.; He, L.F.; Wong, T.L.; Chui, Y.S.; Lee, S.T. Ordered Ag/Si nanowires array: Wide-range surface-enhanced Raman spectroscopy for reproducible biomolecule detection. Nano Lett. 2013, 13, 5039–5045. [Google Scholar] [CrossRef]
- Brolo, A.G.; Arctander, E.; Gordon, R. Nanohole-Enhanced Raman Scattering. Nano. Lett. 2004, 4, 2015–2018. [Google Scholar] [CrossRef]
- Wang, D.; Crozier, K.B.; Zhu, W. Double resonance surface enhanced Raman scattering substrates: An intuitive coupled oscillator model. Opt. Express 2011, 19, 14919–14928. [Google Scholar]
- Xu, B.B.; Ma, Z.C.; Wang, L.; Zhang, R.; Niu, L.G.; Yang, Z.; Zhang, Y.L.; Zheng, W.H.; Zhao, B.; Xu, Y.; et al. Localized flexible integration of high-efficiency surface enhanced Raman scattering (SERS) monitors into microfluidic channels. Lab Chip 2011, 11, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Khosla, R.; Soni, M.; Deva, D.; Sharma, S.K. A highly sensitive, flexible SERS sensor for malachite green detection based on Ag decorated microstructured PDMS substrate fabricated from Taro leaf as template. Sens. Actuators B Chem. 2017, 246, 477–486. [Google Scholar] [CrossRef]
- Aybeke, E.N.; Belliot, G.; Lemaire-Ewing, S.; Estienney, M.; Lacroute, Y.; Pothier, P.; Bourillot, E.; Lesniewska, E. HS-AFM and SERS analysis of murine norovirus infection: Involvement of the lipid rafts. Small 2016, 13, 1600918–1600927. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, R.E.; Aybeke, E.N.; Bourillot, E.; Lacroute, Y.; Lesniewska, E.; Adam, P.; Bijeon, J. Fabrication of annealed gold nanostructures on pre-treated glow-discharge cleaned glasses and their used for localized surface plasmon resonance (LSPR) and surface enhanced Raman spectroscopy (SERS) detection of adsorbed (bio)molecules. Sensors 2017, 17, 236–245. [Google Scholar] [CrossRef]
- Aybeke, E.N.; Lacroute, Y.; Elie-Caille, C.; Bouhelier, A.; Bourillot, E.; Lesniewska, E. Homogeneous large-scale crystalline nanoparticle-covered substrate with high SERS performance. Nanotechnology 2015, 26, 245302–245308. [Google Scholar] [CrossRef]
- Mu, Z.D.; Zhao, X.W.; Xie, Z.Y.; Zhao, Y.J.; Zhong, Q.F.; Bo, L.; Gu, Z.Z. In situ synthesis of gold nanoparticles (AuNPs) in butterfly wings for surface enhanced Raman spectroscopy (SERS). J. Mater. Chem. B 2013, 1, 1607–1613. [Google Scholar] [CrossRef]
- Lv, M.Y.; Teng, H.Y.; Chen, Z.Y.; Zhao, Y.M.; Zhang, X.; Liu, L.; Wu, Z.L.; Liu, L.M.; Xu, H.J. Low-cost Au nanoparticle-decorated cicada wing as sensitive and recyclable substrates for surface enhanced Raman scattering. Sens. Actuators B. Chem. 2015, 209, 820–827. [Google Scholar] [CrossRef]
- Xu, K.C.; Wang, Z.Y.; Tan, C.F.; Kang, N.; Chen, L.W.; Ren, L.; Thian, E.S.; Ho, G.W.; Ji, R.; Hong, M.H. Uniaxially stretched flexible surface plasmon resonance film for versatile surface enhanced Raman scattering diagnostics. ACS Appl. Mater. Interfaces 2017, 9, 26341–26349. [Google Scholar] [CrossRef]
- Lee, C.W.; Ko, H.; Chang, G.S.H.; Huang, C.C. Invisible-ink-assisted pattern and written surfaceenhanced Raman scattering substrates for versatile chem/biosensing platforms. Green Chem. 2018, 20, 5318–5326. [Google Scholar] [CrossRef]
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar] [CrossRef]
- Li, R.F.; Shi, G.C.; Wang, Y.H.; Wang, M.L.; Zhu, Y.Y.; Sun, X.; Xu, H.J.; Chang, C.X. Decoration of Cu films on the microstructural mantis wing as flexible substrates for surface enhanced Raman scattering. Optik 2018, 172, 49–56. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nature 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Liu, B.H.; Han, G.M.; Zhang, Z.P.; Liu, R.Y.; Jiang, C.L.; Wang, S.H.; Han, M.Y. Shell thickness-dependent Raman enhancement for rapid identification and detection of pesticide residues at fruit peels. Anal. Chem. 2012, 84, 255–261. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, J.K. Raman scattering of 4-aminobenzenethiol sandwiched between Ag/Au nanoparticle and macroscopically smooth Au substrate. J. Phys. Chem. B 2005, 109, 20731–20736. [Google Scholar] [CrossRef]
- Oh, S.H.; Im, C.H.; Lindquist, N.C.; Haynes, C.L.; Bantz, S.K. Vertically oriented sub-10-nm plasmonic nanogap arrays. Nano Lett. 2010, 10, 2231–2236. [Google Scholar]
- Zhang, B.H.; Wang, H.S.; Lu, L.H.; Ai, K.L.; Zhang, G.; Cheng, X.L. Large-area silver-coated silicon nanowire arrays for molecular sensing using surface enhanced Raman spectroscopy. Adv. Funct. Mater. 2010, 18, 2348–2355. [Google Scholar] [CrossRef]
- Parsons, H.M.; Ekman, D.R.; Collette, T.W.; Viant, M.R. Spectral relative standard deviation: A practical benchmark in metabolomics. Analyst 2009, 134, 478–485. [Google Scholar] [CrossRef]
- Wang, X.T.; Shi, W.S.; She, G.W.; Mu, L.X. High-performance surface-enhanced Raman scattering sensors based on Ag nanoparticles-coated Si nanowire arrays for quantitative detection of pesticides. Appl. Phys. Lett. 2010, 96, 053104. [Google Scholar] [CrossRef]
- Zhang, M.F.; Meng, J.T.; Wang, D.P.; Tang, Q.; Chen, T.; Rong, S.Z.; Liu, J.Q.; Wu, Y.C. Biomimetic synthesis of hierarchical 3D Ag butterfly wing scale arrays/graphene composites as ultrasensitive SERS substrates for efficient trace chemical detection. J. Mater. Chem. C 2018, 6, 1933–1943. [Google Scholar] [CrossRef]
- Becker, C.; Jochmann, M.A.; Schmidt, T.C. An overview of approaches in liquid chromatography flame ionization detection. TrAC Trend Anal. Chem. 2019, 110, 143–149. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Aloisi, I.; Giocastro, B.; Mondello, L. Current state of comprehensive two-dimensional gas chromatography-mass spectrometry with focus on processes of ionization. TrAC Trend Anal. Chem. 2018, 105, 360–366. [Google Scholar] [CrossRef]
- Pashkova, G.V.; Smagunova, A.N.; Finkelshtein, A.L. X-ray fluorescence analysis of milk and dairy products: A review. TrAC Trend Anal. Chem. 2018, 106, 183–189. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, M.L.; Sun, X.; Shi, G.C.; Zhang, J.Z.; Ma, W.L.; Ren, L.J. Grating-like SERS substrate with tunable gaps based on nanorough Ag nanoislands/moth wing scale arrays for quantitative detection of cypermethrin. Opt. Express 2018, 26, 22168–22181. [Google Scholar] [CrossRef] [PubMed]


| Modes | SERS 532 nm (cm−1) | Band Assignment |
|---|---|---|
| 18a(a1) | 1007 | γCC + γCCC |
| 7a(a1) | 1077 | νCS |
| 9b(b2) | 1140 | δCH |
| 9a(a1) | 1185 | δCH |
| 14b(b2) | 1313 | νCC + δCH |
| 3b(b2) | 1367 | νCC + δCH |
| 19b(b2) | 1453 | νCC + δCH |
| 8b(b2) | 1578 | νCC |
| - | 1650 | δCH |
| Sputtering Time (min) | Peak Intensity I (count) | Increase Factor (IAu-Ag-M.w.-x/IAg-M.w.-x) | |
|---|---|---|---|
| IAu-Ag-M.w.-x | IAg-M.w.-x | ||
| 10 | 106,148 ± 1542.6 | 84,685 ± 748.8 | 1.25 ± 0.025 |
| 20 | 263,045 ± 1146.4.3 | 125,620 ± 1640.5 | 2.09 ± 0.032 |
| 30 | 175,894 ± 1320.3 | 105,630 ± 990.0 | 1.67 ± 0.017 |
| 40 | 157,304 ± 1114.3 | 42,583 ± 909.7 | 3.69 ± 0.097 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Shi, G.; Zhu, J.; Zhu, Y.; Sun, X.; Wang, P.; Jiao, T.; Li, R. Preparation of a Novel SERS Platform Based on Mantis Wing with High-Density and Multi-Level “Hot Spots”. Nanomaterials 2019, 9, 672. https://doi.org/10.3390/nano9050672
Wang M, Shi G, Zhu J, Zhu Y, Sun X, Wang P, Jiao T, Li R. Preparation of a Novel SERS Platform Based on Mantis Wing with High-Density and Multi-Level “Hot Spots”. Nanomaterials. 2019; 9(5):672. https://doi.org/10.3390/nano9050672
Chicago/Turabian StyleWang, Mingli, Guochao Shi, Junlin Zhu, Yanying Zhu, Xin Sun, Peng Wang, Tifeng Jiao, and Ruifeng Li. 2019. "Preparation of a Novel SERS Platform Based on Mantis Wing with High-Density and Multi-Level “Hot Spots”" Nanomaterials 9, no. 5: 672. https://doi.org/10.3390/nano9050672
APA StyleWang, M., Shi, G., Zhu, J., Zhu, Y., Sun, X., Wang, P., Jiao, T., & Li, R. (2019). Preparation of a Novel SERS Platform Based on Mantis Wing with High-Density and Multi-Level “Hot Spots”. Nanomaterials, 9(5), 672. https://doi.org/10.3390/nano9050672