Interaction in Li@Fullerenes and Li+@Fullerenes: First Principle Insights to Li-Based Endohedral Fullerenes
Abstract
1. Introduction
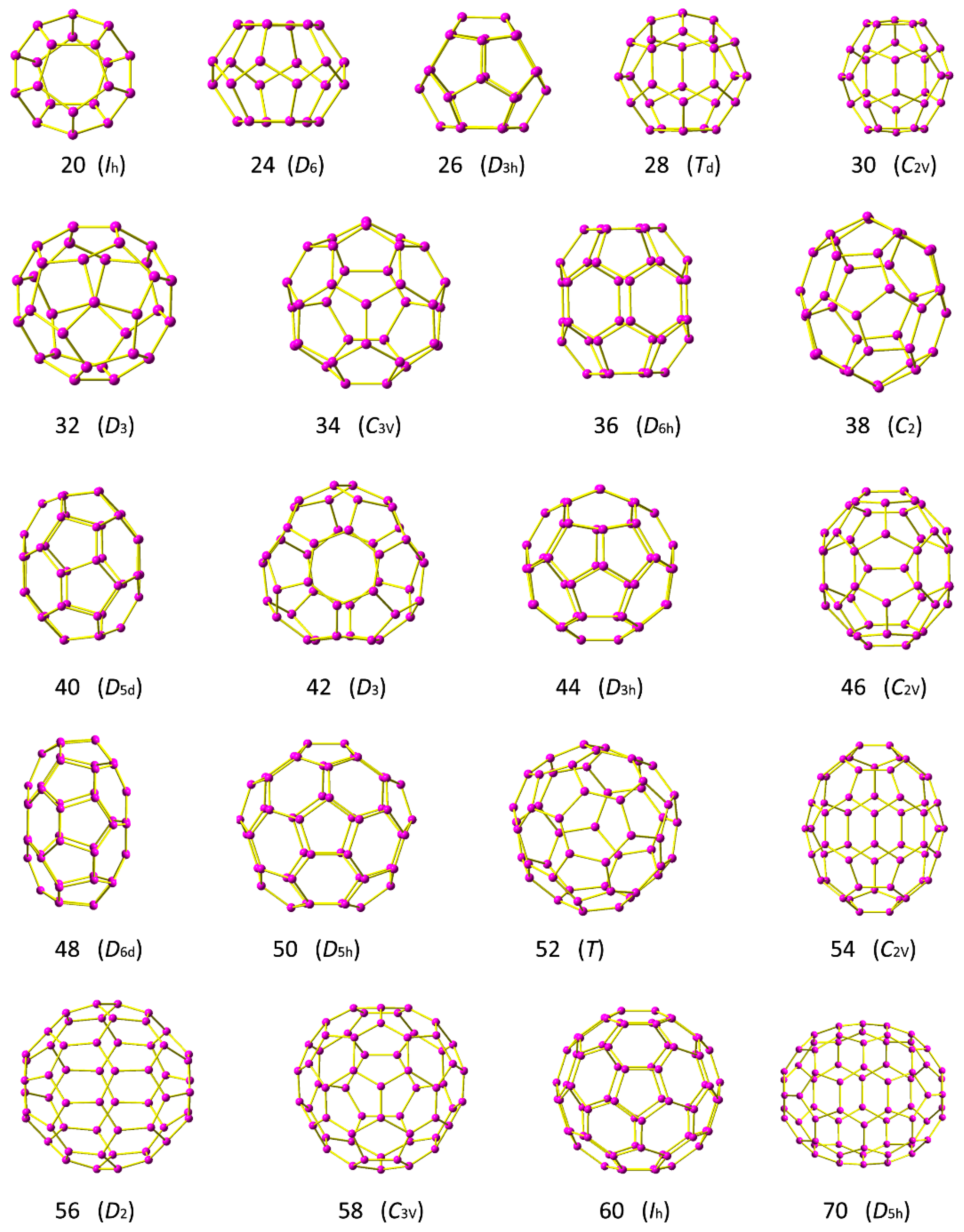
2. Model and Computational Methods
3. Results
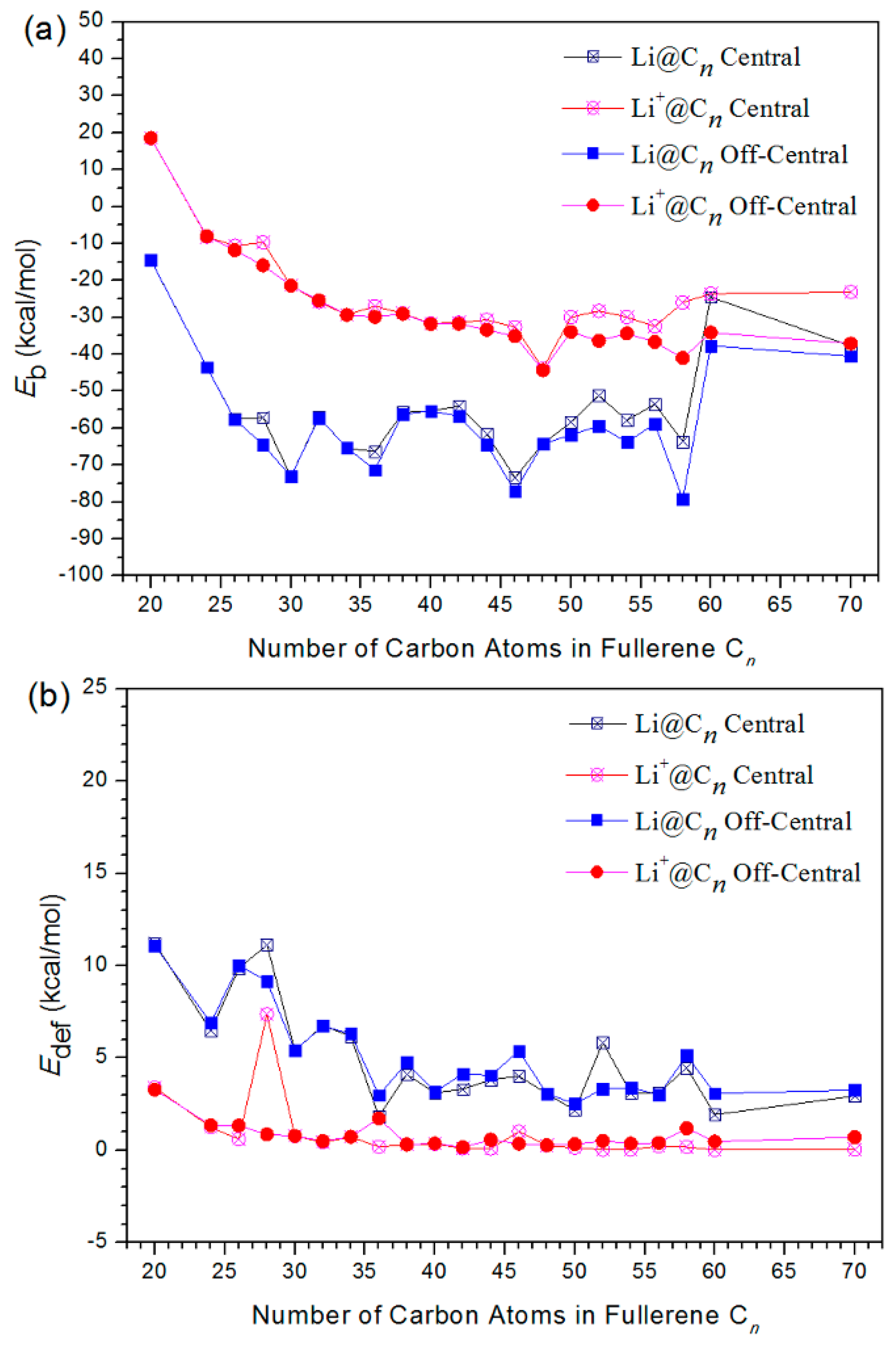
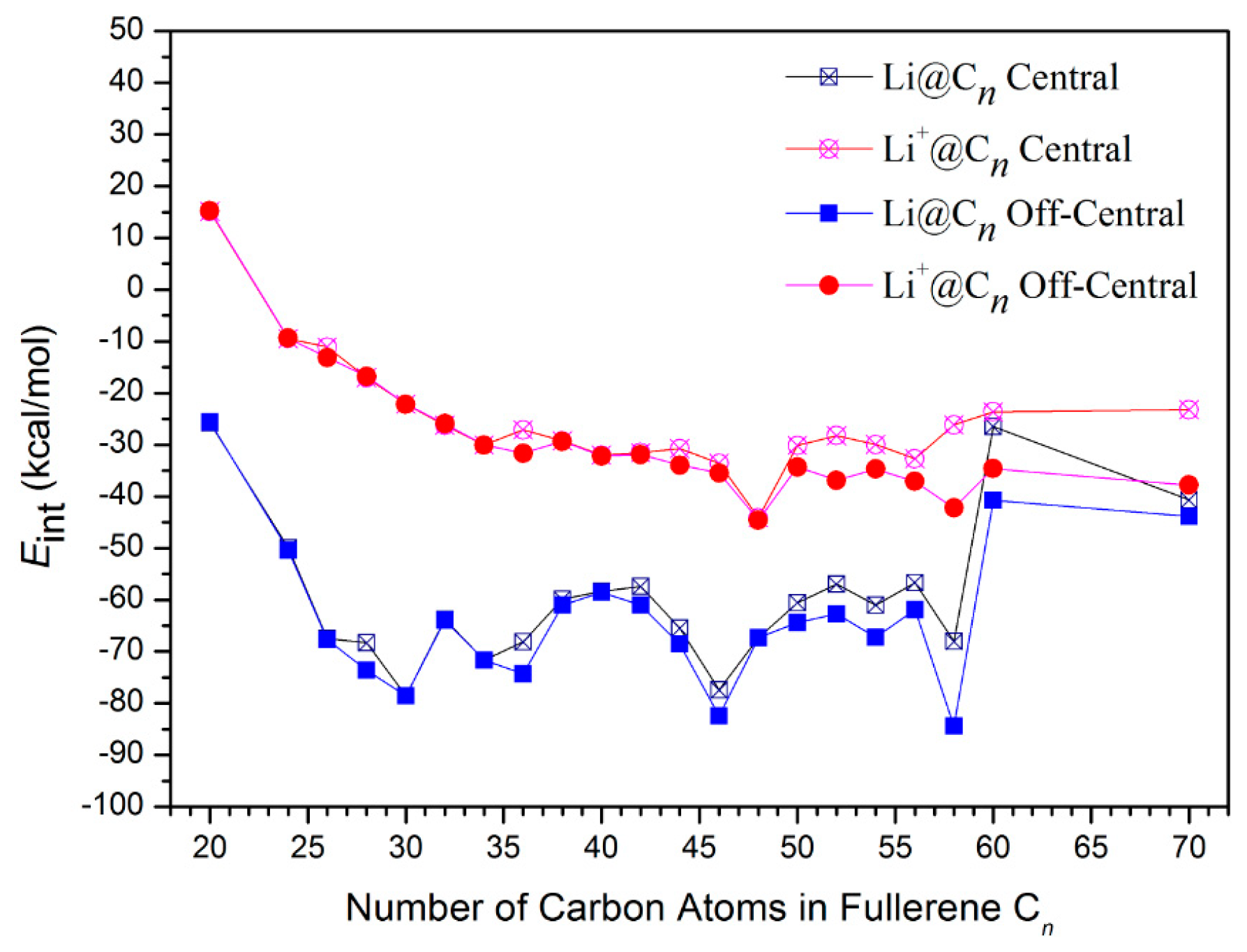
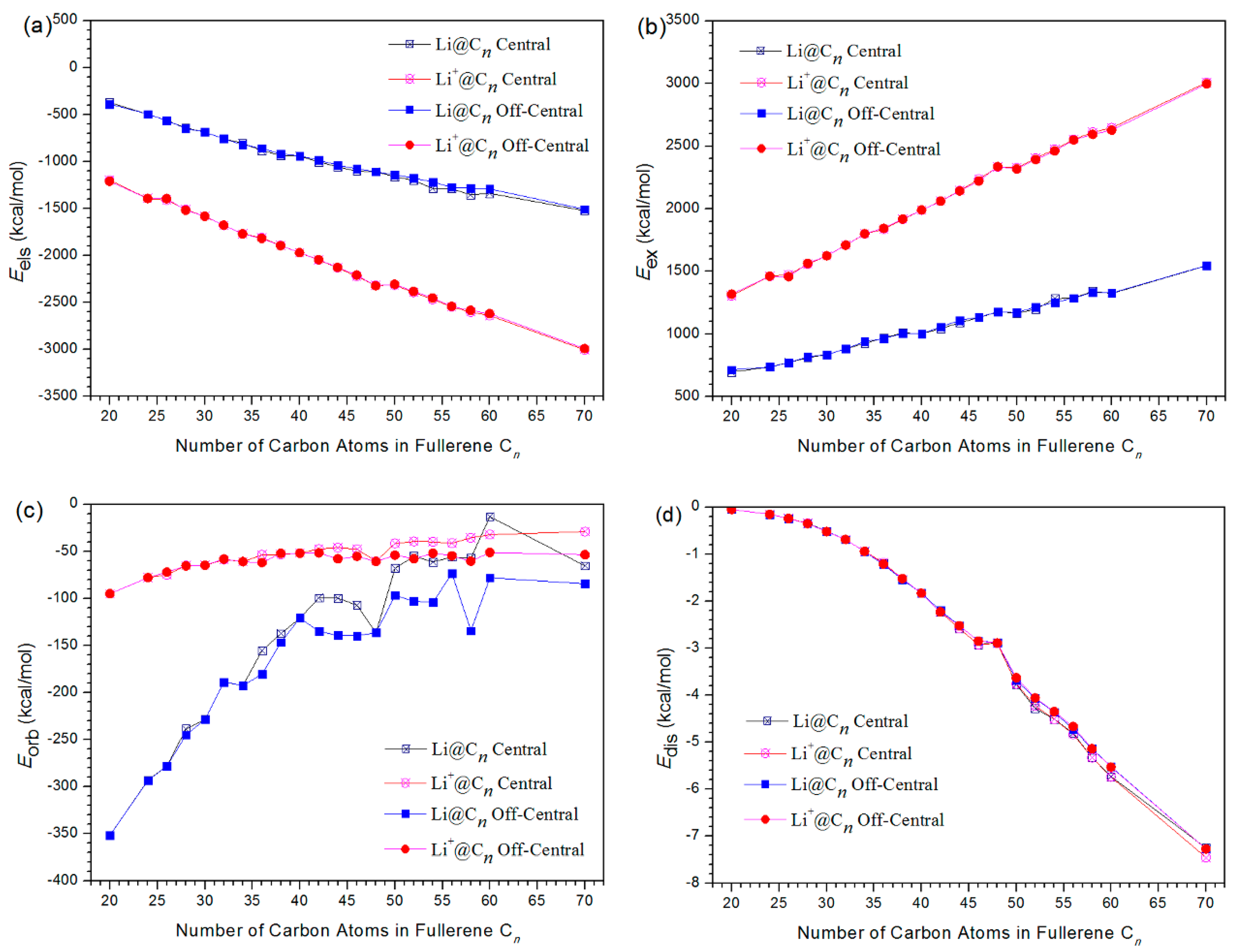
3.1. Energy and Stability
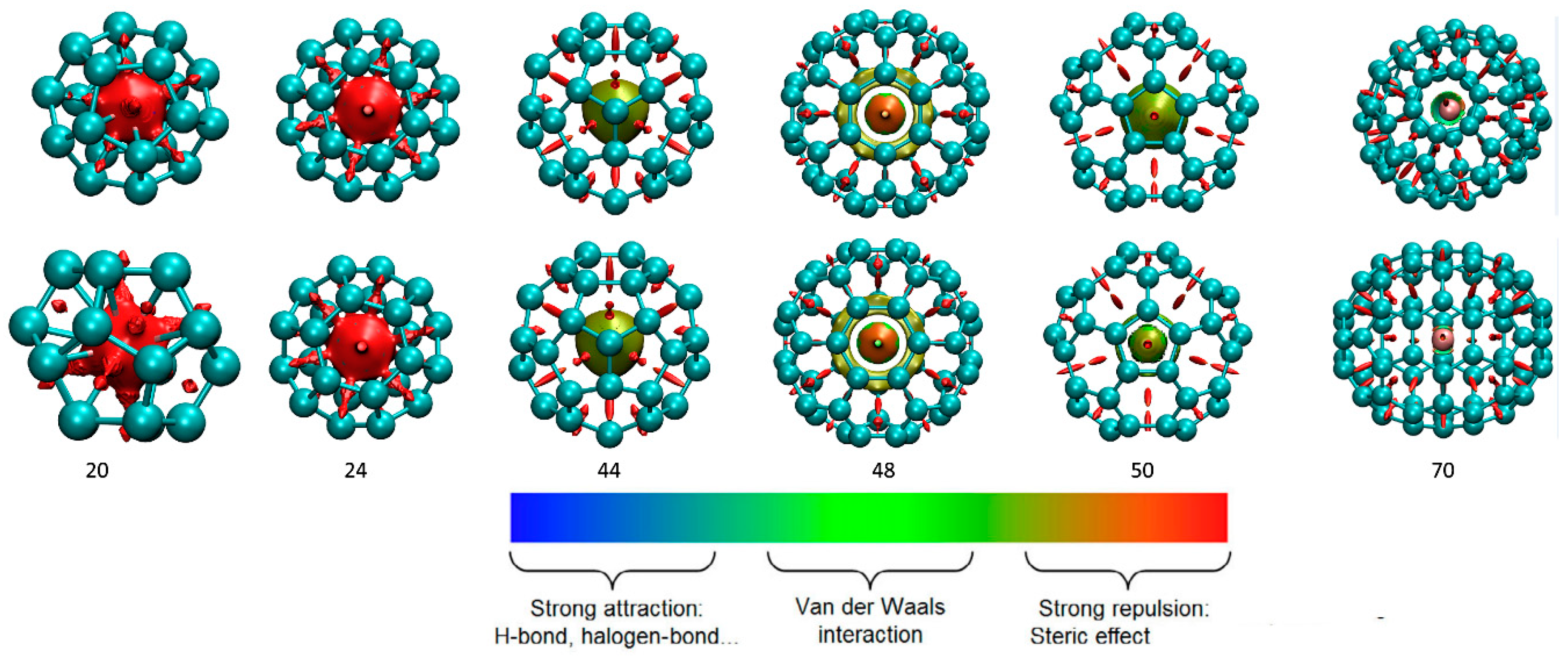
3.2. Interaction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yue, C.; Yu, Y.; Wu, Z.; Sun, S.; He, X.; Li, J. High stability induced by the tin/ti interlayer in three-dimensional si/ge nanorod arrays as anode in micro lithium ion battery. ACS Appl. Mater. Interfaces 2016, 8, 7806–7810. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.B.; Whittingham, M.S.; Huggin, R.A. The iron cyanide bronzes. Mater. Res. Bull. 1972, 7, 101–108. [Google Scholar] [CrossRef]
- Li, W.; Dolocan, A.; Oh, P.; Celio, H.; Park, S.; Cho, J. Dynamic behaviour of interphases and its implication on high-energy-density cathode materials in lithium-ion batteries. Nat. Commun. 2017, 8, 14589. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, J.I.; Tobishima, S.I.; Sakurai, Y.; Saito, K.I.; Hayashi, K. Safety evaluation of rechargeable cells with lithium metal anodes and amorphous V2O5 cathodes. J. Appl. Electrochem. 1998, 28, 135–140. [Google Scholar] [CrossRef]
- Abada, S.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety focused modeling of lithium-ion batteries: A review. J. Power Sour. 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Chi, M.; Liang, C.; Dudney, N.J. Solid electrolyte: The key for high-voltage lithium batteries. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; Obrien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, H. Theoretical study on the peanut-shaped dimers and nanotubes consisted of C50 cages. J. Nanosci. Nanotechnol. 2011, 11, 11104. [Google Scholar]
- Li, Z.; Liu, Z.; Sun, H.; Gao, C. Superstructured Assembly of Nanocarbons: Fullerenes, Nanotubes, and Graphene. Chem. Rev. 2015, 115, 7046–7117. [Google Scholar] [CrossRef]
- Bai, H.; Qiao, W.; Zhu, Y.; Huang, Y. Theoretical study on one-dimensional C50 polymers. Diam. Relat. Mater. 2012, 26, 20–24. [Google Scholar] [CrossRef]
- Fang, Y.; Bi, C.; Wang, D.; Huang, J. The functions of fullerenes in hybrid perovskite solar cells. ACS Energy Lett. 2017, 2, 782–794. [Google Scholar] [CrossRef]
- Hashikawa, Y.; Murata, M.; Wakamiya, A.; Murata, Y. Synthesis and Properties of Endohedral Aza[60] fullerenes: H2O@C59N and H2@C59N as Their Dimers and Monomers. J. Am. Chem. Soc. 2016, 138, 4096–4104. [Google Scholar] [CrossRef] [PubMed]
- Zlatko, B.; Vojtech, V.; Daniel, N.; Peter, M.F. Effects of symmetry breaking on the translation-rotation eigenstates of H2, HF, and H2O inside the fullerene C60. Faraday Discuss. 2018, 212, 547–567. [Google Scholar]
- Wang, L.; Ye, J.T.; Wang, H.Q.; Xie, H.M.; Qiu, Y.Q. Third-order nonlinear optical properties of endohedral fullerene (H2)2@C70 and (H2O)2@C70 accompanied by the prospective of novel (HF)2@C70. J. Phys. Chem. 2018, 122, 6835–6845. [Google Scholar] [CrossRef]
- EL-Barbary, A.A. Potential energy of H2 inside the C116 fullerene dimerization: An atomic analysis. J. Mol. Struct. 2016, 1112, 9–13. [Google Scholar] [CrossRef]
- Chen, C.S.; Kuo, T.S.; Yeh, W.Y. Encapsulation of formaldehyde and hydrogen cyanide in an open-cage fullerene. Chem. Eur. J. 2016, 22, 8773–8776. [Google Scholar] [CrossRef]
- Junghans, K.; Ghiassi, K.B.; Samoylova, N.A.; Deng, Q.; Rosenkranz, M.; Olmstead, M.; Balch, A.; Popov, A. Synthesis and isolation of the titanium–scandium endohedral fullerenes—Sc2TiC@Ih-C80, Sc2TiC@D5h-C80 and Sc2TiC2@Ih-C80: Metal size tuning of the tiiv/tiiii redox potentials. Chem. Eur. J. 2016, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Churilov, G.N.; Popov, A.A.; Vnukova, N.G.; Dudnil, A.I.; Glushchenko, G.A.; Samoylova, N.A.; Dubinina, I.A.; Gulyaeva, U.E. A method and apparatus for high-throughput controlled synthesis of fullerenes and endohedral metal fullerenes. Tech. Phys. Lett. 2016, 42, 475–477. [Google Scholar] [CrossRef]
- Aoyagi, S.; Nishibori, E.; Sawa, H.; Sugimoto, K.; Takata, M.; Miyata, Y.; Kitaure, R.; Shinohara, H.; Okada, H.; Sakai, T.; et al. A layered ionic crystal of polar Li@C60 superatoms. Nat. Chem. 2010, 2, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, M.; Jalbout, A.F.; Trzaskowski, B.; Adamowicz, L. Fullerene as an electron buffer: Charge transfer in Li@C60. Chem. Phys. Lett. 2007, 442, 339–343. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Pandey, S.K.; Misra, N. Prediction of superalkali@C60 endofullerenes, their enhanced stability and interesting properties. Chem. Phys. Lett. 2016, 655, 71–75. [Google Scholar] [CrossRef]
- Noguchi, Y.; Sugino, O.; Okada, H.; Matsuo, Y. First-principles investigation on structural and optical properties of M+@C60 (Where M = H, Li, Na, and K). J. Phys. Chem. 2013, 117, 15362–15368. [Google Scholar] [CrossRef]
- Etindele, A.J.; Maezono, R.; Melono, R.M.; Motapon, O. Influence of endohedral confinement of atoms on structural and dynamical properties of the C60 fullerene. Chem. Phys. Lett. 2017, 685, 395–400. [Google Scholar] [CrossRef]
- Debnath, T.; Saha, J.K.; Banu, T.; Ash, T.; Das, A.K. Structural and thermodynamic aspects of Lin@Cxendohedral metallofullerenes: A DFT approach. Theor. Chem. Acc. 2016, 135, 167. [Google Scholar] [CrossRef]
- Fowler, P.W.; Manolopoulos, D.E. An Atlas of Fullerenes; Clarendon Press: Oxford, UK, 1995. [Google Scholar]
- Bai, H. New solution method of pi-orbital axis vector and its applications in fullerenes and carbon nanotubes. Chin. J. Struct. Chem. 2013, 32, 695. [Google Scholar]
- Zhang, B.L.; Wang, C.Z.; Ho, K.M.; Xu, C.H.; Chan, C.T. The geometry of small fullerene cages: C20 to C70. J. Chem. Phys. 1992, 97, 5007–5011. [Google Scholar] [CrossRef]
- Han, S.S.; Van Duin, A.C.T.; Goddard, W.A.; Lee, H.M. Optimization and application of lithium parameters for the reactive force field, ReaxFF. J. Phys. Chem. 2005, 109, 4575–4582. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Velde, G.T.; Bickelhaupt, F.M.; Baerends, E.J.; Guerra, C.F.; Gisbergen, S.J.A.V.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ziegler, T.; Autschbach, J.; Bashford, D.; Bérces, A.; Bickelhaupt, F.M.; Bo, C.; Boerrigter, P.M.; Cavallo, L.; Chong, D.P.; et al. ADF (2017), SCM, Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Hansen, A.; Brandenburg, J.G.; Bannwarth, C. Dispersion-Corrected Mean-Field Electronic Structure Methods. Chem. Rev. 2016, 116, 5105. [Google Scholar] [CrossRef] [PubMed]
- Goerigk, L.; Hansen, A.; Bauer, C.; Ehrlich, S.; Najibi, A.; Grimme, S. A look at the density functional theory zoo with the advanced GMTKN55 database for general main group thermochemistry, kinetics and noncovalent interactions. Phys. Chem. Chem. Phys. 2017, 19, 32184. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheese, M.J.R.; Montgomery, J.A.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori–Sanchez, P.; Contreras–Garcıa, J.A.; Cohen, J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Hopffgarten, M.V.; Frenking, G. Energy decomposition analysis. Wire Comput. Mol. Sci. 2012, 2, 43–62. [Google Scholar] [CrossRef]
- Li, Y.; Bai, H.; Lin, F.; Huang, Y. Energetics and electronic structures of nitrogen chains encapsulated in zigzag carbon nanotube. Physica 2018, 103, 444–451. [Google Scholar]
- Li, Y.; Bai, H.; Li, L.; Huang, Y. Stabilities and electronic properties of nanowires made of single atomic sulfur chains encapsulated in zigzag carbon nanotubes. Nanotechnology 2018, 29, 415703. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Meher, B.R.; Eustache, D.; Wang, Y. Insight into the interaction between DNA bases and defective graphenes: Covalent or non-covalent. J. Mol. Graph. Model. 2014, 47, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Thomas, V.I.; Żyła, G.; Padmanabhan, A.S.; Mathew, S. Theoretical probing of weak anion−cation interactions in certain pyridinium-based ionic liquid ion pairs and the application of molecular electrostatic potential in their ionic crystal density determination: A comparative study using density functional approach. J. Phys. Chem. 2018, 122, 328–340. [Google Scholar]
- Gao, H.; Feng, W.; Li, X.; Li, N.; Du, Y.; Wu, Y. Insights into the non-covalent interaction between modified nucleobases and graphene nanoflake from first-principles. Physica 2019, 107, 73–79. [Google Scholar] [CrossRef]
- Maximillian, J.S.; Phipps, T.F.; Christofer, S.T.; Skylaris, C.K. Energy decomposition analysis approaches and their evaluation on prototypical protein–drug interaction patterns. Chem. Soc. Rev. 2015, 44, 3177. [Google Scholar]
- Lu, T. Multiwfn—A Multifunctional Wavefunction Analyze—Software Manual. Version 3.6 (dev); 2018; Available online: http://sobereva.com/multiwfn/misc/Multiwfn_3.6(dev).pdf (accessed on 18 April 2019).
- Fowler, P.W.; Manolopoulos, D.E. Magic numbers and stable structures for fullerenes, fullerides and fullerenium ions. Nature 1992, 355, 428. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Gao, H.; Feng, W.; Zhao, Y.; Wu, Y. Interaction in Li@Fullerenes and Li+@Fullerenes: First Principle Insights to Li-Based Endohedral Fullerenes. Nanomaterials 2019, 9, 630. https://doi.org/10.3390/nano9040630
Bai H, Gao H, Feng W, Zhao Y, Wu Y. Interaction in Li@Fullerenes and Li+@Fullerenes: First Principle Insights to Li-Based Endohedral Fullerenes. Nanomaterials. 2019; 9(4):630. https://doi.org/10.3390/nano9040630
Chicago/Turabian StyleBai, Hongcun, Hongfeng Gao, Wei Feng, Yaping Zhao, and Yuhua Wu. 2019. "Interaction in Li@Fullerenes and Li+@Fullerenes: First Principle Insights to Li-Based Endohedral Fullerenes" Nanomaterials 9, no. 4: 630. https://doi.org/10.3390/nano9040630
APA StyleBai, H., Gao, H., Feng, W., Zhao, Y., & Wu, Y. (2019). Interaction in Li@Fullerenes and Li+@Fullerenes: First Principle Insights to Li-Based Endohedral Fullerenes. Nanomaterials, 9(4), 630. https://doi.org/10.3390/nano9040630



