Effects of Poly(Amidoamine) Dendrimer-Coated Mesoporous Bioactive Glass Nanoparticles on Dentin Remineralization
Abstract
1. Introduction
2. Materials and Methods
2.1. Mesoporous Bioactive Glass Nanoparticle (MBN) Synthesis
2.2. PAMAM@MBN Synthesis
2.3. Analysis of Sample Characteristics
2.3.1. X-Ray Diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.3.2. Surface Area and Pore Size Analysis
2.3.3. Analytical Field-Emission Scanning Electron Microscopy (FESEM) and Field-Emission Transmission Electron Microscopy (FETEM)
2.4. In Vitro Ion-Releasing Test
2.5. In Vitro Mineralization Ability Test
2.6. Sensitive Tooth Model Preparation
2.7. Field-Emission Scanning Electron Microscopy Assessment of Dentinal Tubule Occlusion
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Samples
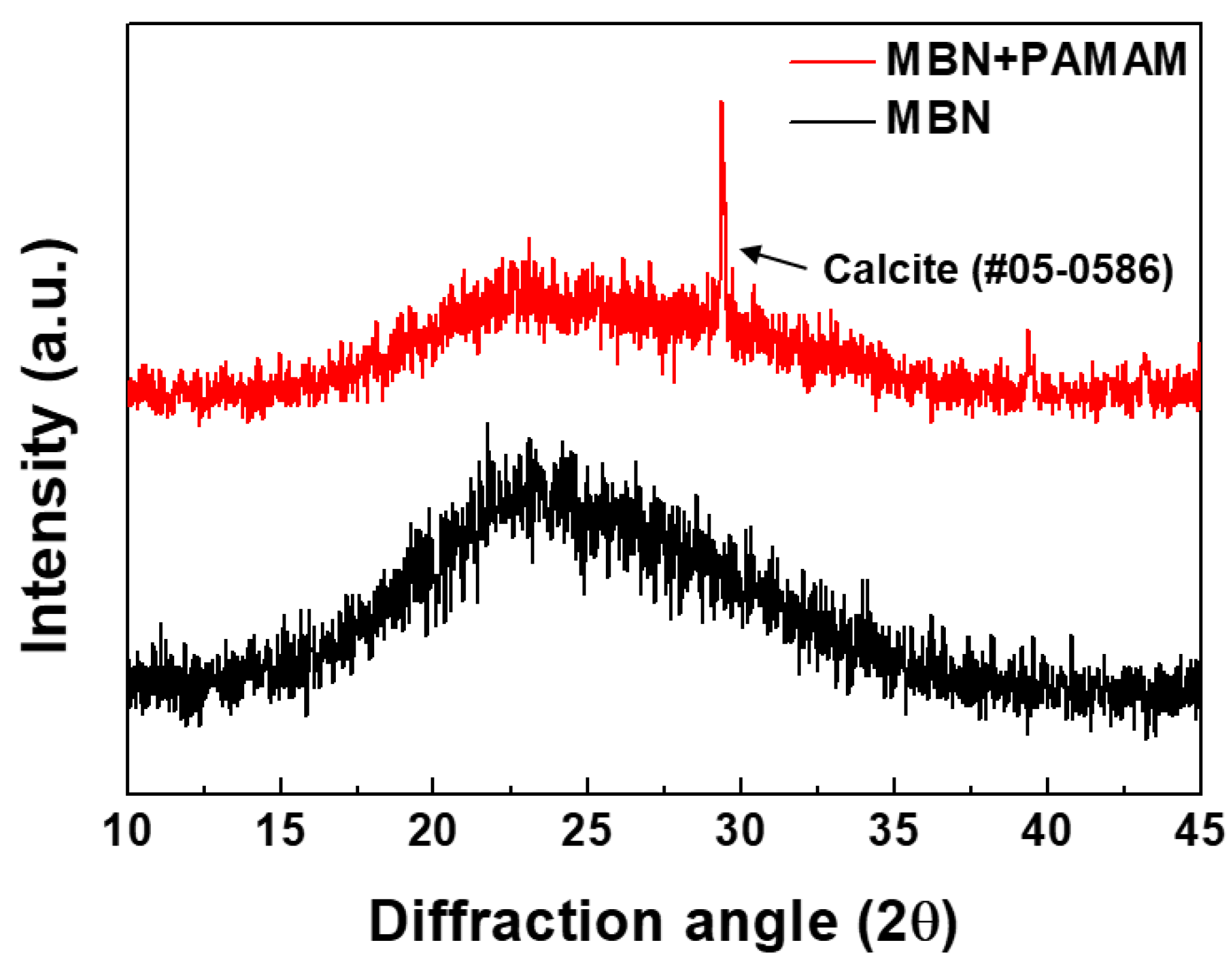
3.1.1. XRD Analysis
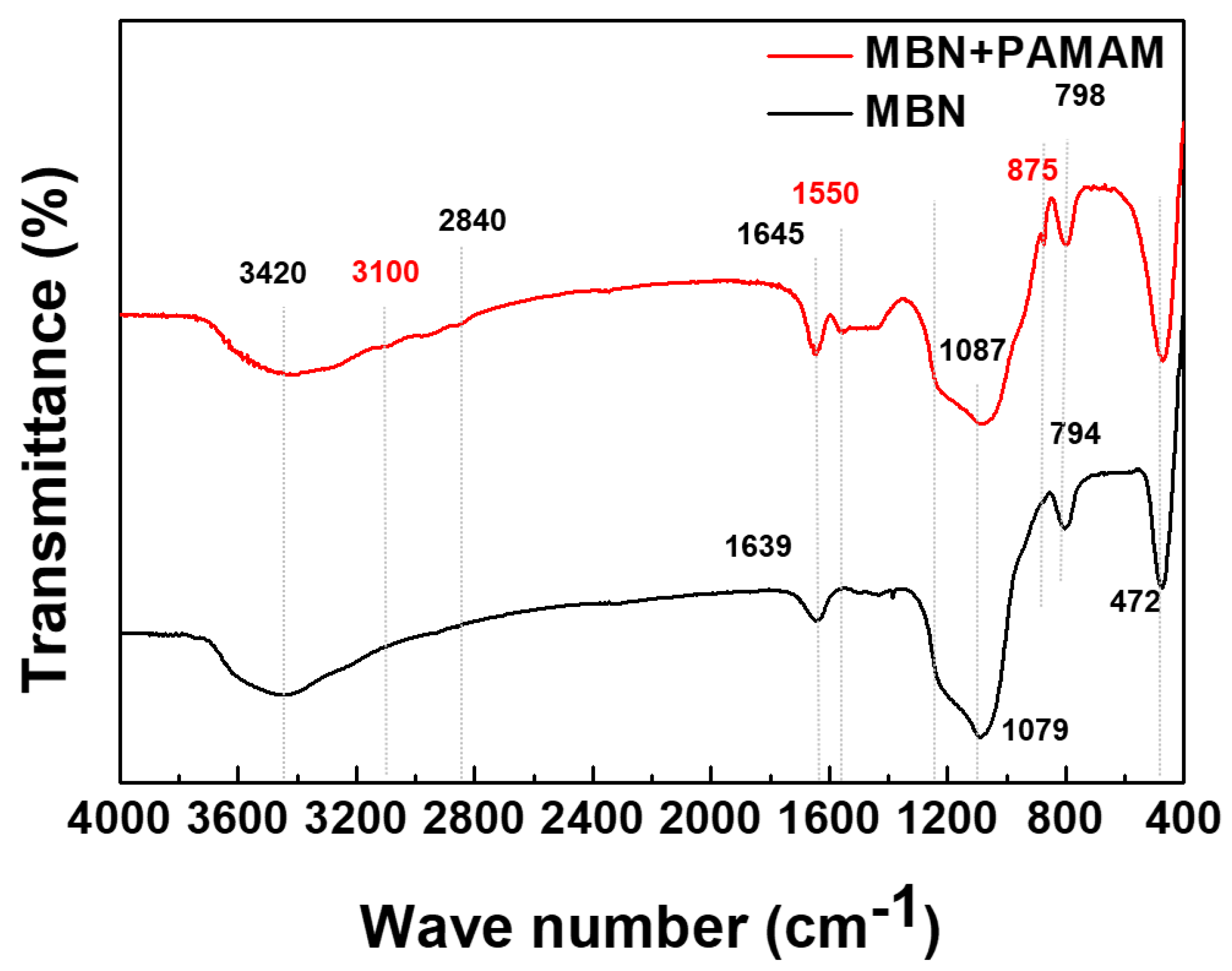
3.1.2. FT-IR
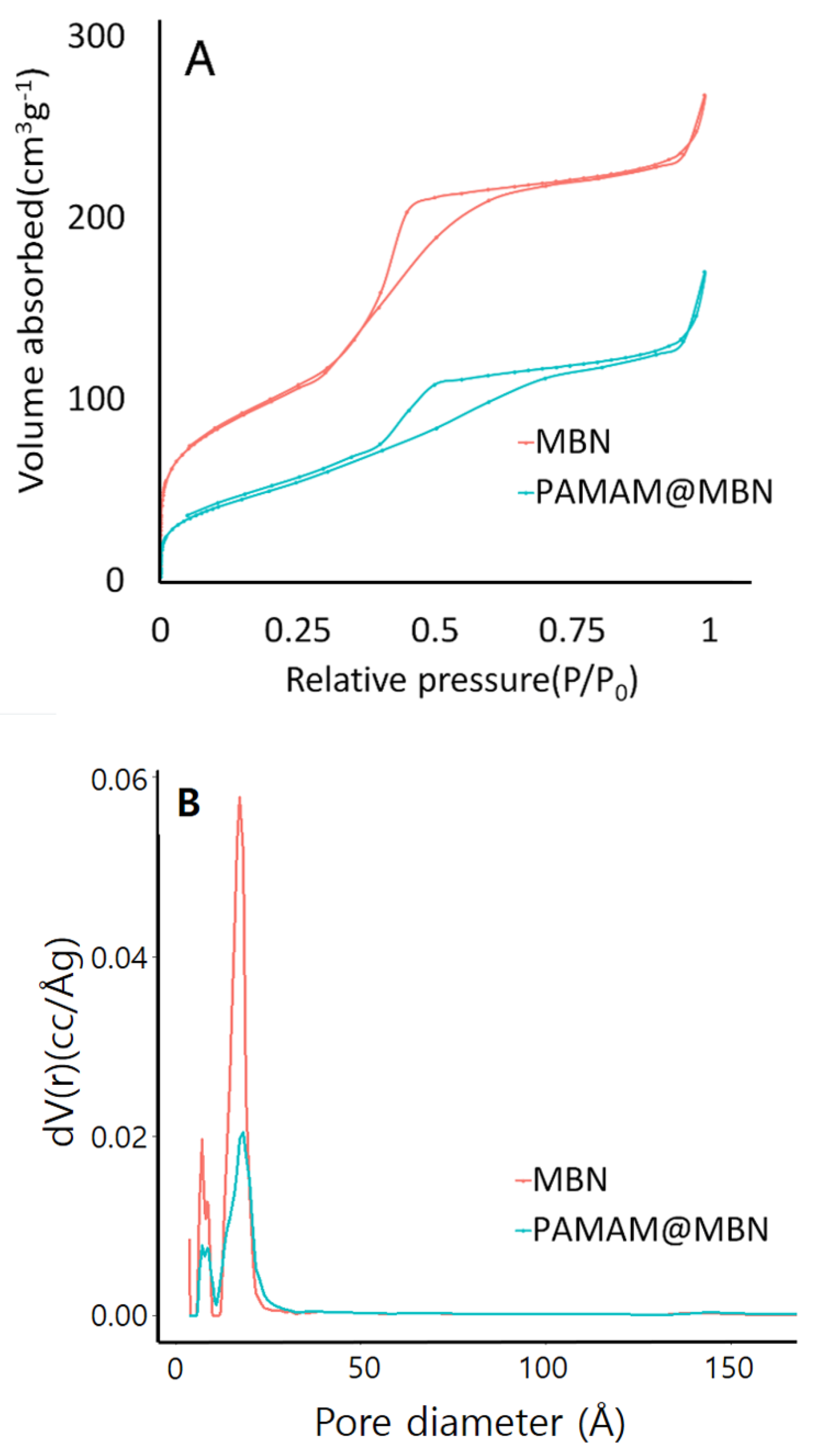
3.1.3. Surface Area and Pore Size Analysis
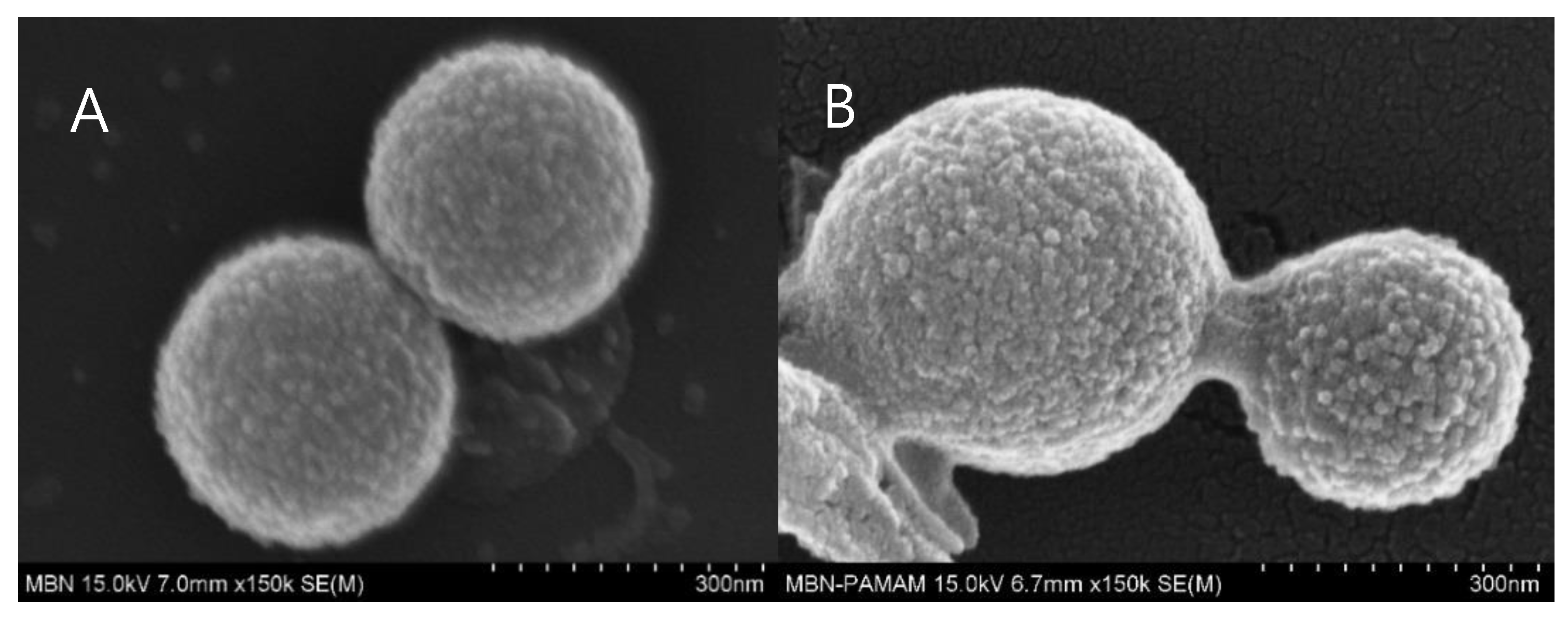
3.1.4. FESEM and FETEM
3.1.5. In Vitro Ion Dissolution Test
3.2. In Vitro Mineralization Ability Test
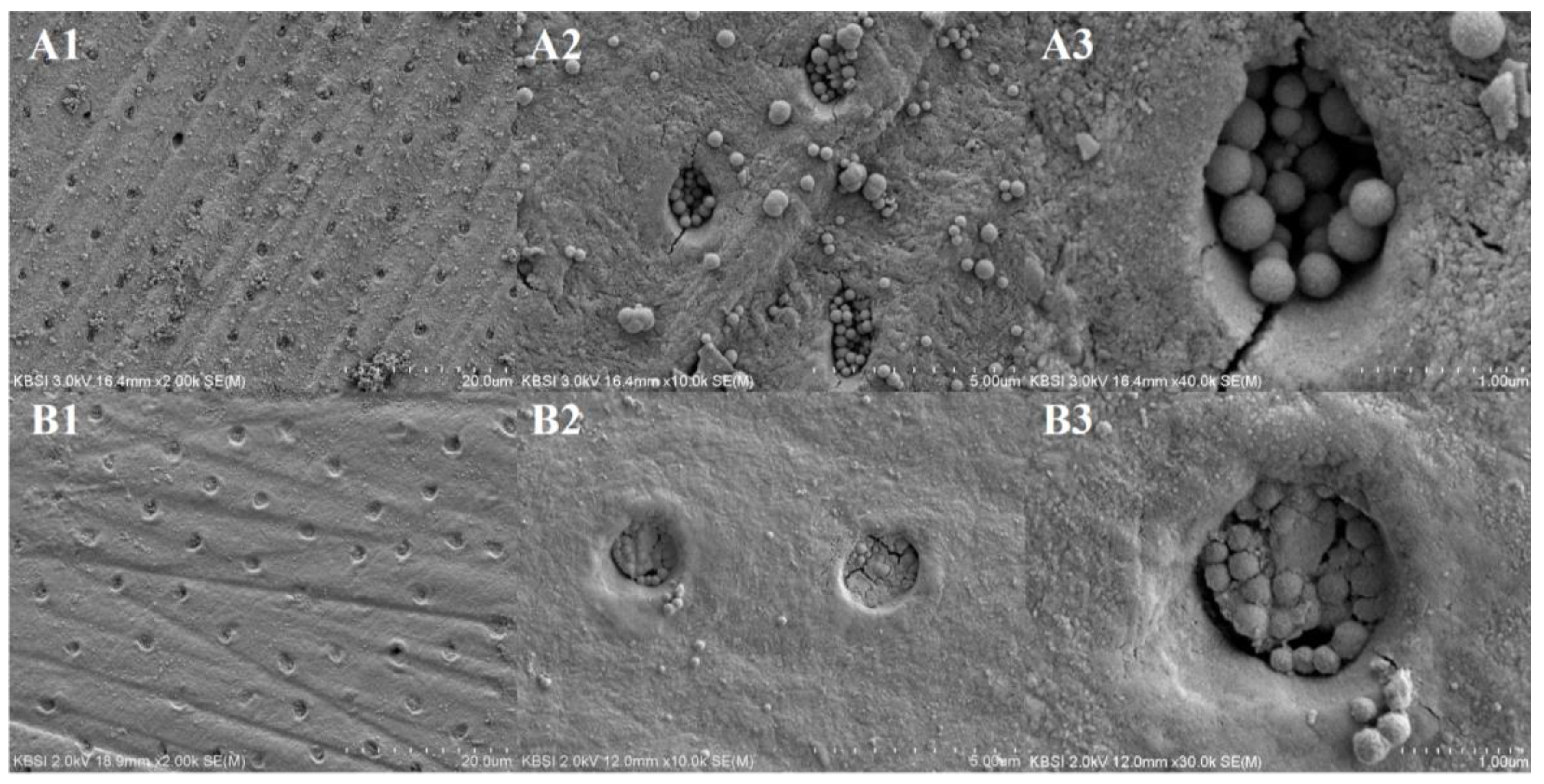
3.3. Comparison of Dentinal Tubule Occlusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brannstrom, M.; Linden, L.A.; Johnson, G. Movement of dentinal and pulpal fluid caused by clinical procedures. J. Dent. Res. 1968, 47, 679–682. [Google Scholar] [CrossRef]
- Absi, E.G.; Addy, M.; Adams, D. Dentine hypersensitivity: A study of the patency of dentinal tubules in sensitive and non sensitive cervical dentine. J. Clin. Periodontol. 1987, 14, 280–284. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Masada, J.; Uchida, A.; Ishida, H. Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J. Dent. Res. 1989, 68, 1498–1502. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Noiri, Y.; Ozaki, K.; Uchida, A.; Ishikawa, Y.; Ishida, H. Transmission electron microscopic characterization of hypersensitive human radicular dentin. J. Dent. Res. 1990, 69, 1293–1297. [Google Scholar] [CrossRef]
- Bartold, P.M. Dentinal hypersensitivity: A review. Aust. Dent. J. 2006, 51, 212–218. [Google Scholar] [CrossRef]
- Cummins, D. Dentin hypersensitivity: From diagnosis to a breakthrough therapy for everyday sensitivity relief. J. Clin. Dent. 2009, 20, 1–9. [Google Scholar]
- Kishore, A.; Mehrotra, K.K.; Saimbi, C.S. Effectiveness of desensitizing agents. J. Endod. 2002, 28, 34–35. [Google Scholar] [CrossRef]
- Jain, P.; Vargas, M.A.; Denehy, G.E.; Boyer, D.B. Dentin desensitizing agents: SEM and X-ray microanalysis assessment. Am. J. Dent. 1997, 10, 21–26. [Google Scholar]
- Frechoso, C.S.; Menendez, M.; Guisasola, C.; Arregui, I.; Tejerina, J.M.; Sicilia, A. Evaluation of the efficacy of two potassium nitrate bioadhesive gels (5% and 10%) in the treatment of dentine hypersensitivity. A randomized clinical trial. J. Clin. Periodontol. 2003, 30, 315–320. [Google Scholar] [CrossRef]
- Lutins, N.D.; Greco, G.W.; McFall, W.T., Jr. Effectiveness of sodium fluoride on tooth hypersensitivity with and without iontophoresis. J. Periodontol. 1984, 55, 285–288. [Google Scholar] [CrossRef]
- Orucoglu, H.; Belli, S. Evaluation of the effect of four self-etching adhesives on dentin permeability. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 110–115. [Google Scholar]
- Ciaramicoli, M.T.; Carvalho, R.C.; Eduardo, C.P. Treatment of cervical dentin hypersensitivity using neodymium:yttrium-aluminum-garnet laser. Clinical evaluation. Lasers Surg. Med. 2003, 33, 358–362. [Google Scholar] [CrossRef]
- Du Min, Q.; Bian, Z.; Jiang, H.; Greenspan, D.C.; Burwell, A.K.; Zhong, J.; Tai, B.J. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (novamin) for the treatment of dentin hypersensitivity. Am. J. Dent. 2008, 21, 210–214. [Google Scholar]
- Chen, W.C.; Kung, J.C.; Chen, C.H.; Hsiao, Y.C.; Shih, C.J.; Chien, C.S. Effects of bioactive glass with and without mesoporous structures on desensitization in dentinal tubule occlusion. Appl. Surf. Sci. 2013, 283, 833–842. [Google Scholar] [CrossRef]
- Schepers, E.J.G.; Ducheyne, P. Bioactive glass particles of narrow size range for the treatment of oral bone defects: A 1–24 months experiment with several materials and particle sizes and size range. J. Oral Rehabil. 1997, 24, 171–181. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Kamitakahara, M.; Miyazaki, T. Bioactive ceramic-based materials with designed reactivity for bone tissue regeneration. J. R. Soc. Interface 2009, 6, 349–360. [Google Scholar] [CrossRef]
- Forsback, A.P.; Areva, S.; Salonen, J.I. Mineralization of dentin induced by treatment with bioactive glass S53P4 in vitro. Acta Odontol. Scand. 2004, 62, 14–20. [Google Scholar] [CrossRef]
- Gillam, D.G.; Tang, J.Y.; Mordan, N.J.; Newman, H.N. The effects of a novel bioglass dentifrice on dentine sensitivity: A scanning electron microscopy investigation. J. Oral Rehabil. 2002, 29, 305–313. [Google Scholar] [CrossRef]
- Efflandt, S.E.; Magne, P.; Douglas, W.H.; Francis, L.F. Interaction between bioactive glasses and human dentin. J. Mater. Sci. Mater. Med. 2002, 13, 557–565. [Google Scholar] [CrossRef]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef]
- Bakry, A.S.; Marghalani, H.Y.; Amin, O.A.; Tagami, J. The effect of a bioglass paste on enamel exposed to erosive challenge. J. Dent. 2014, 42, 1458–1463. [Google Scholar] [CrossRef]
- Farooq, I.; Moheet, I.A.; AlShwaimi, E. In vitro dentin tubule occlusion and remineralization competence of various toothpastes. Arch. Oral Biol. 2015, 60, 1246–1253. [Google Scholar] [CrossRef]
- Liang, K.; Yuan, H.; Li, J.; Yang, J.; Zhou, X.; He, L.; Cheng, L.; Gao, Y.; Xu, X.; Zhou, X.; Li, J. Remineralization of Demineralized Dentin Induced by Amine-Terminated PAMAM Dendrimer. Macromol. Mater. Eng. 2015, 300, 107–117. [Google Scholar] [CrossRef]
- Gaertner, H.F.; Cerini, F.; Kamath, A.; Rochat, A.F.; Siegrist, C.A.; Menin, L.; Hartley, O. Efficient Orthogonal Bioconjugation of Dendrimers for Synthesis of Bioactive Nanoparticles. Bioconjugate Chem. 2011, 22, 1103–1114. [Google Scholar] [CrossRef]
- Lungu, A.; Rusen, E.; Butac, L.; Stancu, I. Epoxy-mediated immobilization of PAMAM dendrimers on methacrylic hydrogels. Dig. J. Nanomater. Biostruct. 2009, 4, 97–107. [Google Scholar]
- Lee, C.C.; MacKay, J.A.; Frechet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Boas, U.; Heegaard, P.M. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Tsetsekou, A.; Kammenou, M.I.; Boukos, N. Controlling the formation of hydroxyapatite nanorods with dendrimers. J. Am. Ceram. Soc. 2011, 94, 2023–2029. [Google Scholar] [CrossRef]
- Donners, J.J.J.M.; Nolte, R.J.M.; Sommerdijk, N.A.J.M. Dendrimer-based hydroxyapatite composites with remarkable materials properties. Adv. Mater. 2003, 15, 313–316. [Google Scholar] [CrossRef]
- Tsortos, A.; Nancollas, G.H. The Adsorption of Polyelectrolytes on Hydroxyapatite Crystals. J. Colloid Interface Sci. 1999, 209, 109–115. [Google Scholar] [CrossRef]
- Tao, S.; Fan, M.; Xu, H.H.K.; Li, J.; He, L.; Zhou, X.; Liang, K.; Li, J. The remineralization effectiveness of PAMAM dendrimer with different terminal groups on demineralized dentin in vitro. RSC Adv. 2017, 7, 54947–54955. [Google Scholar] [CrossRef]
- Reis, C.; De-Deus, G.; Leal, F.; Azevedo, E.; Coutinho-Filho, T.; Paciornik, S. Strong effect on dentin after the use of high concentrations of citric acid: An assessment with co-site optical microscopy and ESEM. Dent. Mater. 2008, 24, 1608–1615. [Google Scholar] [CrossRef]
- Wiegand, A.; Stock, A.; Attin, R.; Werner, C.; Attin, T. Impact of the acid flow rate on dentin erosion. J. Dent. 2007, 35, 21–27. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, M.S.; Mahapatra, C.; Kim, H.W. Effect of aminated mesoporous bioactive glass nanoparticles on the differentiation of dental pulp stem cells. PLoS ONE 2016, 11, e0150727. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Lee, J.H.; Lee, E.J.; Kim, H.W. Collagen hydrogels incorporated with surface-aminated mesoporous nanobioactive glass: Improvement of physicochemical stability and mechanical properties is effective for hard tissue engineering. Acta Biomater. 2013, 9, 9508–9521. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Yu, J.; Yang, H.; Li, K.; Lei, J.; Zhou, L.; Huang, C. A novel application of nanohydroxyapatite/mesoporous silica biocomposite on treating dentin hypersensitivity: An in vitro study. J. Dent. 2016, 50, 21–29. [Google Scholar] [CrossRef]
- Radev, L.; Vladov, D.; Michailova, I.; Cholakova, E.; Fernandes, M.F.; Salvado, I.M. In vitro bioactivity of polycaprolactone/bioglass composites. Int. J. Mater. Chem. 2013, 3, 91–98. [Google Scholar]
- Jung, J.H.; Park, S.B.; Yoo, K.H.; Yoon, S.Y.; Bae, M.K.; Lee, D.J.; Ko, C.C.; Kwon, Y.H.; Kim, Y.I. Effect of different sizes of bioactive glass-coated mesoporous silica nanoparticles on dentinal tubule occlusion and mineralization. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef]
- Maleki, A.; Hayati, B.; Najafi, F.; Gharibi, F.; Joo, S.W. Heavy metal adsorption from industrial wastewater by PAMAM/TiO2 nanohybrid: Preparation, characterization and adsorption studies. J. Mol. Liq. 2016, 224, 95–104. [Google Scholar] [CrossRef]
- Charles, S.; Vasanthan, N.; Kwon, D.; Sekosan, G.; Ghosh, S. Surface modification of poly (amidoamine)(PAMAM) dendrimer as antimicrobial agents. Tetrahedron Lett. 2012, 53, 6670–6675. [Google Scholar] [CrossRef]
- Alothman, Z.A. A review: Fundamental aspects of silica mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Notingher, I.; Jones, J.R.; Verrier, S.; Bisson, I.; Embanga, P.; Edwards, P.; Polak, J.M.; Hench, L.L. Application of FTIR and Raman spectroscopy to characterisation of bioactive materials and living cells. J. Spectroscopy 2003, 17, 275–288. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, Y.; Jia, D. Fabrication of hydroxycarbonate apatite coatings with hierarchically porous structures. Acta Biomater. 2008, 4, 334–342. [Google Scholar] [CrossRef]
- Yu, Y.; Bacsik, Z.; Edén, M. Contrasting In Vitro Apatite Growth from Bioactive Glass Surfaces with that of Spontaneous Precipitation. Materials 2018, 11, 1690. [Google Scholar] [CrossRef]
- Dahlberg, G. Statistical Methods for Medical and Biological Students; George Allen and Unwin: London, UK, 1940; pp. 122–132. [Google Scholar]
- De Oliveira, A.A.R.; de Souza, D.A.; Dias, L.L.S.; de Carvalho, S.M.; Mansur, H.S.; de Magalhaes Pereira, M. Synthesis, characterization and cytocompatibility of spherical bioactive glass nanoparticles for potential hard tissue engineering applications. Biomed. Mater. 2013, 8, 025011–025025. [Google Scholar] [CrossRef]
- Brunner, T.J.; Grass, R.N.; Stark, W.J. Glass and bioglass nanopowders by flame synthesis. Chem. Commun. 2006, 7, 1384–1386. [Google Scholar] [CrossRef]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass composities. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef]
- Sepulveda, J.; Jones, R.; Hench, L.L. In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. J. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef]
- Fernando, D.; Attik, N.; Pladelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Bioactive glass for dentin remineralization: A systematic review. Mater. Sci. Eng. C 2017, 76, 1369–1377. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, T.H.; Kim, M.; Eltohamy, M.; Won, J.E.; Lee, E.J.; Kim, H.W. Capacity of mesoporous bioactive glass nanoparticles to deliver therapeutic molecules. Nanoscale 2012, 4, 7475–7488. [Google Scholar] [CrossRef]
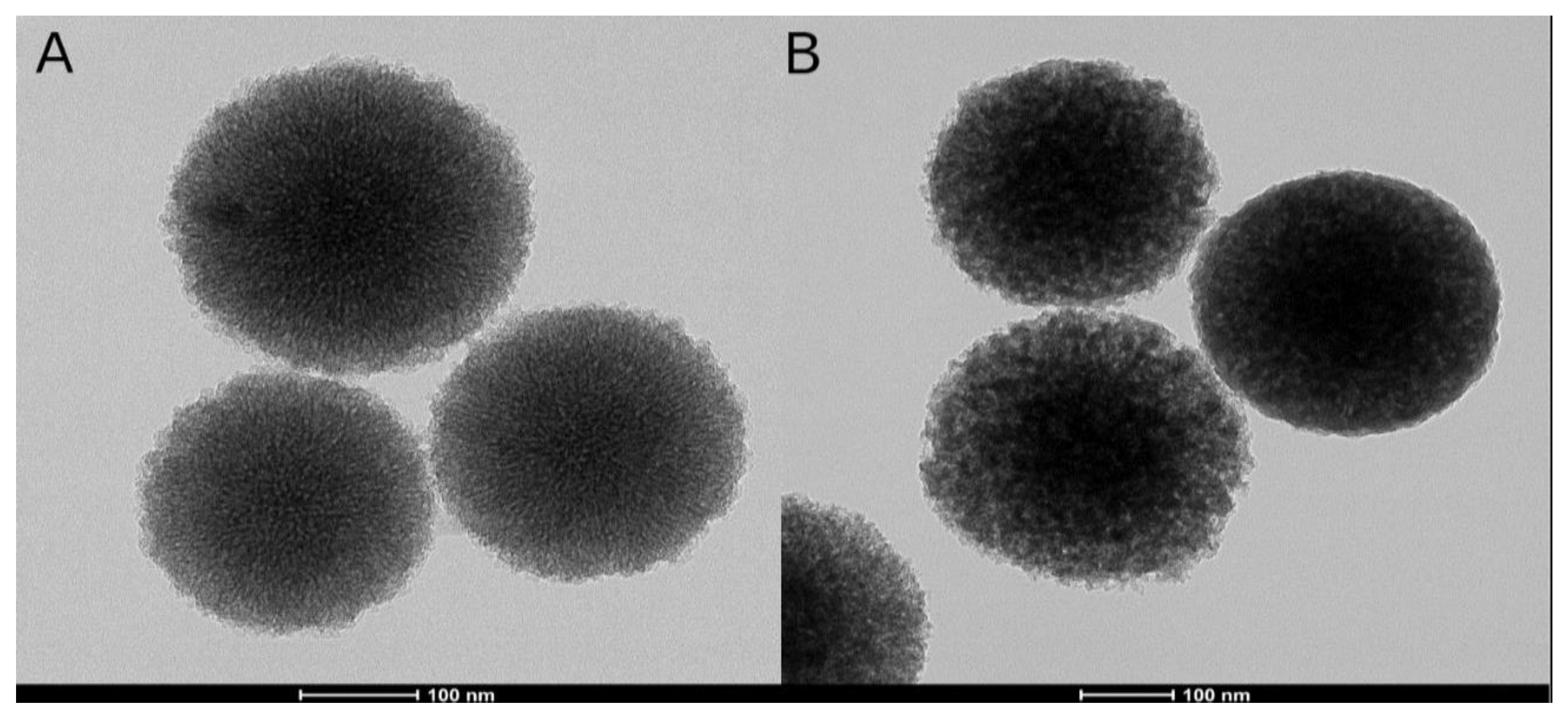
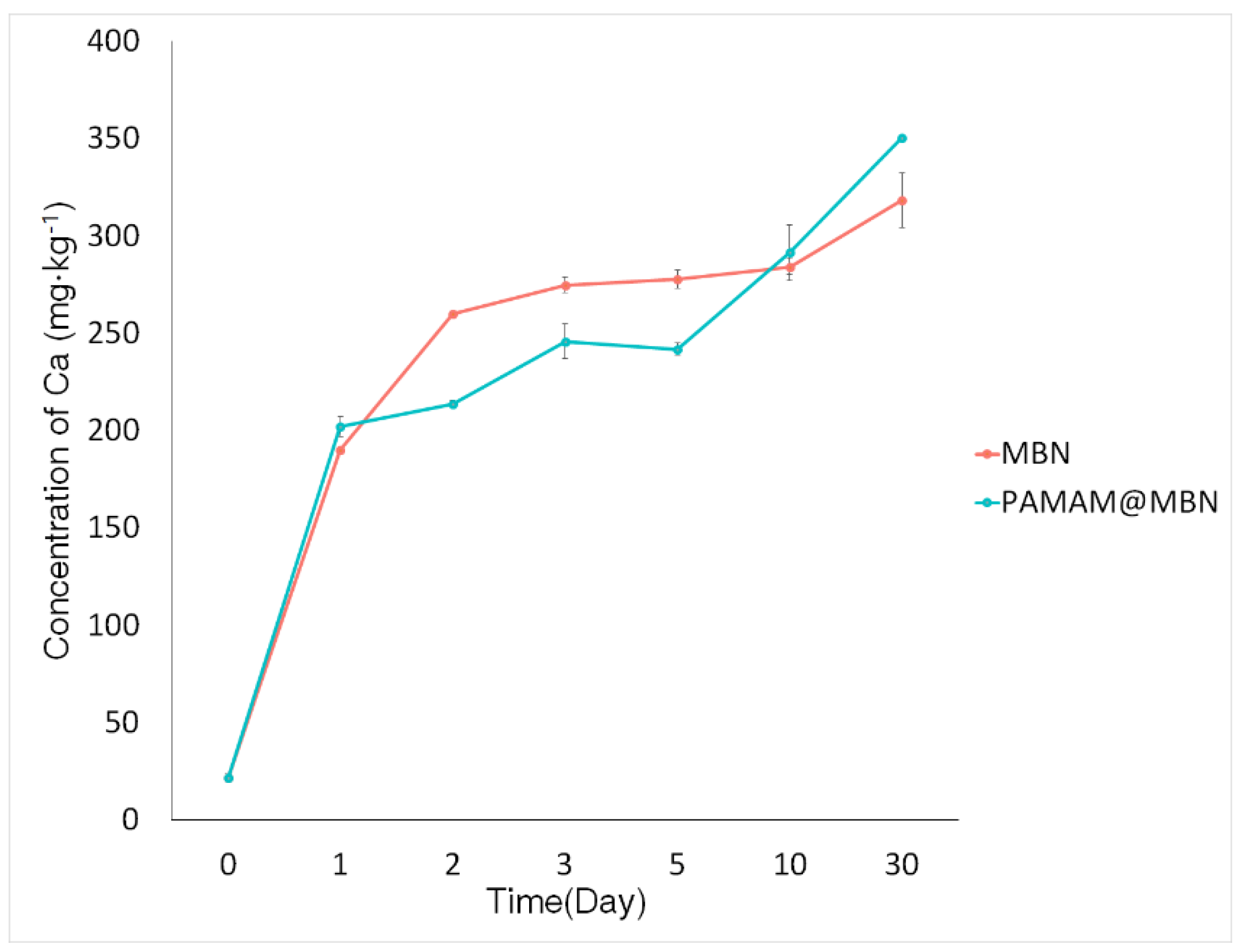
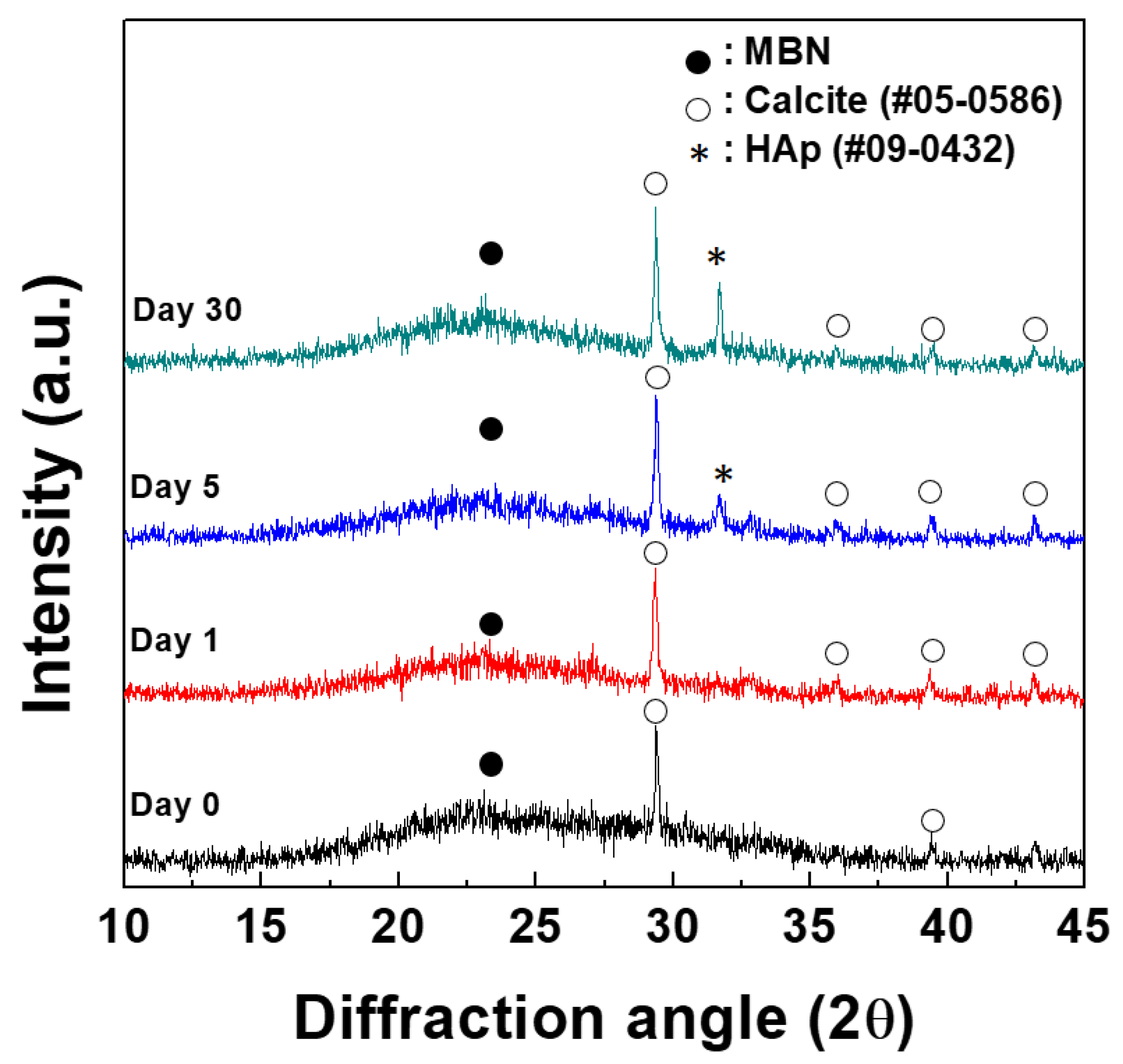
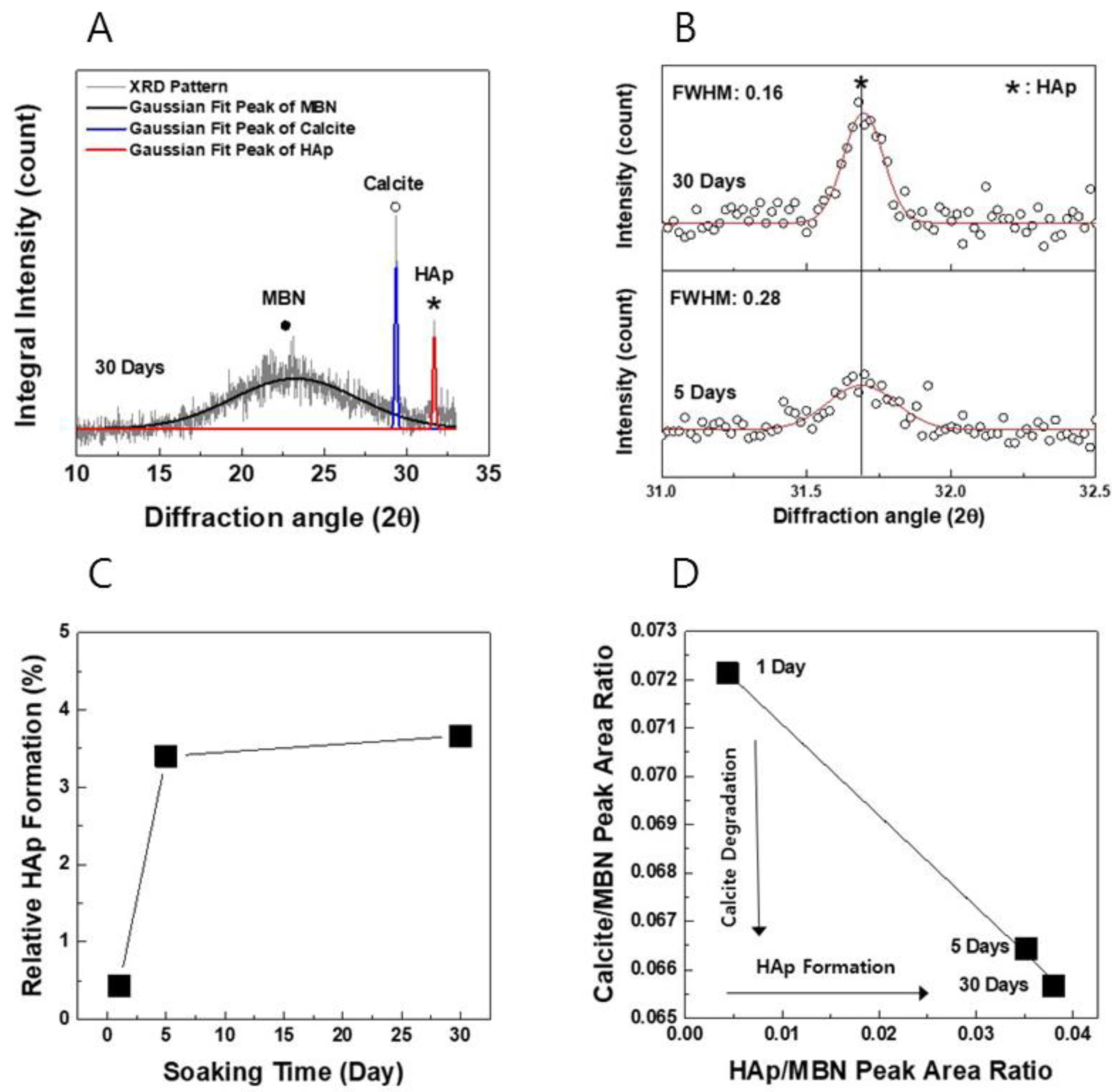

| Samples | SBET (m2/g) | Vp (cm3/g) | Dp (nm) |
|---|---|---|---|
| MBN | 358.45 ± 10.75 | 0.41 ± 0.02 | 23.12 ± 1.39 |
| PAMAM@MBN | 188.30 ± 5.66 | 0.26 ± 0.02 | 28.01 ± 1.68 |
| Groups | Occluded Area (%) | p-Value |
|---|---|---|
| MBN | 80.35 ± 8.89 | 0.003 |
| PAMAM@MBN | 87.86 ± 5.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.; Son, W.-S.; Yoo, K.-H.; Yoon, S.-Y.; Bae, M.-K.; Lee, D.J.; Ko, C.-C.; Choi, Y.-K.; Kim, Y.-I. Effects of Poly(Amidoamine) Dendrimer-Coated Mesoporous Bioactive Glass Nanoparticles on Dentin Remineralization. Nanomaterials 2019, 9, 591. https://doi.org/10.3390/nano9040591
Bae J, Son W-S, Yoo K-H, Yoon S-Y, Bae M-K, Lee DJ, Ko C-C, Choi Y-K, Kim Y-I. Effects of Poly(Amidoamine) Dendrimer-Coated Mesoporous Bioactive Glass Nanoparticles on Dentin Remineralization. Nanomaterials. 2019; 9(4):591. https://doi.org/10.3390/nano9040591
Chicago/Turabian StyleBae, Jungin, Woo-Sung Son, Kyung-Hyeon Yoo, Seog-Young Yoon, Moon-Kyoung Bae, Dong Joon Lee, Ching-Chang Ko, Youn-Kyung Choi, and Yong-Il Kim. 2019. "Effects of Poly(Amidoamine) Dendrimer-Coated Mesoporous Bioactive Glass Nanoparticles on Dentin Remineralization" Nanomaterials 9, no. 4: 591. https://doi.org/10.3390/nano9040591
APA StyleBae, J., Son, W.-S., Yoo, K.-H., Yoon, S.-Y., Bae, M.-K., Lee, D. J., Ko, C.-C., Choi, Y.-K., & Kim, Y.-I. (2019). Effects of Poly(Amidoamine) Dendrimer-Coated Mesoporous Bioactive Glass Nanoparticles on Dentin Remineralization. Nanomaterials, 9(4), 591. https://doi.org/10.3390/nano9040591





