Contrast Agents Delivery: An Up-to-Date Review of Nanodiagnostics in Neuroimaging
Abstract
:1. Introduction
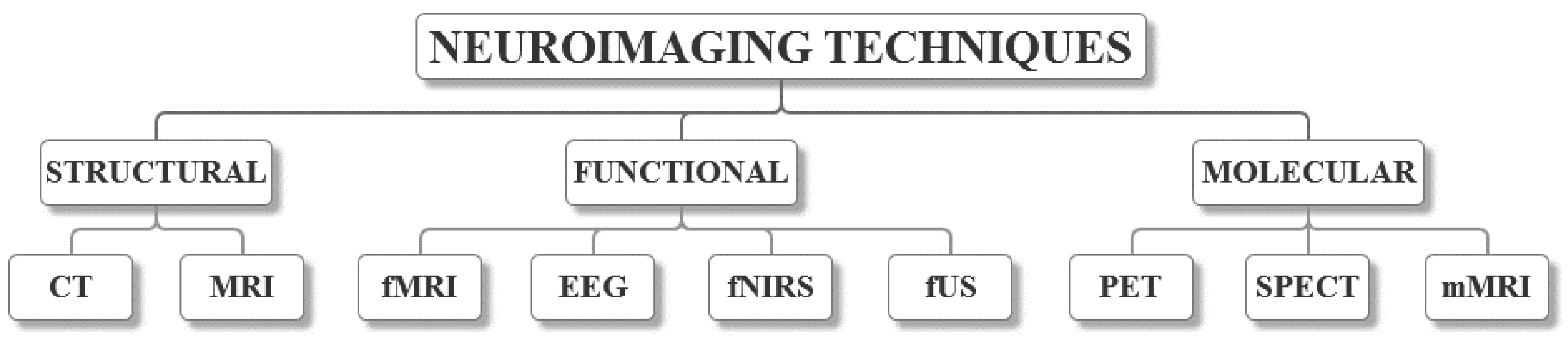
2. Neuroimaging Techniques
2.1. Structural Neuroimaging
2.1.1. Computed Tomography (CT)
2.1.2. Magnetic Resonance Imaging (MRI)
2.2. Functional Neuroimaging
2.2.1. Functional Magnetic Resonance Imaging (fMRI)
2.2.2. Electroencephalography (EEG)
2.2.3. Functional Near-Infrared Spectroscopic Imaging (fNIRS)
2.2.4. Ultrasound-Based Functional Imaging Techniques
2.3. Molecular Neuroimaging
2.3.1. Positron Emission Tomography (PET)
2.3.2. Single Photon Emission Computed Tomography (SPECT)
2.3.3. Molecular Magnetic Resonance Imaging (mMRI)
3. Nanotechnology-Based Approaches for Neuroimaging
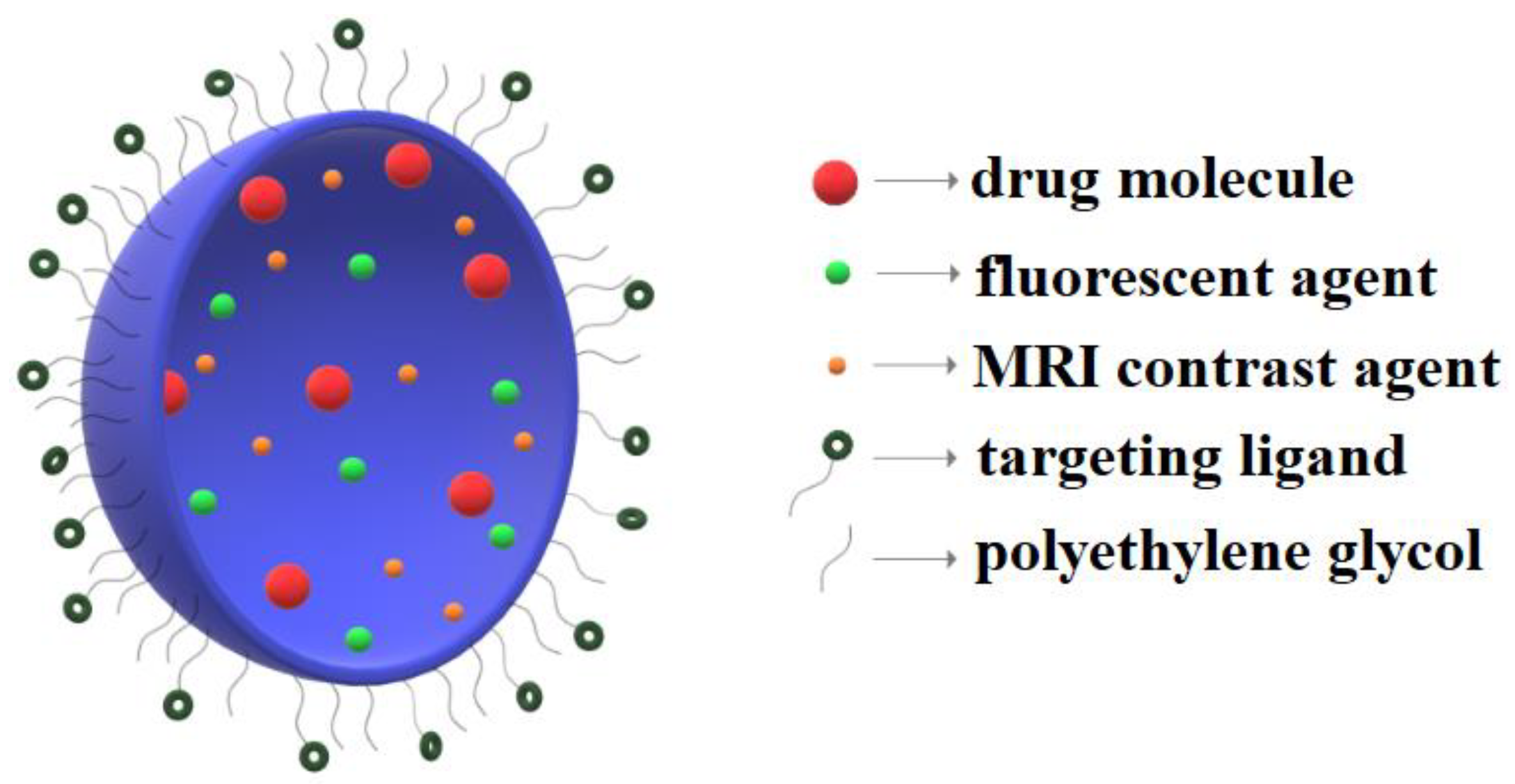
3.1. Nanoparticles
3.2. Liposomes
3.3. Micelles
3.4. Nanobodies
3.5. Quantum Dots
| Nanotechnology-Based Strategy | Imaging Probe | Targeting Strategy | Neuroimaging Technique | Targeted Brain Disease | Experimental Stage | Ref. |
|---|---|---|---|---|---|---|
| Iron-oxide nanoparticles | iron oxide nanoparticles functionalized with caffeic acid | passive—enhanced permeability and retention effect | MRI | glioblastoma | in vivo—orthotopic U87-MG tumour implanted in nude mouse brain | [103] |
| iron oxide nanoparticles functionalized with phosphonate polyethylene glycol chains and covalently coupled to cyclic RGD | active—cyclic RGD peptides | MRI | glioblastoma | in vivo—orthotopic U87-MG tumour implanted in nude mouse brain | [105] | |
| superparamagnetic iron oxide nanoparticles | passive | microwave imaging | emergent stroke | in vivo—New Zealand white rabbits; in vivo—middle aged human male volunteer | [106] | |
| sulphated dextran-coated iron oxide nanoparticles conjugated with the macrocyclic chelator 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid | active—sulphated dextran-coating | PET and MRI | neuroinflammation | in vivo—BALB/c mice | [107] | |
| iron oxide nanoparticles | active—MMP-14 peptide | MRI | glioblastoma | in vivo—NOD scid gamma mice | — | |
| Gold nanoparticles | polyethylene glycol coated gold nanoparticles conjugated with CBP4 peptide | active—CD133 glioma biomarker | laser scanning confocal microscope | glioblastoma | in vivo—U373 glioma cell culture | [111] |
| Manganese oxide nanoparticles | hollow manganese oxide nanoparticles | passive | MRI | hypoxic-ischemic brain injury | in vivo—Sprague Dawley rat pups | [117] |
| N-(trimethoxysilylpropyl) ethylene diamine triacetic acid silane and folic acid-conjugated manganese oxide nanoparticles | active—folic acid, a glioma-specific moiety | MRI | glioblastoma | in vivo—male nude mice (BALB/C) | [116] | |
| Carbon-based nanoparticles | multi-walled carbon nanotubes conjugated with Pittsburgh Compound B and gadolinium complexes | active—Pittsburgh Compound B for binding to Aβ plaques | SPECT and CT | Alzheimer’s disease | in vivo—female C57BL/6 mice | [119] |
| Polysiloxane-based nanoparticles | AGuIX | active—KDKPPR ligand peptide motif | MRI | glioblastoma | in vivo—dorsal skinfold chamber using female nude mice | [120] |
| Liposomes | heptamethine cyanine dye IR780 incorporated into liposomes | active—IR780 dye for tumour targeting | near-infrared fluorescence imaging | glioblastoma | in vivo—T98G and U87MG cells; in vivo—nude mice bearing U87M2/luc tumours | [125] |
| iron oxide nanoparticles and near-infrared fluorescence dye DiR incorporated into polyethylene glycol liposomes functionalized with the F(ab’)2 fragments of PGN635 | active—phosphatidylserine targeting | MRI and near-infrared optical imaging | glioblastoma | in vivo—human glioma U87MG cells; in vivo—BALB/c mice | [126] | |
| paramagnetic chelate gadolinium-diethylenetriaminepentaacetic acid-loaded liposomes coated with polyethylene glycol | passive | MRI | glioblastoma | in vivo—tumour bearing C57BL6 adult male mice | [130] | |
| gadolinium-loaded liposomes | active—GBI-10 aptamer | MRI | glioblastoma | in vitro—MDA-MB-435s human breast duct cell line | [127] | |
| quantum dots and doxorubicin-loaded liposomes | active—focused ultrasound | MRI | glioblastoma | in vivo—Adult male Sprague–Dawley rats | [131] | |
| quantum dots and docetaxel-loaded liposomes | active—RGD-TPGS peptide | — | glioblastoma | in vivo—Charles Foster rats | [132] | |
| Micelles | polyethylene glycol-b-poly(l-lysine-DOTA-gadolinium) micelles | passive—enhanced permeability and retention effect | MRI | ischemia-reperfusion injury | in vivo—Wistar male rats | [137] |
| paramagnetic gadolinium-loaded targeting micelles | active—targeting the vascular cell adhesion molecule | MRI | neuroinflammation | in vivo—C57BL/6J female mice | [138] | |
| gold and superparamagnetic iron oxide-loaded micelles coated with polyethylene glycol and polycaprolactone | Passive | MRI and CT | glioblastoma | in vivo—female athymic nude mice | [139] | |
| Nanobodies | anti-Aβ and anti-pTau VHHs | active—amyloid plaques and neurofibrillary tangles | 2PFI | Alzheimer’s disease | in vivo—PS2APP mice overexpressing hAPP Swedish mutation combined with PS2 N141I mutation and Tg4510 mice with the hMAPT P301L gene mutation | [142] |
| Quantum dots | semiconducting polymer dots encapsulated into poly(styrene-co-maleic anhydride) and conjugated with poly(ethylene glycol) | active—donor-acceptor structure | 2PFI | — | in vivo—ICR female mice | [149] |
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gürsoy-Özdemir, Y.; Bozdağ-Pehlivan, S.; Sekerdag, E. (Eds.) Preface. In Nanotechnology Methods for Neurological Diseases and Brain Tumors; Academic Press: Cambridge, MA, USA, 2017; pp. xv–xvi. [Google Scholar]
- Mollaamin, F. The effect of biointerface of chemicals and inhibitors in the cerebral cortex of brain on language cognition. Biointerface Res. Appl. Chem. 2018, 8, 3628–3634. [Google Scholar]
- Kovacs, G.G. Chapter 21—Concepts and classification of neurodegenerative diseases. In Handbook of Clinical Neurology; Kovacs, G.G., Alafuzoff, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 145, pp. 301–307. [Google Scholar]
- McFaline-Figueroa, J.R.; Lee, E.Q. Brain Tumors. Am. J. Med. 2018, 131, 874–882. [Google Scholar] [CrossRef]
- Das, J.; G K, R. Post stroke depression: The sequelae of cerebral stroke. Neurosci. Biobehav. Rev. 2018, 90, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Khoury, S.; Benavides, R. Pain with traumatic brain injury and psychological disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 87, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; McLeish, A.C.; Shear, P.K.; Privitera, M. Panic and epilepsy in adults: A systematic review. Epilepsy Behav. 2018, 85, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Giovane, R.A.; Lavender, P.D. Central Nervous System Infections. Prim. Care Clin. Off. Pract. 2018, 45, 505–518. [Google Scholar] [CrossRef] [PubMed]
- George, E.; Guenette, J.P.; Lee, T.C. Introduction to Neuroimaging. Am. J. Med. 2018, 131, 346–356. [Google Scholar] [CrossRef]
- Dash, H.H.; Chavali, S. Management of traumatic brain injury patients. Korean J. Anesthesiol. 2018, 71, 12–21. [Google Scholar] [CrossRef]
- Tulay, E.E.; Metin, B.; Tarhan, N.; Arikan, M.K. Multimodal Neuroimaging: Basic Concepts and Classification of Neuropsychiatric Diseases. Clin. EEG Neurosci. 2019, 50, 20–33. [Google Scholar] [CrossRef]
- Furukawa, K.; Ishiki, A.; Tomita, N.; Onaka, Y.; Saito, H.; Nakamichi, T.; Hara, K.; Kusano, Y.; Ebara, M.; Arata, Y.; et al. Introduction and overview of the special issue “Brain imaging and aging”: The new era of neuroimaging in aging research. Ageing Res. Rev. 2016, 30, 1–3. [Google Scholar] [CrossRef]
- Mufford, M.S.; Stein, D.J.; Dalvie, S.; Groenewold, N.A.; Thompson, P.M.; Jahanshad, N. Neuroimaging genomics in psychiatry—A translational approach. Genome Med. 2017, 9, 102. [Google Scholar] [CrossRef]
- Bulut, E.; Akgoz, A.; Oguz, K.K. Chapter 11—Neuroimaging: Techniques and General Applications. In Nanotechnology Methods for Neurological Diseases and Brain Tumors; Gürsoy-Özdemir, Y., Bozdağ-Pehlivan, S., Sekerdag, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 209–224. [Google Scholar]
- Krishnan, K.R.R. Chapter 6—Structural imaging in psychiatric disorders. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 106, pp. 89–95. [Google Scholar]
- Hanganu, A.; Monchi, O. Structural Neuroimaging Markers of Cognitive Decline in Parkinson’s Disease. Parkinson’s Dis. 2016, 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Boly, M.; Gosseries, O.; Massimini, M.; Rosanova, M. Chapter 2—Functional Neuroimaging Techniques. In The Neurology of Conciousness (Second Edition); Laureys, S., Gosseries, O., Tononi, G., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 31–47. [Google Scholar]
- Im, J.J.; Namgung, E.; Choi, Y.; Kim, J.Y.; Rhie, S.J.; Yoon, S. Molecular Neuroimaging in Posttraumatic Stress Disorder. Exp. Neurobiol. 2016, 25, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.W.; Song, H.; Frank, S.J.; Bathala, T.; Venkatesan, A.M.; Anscher, M.; Tang, C.; Bruno, TL.; Wei, W.; Ma, J. Parallel imaging compressed sensing for accelerated imaging and improved signal-to-noise ratio in MRI-based postimplant dosimetry of prostate brachytherapy. Brachytherapy 2018, 17, 816–824. [Google Scholar] [CrossRef]
- Malik, S.; Sachan, M.; Nara, S. Nano-Strategies for Neuro-Imaging and Diagnostics. In Advances in Neurotherapeutic Delivery Technologies; Kumar, P., Pillay, V., Choonara, Y.E., Eds.; OMICS International: Hyderabad, India, 2015. [Google Scholar]
- Sabry, N.M.; Tolba, S.T.M.; Abdel-Gawad, F.K.; Bassem, S.M.; Nassar, H.; El-Taweel, G.E.; Ibrahim, M.A. On the molecular modeling analyses of the interaction between nano zinc oxide and bacteria. Biointerface Res. Appl. Chem. 2018, 8, 3294–3297. [Google Scholar]
- Faisal, N.; Kumar, K. Polymer and metal nanocomposites in biomedical applications. Biointerface Res. Appl. Chem. 2017, 7, 2286–2294. [Google Scholar]
- Kaur, M.; Singh, G.; Khanna, K.; Kaur, N. Nanotechnology: A Review. In Proceedings of the Second National Conference on Advances in Manufacturing Systems, S B S State Technical Campus, Ferozepur, India, 23–24 December 2015. [Google Scholar]
- Sabry, N.M.; Tolba, S.; Abdel-Gawad, F.K.; Bassem, S.M.; Nassar, H.F.; El-Taweel, G.E.; Medhat, A.I. Interaction between nano silver and bacteria: Modeling approach. Biointerface Res. Appl. Chem. 2018, 8, 3570–3574. [Google Scholar]
- Sridhar, S.; Mishra, S.; Gulyas, M.; Padmanabhan, P.; Gulyas, B. An Overview of Multimodal Neuroimaging Using Nanoprobes. Int. J. Mol. Sci. 2017, 18, 311. [Google Scholar] [CrossRef] [PubMed]
- Lafortuna, C.; Tabozzi, S.; Rizzo, G. Functional brain imaging and its application to uncover mechanisms driving food intake in humans. J. Biomed. Graph. Comput. 2014, 4, 10–27. [Google Scholar] [CrossRef]
- Zippo, A.; Castiglioni, I. Integration of 18FDG-PET Metabolic and Functional Connectomes in the Early Diagnosis and Prognosis of the Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 487–497. [Google Scholar] [CrossRef]
- Beckmann, N. In Vivo magnetic resonance techniques and drug discovery. Braz. J. Phys. 2006, 36, 16–22. [Google Scholar] [CrossRef]
- Morton, D.L.; Sandhu, J.S.; Jones, A.K. Brain imaging of pain: State of the art. J. Pain Res. 2016, 9, 613–624. [Google Scholar] [CrossRef]
- Forkel, S.J.; Catani, M. Structural Neuroimaging. In Research Methods in Psycholinguistics and the Neurobiology of Language: A Practical Guide, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 288–308. [Google Scholar]
- Skipper, N.T.; Igra, M.S.; Davidson, A.J. Brain imaging for anaesthetists and intensivists: Part 1—Computed tomography. BJA Educ. 2018, 18, 300–309. [Google Scholar] [CrossRef]
- Pease, A.P.; Nelson, N.C. Chapter 71—Computed Tomography. In Equine Surgery (fifth Edition); Auer, J.A., Stick, J.A., Kümmerle, J.M., Prange, T., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2019; pp. 1193–1201. [Google Scholar]
- Edelstein, W.A.; Mahesh, M.; Carrino, J.A. MRI: Time is dose—And money and versatility. J. Am. Coll. Radiol. JACR 2010, 7, 650–652. [Google Scholar] [CrossRef]
- Copen, W.A.; Lev, M.H.; Rapalino, O. Chapter 6—Brain perfusion: Computed tomography and magnetic resonance techniques. In Handbook of Clinical Neurology; Masdeu, J.C., González, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 135, pp. 117–135. [Google Scholar]
- Heit, J.J.; Wintermark, M. Perfusion Computed Tomography for the Evaluation of Acute Ischemic Stroke. Stroke 2016, 47, 1153–1158. [Google Scholar] [CrossRef]
- Chou, E.T.; Carrino, J.A. Chapter 10—Magnetic Resonance Imaging. In Pain Management; Waldman, S.D., Bloch, J.I., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2007; pp. 106–117. [Google Scholar]
- González-Villà, S.; Oliver, A.; Valverde, S.; Wang, L.; Zwiggelaar, R.; Lladó, X. A review on brain structures segmentation in magnetic resonance imaging. Artif. Intell. Med. 2016, 73, 45–69. [Google Scholar] [CrossRef]
- Rabai, F.; Ramani, R. Chapter 31—Magnetic Resonance Imaging: Anesthetic Implications. In Essentials of Neuroanesthesia; Prabhakar, H., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 519–532. [Google Scholar]
- Wilson, H.; Dervenoulas, G.; Politis, M. Chapter Nine—Structural Magnetic Resonance Imaging in Huntington’s Disease. In International Review of Neurobiology; Politis, M., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 142, pp. 335–380. [Google Scholar]
- Taghizadeh, S.; Labuda, C.; Yang, C.C.; Morris, B.; Kanakamedala, M.R.; Vijayakumar, S.; Rey-Dios, R.; Duggar, W.N.; Florez, E.; Fatemi, A. Optimizing MRI sequences and images for MRI-based stereotactic radiosurgery treatment planning. Rep. Pract. Oncol. Radiother. 2019, 24, 12–19. [Google Scholar] [CrossRef]
- Moser, E.; Laistler, E.; Schmitt, F.; Kontaxis, G. Ultra-High Field NMR and MRI—The Role of Magnet Technology to Increase Sensitivity and Specificity. Front. Phys. 2017, 5, 33. [Google Scholar] [CrossRef]
- Kasliwal, M.K. Functional Neuroimaging: Current Status. OMICS J. Radiol. 2012, 1, e111. [Google Scholar] [CrossRef]
- Medaglia, J.D. Functional Neuroimaging in Traumatic Brain Injury: From Nodes to Networks. Front. Neurol. 2017, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Kropotov, J.D. (Ed.) Chapter 1.3—Functional Magnetic Resonance Imaging. In Functional Neuromarkers for Psychiatry; Academic Press: San Diego, CA, USA, 2016; pp. 17–25. [Google Scholar]
- Buchbinder, B.R. Chapter 4—Functional magnetic resonance imaging. In Handbook of Clinical Neurology; Masdeu, J.C., González, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 135, pp. 61–92. [Google Scholar]
- Fisicaro, R.; Brennan, N.P.; Holodny, A. Chapter 29—Functional Magnetic Resonance Imaging. In Handbook of Neuro-Oncology Neuroimaging (Second Edition); Newton, H.B., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 317–325. [Google Scholar]
- Chen, J.E.; Glover, G.H. Functional Magnetic Resonance Imaging Methods. Neuropsychol. Rev. 2015, 25, 289–313. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A multi-modal parcellation of human cerebral cortex. Nature 2016, 536, 171. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Coalson, T.S.; Bijsterbosch, J.D.; Harrison, S.J.; Harms, M.P.; Anticevic, A.; Van Essen, D.C.; Smith, S.M. Using temporal ICA to selectively remove global noise while preserving global signal in functional MRI data. NeuroImage 2018, 181, 692–717. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Preface. In Radiology Diagnosis. Functional Magnetic Resonance Imaging—Advanced Neuroimaging Applications; Sharma, R., Ed.; Intech Open: London, UK, 2012. [Google Scholar]
- Fröhlich, F. Chapter 13—Imaging Functional Networks with MRI. In Network Neuroscience; Fröhlich, F., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 177–185. [Google Scholar]
- Fukuda, M.; Poplawsky, A.J.; Kim, S.G. Chapter 6—Submillimeter-resolution fMRI: Toward understanding local neural processing. In Progress in Brain Research; Masamoto, K., Hirase, H., Yamada, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 225, pp. 123–152. [Google Scholar]
- Huettel, S.A. Functional MRI (fMRI). In Encyclopedia of Spectroscopy and Spectrometry (Third Edition); Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Oxford, UK, 2017; pp. 778–784. [Google Scholar]
- Abhang, P.A.; Gawali, B.W.; Mehrotra, S.C. Chapter 1—Introduction to Emotion, Electroencephalography and Speech Processing. In Introduction to EEG- and Speech-Based Emotion Recognition; Abhang, P.A., Gawali, B.W., Mehrotra, S.C., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–17. [Google Scholar]
- Kulkarni, N.; Bairagi, V. (Eds.) Chapter Two—Electroencephalogram and Its Use in Clinical Neuroscience. In EEG-Based Diagnosis of Alzheimer Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 25–35. [Google Scholar]
- Proekt, A. Chapter Fifteen—Brief Introduction to Electroencephalography. In Methods in Enzymology; Eckenhoff, R.G., Dmochowski, I.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 603, pp. 257–277. [Google Scholar]
- Wang, Y.; Yan, J.; Wen, J.; Yu, T.; Li, X. An Intracranial Electroencephalography [iEEG] Brain Function Mapping Tool with an Application to Epilepsy Surgery Evaluation. Front. Neuroinform. 2016, 10, 15. [Google Scholar] [CrossRef]
- Nagahama, Y.; Schmitt, A.J.; Nakagawa, D.; Vesole, A.S.; Kamm, J.; Kovach, C.K.; Hasan, D.; Granner, M.; Dlouhy, B.J.; Howard, M.A.; et al. Intracranial EEG for seizure focus localization: Evolving techniques, outcomes, complications and utility of combining surface and depth electrodes. J. Neurosurg. 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Parvizi, J.; Kastner, S. Promises and limitations of human intracranial electroencephalography. Nat. Neurosci. 2018, 21, 474–483. [Google Scholar] [CrossRef]
- Binder, J.R. Chapter 37—Phoneme Perception. In Neurobiology of Language; Hickok, G., Small, S.L., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 447–461. [Google Scholar]
- Adewole, D.O.; Serruya, M.D.; Harris, J.P.; Burrell, J.C.; Petrov, D.; Chen, H.I.; Wolf, J.A.; Cullen, D.K. The Evolution of Neuroprosthetic Interfaces. Crit. Rev. Biomed. Eng. 2016, 44, 123–152. [Google Scholar] [CrossRef]
- Deng, M.; Yang, X.; Silke, M.; Qiu, W.; Xu, M.; Borghs, G.; Wolf, J.A.; Cullen, D.K. Electrochemical deposition of polypyrrole/graphene oxide composite on microelectrodes towards tuning the electrochemical properties of neural probes. Sens. Actuators B Chem. 2011, 158, 176–184. [Google Scholar] [CrossRef]
- Chung, T.; Wang, J.Q.; Wang, J.-Y.; Cao, B.; Li, Y.; Pang, S.W. Electrode modifications to lower electrode impedance and improve neural signal recording sensitivity. J. Neural Eng. 2015, 12, 056018. [Google Scholar] [CrossRef]
- Yücel, M.A.; Selb, J.J.; Huppert, T.J.; Franceschini, M.A.; Boas, D.A. Functional Near Infrared Spectroscopy: Enabling routine functional brain imaging. Curr. Opin. Biomed. Eng. 2017, 4, 78–86. [Google Scholar] [CrossRef]
- Peng, K.; Pouliot, P.; Lesage, F.; Nguyen, D.K. Multichannel continuous electroencephalography-functional near-infrared spectroscopy recording of focal seizures and interictal epileptiform discharges in human epilepsy: A review. Neurophotonics 2016, 3, 031402. [Google Scholar] [CrossRef]
- Herrera-Vega, J.; Treviño-Palacios, C.G.; Orihuela-Espina, F. Neuroimaging with functional near infrared spectroscopy: From formation to interpretation. Infrared Phys. Technol. 2017, 85, 225–237. [Google Scholar] [CrossRef]
- Pfeifer, M.D.; Scholkmann, F.; Labruyère, R. Signal Processing in Functional Near-Infrared Spectroscopy (fNIRS): Methodological Differences Lead to Different Statistical Results. Front. Hum. Neurosci. 2018, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Balardin, J.B.; Zimeo Morais, G.A.; Furucho, R.A.; Trambaiolli, L.; Vanzella, P.; Biazoli, C.; Sato, J.R. Imaging Brain Function with Functional Near-Infrared Spectroscopy in Unconstrained Environments. Front. Hum. Neurosci. 2017, 11, 258. [Google Scholar] [CrossRef]
- Deffieux, T.; Demene, C.; Pernot, M.; Tanter, M. Functional ultrasound neuroimaging: A review of the preclinical and clinical state of the art. Curr. Opin. Neurobiol. 2018, 50, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Gesnik, M.; Blaize, K.; Deffieux, T.; Gennisson, J.-L.; Sahel, J.-A.; Fink, M.; Picaud, S.; Tanter, M. 3D functional ultrasound imaging of the cerebral visual system in rodents. NeuroImage 2017, 149, 267–274. [Google Scholar] [CrossRef]
- Demene, C.; Baranger, J.; Bernal, M.; Delanoe, C.; Auvin, S.; Biran, V.; Alison, M.; Mairesse, J.; Harribaud, E.; Pernot, M.; et al. Functional ultrasound imaging of brain activity in human newborns. Sci. Transl. Med. 2017, 9, eaah6756. [Google Scholar] [CrossRef]
- Hage, B.; Alwatban, M.R.; Barney, E.; Mills, M.; Dodd, M.D.; Truemper, E.J.; Bashford, G.R. Functional Transcranial Doppler Ultrasound for Measurement of Hemispheric Lateralization During Visual Memory and Visual Search Cognitive Tasks. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 2001–2007. [Google Scholar] [CrossRef]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef]
- Zafar, M.; Kratkiewicz, K.; Manwar, R.; Avanaki, M. Development of Low-Cost Fast Photoacoustic Computed Tomography: System Characterization and Phantom Study. Appl. Sci. 2019, 9, 374. [Google Scholar] [CrossRef]
- Wang, L.V.; Xia, J.; Yao, J. Photoacoustic Neuroimaging. In Neurophotonics and Brain Mapping; Chen, Y., Kateb, B., Eds.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lameka, K.; Farwell, M.D.; Ichise, M. Chapter 11—Positron Emission Tomography. In Handbook of Clinical Neurology; Masdeu, J.C., González, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 135, pp. 209–227. [Google Scholar]
- Goel, S.; England, C.G.; Chen, F.; Cai, W. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Adv. Drug Deliv. Rev. 2017, 113, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Moghbel, M.; Newberg, A.; Alavi, A. Chapter 12—Positron emission tomography: Ligand imaging. In Handbook of Clinical Neurology; Masdeu, J.C., González, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 135, pp. 229–240. [Google Scholar]
- Penny, W.D.; Friston, K.J. Functional imaging. Scholarpedia 2007, 2, 1478. [Google Scholar] [CrossRef]
- Du, Y.; Zaidi, H. Single-Photon Emission Computed Tomography: Principles and Applications. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 493–506. [Google Scholar]
- Goffin, K.; van Laere, K. Chapter 13—Single-photon emission tomography. In Handbook of Clinical Neurology; Masdeu., J.C., González, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 135, pp. 241–250. [Google Scholar]
- Kijewski, M.F. Chapter 32—Positron Emission Tomography (PET) and Single-Photon Emission Computed Tomography (SPECT) Physics. In Handbook of Neuro-Oncology Neuroimaging (Second Edition); Newton, H.B., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 353–358. [Google Scholar]
- Yeh, R.; Miloushev, V.Z.; Ichise, M. Chapter 33—Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) Imaging. In Handbook of Neuro-Oncology Neuroimaging (Second Edition); Newton, H.B., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 359–370. [Google Scholar]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. Chapter 17—Single Photon Emission Computed Tomography. In Physics in Nuclear Medicine (Fourth Edition); Cherry, S.R., Sorenson, J.A., Phelps, M.E., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 279–306. [Google Scholar]
- Wang, Z.J.; Chang, T.T.A.; Slauter, R. Chapter 35—Use of Imaging for Preclinical Evaluation. In A Comprehensive Guide to Toxicology in Nonclinical Drug Development (Second Edition); Faqi, A.S., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 921–938. [Google Scholar]
- Hengerer, A.; Grimm, J. Molecular magnetic resonance imaging. Biomed. Imaging Interv. J. 2006, 2, e8. [Google Scholar] [CrossRef] [PubMed]
- Sinharay, S.; Pagel, M.D. Advances in Magnetic Resonance Imaging Contrast Agents for Biomarker Detection. Annu. Rev. Anal. Chem. [Palo Alto, Calif.] 2016, 9, 95–115. [Google Scholar] [CrossRef]
- Haris, M.; Yadav, S.K.; Rizwan, A.; Singh, A.; Wang, E.; Hariharan, H.; Ravinder, R.; Marincola, F.M. Molecular magnetic resonance imaging in cancer. J. Transl. Med. 2015, 13, 313. [Google Scholar] [CrossRef]
- Gauberti, M.; Fournier, A.P.; Docagne, F.; Vivien, D.; Martinez de Lizarrondo, S. Molecular Magnetic Resonance Imaging of Endothelial Activation in the Central Nervous System. Theranostics 2018, 8, 1195–1212. [Google Scholar] [CrossRef]
- Shuhendler, A.J.; Ye, D.; Brewer, K.D.; Bazalova-Carter, M.; Lee, K.-H.; Kempen, P.; Wittrup, K.D.; Graves, E.E.; Rutt, B.; Rao, J. Molecular Magnetic Resonance Imaging of Tumor Response to Therapy. Sci. Rep. 2015, 5, 14759. [Google Scholar] [CrossRef]
- Li, J.; You, J.; Wu, C.; Dai, Y.; Shi, M.; Dong, L.; Xu, K. T1-T2 molecular magnetic resonance imaging of renal carcinoma cells based on nano-contrast agents. Int. J. Nanomed. 2018, 13, 4607–4625. [Google Scholar] [CrossRef]
- Bonnet, C.S.; Tóth, É. Molecular Magnetic Resonance Imaging Probes Based on Ln3+ Complexes. Adv. Org. Chem. 2016, 68, 43–96. [Google Scholar]
- Bhargava, A.; Cheung, J.; Eshaghian-Wilner, M.M.; Lee, W.; Ravicz, K.; Schlesinger, M.; Shah, Y.; Uppal, A. An introduction to nanomedicine. In Wireless Computing in Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zhang, W.; Wang, W.; Yu, D.X.; Xiao, Z.; He, Z. Application of nanodiagnostics and nanotherapy to CNS diseases. Nanomedicine 2018, 13, 2341–2371. [Google Scholar] [CrossRef]
- Kumar, A.; Tan, A.; Wong, J.; Spagnoli, J.C.; Lam, J.; Blevins, B.D.; G, N.; Thorne, L.; Ashkan, K.; Xie, J.; et al. Nanotechnology for Neuroscience: Promising Approaches for Diagnostics, Therapeutics and Brain Activity Mapping. Adv. Funct. Mater. 2017, 27, 1700489. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bisht, R.; Pal, D.; Mitra, A.K. Chapter 4—Diagnosis and Drug Delivery to the Brain: Novel Strategies. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 59–83. [Google Scholar]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef]
- Posadas, I.; Monteagudo, S.; Ceña, V. Nanoparticles for brain-specific drug and genetic material delivery, imaging and diagnosis. Nanomedicine 2016, 11, 833–849. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Archunan, G.; Sivakumar, M.; Tamil Selvan, S.; Fred, A.L.; Kumar, S.; Gulyás, B.; Padmanabhan, P. Theranostic applications of nanoparticles in neurodegenerative disorders. Int. J. Nanomed. 2018, 13, 5561–5576. [Google Scholar] [CrossRef] [PubMed]
- Nedyalkova, M.; Donkova, B.; Romanova, J.; Tzvetkov, G.; Madurga, S.; Simeonov, V. Iron oxide nanoparticles—In vivo/in vitro biomedical applications and in silico studies. Adv. Colloid Interface Sci. 2017, 249, 192–212. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef]
- Richard, S.; Saric, A.; Boucher, M.; Slomianny, C.; Geffroy, F.; Mériaux, S.; Lalatonne, Y.; Petit, P.X.; Motte, L. Antioxidative Theranostic Iron Oxide Nanoparticles toward Brain Tumors Imaging and ROS Production. ACS Chem. Biol. 2016, 11, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Chen, Z.; Li, K.; Morais, G.R.; Klockow, J.; Yerneni, K.; Pisani, L.; Chin, F.T.; Mitra, S.; Cheshier, S.; et al. A Novel Theranostic Strategy for MMP-14-Expressing Glioblastomas Impacts Survival. Mol. Cancer Ther. 2017, 16, 1909–1921. [Google Scholar] [CrossRef]
- Richard, S.; Boucher, M.; Lalatonne, Y.; Mériaux, S.; Motte, L. Iron oxide nanoparticle surface decorated with cRGD peptides for magnetic resonance imaging of brain tumours. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2017, 1861, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.S.; Chung, T.K.; Prout, B.S.; Nagahama, Y.; Raghavan, M.L.; Hasan, D.M. Iron nanoparticle contrast enhanced microwave imaging for emergent stroke: A pilot study. J. Clin. Neurosci. 2019, 59, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Valenzuela, A.; Petit, F.; Chow, S.; Leung, K.; Gorin, F.; Louie, A.Y.; Dhenain, M. In Vivo MRI of Functionalized Iron Oxide Nanoparticles for Brain Inflammation. Contrast Media Mol. Imaging 2018, 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Hsiao, J.-K.; Liu, H.-M.; Wu, C.-H. Characterization of an iron oxide nanoparticle labelling and MRI-based protocol for inducing human mesenchymal stem cells into neural-like cells. Sci. Rep. 2017, 7, 3587. [Google Scholar] [CrossRef]
- Meola, A.; Rao, J.; Chaudhary, N.; Sharma, M.; Chang, S.D. Gold Nanoparticles for Brain Tumor Imaging: A Systematic Review. Front. Neurol. 2018, 9, 328. [Google Scholar] [CrossRef]
- Mahan, M.M.; Doiron, A.L. Gold Nanoparticles as X-ray, CT and Multimodal Imaging Contrast Agents: Formulation, Targeting and Methodology. J. Nanomater. 2018, 2018, 15. [Google Scholar] [CrossRef]
- Cho, J.-H.; Kim, A.R.; Kim, S.-H.; Lee, S.-J.; Chung, H.; Yoon, M.-Y. Development of a novel imaging agent using peptide-coated gold nanoparticles toward brain glioma stem cell marker CD133. Acta Biomater. 2017, 47, 182–192. [Google Scholar] [CrossRef]
- Nicholls, F.J.; Rotz, M.W.; Ghuman, H.; MacRenaris, K.W.; Meade, T.J.; Modo, M. DNA–gadolinium–gold nanoparticles for in vivo T1 MR imaging of transplanted human neural stem cells. Biomaterials 2016, 77, 291–306. [Google Scholar] [CrossRef]
- Betzer, O.; Perets, N.; Barnoy, E.; Offen, D.; Popovtzer, R. Labeling and tracking exosomes within the brain using gold nanoparticles. Proc. SPIE 2018, 10506, 1050618. [Google Scholar]
- Azria, D.; Blanquer, S.; Verdier, J.-M.; Belamie, E. Nanoparticles as contrast agents for brain nuclear magnetic resonance imaging in Alzheimer’s disease diagnosis. J. Mater. Chem. B 2017, 5, 7216–7237. [Google Scholar] [CrossRef]
- Gale, E.M.; Caravan, P. Gadolinium-Free Contrast Agents for Magnetic Resonance Imaging of the Central Nervous System. ACS Chem. Neurosci. 2018, 9, 395–397. [Google Scholar] [CrossRef]
- Chen, N.; Shao, C.; Qu, Y.; Li, S.; Gu, W.; Zheng, T.; Ye, L.; Yu, C. Folic Acid-Conjugated MnO Nanoparticles as a T1 Contrast Agent for Magnetic Resonance Imaging of Tiny Brain Gliomas. ACS Appl. Mater. Interfaces 2014, 6, 19850–19857. [Google Scholar] [CrossRef]
- Jeon, T.Y.; Kim, J.H.; Im, G.H.; Kim, J.H.; Yang, J.; Yoo, S.Y.; Lee, J.H. Hollow manganese oxide nanoparticle-enhanced MRI of hypoxic-ischaemic brain injury in the neonatal rat. Br. J. Radiol. 2016, 89, 20150806. [Google Scholar] [CrossRef]
- Servant, A.; Jacobs, I.; Bussy, C.; Fabbro, C.; da Ros, T.; Pach, E.; Ballesteros, B.; Prato, M.; Nicolay, K.; Kostarelos, K. Gadolinium-functionalised multi-walled carbon nanotubes as a T1 contrast agent for MRI cell labelling and tracking. Carbon 2016, 97, 126–133. [Google Scholar] [CrossRef]
- Costa, P.M.; Wang, J.T.-W.; Morfin, J.-F.; Khanum, T.; To, W.; Sosabowski, J.; Tóth, E.; Al-Jamal1, KT. Functionalised Carbon Nanotubes Enhance Brain Delivery of Amyloid-Targeting Pittsburgh Compound B (PiB)-Derived Ligands. Nanotheranostics 2018, 2, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Colombeau, L.; Gries, M.; Peterlini, T.; Mathieu, C.; Thomas, N.; Boura, C.; Frochot, C.; Vanderesse, R.; Lux, F.; et al. Ultrasmall AGuIX theranostic nanoparticles for vascular-targeted interstitial photodynamic therapy of glioblastoma. Int. J. Nanomed. 2017, 12, 7075–7088. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Mukaya, H.E. Chapter 3—Polymer Therapeutics: Design, Application and Pharmacokinetics. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 33–48. [Google Scholar]
- Dimov, N.; Kastner, E.; Hussain, M.; Perrie, Y.; Szita, N. Formation and purification of tailored liposomes for drug delivery using a module-based micro continuous-flow system. Sci. Rep. 2017, 7, 12045. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood-brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef]
- Li, S.; Johnson, J.; Peck, A.; Xie, Q. Near infrared fluorescent imaging of brain tumour with IR780 dye incorporated phospholipid nanoparticles. J. Transl. Med. 2017, 15, 18. [Google Scholar] [CrossRef]
- Zhang, L.; Habib, A.A.; Zhao, D. Phosphatidylserine-targeted liposome for enhanced glioma-selective imaging. Oncotarget 2016, 7, 38693–38706. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Li, K.-F.; Wang, H.; Gu, M.-J.; Liu, L.-S.; Zheng, Z.-Z.; Han, N.Y.; Yang, Z.J.; Fan, T.Y. Preparation and In Vitro Evaluation of a MRI Contrast Agent Based on Aptamer-Modified Gadolinium-Loaded Liposomes for Tumor Targeting. AAPS PharmSciTech 2017, 18, 1564–1571. [Google Scholar] [CrossRef]
- Tomitaka, A.; Arami, H.; Huang, Z.; Raymond, A.; Rodriguez, E.; Cai, Y.; Febo, M.; Takemura, Y.; Nair, M. Hybrid magneto-plasmonic liposomes for multimodal image-guided and brain-targeted HIV treatment. Nanoscale 2018, 10, 184–194. [Google Scholar] [CrossRef]
- Fülöp, A.; Sammour, D.A.; Erich, K.; von Gerichten, J.; van Hoogevest, P.; Sandhoff, R.; Hopf, C. Molecular imaging of brain localization of liposomes in mice using MALDI mass spectrometry. Sci. Rep. 2016, 6, 33791. [Google Scholar] [CrossRef]
- Pacheco-Torres, J.; Mukherjee, N.; Walko, M.; Lopez-Larrubia, P.; Ballesteros, P.; Cerdan, S.; Kocer, A. Image guided drug release from pH-sensitive Ion channel-functionalized stealth liposomes into an in vivo glioblastoma model. Nanomedicine 2015, 11, 1345–1354. [Google Scholar] [CrossRef]
- Lin, Q.; Mao, K.L.; Tian, F.R.; Yang, J.J.; Chen, P.P.; Xu, J.; Fan, Z.L.; Zhao, Y.P.; Li, W.F.; Zheng, L.; et al. Brain tumour-targeted delivery and therapy by focused ultrasound introduced doxorubicin-loaded cationic liposomes. Cancer Chemother. Pharmacol. 2016, 77, 269–280. [Google Scholar] [CrossRef]
- Sonali; Singh, R.P.; Sharma, G.; Kumari, L.; Koch, B.; Singh, S.; Bharti, S.; Rajinikanth, P.S.; Pandey, B.L.; Muthu, M.S. RGD-TPGS decorated theranostic liposomes for brain targeted delivery. Colloids Surf. B Biointerfaces 2016, 147, 129–141. [Google Scholar] [CrossRef]
- Joseph, M.; Trinh, H.M.; Mitra, A.K. Chapter 7—Peptide and Protein-Based Therapeutic Agents*. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 145–167. [Google Scholar]
- Rana, S.; Bhattacharjee, J.; Barick, K.C.; Verma, G.; Hassan, P.A.; Yakhmi, J.V. Chapter 7—Interfacial engineering of nanoparticles for cancer therapeutics. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 177–209. [Google Scholar]
- Priya, L.B.; Baskaran, R.; Padma, V.V. Chapter 21—Phytonanoconjugates in oral medicine. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 639–668. [Google Scholar]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef]
- Shiraishi, K.; Wang, Z.; Kokuryo, D.; Aoki, I.; Yokoyama, M. A polymeric micelle magnetic resonance imaging [MRI] contrast agent reveals blood–brain barrier (BBB) permeability for macromolecules in cerebral ischemia-reperfusion injury. J. Control. Release 2017, 253, 165–171. [Google Scholar] [CrossRef]
- Garello, F.; Pagoto, A.; Arena, F.; Buffo, A.; Blasi, F.; Alberti, D.; Terreno, E. MRI visualization of neuroinflammation using VCAM-1 targeted paramagnetic micelles. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2341–2350. [Google Scholar] [CrossRef]
- Sun, L.; Joh, D.Y.; Al-Zaki, A.; Stangl, M.; Murty, S.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Kaol, G.D.; Tsourkas, A. Theranostic Application of Mixed Gold and Superparamagnetic Iron Oxide Nanoparticle Micelles in Glioblastoma Multiforme. J. Biomed. Nanotechnol. 2016, 12, 347–356. [Google Scholar] [CrossRef]
- Steeland, S.; Vandenbroucke, R.E.; Libert, C. Nanobodies as therapeutics: Big opportunities for small antibodies. Drug Discov. Today 2016, 21, 1076–1113. [Google Scholar] [CrossRef]
- Van Audenhove, I.; Gettemans, J. Nanobodies as Versatile Tools to Understand, Diagnose, Visualize and Treat Cancer. EBioMedicine 2016, 8, 40–48. [Google Scholar] [CrossRef]
- Li, T.; Vandesquille, M.; Koukouli, F.; Dudeffant, C.; Youssef, I.; Lenormand, P.; Ganneau, C.; Maskos, U.; Czech, C.; Grueninger, F.; et al. Camelid single-domain antibodies: A versatile tool for in vivo imaging of extracellular and intracellular brain targets. J. Control. Release 2016, 243, 1–10. [Google Scholar] [CrossRef]
- Reshma, V.G.; Mohanan, P.V. Quantum dots: Applications and safety consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, J. Chapter 3—Quantum Dots and Nanoclusters. In Nano-Inspired Biosensors for Protein Assay with Clinical Applications; Li, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–90. [Google Scholar]
- Zhu, R.; Chen, H.; Wu, S.-T.; Dong, Y. Quantum Dot Light Emitting Diodes. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Yamamoto, K. Application 13—Bioimaging with Quantum Dots. In Nanoparticle Technology Handbook (Third Edition); Naito, M., Yokoyama, T., Hosokawa, K., Nogi, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 473–477. [Google Scholar]
- Gao, L.; Zhao, X.; Wang, J.; Wang, Y.; Yu, L.; Peng, H.; Jianzhong, Z. Multiple functionalized carbon quantum dots for targeting glioma and tissue imaging. Opt. Mater. 2018, 75, 764–769. [Google Scholar] [CrossRef]
- Huang, N.; Cheng, S.; Zhang, X.; Tian, Q.; Pi, J.; Tang, J.; Huang, Q.; Wang, F.; Chen, J.; Xie, Z.; et al. Efficacy of NGR peptide-modified PEGylated quantum dots for crossing the blood–brain barrier and targeted fluorescence imaging of glioma and tumour vasculature. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 83–93. [Google Scholar] [CrossRef]
- Alifu, N.; Sun, Z.; Zebibula, A.; Zhu, Z.; Zhao, X.; Wu, C.; Wang, Y.; Qian, J. Deep-red polymer dots with bright two-photon fluorescence and high biocompatibility for in vivo mouse brain imaging. Opt. Commun. 2017, 399, 120–126. [Google Scholar] [CrossRef]
- Isherwood, B.; Timpson, P.; McGhee, E.J.; Anderson, K.I.; Canel, M.; Serrels, A.; Brunton, V.G.; Carragher, N.O. Live cell in vitro and in vivo imaging applications: Accelerating drug discovery. Pharmaceutics 2011, 3, 141–170. [Google Scholar] [CrossRef]
- Han, H.-S.; Niemeyer, E.; Huang, Y.; Kamoun, W.S.; Martin, J.D.; Bhaumik, J.; Chen, Y.; Roberge, S.; Cui, K.; Martin, M.R.; et al. Quantum dot/antibody conjugates for in vivo cytometric imaging in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 1350–1355. [Google Scholar] [CrossRef]
- Barry, J.F.; Turner, M.J.; Schloss, J.M.; Glenn, D.R.; Song, Y.; Lukin, M.D.; Park, H.; Walsworth, R.L. Optical magnetic detection of single-neuron action potentials using quantum defects in diamond. Proc. Natl. Acad. Sci. USA 2016, 113, 14133–14138. [Google Scholar] [CrossRef]
- Zhu, H.; Zou, G.; Wang, N.; Zhuang, M.; Xiong, W.; Huang, G. Single-neuron identification of chemical constituents, physiological changes and metabolism using mass spectrometry. Proc. Natl. Acad. Sci. USA 2017, 114, 2586–2591. [Google Scholar] [CrossRef]
- Croton, L.C.P.; Morgan, K.S.; Paganin, D.M.; Kerr, L.T.; Wallace, M.J.; Crossley, K.J.; Miller, S.L.; Yagi, N.; Uesugi, K.; Hooper, S.B.; et al. In situ phase contrast X-ray brain CT. Sci. Rep. 2018, 8, 11412. [Google Scholar] [CrossRef]
- Amirav, L.; Berlin, S.; Olszakier, S.; Pahari, S.K.; Kahn, I. Multi-Modal Nano Particle Labeling of Neurons. Front. Neurosci. 2019, 13, 12. [Google Scholar] [CrossRef]
- Deans, C.; Marmugi, L.; Hussain, S.; Renzoni, F. Optical atomic magnetometry for magnetic induction imaging of the heart. Proc. SPIE 2016, 99000F. [Google Scholar] [CrossRef]
- Bravin, A.; Coan, P.; Suortti, P. X-ray phase-contrast imaging: From pre-clinical applications towards clinics. Phys. Med. Biol. 2013, 58, R1–R35. [Google Scholar] [CrossRef]
- Xi, Y.; Lin, X.; Yuan, F.; Yang, G.-Y.; Zhao, J. High-Resolution and Quantitative X-ray Phase-Contrast Tomography for Mouse Brain Research. Comput. Math. Methods Med. 2015, 2015, 12. [Google Scholar] [CrossRef]
| CT | MRI | fMRI | EEG | iEEG | fNIRS | fUS | PET | SPECT | mMRI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cost | $–$$ | $$ | — | $ | — | $–$$ | — | $$$ | $$–$$$ | — |
| Invasiveness | minimal | non-invasive | non-invasive | non-invasive | requires surgery | non-invasive | non-invasive | minimal | minimal | non-invasive |
| Acquisition time | minutes | minutes to hours | minutes to hours | minutes | hours | minutes | — | minutes | minutes | minutes to hours |
| Portability | not portable | not portable | not portable | portable | not portable | portable | portable | not portable | not portable | not portable |
| Personnel requirements | qualified personnel | qualified personnel | qualified personnel | qualified personnel—optional | qualified personnel | qualified personnel—optional | qualified personnel—optional | qualified personnel | qualified personnel | qualified personnel |
| Spatial resolution | 0.5–0.625 mm | 1–2 mm | 1–2 mm | 5–9 cm | 4.5 mm | 1 cm | 50–200 µm | 3–5 mm | 6–8 mm | — |
| Temporal resolution | 85–135 ms | 20–50 ms | 1–3 s | 130 ms | 0.8 ms | 330 ms | 1–100 ms | 5 s to 5 min | 15 min | — |
| Penetration depth | no limit | no limit | 1.2 mm | — | — | 3 cm | no limit | no limit | no limit | — |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Contrast Agents Delivery: An Up-to-Date Review of Nanodiagnostics in Neuroimaging. Nanomaterials 2019, 9, 542. https://doi.org/10.3390/nano9040542
Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Contrast Agents Delivery: An Up-to-Date Review of Nanodiagnostics in Neuroimaging. Nanomaterials. 2019; 9(4):542. https://doi.org/10.3390/nano9040542
Chicago/Turabian StyleTeleanu, Daniel Mihai, Cristina Chircov, Alexandru Mihai Grumezescu, Adrian Volceanov, and Raluca Ioana Teleanu. 2019. "Contrast Agents Delivery: An Up-to-Date Review of Nanodiagnostics in Neuroimaging" Nanomaterials 9, no. 4: 542. https://doi.org/10.3390/nano9040542
APA StyleTeleanu, D. M., Chircov, C., Grumezescu, A. M., Volceanov, A., & Teleanu, R. I. (2019). Contrast Agents Delivery: An Up-to-Date Review of Nanodiagnostics in Neuroimaging. Nanomaterials, 9(4), 542. https://doi.org/10.3390/nano9040542








