MOF-Derived FeS/C Nanosheets for High Performance Lithium Ion Batteries
Abstract
1. Introduction
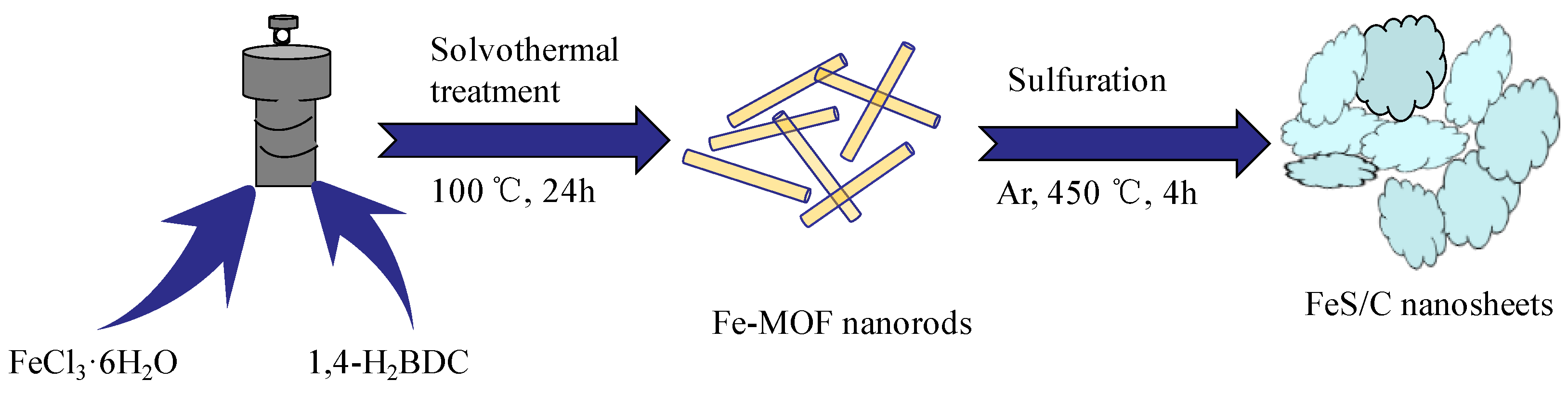
2. Experimental
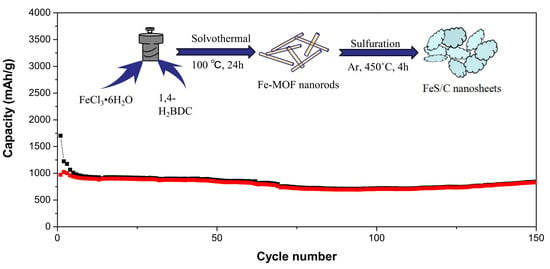
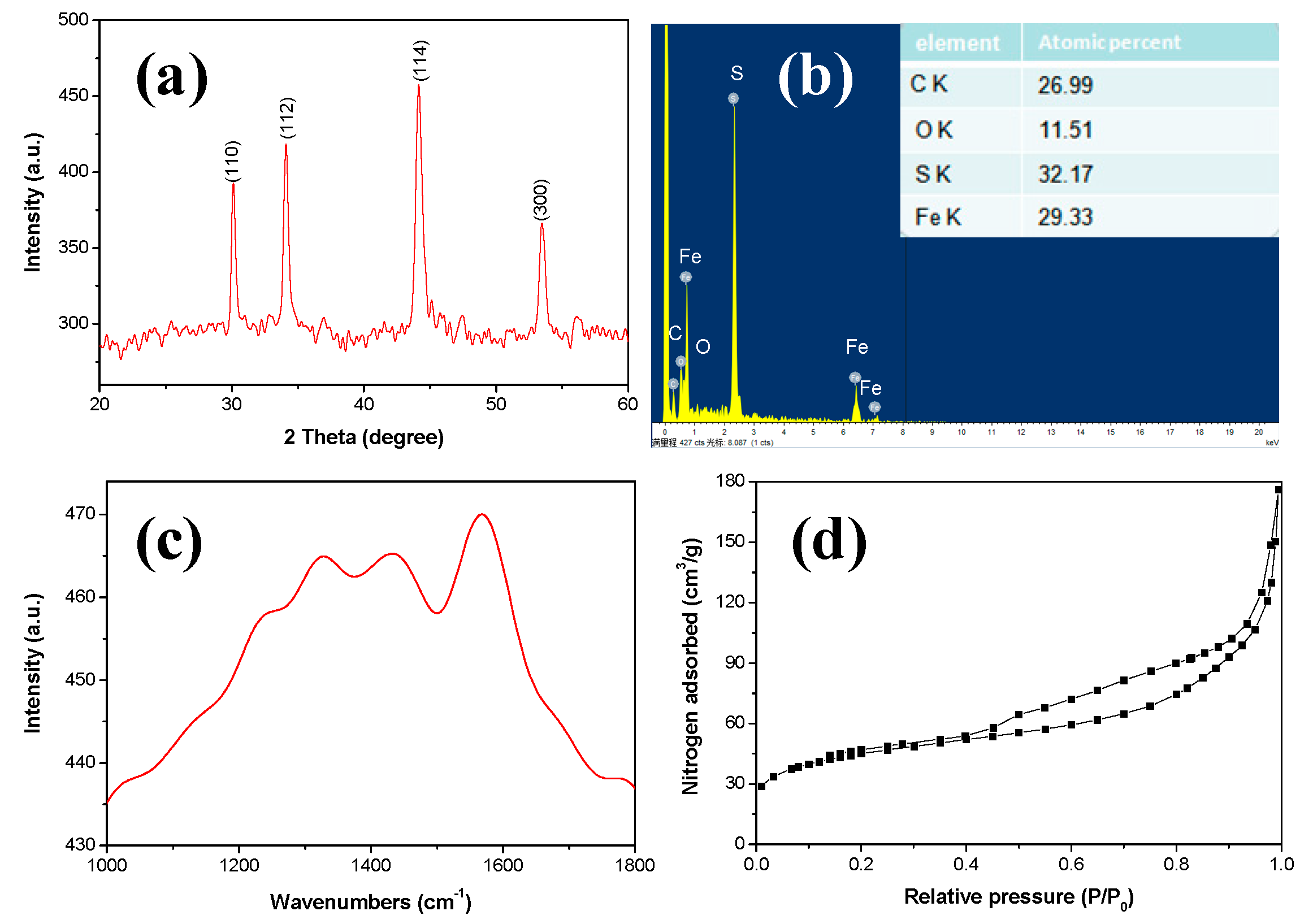
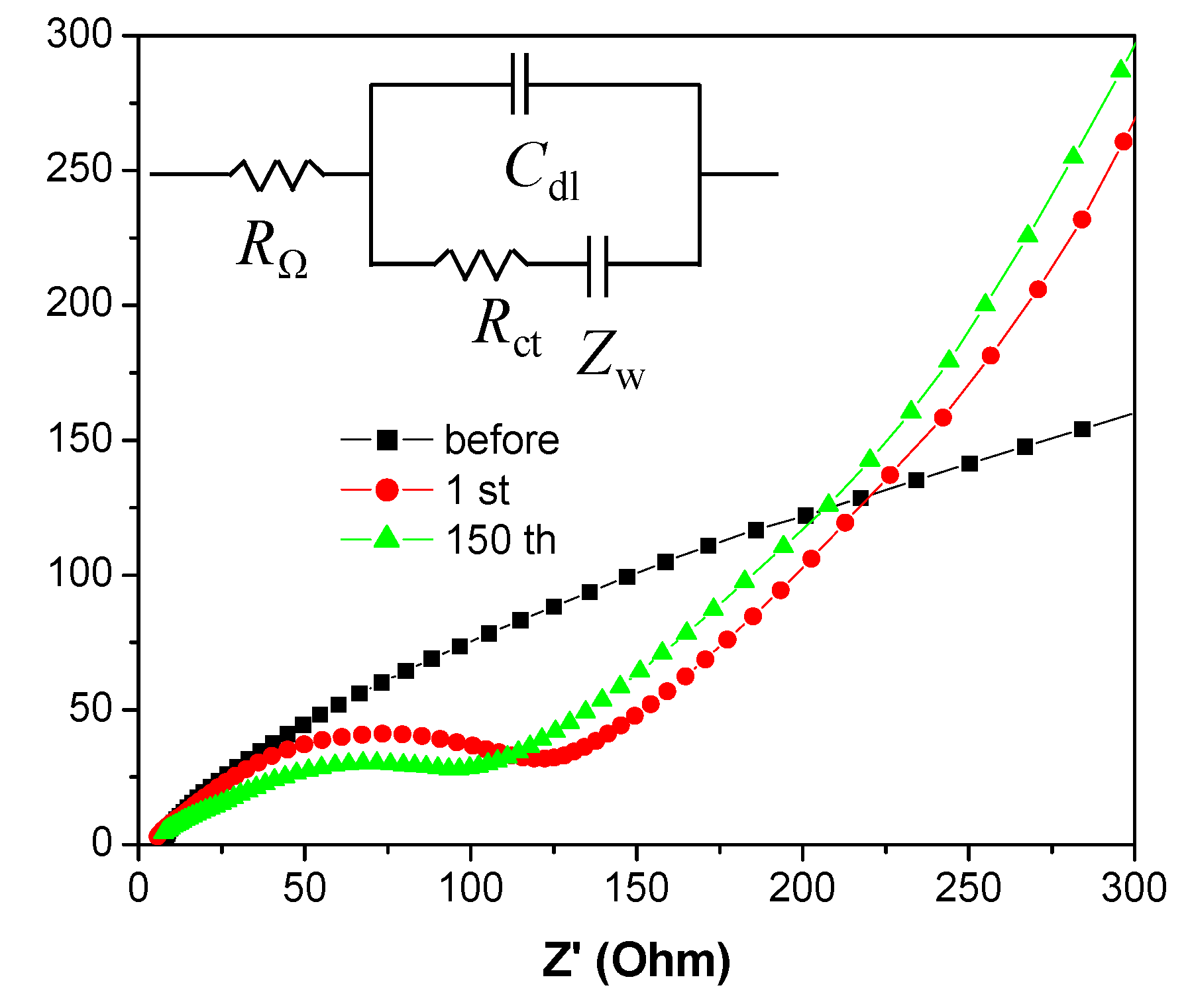
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, L.; Zheng, B. Wind energy generation technological paradigm diffusion. Renew. Sustain. Energy Rev. 2016, 59, 436–449. [Google Scholar] [CrossRef]
- Rickels, W.; Dovern, J.; Quaas, M. Beyond fisheries: Common-pool resource problems in oceanic resources and services. Glob. Environ. Chang. 2016, 40, 37–49. [Google Scholar] [CrossRef]
- Shen, L.; Yu, L.; Yu, X.Y.; Zhang, X.; Lou, X.W. Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Liu, Q.; Duan, H. Recent progress in Zn-based anodes for advanced lithium ion batteries. Mater. Chem. Front. 2018, 2, 1414–1435. [Google Scholar] [CrossRef]
- Rodrigues, M.F.; Bahu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2017, 2, 17108. [Google Scholar] [CrossRef]
- Wang, S.; Quan, W.; Zhu, Z.; Yang, Y.; Liu, Q.; Ren, Y.; Zhang, X.; Xu, R.; Hong, Y.; Zhang, Z.; et al. Lithium titanate hydrates with superfast and stable cycling in lithium ion batteries. Nat. Commun. 2017, 8, 627. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Qiao, X.; Shen, J.; Liu, G.; Sun, T.; Wu, H.; Hou, H.; Liu, X.; Zhang, Y.; Ji, X. Anatase inverse opal TiO2−x@N-doped C induced the dominant pseudocapacitive effect for durable and fast lithium/sodium storage. Electrochim. Acta 2019, 299, 540–548. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for lithium insertion in carbonaceous materials. Science 1995, 27, 590–593. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Lou, X.W. Synthesis of highly uniform molybdenum-glycerate spheres and their conversion into hierarchical MoS2 hollow nanospheres for lithium-ion batteries. Angew. Chem. Int. Ed. 2016, 55, 7423–7426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Yan, B.; Xiong, D.; Li, D.; Lawes, S.; Sun, X. Recent developments and understanding of novel mixed transition-metal oxides as anodes in lithium ion batteries. Adv. Energy Mater. 2016, 6, 1502175. [Google Scholar] [CrossRef]
- Wu, N.; Du, W.; Gao, X.; Zhao, L.; Liu, G.; Liu, X.; Wu, H.; He, Y.B. Hollow SnO2 nanospheres with oxygen vacancies entrapped by a N-doped graphene network as robust anode materials for lithium-ion batteries. Nanoscale 2018, 10, 11460–11466. [Google Scholar] [CrossRef]
- Chen, S.; Shen, L.; van Aken, P.A.; Maier, J.; Yu, Y. Dual-functionalized double carbon shells coated silicon nanoparticles for high performance lithium-ion batteries. Adv. Mater. 2017, 29, 1605650. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kwon, T.; Coskun, A.; Choi, J.W. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 2017, 21, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Wang, J.; Yu, X.; Ge, J.; Zhang, H.; Lu, B. Semimetallic vanadium molybdenum sulfide for high-performance battery electrodes. J. Mater. Chem. A 2018, 6, 9411–9419. [Google Scholar] [CrossRef]
- Lou, P.; Tan, Y.; Lu, P.; Cui, Z.; Guo, X. Novel one-step gas-phase reaction synthesis of transition metal sulfide nanoparticles embedded in carbon matrices for reversible lithium storage. J. Mater. Chem. A 2016, 4, 16849–16855. [Google Scholar] [CrossRef]
- Liu, J.; Wen, Y.; Wang, Y.; van Aken, P.A.; Maier, J.; Yu, Y. Carbon-Encapsulated Pyrite as Stable and Earth-Abundant High Energy Cathode Material for Rechargeable Lithium Batteries. Adv. Mater. 2014, 26, 6025–6030. [Google Scholar] [CrossRef]
- Yu, X.Y.; Yu, L.; Lou, X.W. Metal Sulfide Hollow Nanostructures for Electrochemical Energy Storage. Adv. Energy Mater. 2016, 6, 1501333. [Google Scholar] [CrossRef]
- Liu, D.H.; Li, W.H.; Zheng, Y.P.; Cui, Z.; Yan, X.; Liu, D.S.; Wang, J.; Zhang, Y.; Lv, H.Y.; Bai, F.Y.; et al. In Situ Encapsulating α- MnS into N,S-Codoped Nanotube-Like Carbon as Advanced Anode Material: α→β Phase Transition Promoted Cycling Stability and Superior Li/Na-Storage Performance in Half/Full Cells. Adv. Mater. 2018, 30, 1706317. [Google Scholar] [CrossRef]
- Fan, H.H.; Li, H.H.; Guo, J.Z.; Zheng, Y.P.; Huang, K.C.; Fan, C.Y.; Li, H.F.; Wu, X.L.; Zhang, J.P. Target construction of ultrathin graphitic carbon encapsulated FeS hierarchical microspheres featuring superior low-temperature lithium/sodium storage properties. J. Mater. Chem. A 2018, 6, 7997–8005. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Zhang, F.; Zhang, X.; Zhang, W.; Lee, C.S.; Tang, Y. In-situ incorporation of FeS nanoparticles/carbon nanosheets composite with an interconnected porous structure as a high-performance anode for lithium ion batteries. J. Mater. Chem. A 2016, 4, 3697–3703. [Google Scholar] [CrossRef]
- Zhu, C.; Wen, Y.; van Aken, P.A.; Maier, J.; Yu, Y. High lithium storage performance of FeS nanodots in porous graphitic carbon nanowires. Adv. Funct. Mater. 2015, 25, 2335–2342. [Google Scholar] [CrossRef]
- Hou, B.H.; Wang, Y.Y.; Guo, J.Z.; Ning, Q.L.; Xi, X.T.; Pang, W.L.; Cao, A.M.; Wang, X.; Zhang, J.P.; Wu, X.L. Pseudocapacitance-boosted ultrafast Na storage in a pie-like FeS@C nanohybrid as an advanced anode material for sodium-ion full batteries. Nanoscale 2018, 10, 9218–9225. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Zhang, S.S. Chemical stability and electrochemical characteristics of FeS microcrystals as the cathode material of rechargeable lithium battery. J. Mater. Chem. A 2015, 3, 12240–12246. [Google Scholar] [CrossRef]
- Li, X.W.W.; Shi, J.; Gu, L.; Yu, Y. FeS@C on carbon cloth as flexible electrode for both lithium and sodium storage. ACS Appl. Mater. Interfaces 2015, 7, 27804–27809. [Google Scholar]
- Li, L.; Gao, C.; Kovalchuk, A.; Peng, Z.; Ruan, G.; Yang, Y.; Fei, H.; Zhong, Q.; Li, Y.; Tour, J.M. Sandwich structured graphene-wrapped FeS-graphene nanoribbons with improved cycling stability for lithium ion batteries. Nano Res. 2016, 9, 2904–2911. [Google Scholar] [CrossRef]
- Nelson, A.P.; Farha, O.K.; Mulfort, K.L.; Hupp, J.T. Supercritical processing as a route to high internal surface areas and permanent microporosity in metal-organic framework materials. J. Am. Chem. Soc. 2009, 131, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Fortea-Pérez, F.R.; Mon, M.; Ferrando-Soria, J.; Boronat, M.; Pérez, A.L.; Corma, A.; Herrera, J.M.; Osadchii, D.; Gascon, J.; Armentano, D.; Pardo, E. The MOF-driven synthesis of supported palladium clusters with catalytic activity for carbene-mediated chemistry. Nat. Mater. 2017, 16, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- He, H.; Ma, E.; Cui, Y.; Yu, J.; Yang, Y.; Song, T.; Wu, C.D.; Chen, X.; Chen, B.; Qian, G. Polarized three-photon-pumped laser in a single MOF microcrystal. Nat. Commun. 2016, 7, 11087. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, X.B. Metal-organic frameworks for electrochemical applications. TrAC Trends Anal. Chem. 2016, 75, 86–96. [Google Scholar] [CrossRef]
- Kim, S.B.; Kim, J.Y.; Jeong, N.C.; Ok, K.M. Anisotropic Li+ ion conductivity in a large single crystal of a Co(III) coordination complex. Inorg. Chem. Front. 2107, 4, 79–83. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, E.J.; Song, D.; Jeong, N.C. High Proton Mobility with High Directionality in Isolated Channels of MOF-74. ACS Appl. Mater. Interfaces 2018, 10, 35354–35360. [Google Scholar] [CrossRef]
- Li, W.; Hu, S.; Luo, X.; Li, Z.; Sun, X.; Li, M.; Liu, F.; Yu, Y. Confined amorphous red phosphorus in MOF-Derived N-doped microporous carbon as a superior anode for sodium-ion battery. Adv. Mater. 2017, 29, 1605820. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zeng, X.; Zhang, Y.; Dai, Z.; Fan, H.; Huang, Y.; Zhang, W.; Zhang, H.; Lu, J.; Huo, F.; et al. Sn nanoparticles encapsulated in 3D nanoporous carbon derived from a metal-organic framework for anode material in lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 17172–17177. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Zhou, J.; Liang, C.; Pan, A.; Zhang, C.; Tang, Y.; Tan, X.; Liu, J.; Liang, S. MOFs nanosheets derived porous metal oxide-coated three-dimensional substrates for lithium-ion battery applications. Nano Energy 2016, 26, 57–65. [Google Scholar] [CrossRef]
- Liu, J.; Wu, C.; Xiao, D.; Kopold, P.; Gu, L.; van Aken, P.A.; Maier, J.; Yu, Y. MOF-Derived Hollow Co9S8 Nanoparticles embedded in graphitic carbon nanocages with superior Li-ion storage. Small 2016, 12, 2354–2364. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Liu, J.; Wang, C.; Zhao, Y.; Yue, G. MOF-Derived ZnO/Ni3ZnC0.7/C hybrids yolk-shell microspheres with excellent electrochemical performances for lithium ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 6472–6480. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Xu, X.; Zhang, L.; Hu, R.; Liu, J.; Ouyang, L.; Yang, L.; Zhu, M. Ilmenite nanotubes for high stability and high rate sodium-ion battery anodes. ACS Nano 2017, 11, 5120–5129. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef]
- Huang, W.; Li, S.; Cao, X.; Hou, C.; Zhang, Z.; Feng, J.; Ci, L.; Si, P.; Chi, Q. Metal−Organic Framework Derived Iron Sulfide−Carbon Core−Shell Nanorods as a Conversion-Type Battery Material. ACS Sustain. Chem. Eng. 2017, 5, 5039–5048. [Google Scholar] [CrossRef]
- Xu, X.; Tan, H.; Xi, K.; Ding, S.; Yu, D.; Cheng, S.; Yang, G.; Peng, X.; Fakeeh, A.; Kumar, R.V. Bamboo-like amorphous carbon nanotubes clad in ultrathin nickel oxide nanosheets for lithium-ion battery electrodes with long cycle life. Carbon 2015, 84, 491–499. [Google Scholar] [CrossRef]
- Veres, M.; Tóth, S.; Koós, M. New aspects of Raman scattering in carbon-based amorphous materials. Diam. Relat. Mater. 2008, 17, 1692–1696. [Google Scholar] [CrossRef]
- He, J.; Wang, N.; Cui, Z.; Du, H.; Fu, L.; Huang, C.; Yang, Z.; Shen, X.; Yi, Y.; Tu, Z.; et al. Hydrogen substituted graphdiyne as carbon-rich flexible electrode for lithium and sodium ion batteries. Nat. Commun. 2017, 8, 1172. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Lin, Q.L.; Yuan, B.; Chen, G.; Xie, P.; Li, Y.L.; Xu, Y.; Deng, S.D.; Smirnov, S.; Luo, H.M. Reduced graphene oxide wrapped FeS nanocomposite for lithium-ion battery anode with improved performance. ACS Appl. Mater. Interfaces 2013, 5, 5330–5335. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.P.; Li, J.C.; Ma, Z.; Chi, Y.; Xue, H.G. A facile method to prepare FeS/porous carbon composite as advanced anode material for lithium-ion batteries. J. Mater. Sci. 2017, 52, 2345–2355. [Google Scholar] [CrossRef]
- Yang, C.; Feng, J.; Lv, F.; Zhou, J.; Lin, C.; Wang, K.; Zhang, Y.; Yang, Y.; Wang, W.; Li, J.; et al. Metallic graphene-like VSe2 ultrathin nanosheets: Superior potassium-ion storage and their working mechanism. Adv. Mater. 2018, 30, 1800036. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Lu, B. Reactive oxygen-doped 3D interdigital carbonaceous materials for Li and Na ion batteries. Small 2016, 12, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Li, W.; Wang, F.; Bongard, H.; Spliethoff, B.; Schmidt, W.; Weidenthaler, C.; Xia, Y.; Zhao, D.; Schüth, F. Controllable synthesis of mesoporous peapod-like Co3O4@carbon nanotube arrays for high-performance lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 7060–7064. [Google Scholar] [CrossRef]
- Shangguan, E.; Guo, L.; Li, F.; Wang, Q.; Li, J.; Li, Q.; Chang, Z.; Yuan, X.Z. FeS anchored reduced graphene oxide nanosheets as advanced anode material with superior high-rate performance for alkaline secondary batteries. J. Power Sources 2016, 327, 187–195. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Hu, Z.; Sun, D.; Jia, H.; Liu, X. MOF-Derived FeS/C Nanosheets for High Performance Lithium Ion Batteries. Nanomaterials 2019, 9, 492. https://doi.org/10.3390/nano9040492
Zhao J, Hu Z, Sun D, Jia H, Liu X. MOF-Derived FeS/C Nanosheets for High Performance Lithium Ion Batteries. Nanomaterials. 2019; 9(4):492. https://doi.org/10.3390/nano9040492
Chicago/Turabian StyleZhao, Jianguo, Zhuan Hu, Dezhu Sun, Hong Jia, and Xianming Liu. 2019. "MOF-Derived FeS/C Nanosheets for High Performance Lithium Ion Batteries" Nanomaterials 9, no. 4: 492. https://doi.org/10.3390/nano9040492
APA StyleZhao, J., Hu, Z., Sun, D., Jia, H., & Liu, X. (2019). MOF-Derived FeS/C Nanosheets for High Performance Lithium Ion Batteries. Nanomaterials, 9(4), 492. https://doi.org/10.3390/nano9040492