Wall Thickness of Industrial Multi-Walled Carbon Nanotubes Is Not a Crucial Factor for Their Degradation by Sodium Hypochlorite
Abstract
1. Introduction
2. Materials and Methods
2.1. MWCNTs
2.2. Incubation of MWCNTs with Hypochlorite
2.3. Electron Microscopy Analysis
2.4. Morphometric Analysis
2.5. Raman Spectroscopy
2.6. Energy-Dispersive X-Ray Spectroscopy (EDS)
3. Results and Discussion
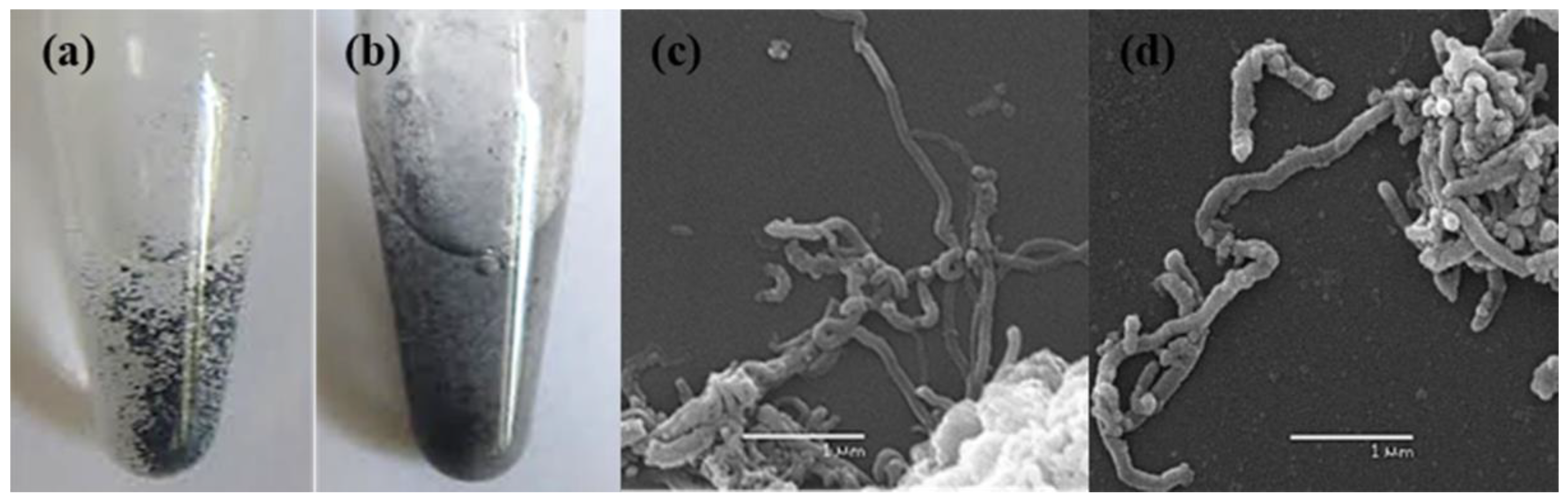
3.1. Aqueous Suspension of MWCNTs
3.2. Characterization of Intact MWCNTs by Scanning Electron Microscopy
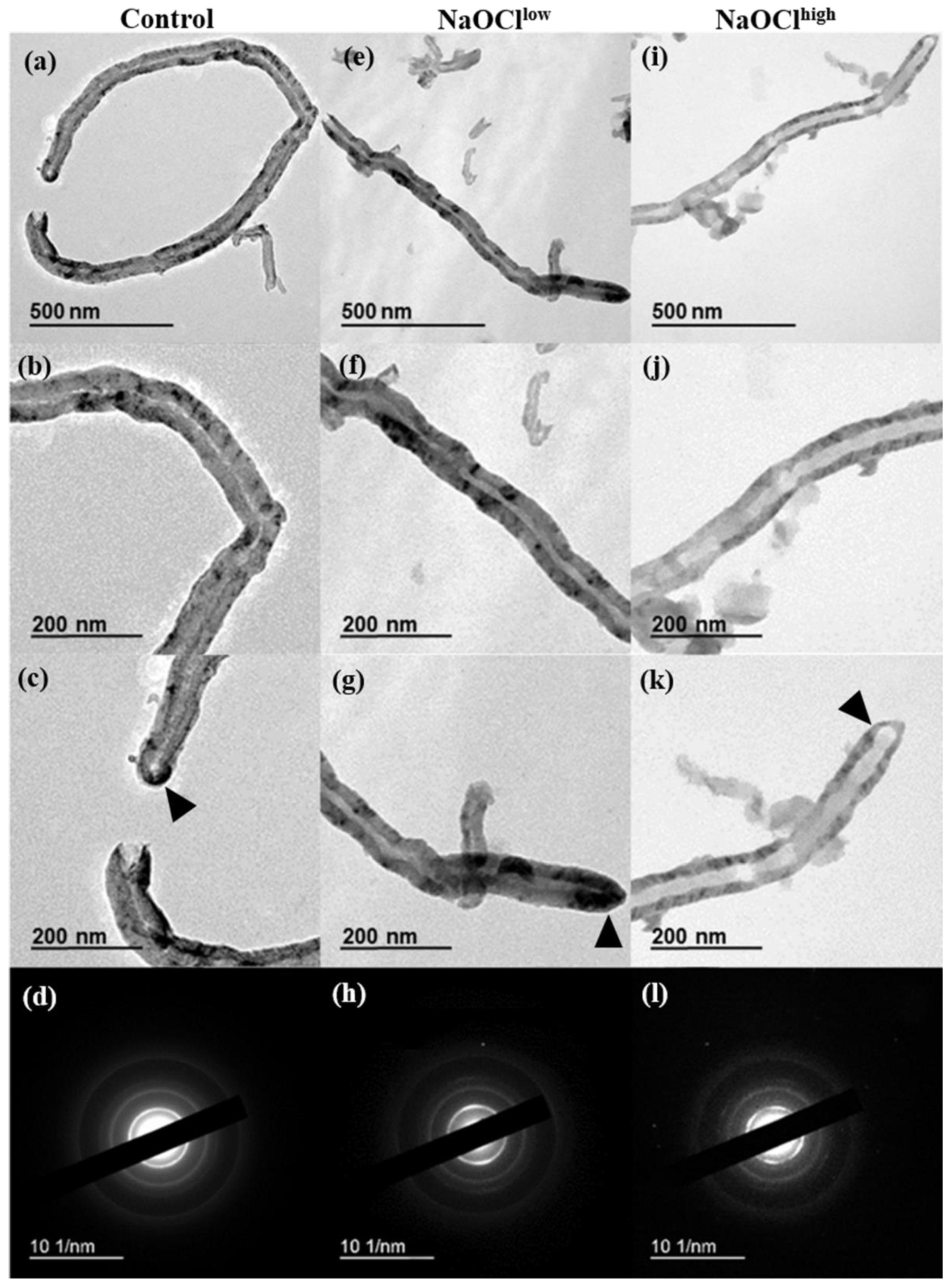
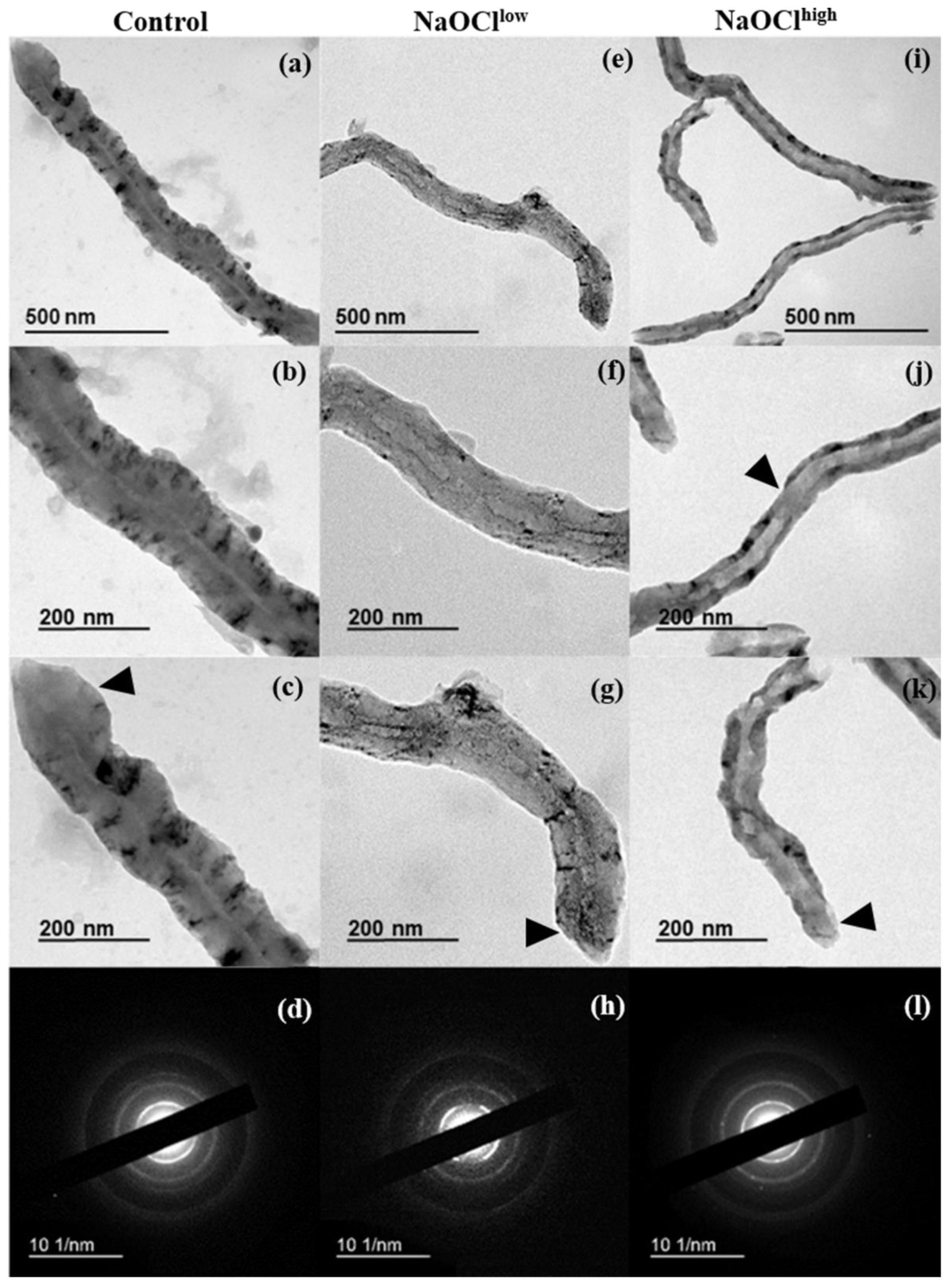
3.3. Characterization of Hypochlorite-Induced Degradation of MWCNTs by Transmission Electron Microscopy (TEM)
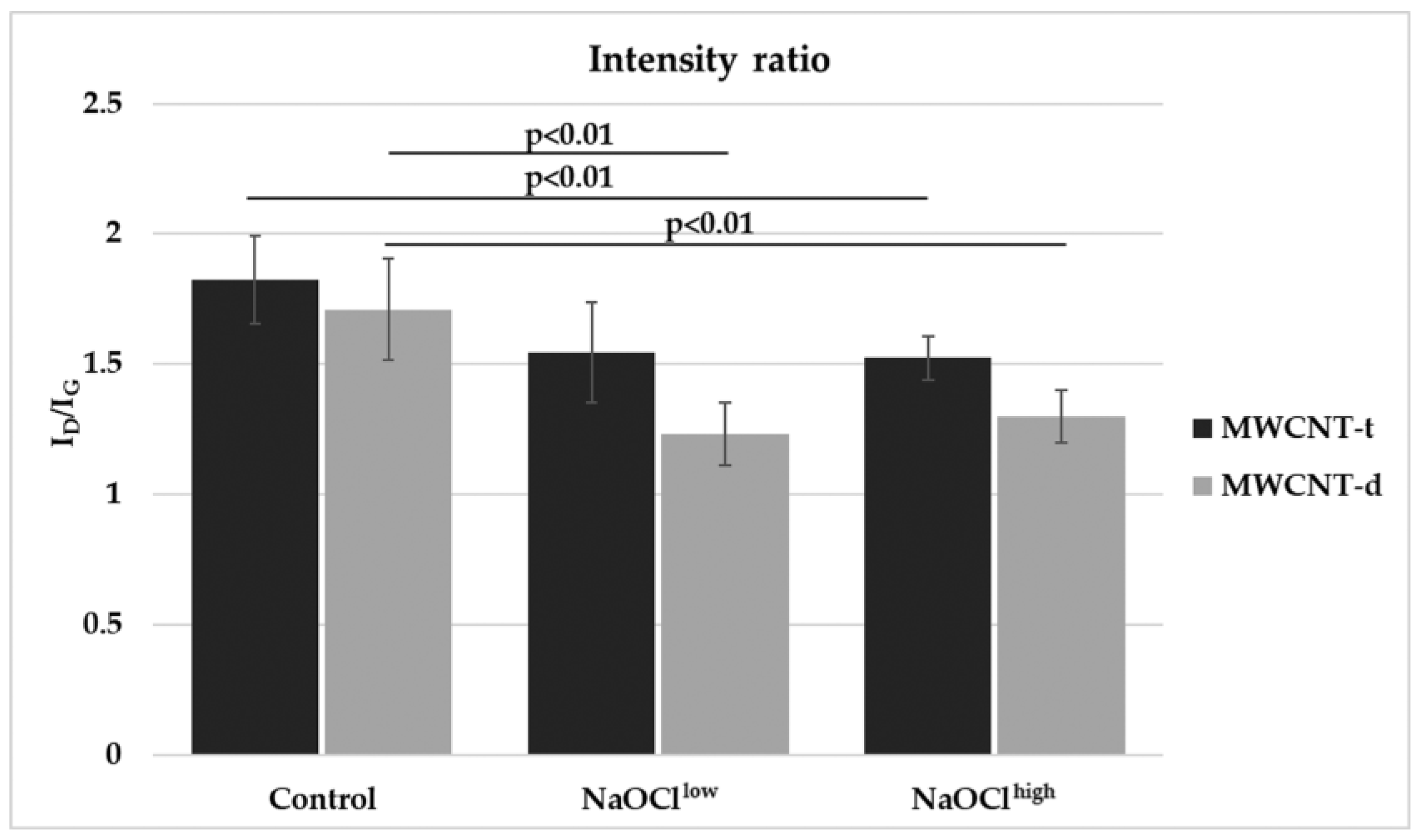
3.4. Characterization of Hypochlorite-Induced Degradation of MWCNTs Using Raman Spectroscopy
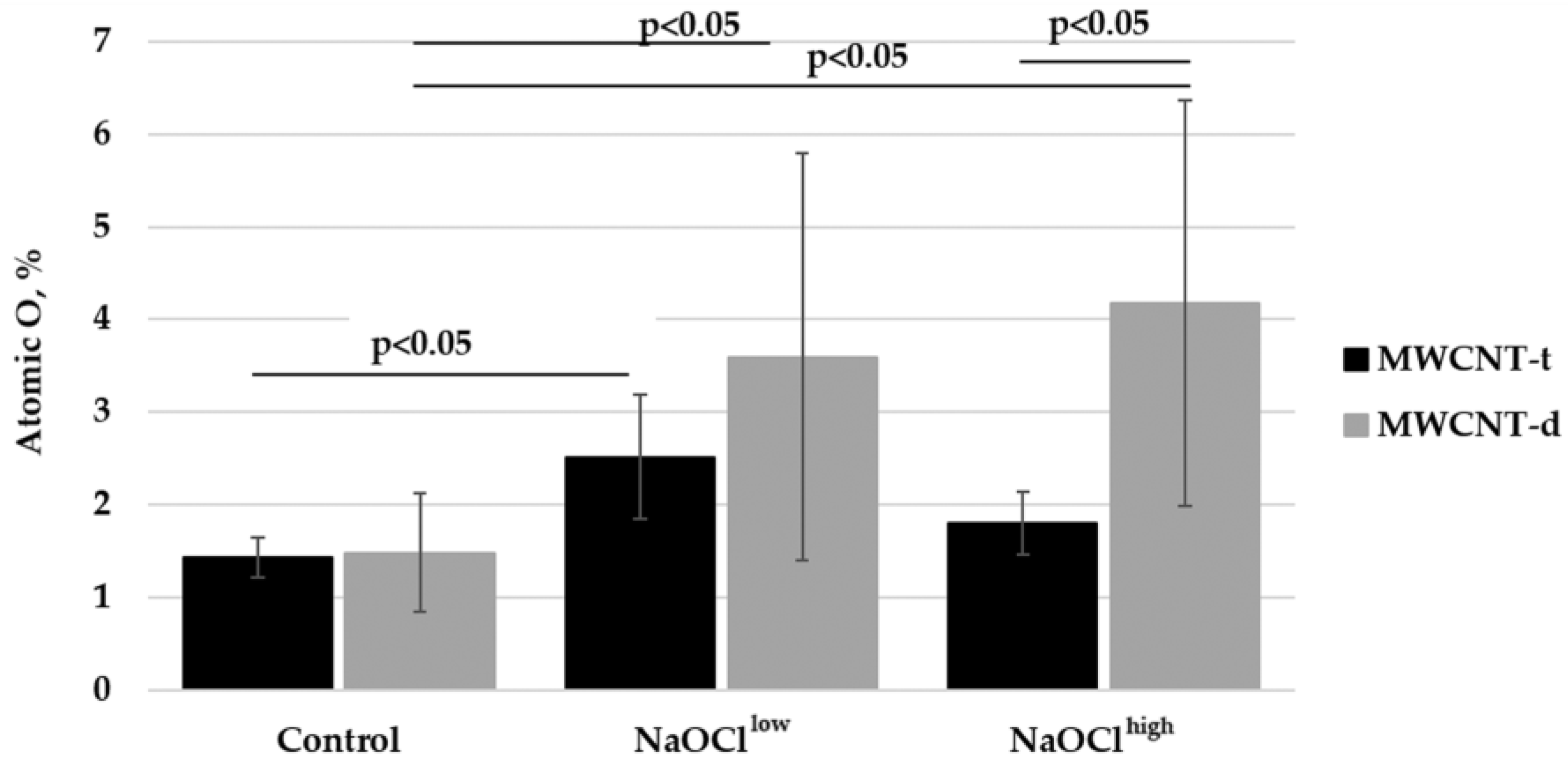
3.5. Characterization of Hypochlorite-Induced Degradation of MWCNTs Using Energy-Dispersive X-Ray Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kausar, A.; Rafique, I.; Muhammad, B. Review of applications of polymer/carbon nanotubes and epoxy/CNT composites. Polym. Plast. Technol. Eng. 2016, 55, 1167–1191. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Wang, Y.; Zhang, Q. Roles of carbon nanotubes in novel energy storage devices. Carbon 2017, 122, 462–474. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, Y.; Zhang, M.; Haniu, H.; Okamoto, M.; Aoki, K.; Takizawa, T.; Yoshida, K.; Sobajima, A.; Kamanaka, T.; et al. In vitro and In vivo evaluation of a three-dimensional porous multi-walled carbon nanotube scaffold for bone regeneration. Nanomaterials 2017, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tao, L.; Wen, S.; Hou, W.; Shi, X. Hyaluronic acid-modified multiwalled carbon nanotubes for targeted delivery of doxorubicin into cancer cells. Carbohydr. Res. 2015, 405, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Andón, F.T.; El-Sayed, R.; Fadeel, B. Mechanisms of carbon nanotube-induced toxicity: Focus on pulmonary inflammation. Adv. Drug Deliv. Rev. 2013, 65, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Fatkhutdinova, L.M.; Khaliullin, T.O.; Vasil’yeva, O.L.; Zalyalov, R.R.; Mustafin, I.G.; Kisin, E.R.; Birch, M.E.; Yanamala, N.; Shvedova, A.A. Fibrosis biomarkers in workers exposed to MWCNTs. Toxicol. Appl. Pharmacol. 2016, 299, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Kichambare, P.D.; Gou, P.; Vlasova, I.I.; Kapralov, A.A.; Konduru, N.; Kagan, V.E.; Star, A. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008, 8, 2903–3899. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Konduru, N.V.; Feng, W.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 2010, 5, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, I.I.; Kapralov, A.A.; Michael, Z.P.; Burkert, S.C.; Shurin, M.R.; Star, A.; Shvedova, A.A.; Kagan, V.E. Enzymatic oxidative biodegradation of nanoparticles: Mechanisms, significance and applications. Toxicol. Appl. Pharmacol. 2016, 299, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Kotchey, G.P.; Chen, Y.; Yanamala, N.V.K.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 17194–17205. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; El-Sayed, R.; Andón, F.T.; Mukherjee, S.P.; Gregory, J.; Li, H.; Zhao, Y.; Seo, W.; Fornara, A.; Brandner, B.; et al. Lactoperoxidase-mediated degradation of single-walled carbon nanotubes in the presence of pulmonary surfactant. Carbon 2015, 91, 506–517. [Google Scholar] [CrossRef]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [PubMed]
- Weiss, S.J.; Klein, R.; Slivka, A.; Wei, M. Chlorination of taurine by human neutrophils: Evidence for hypochlorous acid generation. J. Clin. Investig. 1982, 70, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, I.I.; Vakhrusheva, T.V.; Sokolov, A.V.; Kostevich, V.A.; Gusev, A.A.; Gusev, S.A.; Melnikova, V.I.; Lobach, A.S. PEGylated single-walled carbon nanotubes activate neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Toxicol. Appl. Pharmacol. 2012, 264, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hurt, R.H.; Kane, A.B. Biodurability of single-walled carbon nanotubes depends on surface functionalization. Carbon 2010, 48, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Russier, J.; Ménard-Moyon, C.; Venturelli, E.; Gravel, E.; Marcolongo, G.; Meneghetti, M.; Doris, E.; Bianco, A. Oxidative biodegradation of single- and multi-walled carbon nanotubes. Nanoscale 2011, 3, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G. Defects and Disorder in Carbon Nanotubes; Oxford University Press: Oxford, UK, 2009; pp. 1–73. [Google Scholar]
- Zhao, Y.; Allen, B.L.; Star, A. Enzymatic degradation of multiwalled carbon nanotubes. J. Phys. Chem. A 2011, 115, 9536–9544. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.; Lozano, N.; Zhang, M.; Iijima, S.; Yudasaka, M.; Bussy, C.; Kostarelos, K. Hypochlorite degrades 2D graphene oxide sheets faster than 1D oxidised carbon nanotubes and nanohorns. npj 2D Mater. Appl. 2017, 1, 39. [Google Scholar] [CrossRef]
- Elgrabli, D.; Dachraoui, W.; Ménard-Moyon, C.; Liu, X.J.; Bégin, D.; Bégin-Colin, S.; Bianco, A.; Gazeau, F.; Alloyeau, D. Carbon nanotube degradation in macrophages: Live nanoscale monitoring and understanding of biological pathway. ACS Nano 2015, 9, 10113–10124. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, B.; Bianco, A.; Ménard-Moyon, C. Designing multimodal carbon nanotubes by covalent multi-functionalization. Nanoscale 2016, 8, 18596–18611. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, A.R.; Kurapati, R.; Russier, J.; Ménard-Moyon, C.; Bartolini, I.; Meneghetti, M.; Kostarelos, K.; Bianco, A. Degradation-by-design: Surface modification with functional substrates that enhance the enzymatic degradation of carbon nanotubes. Biomaterials 2015, 72, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Modugno, G.; Ksar, F.; Battigelli, A.; Russier, J.; Lonchambon, P.; Eleto Da Silva, E.; Ménard-Moyon, C.; Soula, B.; Galibert, A.M.; Pinault, M.; et al. A comparative study on the enzymatic biodegradability of covalently functionalized double- and multi-walled carbon nanotubes. Carbon 2016, 100, 367–374. [Google Scholar] [CrossRef]
- Bussy, C.; Hadad, C.; Prato, M.; Bianco, A.; Kostarelos, K. Intracellular degradation of chemically functionalized carbon nanotubes using a long-term primary microglial culture model. Nanoscale 2016, 8, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Bussy, C.; Gherardini, L.; Meneghetti, M.; Herrero, M.A.; Bianco, A.; Prato, M.; Pizzorusso, T.; Al-Jamal, K.T.; Kostarelos, K. In vivo degradation of functionalized carbon nanotubes after stereotactic administration in the brain cortex. Nanomedicine 2012, 7, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.F.; Martinez, D.S.T.; Paula, A.J.; Silveira, J.V.; Alves, O.L.; Souza Filho, A.G. Temperature effects on the nitric acid oxidation of industrial grade multiwalled carbon nanotubes. J. Nanopart. Res. 2013, 15, 1761. [Google Scholar] [CrossRef]
- Masyutin, A.; Erokhina, M.; Sychevskaya, K.; Gusev, A.; Vasyukova, I.; Smirnova, E.; Onishchenko, G. Multi-walled carbon nanotubes: Biodegradation by gastric agents in vitro and effect on murine intestinal system. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 3rd International Youth Conference on Interdisciplinary Problems of Nanotechnology, Biomedicine and Nanotoxicology (Nanobiotech 2015), Tambov, Russia, 21–22 May 2015; IOP Publishing Ltd.: Bristol, UK; Volume 98.
- Wu, C.H. Studies of the equilibrium and thermodynamics of the adsorption of Cu2+ onto as-produced and modified carbon nanotubes. J. Colloid Interface Sci. 2007, 311, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Yen, C.H.; Wang, W.J.; Horng, J.J.; Tsai, Y.P. Assessment of adequate sodium hypochlorite concentration for pre-oxidization of multi-walled carbon nanotubes. J. Chem. Technol. Biotechnol. 2010, 85, 699–707. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I. Variations of interlayer spacing in carbon nanotubes. RSC Adv. 2014, 4, 30807–30815. [Google Scholar] [CrossRef]
- Gerard Lavin, J.; Subramoney, S.; Ruoff, R.S.; Berber, S.; Tománek, D. Scrolls and nested tubes in multiwall carbon nanotubes. Carbon 2002, 40, 1123–1130. [Google Scholar] [CrossRef]
- Elgrabli, D.; Dachraoui, W.; De Marmier, H.; Ménard-Moyon, C.; Bégin, D.; Bégin-Colin, S.; Bianco, A.; Alloyeau, D.; Gazeau, F. Intracellular degradation of functionalized carbon nanotube/iron oxide hybrids is modulated by iron via Nrf2 pathway. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, D.; Chatelain, A.; de Heer, W. Nanocapillarity and Chemistry in Carbon Nanotubes. Science 1996, 274, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Iyer, P.K.; Giri, P.K. Diameter dependence of oxidative stability in multiwalled carbon nanotubes: Role of defects and effect of vacuum annealing. J. Appl. Phys. 2010, 108, 084313. [Google Scholar] [CrossRef]
- Lalwani, G.; Xing, W.; Sitharaman, B. Enzymatic degradation of oxidized and reduced graphene nanoribbons by lignin peroxidase. J. Mater. Chem. B 2014, 2, 6354–6362. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bai, Y.; Zhang, Y.; Sun, M.; Cheng, R.; Xu, X.; Chen, Y.; Mo, Y. Effect of hydroxyl radical on the structure of multi-walled carbon nanotubes. Synth. Met. 2005, 155, 509–515. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006, 92, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.; Mast, J.; De Temmerman, P.-J. Multi-Walled Carbon Nanotubes, Nm-400, Nm-401, Nm-402, Nm-403: Characterisation And Physico-Chemical Properties; Publications Office of the European Union: Ispra, Italy, 2014. [Google Scholar]
- Kagan, V.E.; Tyurina, Y.Y.; Tyurin, V.A.; Konduru, N.V.; Potapovich, A.I.; Osipov, A.N.; Kisin, E.R.; Schwegler-Berry, D.; Mercer, R.; Castranova, V.; et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: Role of iron. Toxicol. Lett. 2006, 165, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Pulskamp, K.; Diabaté, S.; Krug, H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Aldieri, E.; Fenoglio, I.; Cesano, F.; Gazzano, E.; Gulino, G.; Scarano, D.; Attanasio, A.; Mazzucco, G.; Ghigo, D.; Fubini, B. The role of iron impurities in the toxic effects exerted by short multiwalled carbon nanotubes (MWCNT) in murine alveolar macrophages. J. Toxicol. Environ. Health Part A 2013, 76, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Huang, S.; Wu, C. Multi-walled carbon nanotube structural instability with/without metal nanoparticles under electron beam irradiation. New J. Phys. 2017, 19, 123016. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Hong, C.K.; Shim, S.E. Measurement of the dispersion stability of pristine and surface-modified multiwalled carbon nanotubes in various nonpolar and polar solvents. Meas. Sci. Technol. 2007, 18, 3707–3712. [Google Scholar] [CrossRef]
- Kuroda, C.; Haniu, H.; Ajima, K.; Tanaka, M.; Sobajima, A.; Ishida, H.; Tsukahara, T.; Matsuda, Y.; Aoki, K.; Kato, H.; et al. The dispersion state of tangled multi-walled carbon nanotubes affects their cytotoxicity. Nanomaterials 2016, 6, 219. [Google Scholar] [CrossRef] [PubMed]
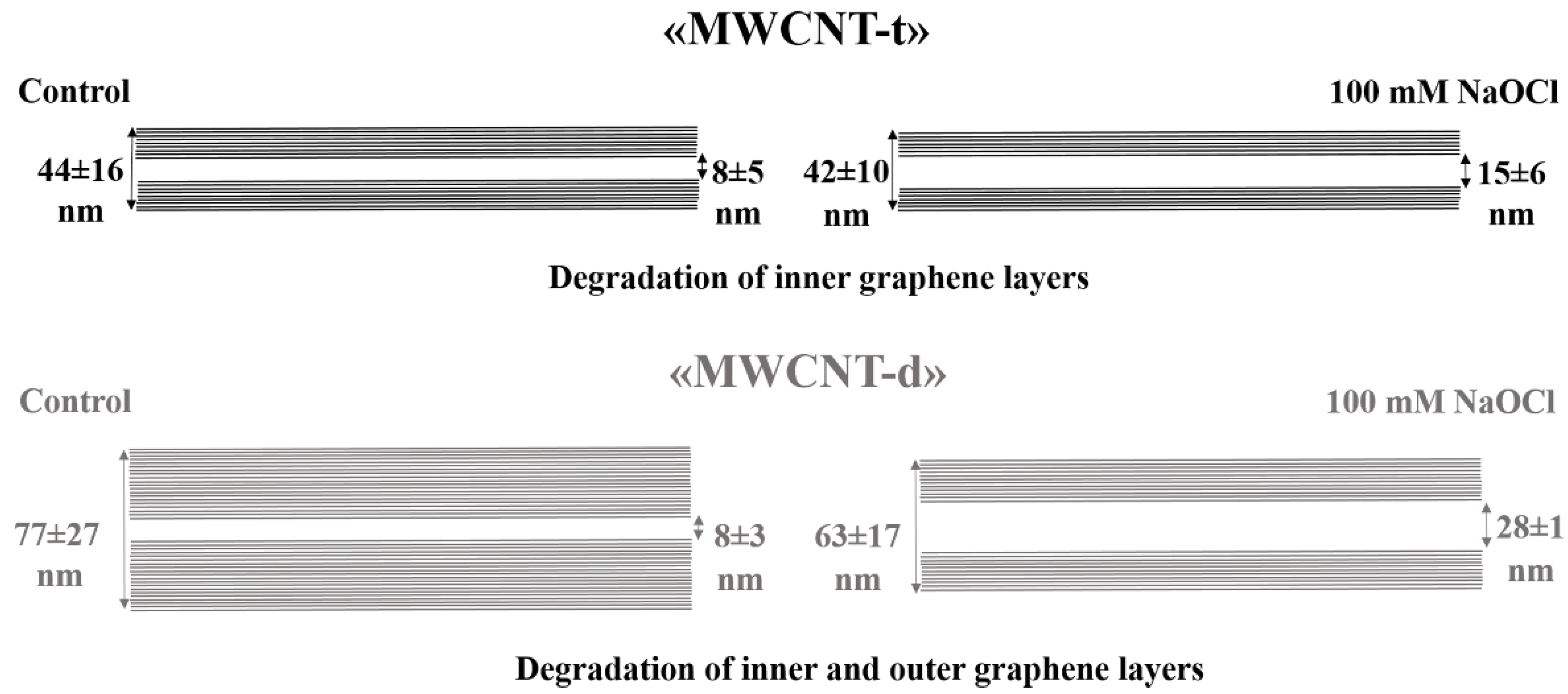
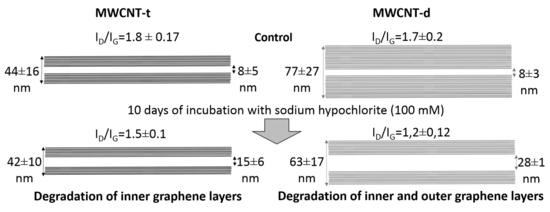
| Parameter | Dout, nm | Din, nm | Avg. Wall Thickness, nm (%) | |||
|---|---|---|---|---|---|---|
| Sample | MWCNT-t | MWCNT-d | MWCNT-t | MWCNT-d | MWCNT-t | MWCNT-d |
| Control | 44 ± 16 | 77 ± 27 | 8 ± 5 * | 8 ± 3 # | 18 ± 11 (100%) | 35 ± 12 (100%) |
| NaOCllow | 44 ± 12 | 76 ± 22 $ | 12 ± 6 * | 13 ± 3 # | 16 ± 9 (89%) | 32 ± 13 (91%) |
| NaOClhigh | 42 ± 10 | 63 ± 17 $ | 15 ± 6 * | 28 ± 1 # | 14 ± 8 (78%) | 18 ± 9 (51%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masyutin, A.G.; Bagrov, D.V.; Vlasova, I.I.; Nikishin, I.I.; Klinov, D.V.; Sychevskaya, K.A.; Onishchenko, G.E.; Erokhina, M.V. Wall Thickness of Industrial Multi-Walled Carbon Nanotubes Is Not a Crucial Factor for Their Degradation by Sodium Hypochlorite. Nanomaterials 2018, 8, 715. https://doi.org/10.3390/nano8090715
Masyutin AG, Bagrov DV, Vlasova II, Nikishin II, Klinov DV, Sychevskaya KA, Onishchenko GE, Erokhina MV. Wall Thickness of Industrial Multi-Walled Carbon Nanotubes Is Not a Crucial Factor for Their Degradation by Sodium Hypochlorite. Nanomaterials. 2018; 8(9):715. https://doi.org/10.3390/nano8090715
Chicago/Turabian StyleMasyutin, Alexander G., Dmitry V. Bagrov, Irina I. Vlasova, Igor I. Nikishin, Dmitry V. Klinov, Ksenia A. Sychevskaya, Galina E. Onishchenko, and Maria V. Erokhina. 2018. "Wall Thickness of Industrial Multi-Walled Carbon Nanotubes Is Not a Crucial Factor for Their Degradation by Sodium Hypochlorite" Nanomaterials 8, no. 9: 715. https://doi.org/10.3390/nano8090715
APA StyleMasyutin, A. G., Bagrov, D. V., Vlasova, I. I., Nikishin, I. I., Klinov, D. V., Sychevskaya, K. A., Onishchenko, G. E., & Erokhina, M. V. (2018). Wall Thickness of Industrial Multi-Walled Carbon Nanotubes Is Not a Crucial Factor for Their Degradation by Sodium Hypochlorite. Nanomaterials, 8(9), 715. https://doi.org/10.3390/nano8090715