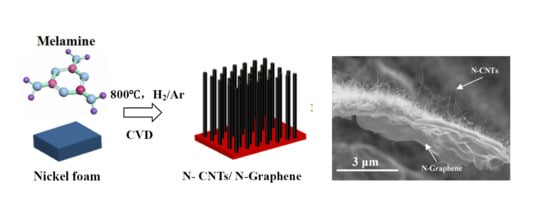
One-Step Chemical Vapor Deposition Synthesis of 3D N-doped Carbon Nanotube/N-doped Graphene Hybrid Material on Nickel Foam
Abstract
1. Introduction
2. Materials and Methods
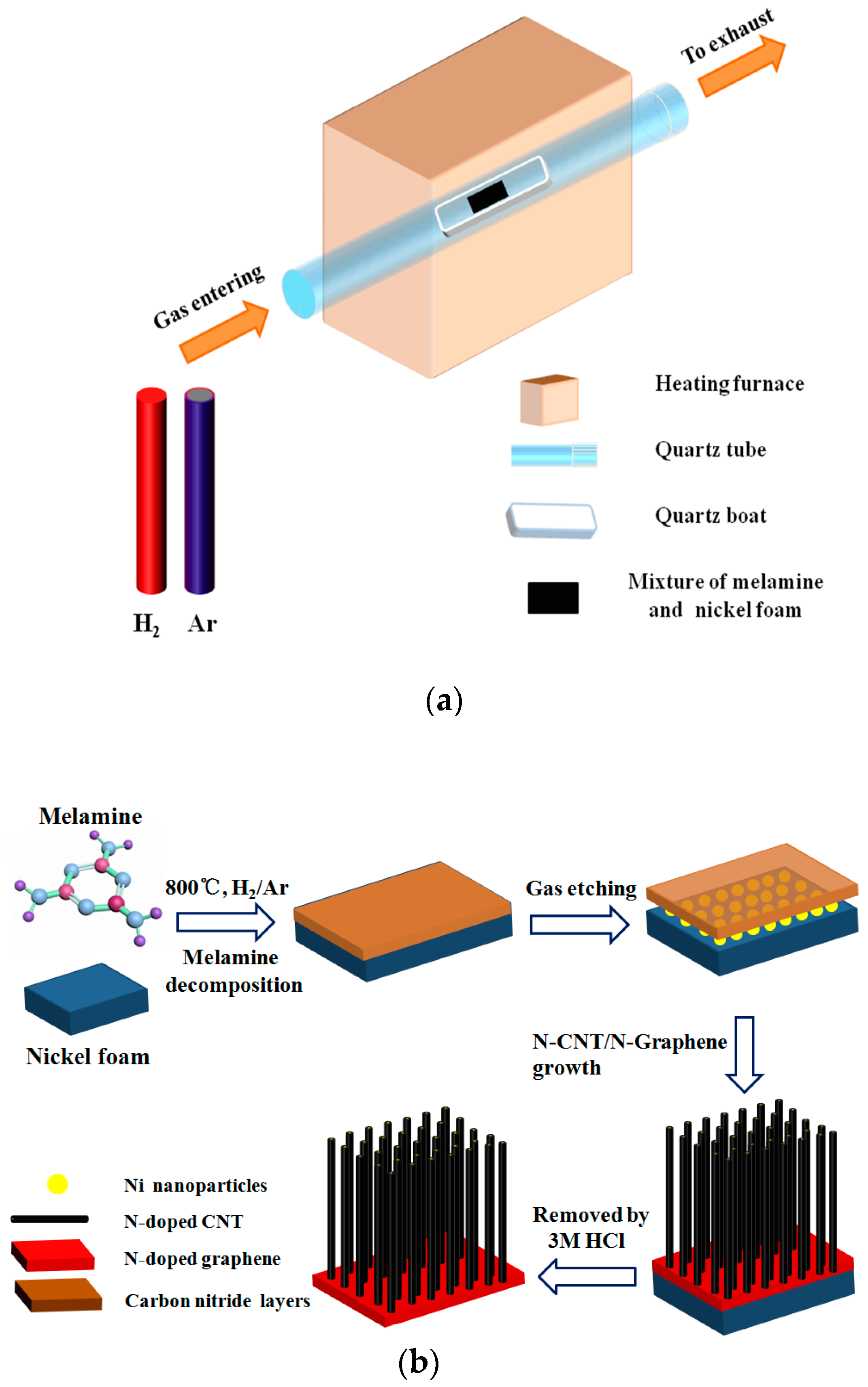
2.1. Synthetic Procedures of N-CNTs/N-Graphene
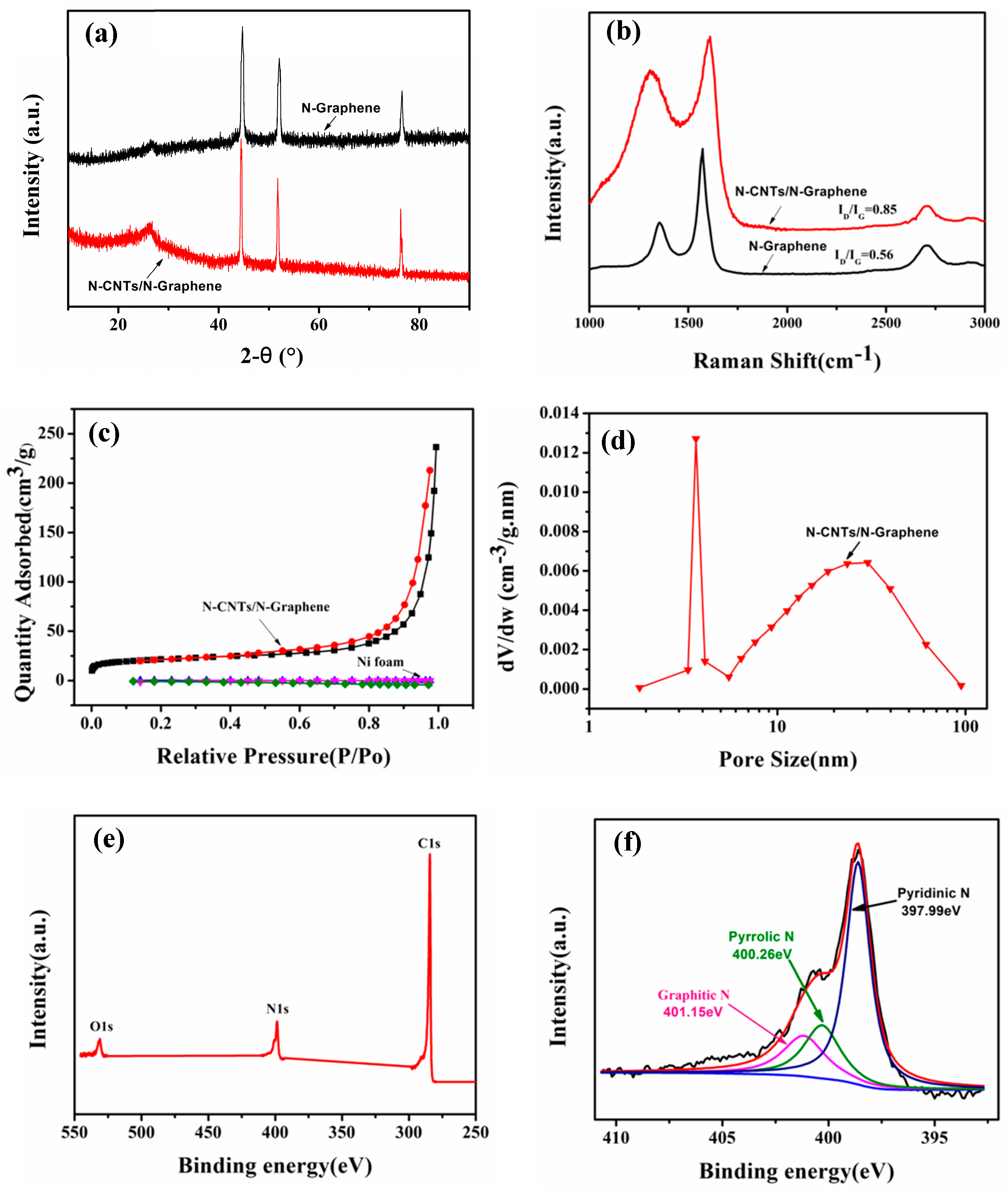
2.2. Characterization
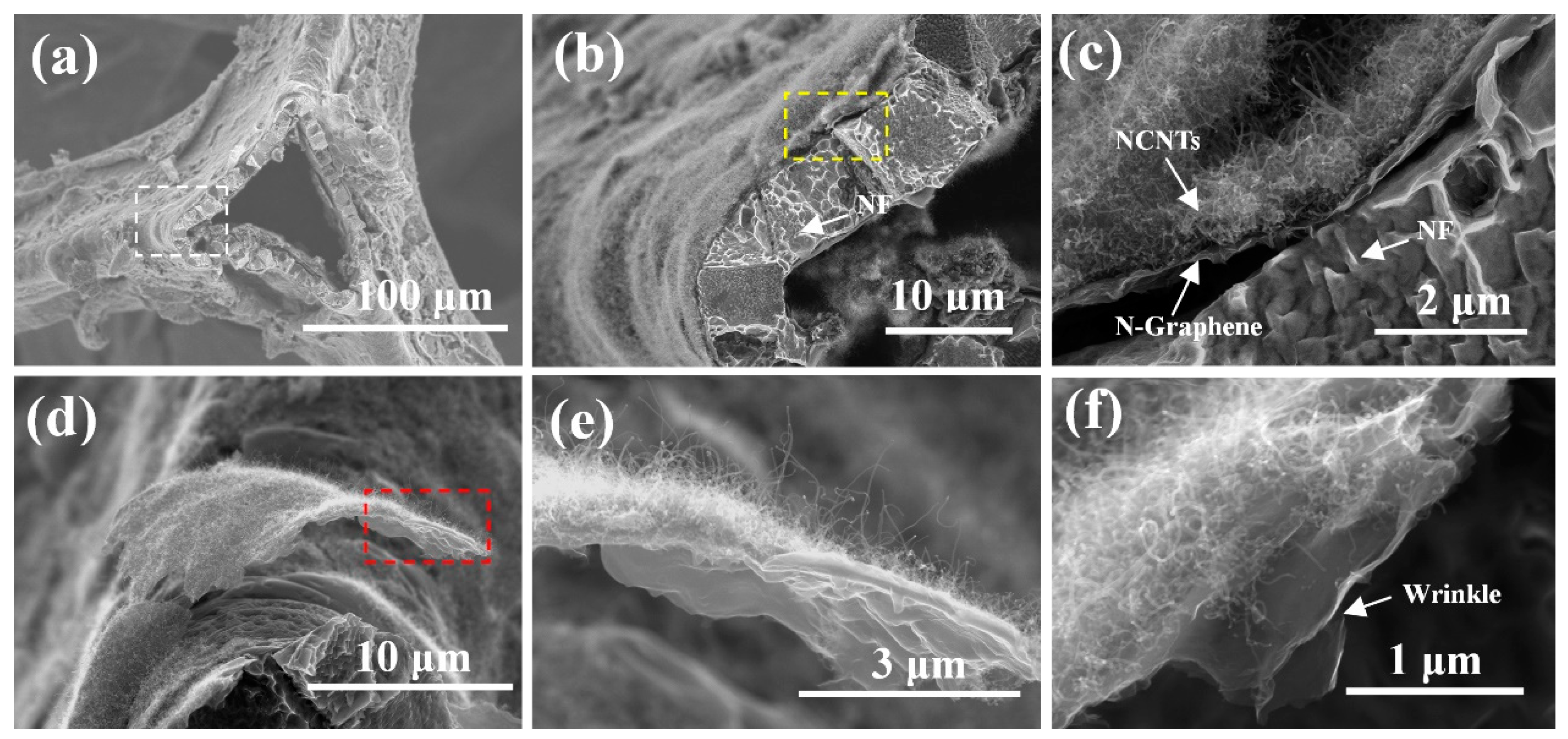
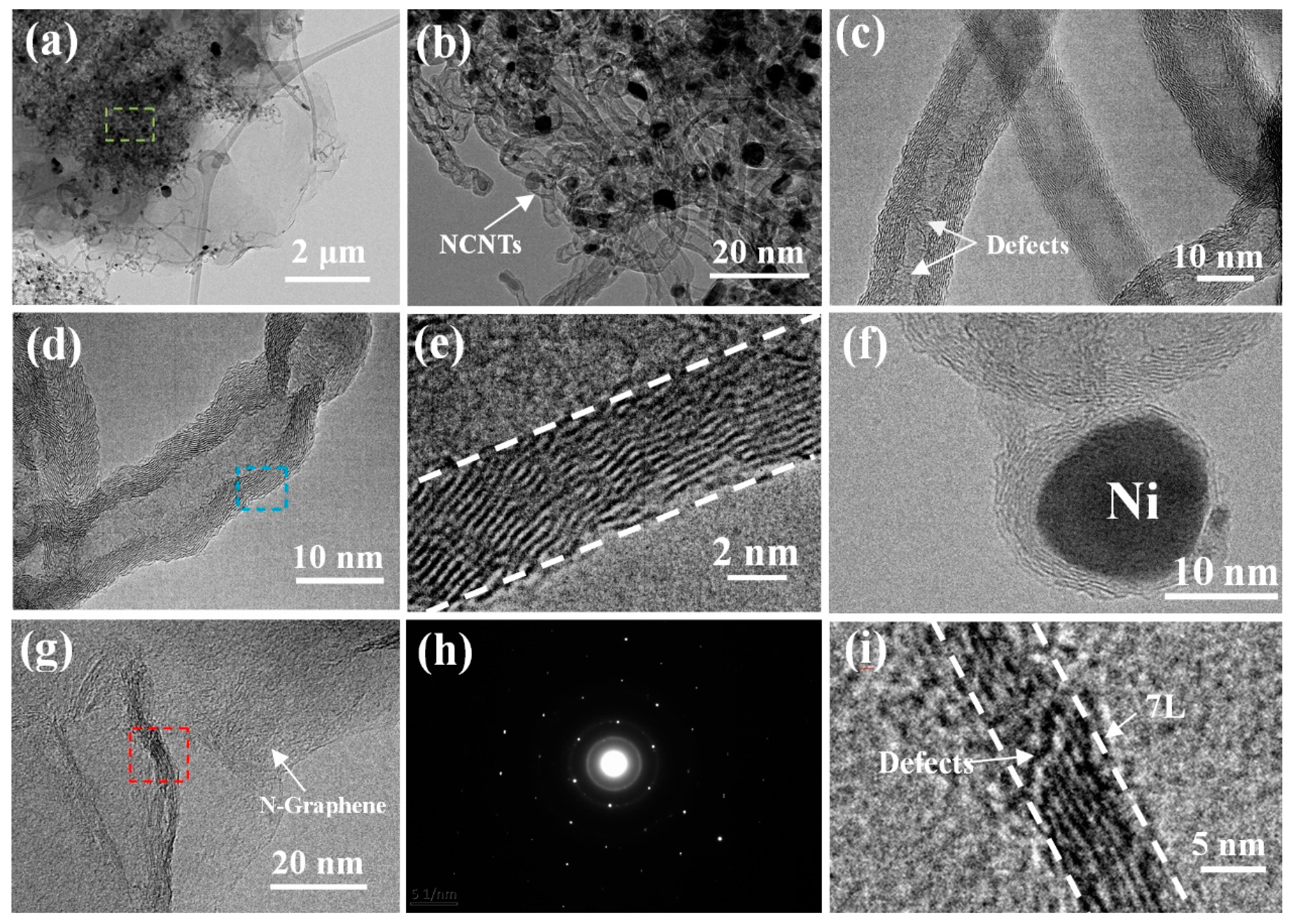
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fan, H.; Yang, k.; Boye, D.M.; Sigmon, T.; Malloy, K.J.; Xu, H.; Lopez, G.P.; Brinker, C.J. Self-Assembly of ordered, robust, three-dimensional gold Nanocrystal/Silica arrays. Science 2004, 304, 567. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, W.X.; Suk, J.W.; Potts, J.W.; Ruoff, R.S. Graphene and graphene oxide: synthesis, properties and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Bunch, J.S.; Am, V.D.; Verbridge, S.S.; Frank, I.W.; Tanenbaum, D.M.; Parpia, J.M.; Craighead, H.G.; Mceuen, P.L. Electromechanical resonators from graphene sheets. Science 2007, 315, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Ma, L.; Zhu, Y.; Lahiri, I.; Hahm, M.G.; Liu, Z.; Yang, S.; Xiang, C.; Lu, W.; Peng, Z. Three-dimensional metal-graphene-nanotube multifunctional hybrid materials. ACS Nano 2013, 7, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.J.; Yan, J.; Zhi, L.J.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.L.; Qian, W.Z.; Fei, W. A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.L.; Kopold, P.; Hahn, K.; van Aken, P.A.; Maier, J.; Yu, Y. Facile Solid-State Growth of 3D Well-Interconnected Nitrogen-Rich Carbon Nanotube-Graphene Hybrid Architectures for Lithium–Sulfur Batteries. Adv. Funct. Mater. 2016, 26, 1112–1119. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruo, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Wu, P.; Wang, C.X.; Fan, X.D.; Zhang, W.; Zhai, X.; Zeng, C.; Li, Z.; Yang, J.; Hou, J.G.; et al. Low-temperature growth of graphene by chemical vapor deposition using solid and liquid carbon sources. ACS Nano 2011, 5, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Kumar, K.; Fisher, F.T.; Yang, E.-H. Out-of-plane growth of CNTs on graphene for supercapacitor applications. Nanotechnology 2012, 23, 15301–15307. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Shen, S.; Zhuang, J.; Wang, X. Noble-metal-promoted three-dimensional macroassembly of single-layered graphene oxide. Angew. Chem. Int. Ed. 2010, 49, 4603–4607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Zhang, T.; Chang, H.; Xiao, P.; Chen, H.; Huang, Z.; Chen, Y. Broadband and tunable high-performance microwave absorption of an ultralight and highly compressible graphene foam. Adv. Mater. 2015, 27, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; Dileo, R.A.; Raffaelle, R.P. Carbon nanotubes for lithium ion batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Gomez, L.M.; Kumar, A.; Zhang, Y.; Ryu, K.; Badmaev, A.; Zhou, C. Scalable light-induced metal to semiconductor conversion of carbon nanotubes. Nano Lett. 2009, 9, 3592. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium-sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, Y.H.; Lv, W.; Yu, W.J.; Wu, S.; Hou, P.X.; Yang, Q.H.; Meng, Q.B.; Liu, C.; Cheng, H.M. Vertically Aligned Carbon Nanotubes Grown on Graphene Paper as Electrodes in Lithium-Ion Batteries and Dye-Sensitized Solar Cells. Adv. Energy Mater. 2011, 1, 486–490. [Google Scholar] [CrossRef]
- Dai, G.P.; Wu, M.H.; Taylor, D.K.; Brennaman, M.K.; Vinodgopal, K. Hybrid 3D graphene and aligned carbon nanofiber array architectures. RSC Adv. 2012, 2, 8965–8968. [Google Scholar] [CrossRef]
- Lv, R.T.; Cui, T.X.; Jun, M.S.; Zhang, Q.; Cao, A.Y.; Su, D.S.; Zhang, Z.J.; Yoon, S.H.; Miyawaki, J.; Mochida, I.; et al. Open-Ended, N-doped Carbon Nanotube-Graphene Hybrid Nanostructures as High-Performance Catalyst Support. Adv. Funct. Mater. 2015, 21, 999–1006. [Google Scholar] [CrossRef]
- Du, F.; Yu, D.; Dai, L.; Ganguli, S.; Varshney, V.; Roy, A.K. Preparation of Tunable 3D Pillared Carbon Nanotube-Graphene Networks for High-Performance Capacitance. Chem. Mater. 2011, 23, 4810–4816. [Google Scholar] [CrossRef]
- Su, D.; Cortie, M.; Wang, G. Fabrication of N-doped graphene-carbon nanotube hybrids from prussian blue for lithium-sulfur batteries. Adv. Energy Mater. 2017, 7, 1602014. [Google Scholar] [CrossRef]
- Wu, C.; Cai, J.J.; Zhu, Y.; Zhang, K.L. Hybrid reduced graphene oxide nanosheet supported Mn-Ni-Co Ternary Oxides for Aqueous Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 19114–19123. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, X.F.; X, L.Y.; J, P.K. Graphene oxide-encapsulated carbon nanotube hybrids for high dielectric performance nanocomposites with enhanced energy storage density. Nanoscale 2013, 5, 3847–3855. [Google Scholar] [CrossRef] [PubMed]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. BoroN-doped carbon nanotubes as metal-free electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 123, 7270. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2012, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, S.; Ma, Z.; Du, Z.; Li, C.; Song, J.; Wang, G.; Shao, G. Novel nitrogen-doped hierarchically porous coralloid carbon materials as host matrixes for lithium–sulfur batteries. Electrochim. Acta 2015, 159, 8–15. [Google Scholar] [CrossRef]
- Zhu, Y.; L, L.; Zhang, C.; Casillas, G.; Sun, Z.; Yan, Z.; Ruan, G.; Peng, Z.; Raji, A.-R.O.; Kittrell, C.R.; Hauge, R.H.; Tour, J.M. A seamless three-dimensional carbon nanotube /graphene hybrid material. Nat. Commun. 2012, 3, 1225. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.L.; Li, H.F.; Wang, C.; Jing, B.B.; Hu, H.Y.; Xie, N.; Wu, M.H.; Vinodgopal, K.; Dai, G.P. Melamine as a single source for fabrication of mesoscopic 3D composites of N-doped carbon nanotubes on graphene. RSC Adv. 2018, 8, 12157–12164. [Google Scholar] [CrossRef]
- Li, D.J.; Maiti, U.N.; Lim, J.; Choi, D.S.; Lee, W.J.; Oh, Y.; Lee, G.Y.; Kim, S.O. Molybdenum sulfide/N-doped CNT forest hybrid catalysts for high-performance hydrogen evolution reaction. Nano Lett. 2014, 14, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.L.; Ma, Z.K.; Xie, Y.E.; Zhang, M.; Zhao, Na.; Song, H.H. Fe/N-doped graphene with rod-like CNTs as an air-cathode catalyst in microbial fuel cells. RSC Adv. 2018, 8, 1203. [Google Scholar] [CrossRef]
- Samad, Y.A.; Li, Y.Q.; Schiffer, A.; Alhassan, S.M.; Liao, K. Graphene foam developed with a novel two-Step technique for low and high strains and pressure-sensing applications. Small 2015, 20, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Zhang, J.; Shen, L.H.; Ma, Y.M.; Lei, W.W.; Cui, Q.L.; Zou, G.T. Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine. Appl. Phys. A 2009, 94, 387–392. [Google Scholar] [CrossRef]
- Robertson, J. Heterogeneous catalysis model of growth mechanisms of carbon nanotubes, graphene and silicon nanowires. J. Mater. Chem. 2012, 22, 19858. [Google Scholar] [CrossRef]
- Chae, S.J.; Güneş, F.; Kim, K.K.; Kim, E.S.; Han, G.H.; Kim, S.M. Synthesis of Large-Area Graphene Layers on Poly-Nickel Substrate by Chemical Vapor Deposition: Wrinkle Formation. Adv. Mater. 2009, 21, 2328–2333. [Google Scholar] [CrossRef]
- Zhang, S.M.; Zhang, H.Y.; Liu, Q.; Chen, S. Fe-N doped carbon nanotube/graphene composite: Facile synthesis and superior electrocatalytic activity. J. Mater. Chem. A 2013, 1, 3302. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, T.; Nitze, F.; Barzegar, H.R.; Tai, C.W.; Mazurkiewicz, M.; Malolepszy, A.; Stobinski, L.; Wågberg, T. Nitrogen doped multi walled carbon nanotubes produced by CVD-correlating XPS and Raman spectroscopy for the study of nitrogen inclusion. Carbon 2012, 50, 3535. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Barrejón, M.; Primo, A.; Gómez-Escalonilla, M.J.; Fierro, J.L.; García, H.; Barrejón, M. Covalent functionalization of N-doped graphene by N-alkylation. Chem. Commun. 2015, 51, 16916. [Google Scholar]
- Min, S.; Zhao, C.; Chen, G.; Qian, X. One-pot hydrothermal synthesis of reduced graphene oxide/Ni(OH)2, films on nickel foam for high performance supercapacitors. Electrochim. Acta. 2014, 115, 155–164. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 6, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Liu, Y.; Baek, J.B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- De Arco, L.G.; Zhang, Y.; Kumar, A.; Zhou, C. Synthesis, transfer and devices of single- and few-layer graphene by chemical vapor deposition. IEEE Trans. Nanotechnol. 2009, 8, 135–138. [Google Scholar] [CrossRef]
- Wu, H.; Xia, L.; Ren, J.; Zheng, Q.; Xu, C.; Lin, D. A high-efficiency N/P co-doped graphene/CNT@porous carbon hybrid matrix as a cathode host for high performance lithium–sulfur batteries. J. Mater. Chem. A. 2017, 5, 20458–20472. [Google Scholar] [CrossRef]
- Chen, S.; Yeoh, W.; Liu, Q.; Wang, G. Chemical-free synthesis of graphene-carbon nanotube hybrid materials for reversible lithium storage in lithium-ion batteries. Carbon 2012, 50, 4557–4565. [Google Scholar] [CrossRef]
- Czerw, R.; Terrones, M.; Charlier, J.C.; Blase, X.; Foley, B.; Kamalakaran, R.; Grobert, N.; Terrones, H.; Tekleab, D.; Ajayan, P.M.; et al. Identification of electron donor states in N-doped carbon nanotubes. Nano Lett. 2000, 1, 457–460. [Google Scholar] [CrossRef]
- Tian, G.-L.; Zhao, M.-Q.; Yu, D.; Kong, X.-Y.; Huang, J.-Q.; Zhang, Q.; Wei, F. Nitrogen-doped graphene/carbon nanotube hybrids: in-situ formation on bifunctional catalysts and their superior electrocatalytic activity for oxygen evolution/reduction reaction. Small 2014, 11, 2251–2259. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-F.; Wu, F.; Wang, C.; Zhang, P.-X.; Hu, H.-Y.; Xie, N.; Pan, M.; Zeng, Z.; Deng, S.; Wu, M.H.; et al. One-Step Chemical Vapor Deposition Synthesis of 3D N-doped Carbon Nanotube/N-doped Graphene Hybrid Material on Nickel Foam. Nanomaterials 2018, 8, 700. https://doi.org/10.3390/nano8090700
Li H-F, Wu F, Wang C, Zhang P-X, Hu H-Y, Xie N, Pan M, Zeng Z, Deng S, Wu MH, et al. One-Step Chemical Vapor Deposition Synthesis of 3D N-doped Carbon Nanotube/N-doped Graphene Hybrid Material on Nickel Foam. Nanomaterials. 2018; 8(9):700. https://doi.org/10.3390/nano8090700
Chicago/Turabian StyleLi, Hua-Fei, Fan Wu, Chen Wang, Pei-Xin Zhang, Hai-Yan Hu, Ning Xie, Ming Pan, Zheling Zeng, Shuguang Deng, Marvin H. Wu, and et al. 2018. "One-Step Chemical Vapor Deposition Synthesis of 3D N-doped Carbon Nanotube/N-doped Graphene Hybrid Material on Nickel Foam" Nanomaterials 8, no. 9: 700. https://doi.org/10.3390/nano8090700
APA StyleLi, H.-F., Wu, F., Wang, C., Zhang, P.-X., Hu, H.-Y., Xie, N., Pan, M., Zeng, Z., Deng, S., Wu, M. H., Vinodgopal, K., & Dai, G.-P. (2018). One-Step Chemical Vapor Deposition Synthesis of 3D N-doped Carbon Nanotube/N-doped Graphene Hybrid Material on Nickel Foam. Nanomaterials, 8(9), 700. https://doi.org/10.3390/nano8090700