Recent Progress in Upconversion Photodynamic Therapy
Abstract
:1. Introduction
2. Principles of Photosensitization
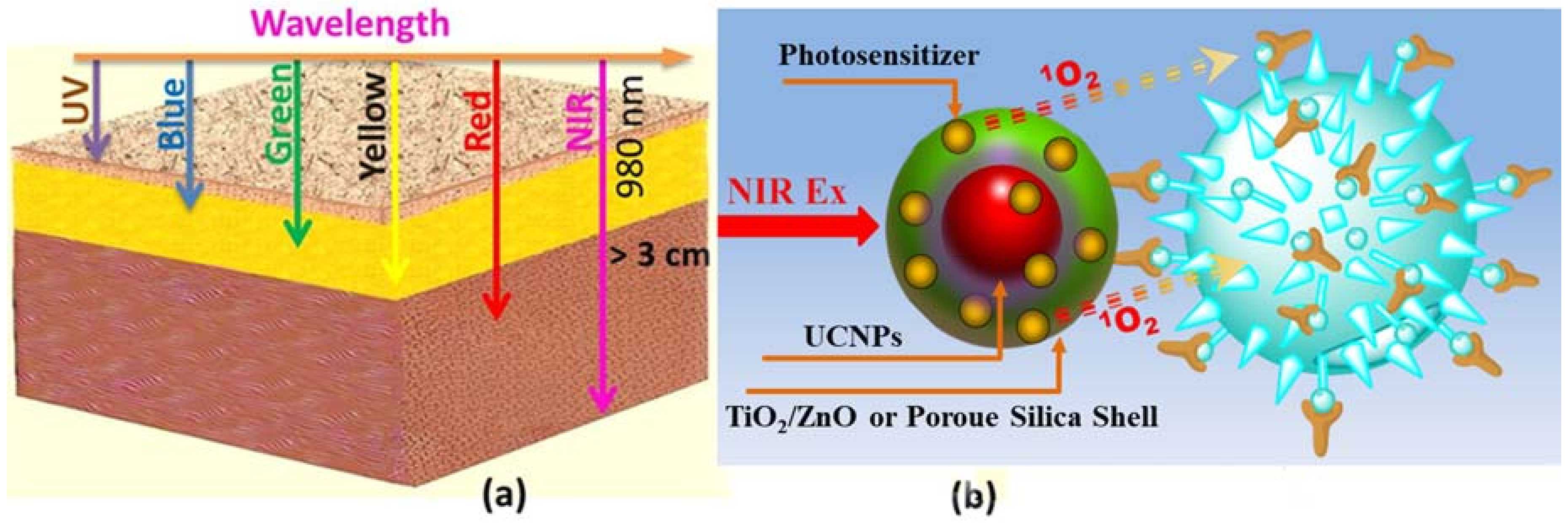
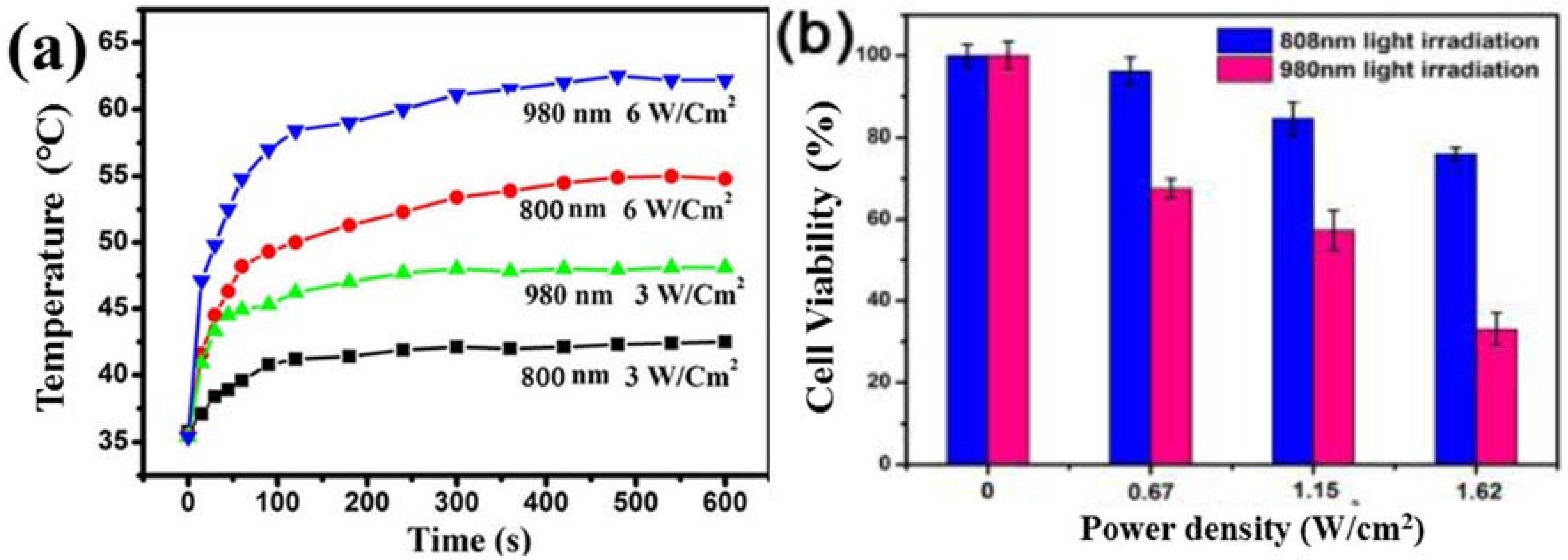
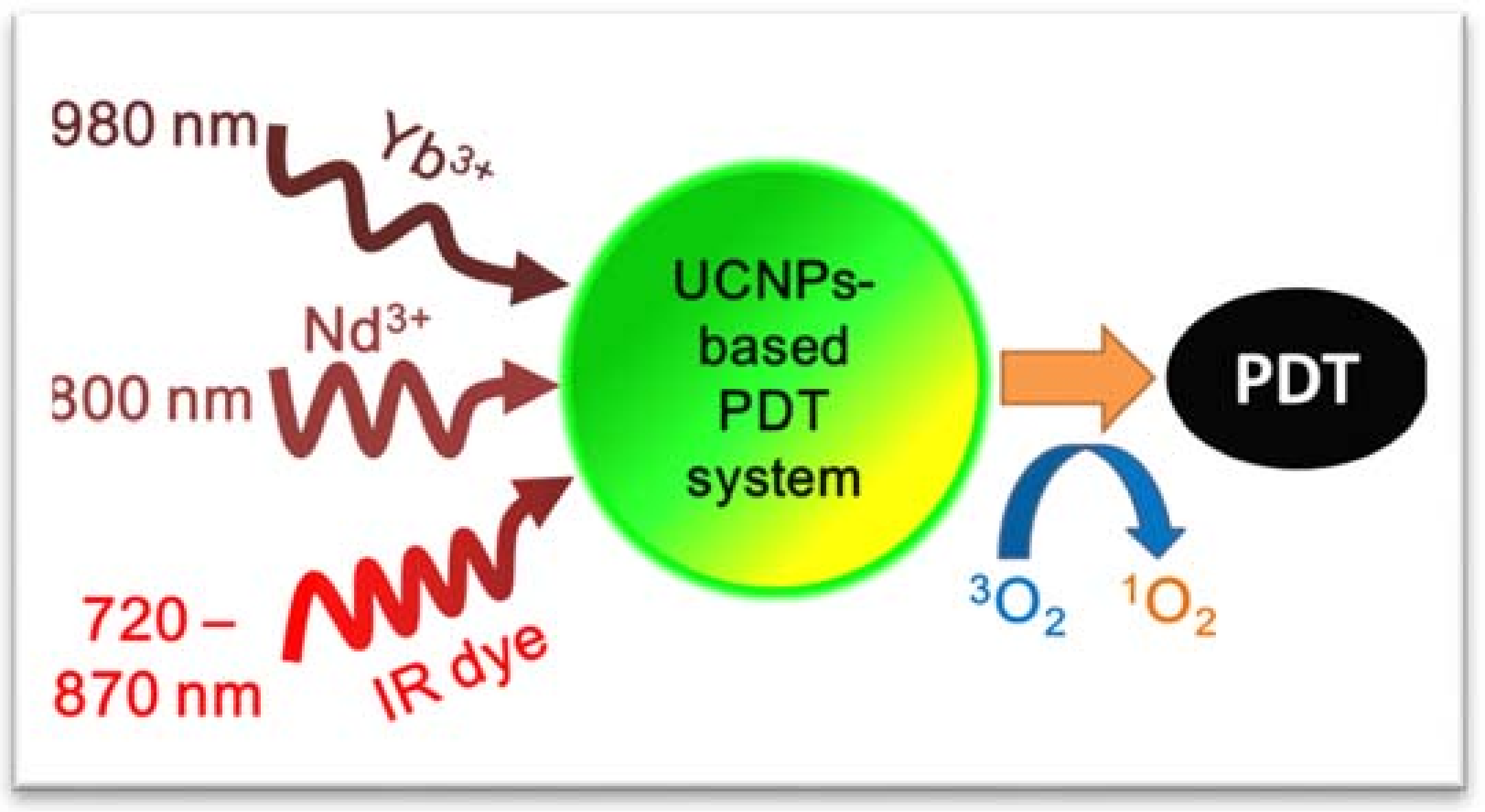
3. Available Excitation Wavelengths to Excite Lanthanide-Doped UCNPs for PDT Application
4. Emission Wavelengths Offered by Lanthanide-Doped UCNPs for PDT Application
5. Surface Modification and Bioconjugation of Lanthanide Doped UCNPs
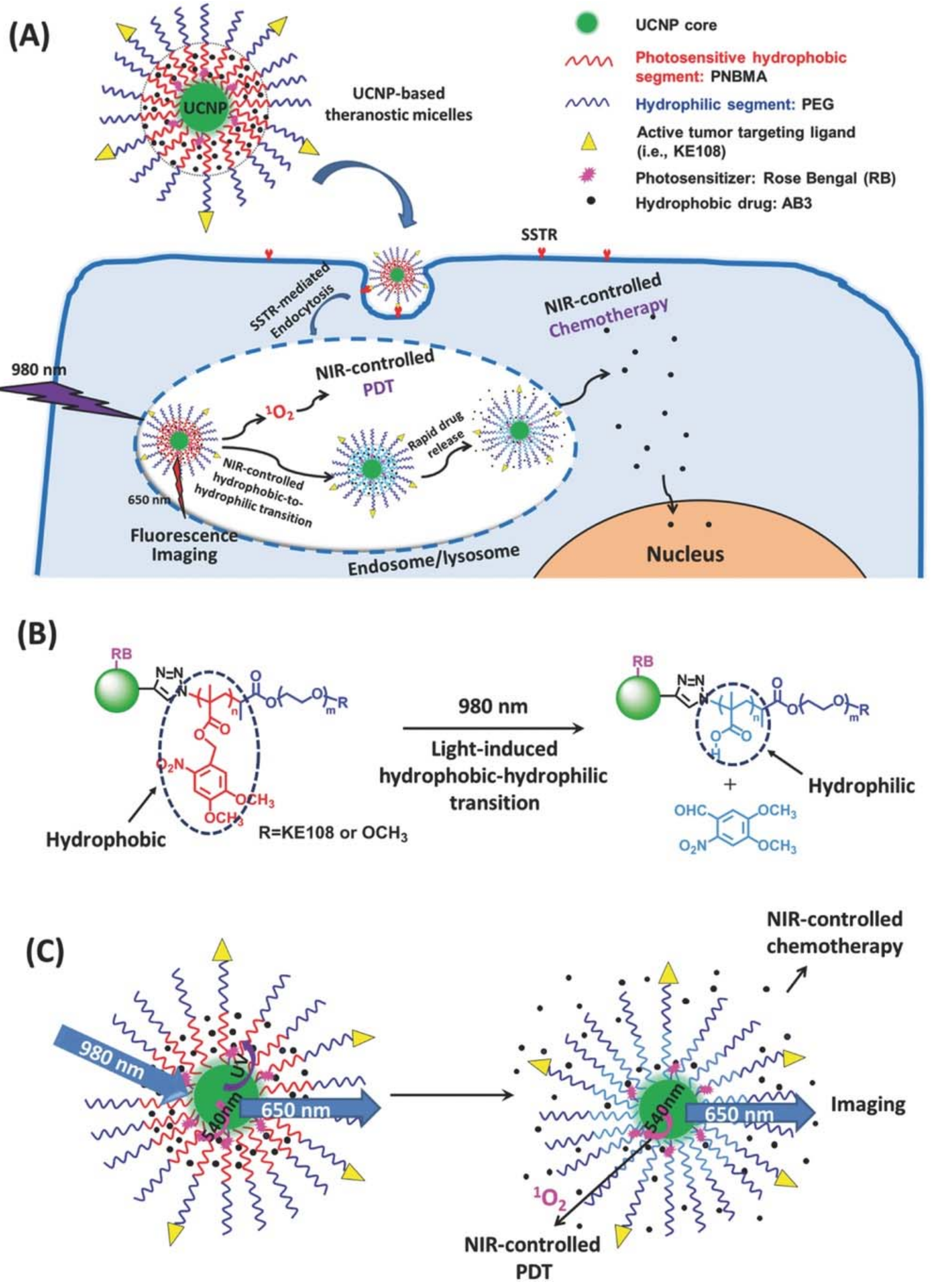
6. In Vitro and In Vivo PDT by Lanthanide Doped UCNPs
7. Conclusions and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Valiev, R.R.; Ohulchanskyy, T.Y.; Agren, H.; Yang, C.H.; Chen, G.Y. Dye-sensitized lanthanide-doped upconversion nanoparticles. Chem. Soc. Rev. 2017, 46, 4150–4167. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.H.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.F.; Wu, S.N.; Wu, B.Y.; Chen, W.R.; Xing, D. Mitochondria-Targeting Single-Walled Carbon Nanotubes for Cancer Photothermal Therapy. Small 2011, 7, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Shafirstein, G.; Bellnier, D.; Oakley, E.; Hamilton, S.; Potasek, M.; Beeson, K.; Parilov, E. Interstitial Photodynamic Therapy—A Focused Review. Cancers 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.N. Introduction to Nanomedcine Nanobioengineering; Wiley-Interscience: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kim, S.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Pandey, R.K.; Prasad, P.N. Organically modified silica nanoparticles co-encapsulating photosensitizing drug and aggregation-enhanced two-photon absorbing fluorescent dye aggregates for two-photon photodynamic therapy. J. Am. Chem. Soc. 2007, 129, 2669–2675. [Google Scholar] [CrossRef] [PubMed]
- He, G.S.; Tan, L.S.; Zheng, Q.; Prasad, P.N. Multiphoton absorbing materials: Molecular designs, characterizations, and applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef] [PubMed]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X.G. Recent Advances in the Chemistry of Lanthanide-Doped Upconversion Nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Schafer, H. Upconverting Nanoparticles. Angew. Chem.-Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Peng, Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003, 23, 3591–3600. [Google Scholar] [PubMed]
- Spikes, J.D. Photodynamic Action: From Paramecium to Photochemotherapy. Photochem. Photobiol. 1997, 65, 142s–147s. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Thiele, J. The role of oxygen in cutaneous photodynamic therapy. Free Radic. Biol. Med. 1998, 24, 835–847. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233, 351–371. [Google Scholar] [CrossRef]
- Niedre, M.; Patterson, M.S.; Wilson, B.C. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem. Photobiol. 2002, 75, 382–391. [Google Scholar] [CrossRef]
- Krasnovsky, A.A. Photoluminescence of singlet oxygen in pigment solutions. Photochem. Photobiol. 1979, 29, 29–36. [Google Scholar] [CrossRef]
- Ohulchanskyy, T.Y.D.; David, J.; Detty, M.R.; Prasad, P.N. Heteroatom Substitution Induced Changes in Excited-State Photophysics and Singlet Oxygen Generation in Chalcogenoxanthylium Dyes: Effect of Sulfur and Selenium Substitutions. J. Phys. Chem. B 2004, 108, 8668–8672. [Google Scholar] [CrossRef]
- Chin, K.K.; Trevithick-Sutton, C.C.; McCallum, J.; Jockusch, S.; Turro, N.J.; Scaiano, J.C.; Foote, C.S.; Garcia-Garibay, M.A. Quantitative determination of singlet oxygen generated by excited state aromatic amino acids, proteins, and immunoglobulins. J. Am. Chem. Soc. 2008, 130, 6912–6913. [Google Scholar] [CrossRef] [PubMed]
- Jockusch, S.; Turro, N.J.; Thompson, E.K.; Gouterman, M.; Callis, J.B.; Khalil, G.E. Singlet molecular oxygen by direct excitation. PhotoChem. Photobiol. Sci. 2008, 7, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Kroncke, K.D.; Sies, H. Singlet oxygen-induced signaling effects in mammalian cells. PhotoChem. Photobiol. Sci. 2003, 2, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S. Mechanisms of Photosensitized Oxidation. Science 1968, 162, 963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Steelant, W.; Kumar, M.; Scholfield, M. Versatile Photosensitizers for Photodynamic Therapy at Infrared Excitation. J. Am. Chem. Soc. 2007, 129, 4526–4527. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, Y.; Yang, T.S.; Feng, W.; Li, C.G.; Li, F.Y. Sub-10 nm Hexagonal Lanthanide-Doped NaLuF4 Upconversion Nanocrystals for Sensitive Bioimaging in Vivo. J. Am. Chem. Soc. 2011, 133, 17122–17125. [Google Scholar] [CrossRef] [PubMed]
- Gnanasammandhan, M.K.; Idris, N.M.; Bansal, A.; Huang, K.; Zhang, Y. Near-IR photoactivation using mesoporous silica-coated NaYF4:Yb,Er/Tm upconversion nanoparticles. Nat. Protoc. 2016, 11, 688–713. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Shao, W.; Valiev, R.R.; Ohulchanskyy, T.Y.; He, G.S.; Agren, H.; Prasad, P.N. Efficient Broadband Upconversion of Near-Infrared Light in Dye-Sensitized Core/Shell Nanocrystals. Adv. Opt. Mater. 2016, 4, 1760–1766. [Google Scholar] [CrossRef]
- Hou, Z.Y.; Zhang, Y.X.; Deng, K.R.; Chen, Y.Y.; Li, X.J.; Deng, X.R.; Cheng, Z.Y.; Lian, H.Z.; Li, C.X.; Lin, J. UV-Emitting Upconversion-Based TiO2 Photosensitizing Nanoplatform: Near-Infrared Light Mediated In Vivo Photodynamic Therapy via Mitochondria-Involved Apoptosis Pathway. ACS Nano 2015, 9, 2584–2599. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.Q.; Rengaramchandran, A.; Selvan, S.T.; Paulmurugan, R.; Zhang, Y. Core—shell upconversion nanoparticle—Semiconductor heterostructures for photodynamic therapy. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.C.; Qian, H.S.; Idris, N.M.; Zhang, Y. Singlet oxygen-induced apoptosis of cancer cells using upconversion fluorescent nanoparticles as a carrier of photosensitizer. NanoMed.-Nanotechnol. Biol. Med. 2010, 6, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tao, H.Q.; Cheng, L.; Liu, Z. Near-Infrared Light Induced In Vivo Photodynamic Therapy of Cancer Based on Upconversion Nanoparticles. BioMaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.N.; Budijono, S.J.; Hu, G.H.; Yao, N.; Kang, Y.B.; Ju, Y.G.; Prud’homme, R.K. Pegylated Composite Nanoparticles Containing Upconverting Phosphors and meso-Tetraphenyl porphine (TPP) for Photodynamic Therapy. Adv. Funct. Mater. 2011, 21, 2488–2495. [Google Scholar] [CrossRef]
- Qiao, X.F.; Zhou, J.C.; Xiao, J.W.; Wang, Y.F.; Sun, L.D.; Yan, C.H. Triple-functional core-shell structured upconversion luminescent nanoparticles covalently grafted with photosensitizer for luminescent, magnetic resonance imaging and photodynamic therapy in vitro. Nanoscale 2012, 4, 4611–4623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.G.; Wei, Y.C.; Wu, B.Y.; Chen, Q.; Xing, D. Pyropheophorbide A and c(RGDyK) Comodified Chitosan-Wrapped Upconversion Nanoparticle for Targeted Near-Infrared Photodynamic Therapy. Mol. Pharm. 2012, 9, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, X.M.; Zeng, Q.H.; Zhang, Y.L.; Tu, L.P.; Liu, T.; Kong, X.G.; Wang, Y.H.; Cao, F.; Lambrechts, S.A.G.; et al. Covalently Assembled NIR Nanoplatform for Simultaneous Fluorescence Imaging and Photodynamic Therapy of Cancer Cells. ACS Nano 2012, 6, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.G.; Wang, C.Y.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.L.; Zhu, W.W.; Peng, R.; Liu, Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Z.; Xu, L.G.; Ma, W.; Wu, X.L.; Kuang, H.; Wang, L.B.; Xu, C.L. Hierarchical Plasmonic Nanorods and Upconversion Core-Satellite Nanoassemblies for Multimodal Imaging-Guided Combination Phototherapy. Adv. Mater. 2016, 28, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Shen, J.; Ohulchanskyy, T.Y.; Patel, N.J.; Kutikov, A.; Li, Z.P.; Song, J.; Pandey, R.K.; Agren, H.; Prasad, P.N.; et al. (alpha-NaYbF4:Tm3+)/CaF2 Core/Shell Nanoparticles with Efficient Near-Infrared to Near-Infrared Upconversion for High-Contrast Deep Tissue Bioimaging. ACS Nano 2012, 6, 8280–8287. [Google Scholar] [CrossRef] [PubMed]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In Vivo Photodynamic Therapy Using Upconversion Nanoparticles as Remote-Controlled Nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Ungun, B.; Prud’homme, R.K.; Budijono, S.J.; Shan, J.N.; Lim, S.F.; Ju, Y.G.; Austin, R. Nanofabricated upconversion nanoparticles for photodynamic therapy. Opt. Exp. 2009, 17, 80–86. [Google Scholar] [CrossRef]
- Qian, H.S.; Guo, H.C.; Ho, P.C.L.; Mahendran, R.; Zhang, Y. Mesoporous-Silica-Coated Up-Conversion Fluorescent Nanoparticles for Photodynamic Therapy. Small 2009, 5, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, H.M.; Kim, J.H.; Moon, K.C.; Yoo, B.; Lee, K.T.; Lee, N.; Choi, Y.; Park, W.; Ling, D.; et al. Theranostic Probe Based on Lanthanide-Doped Nanoparticles for Simultaneous In Vivo Dual-Modal Imaging and Photodynamic Therapy. Adv. Mater. 2012, 24, 5755–5761. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.S.; Chen, H.Y.; Zhu, H.Y.; Tian, J.M.; Chi, X.M.; Qian, Z.Y.; Achilefu, S.; Gu, Y.Q. Amphiphilic chitosan modified upconversion nanoparticles for in vivo photodynamic therapy induced by near-infrared light. J. Mater. Chem. 2012, 22, 4861–4873. [Google Scholar] [CrossRef]
- Lim, M.E.; Lee, Y.L.; Zhang, Y.; Chu, J.J.H. Photodynamic Inactivation of Viruses Using Upconversion Nanoparticles. Biomaterials 2012, 33, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.Y.; Pan, Y.W.; Tian, Y.; Wang, X.; Ren, W.Z.; Wang, S.J.; Lu, G.M.; Wu, A.G. Doxorubicin-loaded NaYF4:Yb/Tm-TiO2 inorganic photosensitizers for NIR-triggered photodynamic therapy and enhanced chemotherapy in drug-resistant breast cancers. Biomaterials 2015, 57, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Idris, N.M.; Li, Z.Q.; Huang, K.; Soo, K.C.; Zhang, Y. Titania Coated Upconversion Nanoparticles for Near-Infrared Light Triggered Photodynamic Therapy. ACS Nano 2015, 9, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.E.; Zeng, L.Y.; Pan, Y.W.; Luo, S.; Ren, W.Z.; Gong, A.; Ma, X.H.; Liang, H.Z.; Lu, G.M.; Wu, A.G. Inorganic photosensitizer coupled Gd-based upconversion luminescent nanocomposites for in vivo magnetic resonance imaging and near-infrared-responsive photodynamic therapy in cancers. Biomaterials 2015, 44, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.L.; Ju, E.G.; Chen, Z.W.; Li, Z.H.; Ren, J.S.; Qu, X.G. Upconverting Nanoparticles with a Mesoporous TiO2 Shell for Near-Infrared-Triggered Drug Delivery and Synergistic Targeted Cancer Therapy. Chem.-A Eur. J. 2014, 20, 14012–14017. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.Q.; Qian, J.; Liang, H.J.; Somesfalean, G.; Wang, D.; He, S.L.; Zhang, Z.G.; Andersson-Engels, S. Using 915 nm Laser Excited Tm3+/Er3+/Ho3+-Doped NaYbF4 Upconversion Nanoparticles for in Vitro and Deeper in Vivo Bioimaging without Overheating Irradiation. ACS Nano 2011, 5, 3744–3757. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Ohulchanskyy, T.Y.; Liu, S.; Law, W.C.; Wu, F.; Swihart, M.T.; Agren, H.; Prasad, P.N. Core/Shell NaGdF4:Nd3+/NaGdF4 Nanocrystals with Efficient Near-Infrared to Near-Infrared Downconversion Photoluminescence for Bioimaging Applications. ACS Nano 2012, 6, 2969–2977. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Tian, G.; Gu, Z.; Yang, Y.; Gu, L.; Zhao, Y.; Ma, Y.; Yao, J. Elimination of Photon Quenching by a Transition Layer to Fabricate a Quenching-Shield Sandwich Structure for 800 nm Excited Upconversion Luminescence of Nd3+-Sensitized Nanoparticles. Adv. Mater. 2014. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Wang, R.; Zhang, F.; Zhou, L.; Shen, D.K.; Yao, C.; Zhao, D.Y. Nd3+ Sensitized Up/Down Converting Dual-Mode Nanomaterials for Efficient In-vitro and In-vivo Bioimaging Excited at 800 nm. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xue, B.; Kong, X.G.; Tu, L.P.; Liu, X.M.; Zhang, Y.L.; Chang, Y.L.; Luo, Y.S.; Zhao, H.Y.; Zhang, H. 808 nm driven Nd3+-sensitized upconversion nanostructures for photodynamic therapy and simultaneous fluorescence imaging. Nanoscale 2015, 7, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Ai, F.J.; Ju, Q.; Zhang, X.M.; Chen, X.; Wang, F.; Zhu, G.Y. A core-shell-shell nanoplatform upconverting near-infrared light at 808 nm for luminescence imaging and photodynamic therapy of cancer. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.X.; Yang, D.; Yang, P.P.; Lv, R.C.; Li, C.X.; Zhong, C.N.; He, F.; Gai, S.L.; Lin, J. A Single 808 nm Near-Infrared Light-Mediated Multiple Imaging and Photodynamic Therapy Based on Titania Coupled Upconversion Nanoparticles. Chem. Mater. 2015, 27, 7957–7968. [Google Scholar] [CrossRef]
- Lv, R.C.; Yang, D.; Yang, P.P.; Xu, J.T.; He, F.; Gai, S.L.; Li, C.X.; Dai, Y.L.; Yang, G.X.; Lin, J. Integration of Upconversion Nanoparticles and Ultrathin Black Phosphorus for Efficient Photodynamic Theranostics under 808 nm Near-Infrared Light Irradiation. Chem. Mater. 2016, 28, 4724–4734. [Google Scholar] [CrossRef]
- Lu, F.; Yang, L.; Ding, Y.J.; Zhu, J.J. Highly Emissive Nd3+-Sensitized Multilayered Upconversion Nanoparticles for Efficient 795 nm Operated Photodynamic Therapy. Adv. Funct. Mater. 2016, 26, 4778–4785. [Google Scholar] [CrossRef]
- Chen, G.Y.; Damasco, J.; Qiu, H.L.; Shao, W.; Ohulchanskyy, T.Y.; Valiev, R.R.; Wu, X.; Han, G.; Wang, Y.; Yang, C.H.; et al. Energy-Cascaded Upconversion in an Organic Dye-Sensitized Core/Shell Fluoride Nanocrystal. Nano Lett. 2015, 15, 7400–7407. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.W.; Takle, K.; Bilsel, O.; Li, Z.J.; Lee, H.; Zhang, Z.J.; Li, D.S.; Fan, W.; Duan, C.Y.; et al. Dye-Sensitized Core/Active Shell Upconversion Nanoparticles for Optogenetics and Bioimaging Applications. Acs Nano 2016, 10, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Yang, P.P.; Sun, M.D.; Bi, H.T.; Liu, B.; Yang, D.; Gai, S.L.; He, F.; Lin, J. Highly Emissive Dye-Sensitized Upconversion Nanostructure for Dual-Photosensitizer Photodynamic Therapy and Bioimaging. ACS Nano 2017, 11, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, W.B.; Ma, H.H.; Jiang, R.C.; Tang, Y.F.; Ji, Y.; Lu, X.M.; Hou, B.; Deng, W.X.; Huang, W.; et al. Photo-Induced Charge-Variable Conjugated Polyelectrolyte Brushes Encapsulating Upconversion Nanoparticles for Promoted siRNA Release and Collaborative Photodynamic Therapy under NIR Light Irradiation. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Liu, X.M.; Zheng, M.; Kong, X.G.; Zhang, Y.L.; Zeng, Q.H.; Sun, Z.C.; Buma, W.J.; Zhang, H. Separately doped upconversion-C-60 nanoplatform for NIR imaging-guided photodynamic therapy of cancer cells. Chem. Commun. 2013, 49, 3224–3226. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Jaskula-Sztul, R.; Esquibel, C.R.; Lou, I.; Zheng, Q.F.; Dammalapati, A.; Harrison, A.; Eliceiri, K.W.; Tang, W.P.; Chen, H.; et al. Neuroendocrine Tumor-Targeted Upconversion Nanoparticle-Based Micelles for Simultaneous NIR-Controlled Combination Chemotherapy and Photodynamic Therapy, and Fluorescence Imaging. Adv. Funct. Mater. 2017, 27, 1604671. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Uehara, M.; Nakamura, H.; Miyazaki, M.; Maeda, H. Synthesis of Well-Dispersed Y2O3: Eu Nanocrystals and Self-Assembled Nanodisks Using a Simple Non-Hydrolytic Route. Adv. Mater. 2005, 17, 2506–2511. [Google Scholar] [CrossRef]
- Shan, J.N.; Kong, W.J.; Wei, R.; Yao, N.; Ju, Y.G. An Investigation of the Thermal Sensitivity and Stability of The Beta-NaYF4:Yb, Er Upconversion Nanophosphors. J. Appl. Phys. 2010, 107, 054901. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.W.; Du, Y.P.; Yan, Z.G.; Si, R.; You, L.P.; Yan, C.H. From Trifluoroacetate Complex Precursors to Monodisperse Rare-Earth Fluoride and Oxyfluoride Nanocrystals with Diverse Shapes through Controlled Fluorination in Solution Phase. Chem.-A Eur. J. 2007, 13, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, H.M.; Cui, L.; An, N.; Tong, R.H.; Chen, Y.H.; Yang, C.Y.; Li, X.; Liu, J.Y.; Qu, F.Y. Near Infrared Light Triggered Reactive Oxygen Species Responsive Upconversion Nanoplatform for Drug Delivery and Photodynamic Therapy. Eur. J. Inorg. Chem. 2016, 1206–1213. [Google Scholar] [CrossRef]
- Xiong, L.Q.; Yang, T.S.; Yang, Y.; Xu, C.J.; Li, F.Y. Long-Term In Vivo Biodistribution Imaging and Toxicity of Polyacrylic Acid-Coated Upconversion Nanophosphors. Biomaterials 2010, 31, 7078–7085. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, L.A.; Liu, Z.A. Drug Delivery with Upconversion Nanoparticles for Multi-Functional Targeted Cancer Cell Imaging and Therapy. Biomaterials 2011, 32, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Gu, Z.J.; Zhou, L.J.; Yin, W.Y.; Liu, X.X.; Yan, L.; Jin, S.; Ren, W.L.; Xing, G.M.; Li, S.J.; et al. Mn2+ Dopant-Controlled Synthesis of NaYF4:Yb/Er Upconversion Nanoparticles for in vivo Imaging and Drug Delivery. Adv. Mater. 2012, 24, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
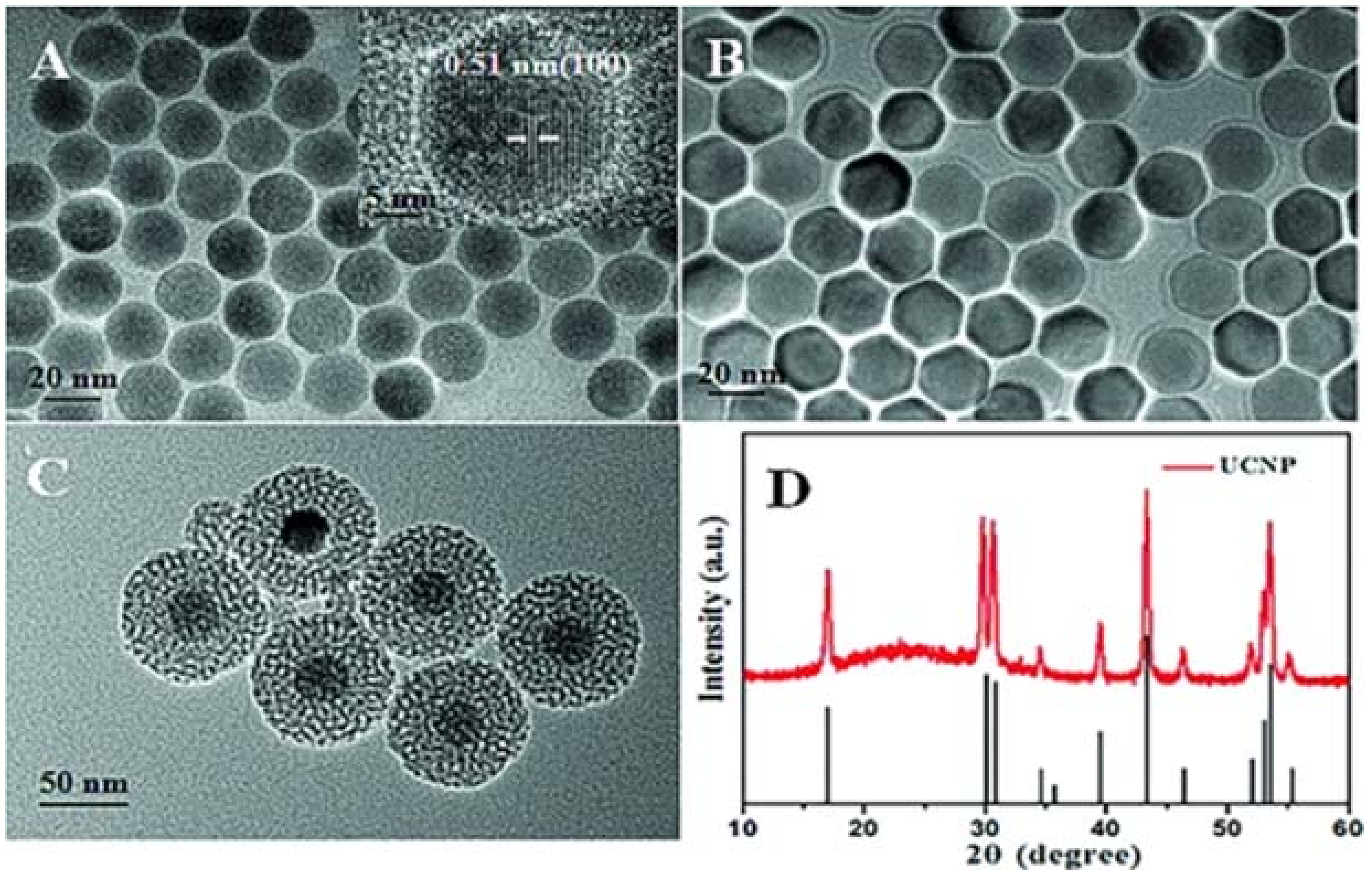
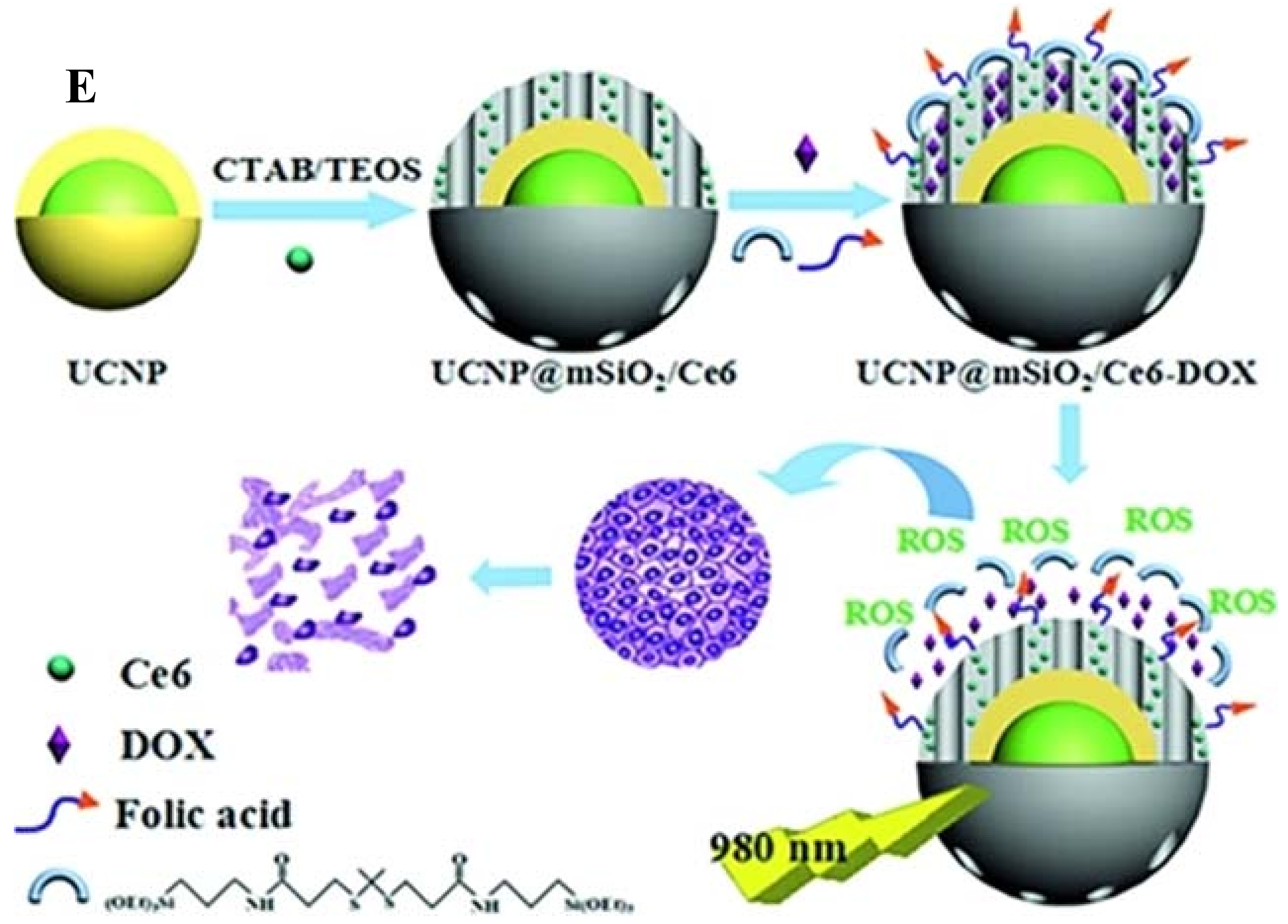

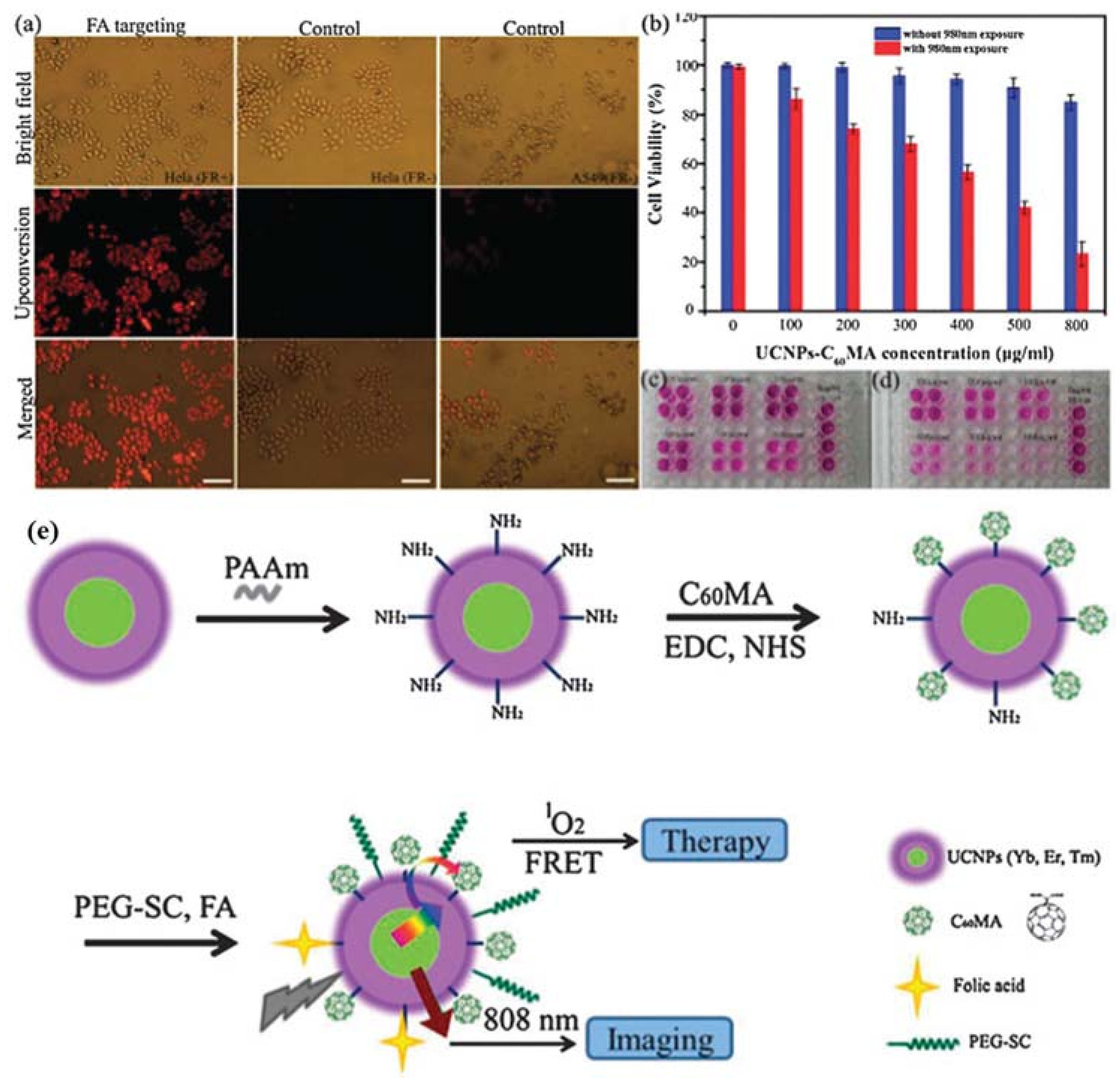

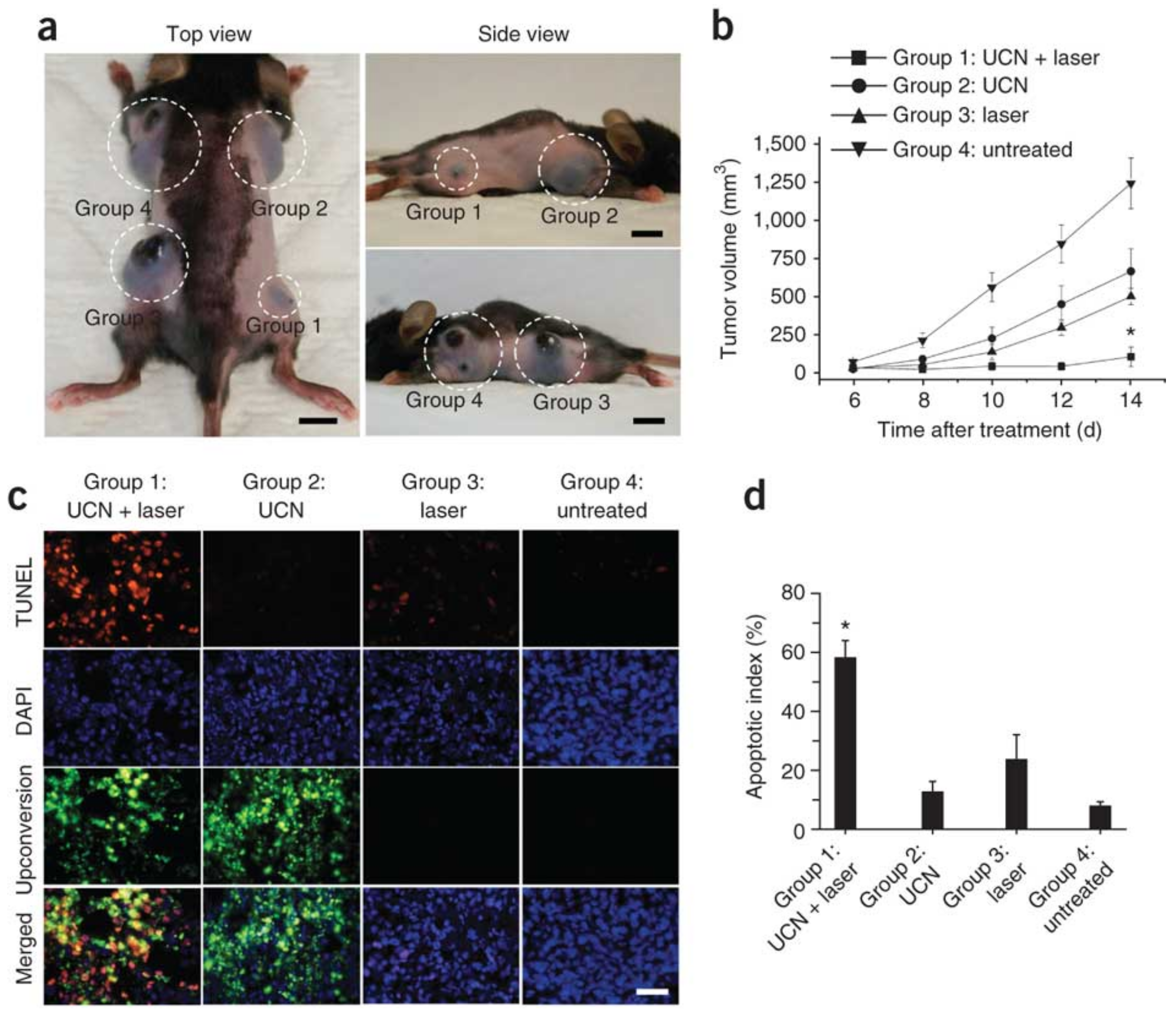
| UCNPs | Coating | Emission (nm) | PS | In Vitro | In Vivo | Ref. |
|---|---|---|---|---|---|---|
| NaYF4:Yb/Er | Silica | 540 | MC 540 | MCF-7/AZ breast cancer cells | N/A | [29] |
| NaYF4:Yb/Er | Mesoporous silica | 540, 660 | MC 540, ZnPc | B16-F0 melanoma cells | B16-F0 cells-bearing C57BL/6 mice. | [45] |
| NaGdF4:Yb/Er@CaF2 | SiO2 | 660 | SPCD | HeLa cells | N/A | [39] |
| NaYF4:Yb/Er | Silica | 660 | ZnPc | MB49-PSA bladder cancer cells | N/A | [36] |
| NaYF4:Yb/Er | PEG-b-PCL | 540, 660 | TPP | HeLa cells | N/A | [38] |
| NaYF4:Yb/Er | PEG | 540 | TPP | N/A | N/A | [46] |
| NaYF4:Yb/Er | Mesoporous silica | 540 | MC 540 | murine bladder cancer cells (MB49) | N/A | [47] |
| NaYF4:Yb/Er | PEG | 660 | Ce6 | HeLa cells | tumor-bearing mice | [37] |
| NaYF4:Yb/Er | PEI-OCMC | 660 | Ppa | integrin positive cells(U87-MG), integrin negative cells (MCF-7) | N/A | [40] |
| NaYF4:Yb/Er@NaGdF4 | PEG | 660 | Ce6 | U87MG glioblastoma cells | U87MG tumor-bearing nude mice | [48] |
| NaYF4:Yb/Er@NaYF4:Yb/Tm | PEG-SC | 450, 475, 540, 660 | C60MA | HeLa cells | N/A | [37] |
| NaYF4:Yb/Er | SOC | 660 | ZnPc | MCF-7 cancer cells | S180 tumor-bearing Female Kunming mice | [49] |
| NaYF4:Yb/Er | AEP | 540 | RB | JAR choriocarcinoma cells | N/A | [41] |
| NaYF4:Yb/Er | PEI | 660 | ZnPc | DENV2-infected HepG2 cells | DENV2 virus-bearing BALB/c mice | [50] |
| NaYF4:Yb/Tm@NaGdF4:Yb | PVP | 345, 360, 450, 475 | TiO2 | HeLa cells | HeLa tumor-bearing Balbc/c nude mice | [34] |
| NaYF4:Yb/Tm | PEG-1500, APTS | 345, 360, 450, 475 | TiO2 | MCF-7 and MCF-7/ADR cells | Female balb/c nude mice | [51] |
| NaYF4:Yb/Tm | Silica and Mal-PEG-silane | 345, 360, 450, 475 | TiO2 | OSCC | Female balb/c nude mice | [52] |
| NaGdF4:Yb/Tm | Silica and APTS | 345, 360, 450, 475 | TiO2 | HeLa and MCF-7 cells | MCF-7 tumor-bearing nude mice | [53] |
| NaGdF4:Yb/Tm | Silica and hyaluronic acid | 345, 360, 450, 475 | TiO2 | MDA-MB-231 cancer cells | N/A | [54] |
| NaYF4:Yb/Tm | Sodium citrate | 450, 475 | ZnO | MDA-MB-231 breast cancer cell | N/A | [35] |
| UCNPs | Coating | Emission (nm) | PS | In Vitro | In Vivo | Ref. |
|---|---|---|---|---|---|---|
| NaYF4:Yb/Ho@NaYF4:Nd@NaYF4 | PAAm | 540 | RB | HeLa cells | N/A | [59] |
| NaYbF4:Nd@NaGdF4:Yb/Er@NaGdF4 | AEP, PEG | 660 | Ce6 | A549 and KB cells | N/A | [60] |
| NaGdF4:Yb/Tm@NaGdF4:Yb@NaNdF4:Yb@NaGdF4 | mSiO2 | 345, 360, 450, 475 | TiO2 | HeLa cells | Female Kunming tumor-bearing mice | [61] |
| IR-808-dye sensitized NaGdF4:Yb,Er@NaGdF4:Yb @NaNdF4:Yb | mSiO2 | 540, 660 | MC540, Ce6 | HeLa cells | Female Balb/c | [61] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Tan, M.; Ohulchanskyy, T.Y.; Lovell, J.F.; Chen, G. Recent Progress in Upconversion Photodynamic Therapy. Nanomaterials 2018, 8, 344. https://doi.org/10.3390/nano8050344
Qiu H, Tan M, Ohulchanskyy TY, Lovell JF, Chen G. Recent Progress in Upconversion Photodynamic Therapy. Nanomaterials. 2018; 8(5):344. https://doi.org/10.3390/nano8050344
Chicago/Turabian StyleQiu, Hailong, Meiling Tan, Tymish Y. Ohulchanskyy, Jonathan F. Lovell, and Guanying Chen. 2018. "Recent Progress in Upconversion Photodynamic Therapy" Nanomaterials 8, no. 5: 344. https://doi.org/10.3390/nano8050344
APA StyleQiu, H., Tan, M., Ohulchanskyy, T. Y., Lovell, J. F., & Chen, G. (2018). Recent Progress in Upconversion Photodynamic Therapy. Nanomaterials, 8(5), 344. https://doi.org/10.3390/nano8050344








