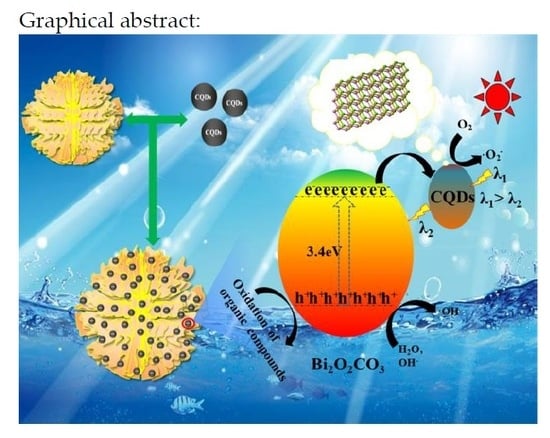
Enhanced Photocatalytic Activity toward Organic Pollutants Degradation and Mechanism Insight of Novel CQDs/Bi2O2CO3 Composite
Abstract
:1. Introduction
2. Experimental Methods
2.1. Photocatalyst Synthesis
2.1.1. Synthesis of the 3D Flower-Like Bi2O2CO3
2.1.2. Preparation of Carbon Quantum Dots (CQDs)
2.1.3. Preparation of CQD/Bi2O2CO3 Photocatalysts
2.2. Photocatalyst Characterization
2.3. Photocatalytic Activity
2.4. Photoelectrocatalytic Activity
3. Results and Discussion
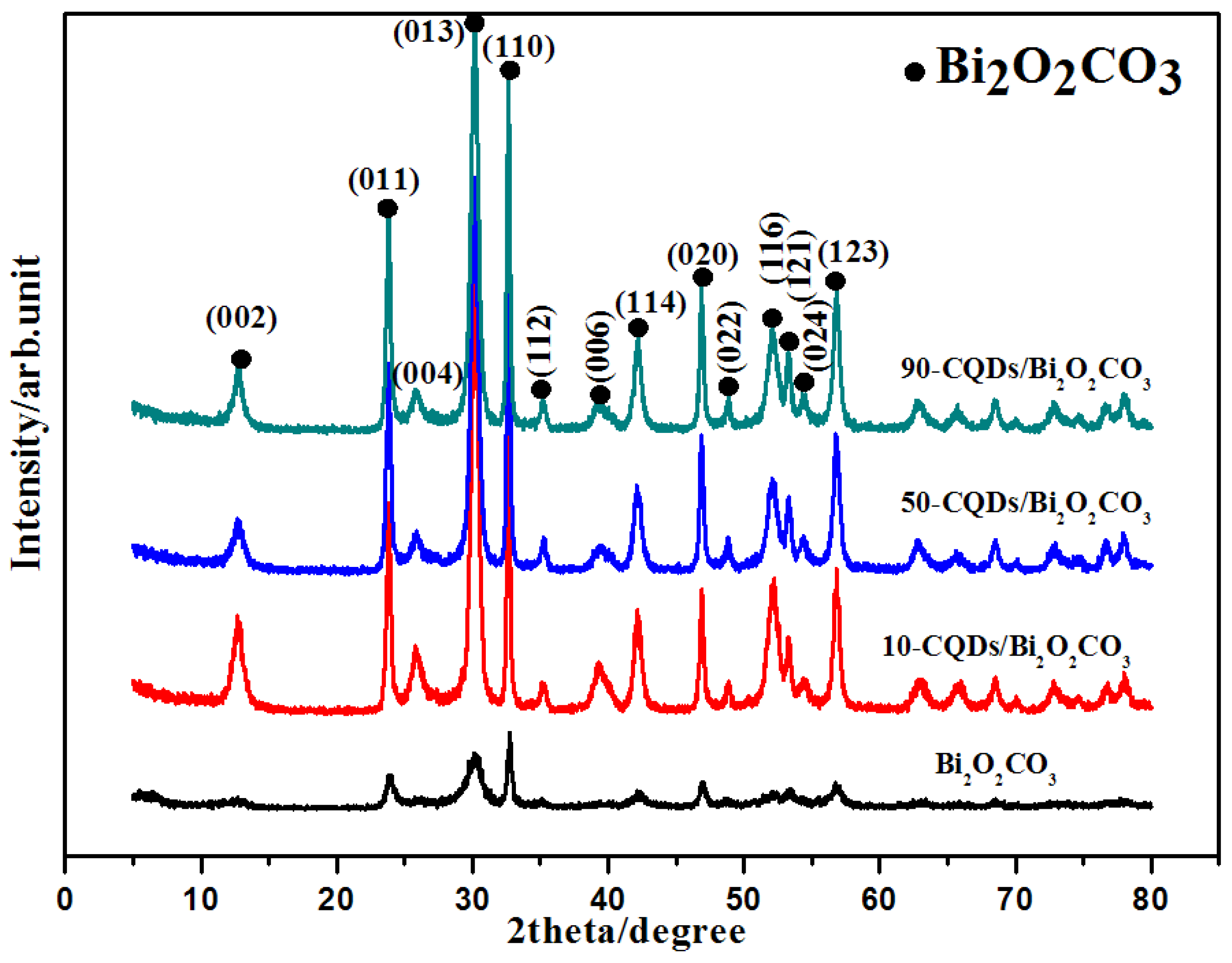
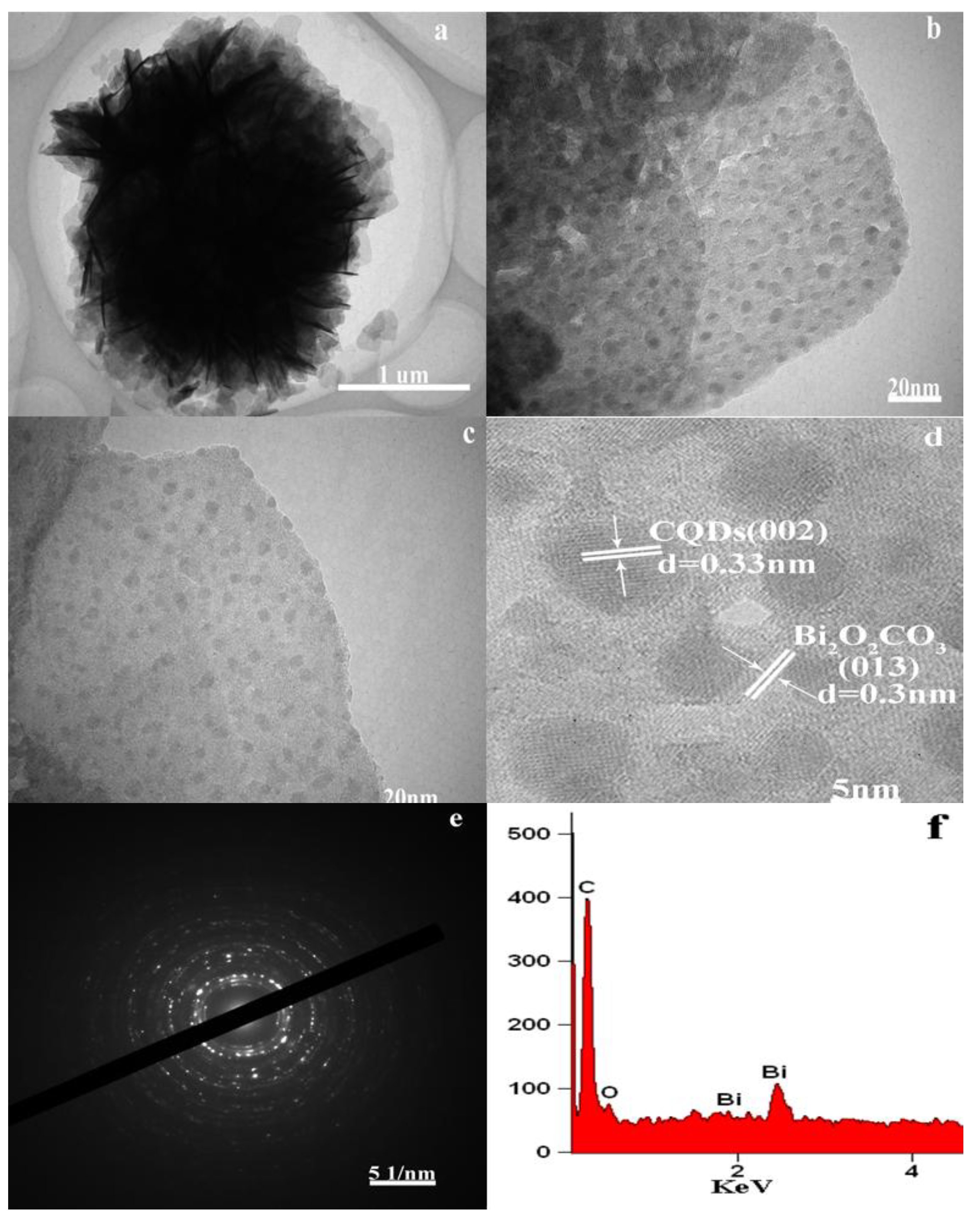
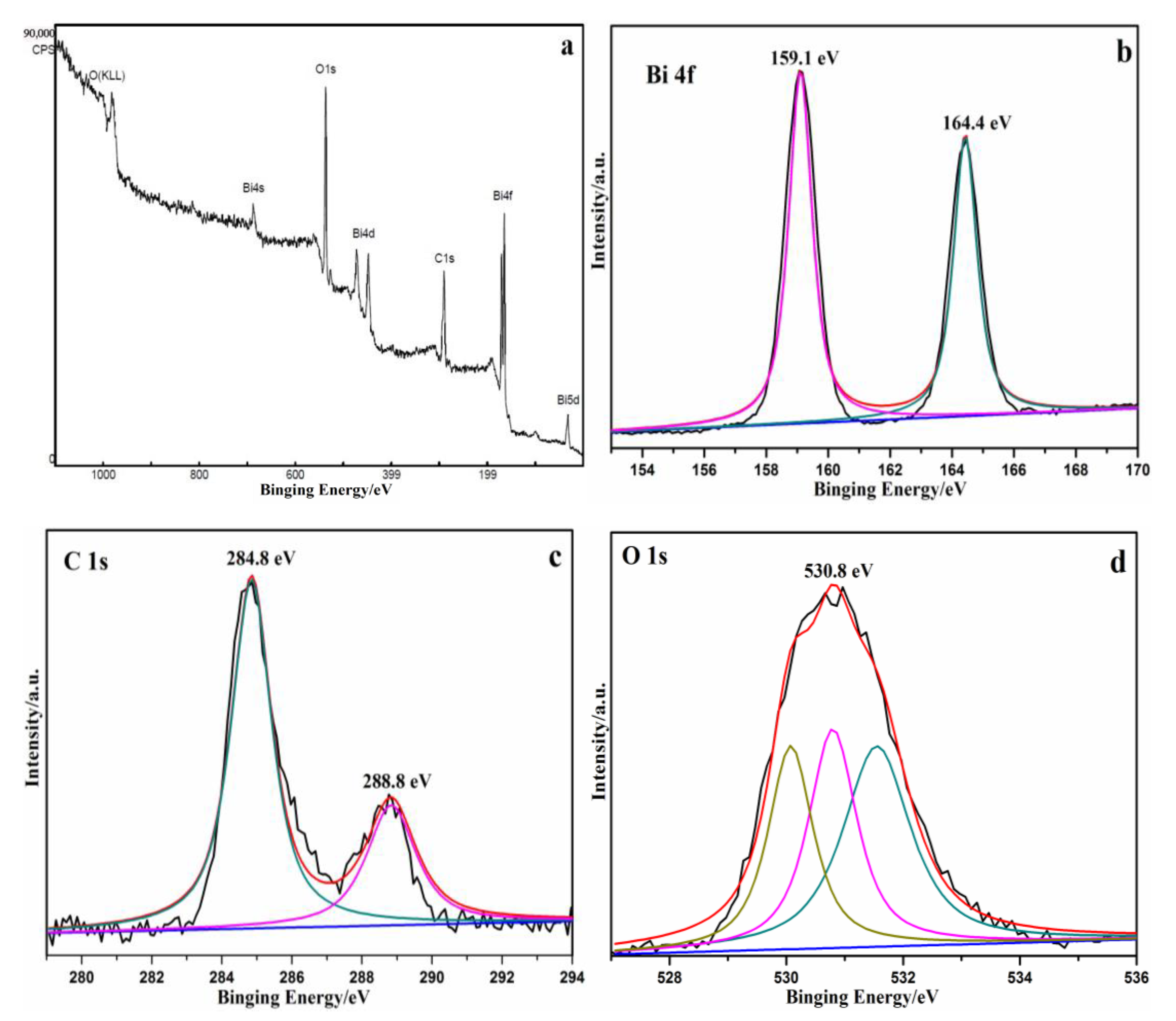
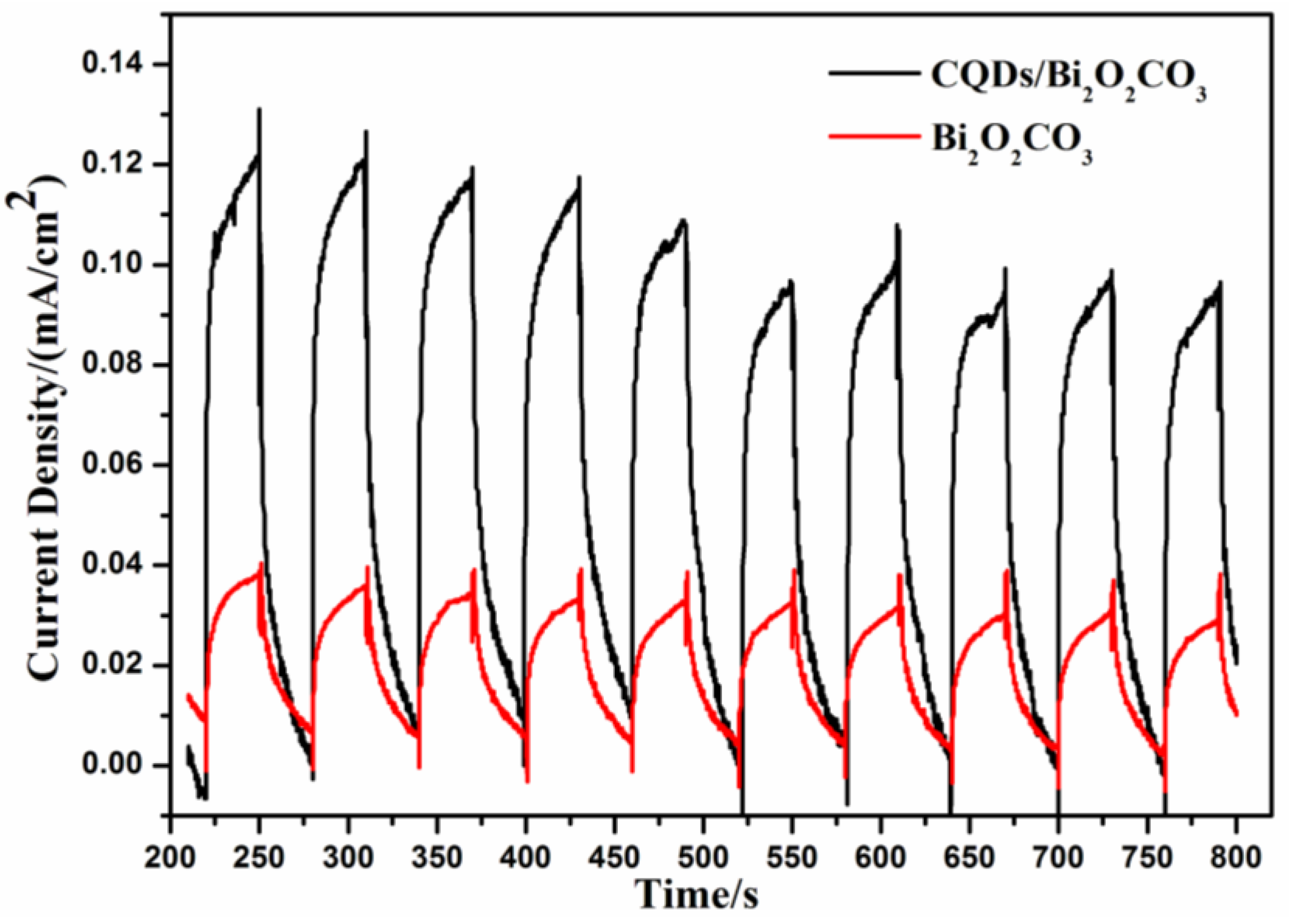
3.1. Catalyst Characterization
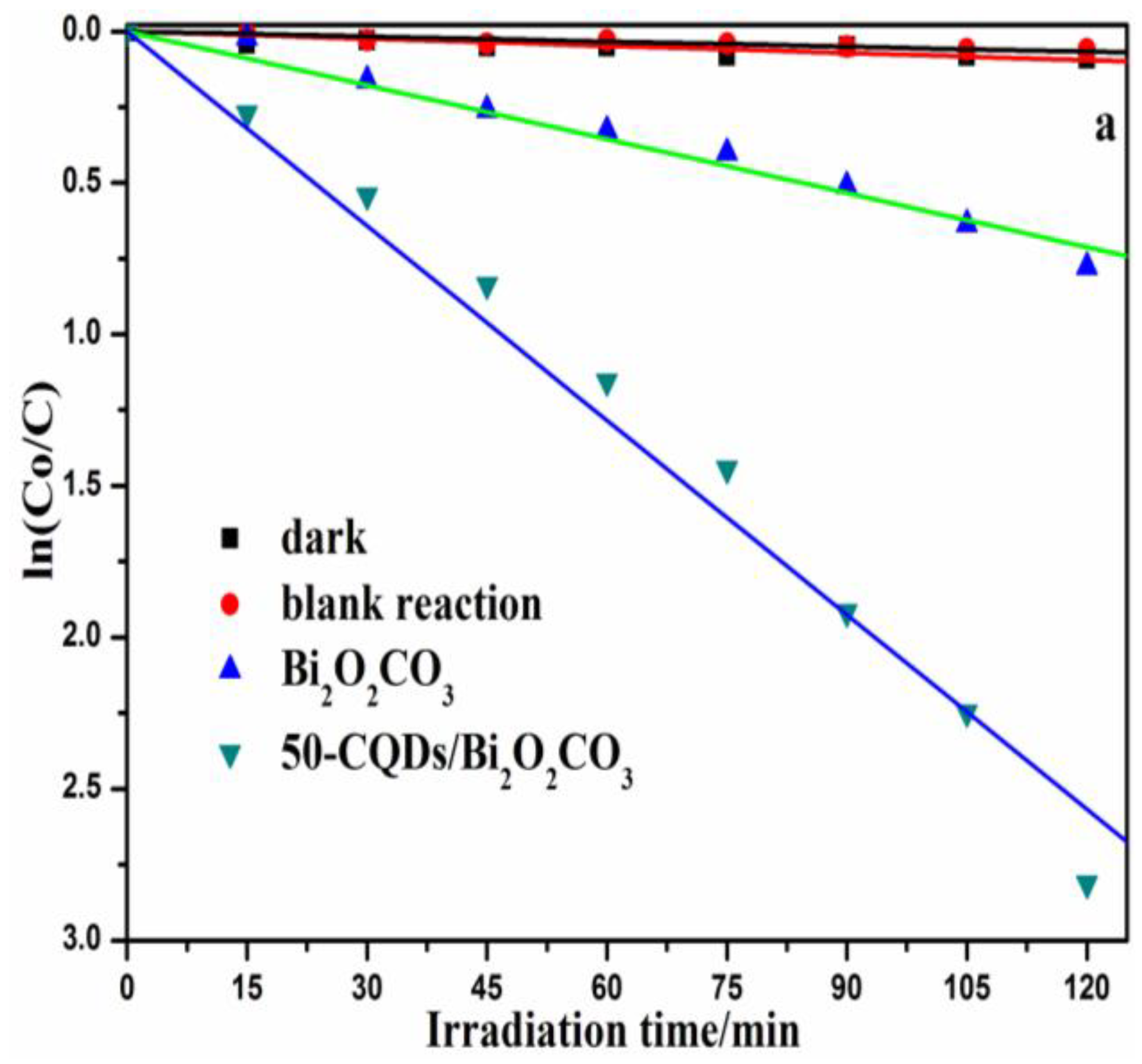
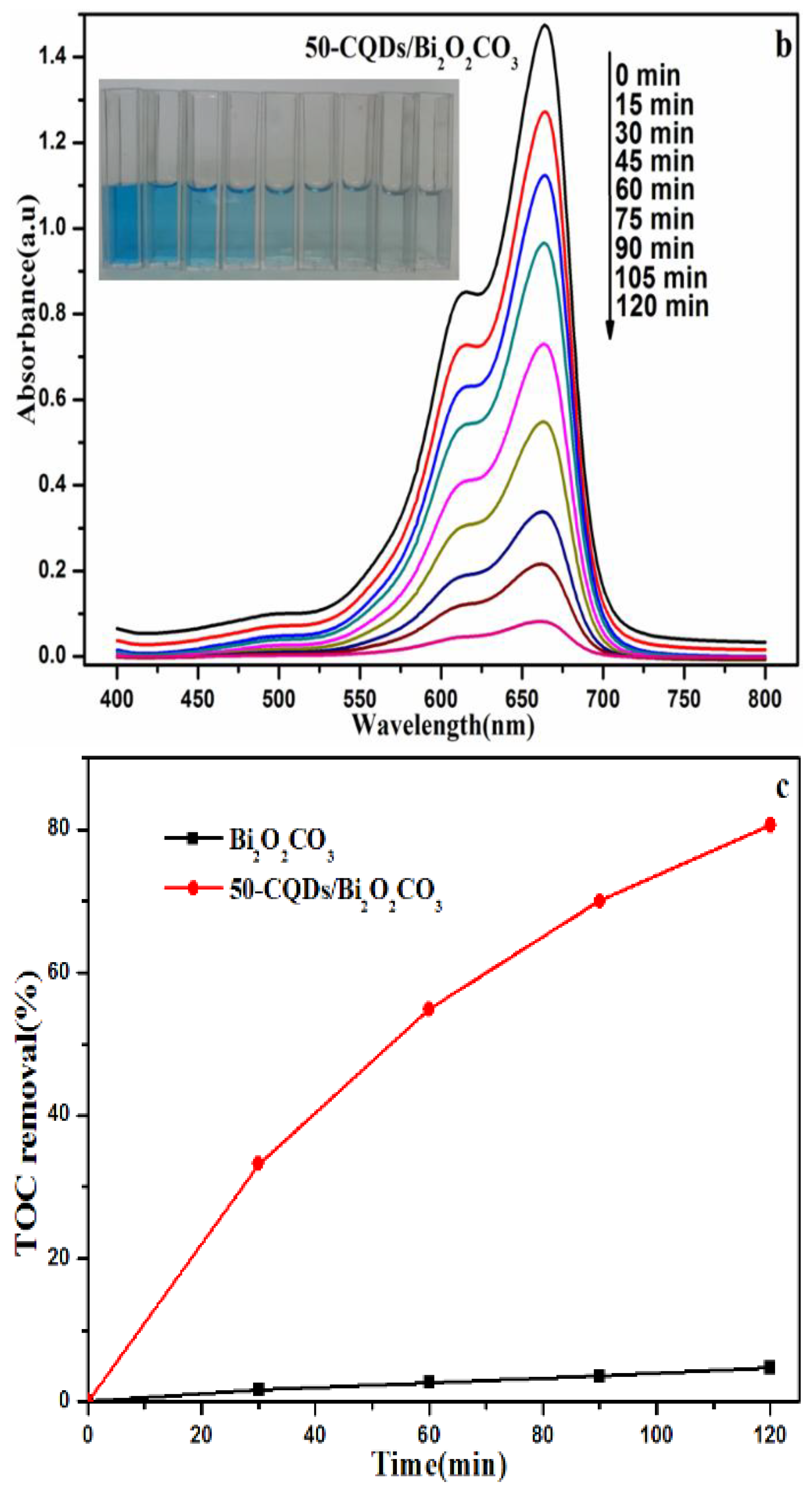
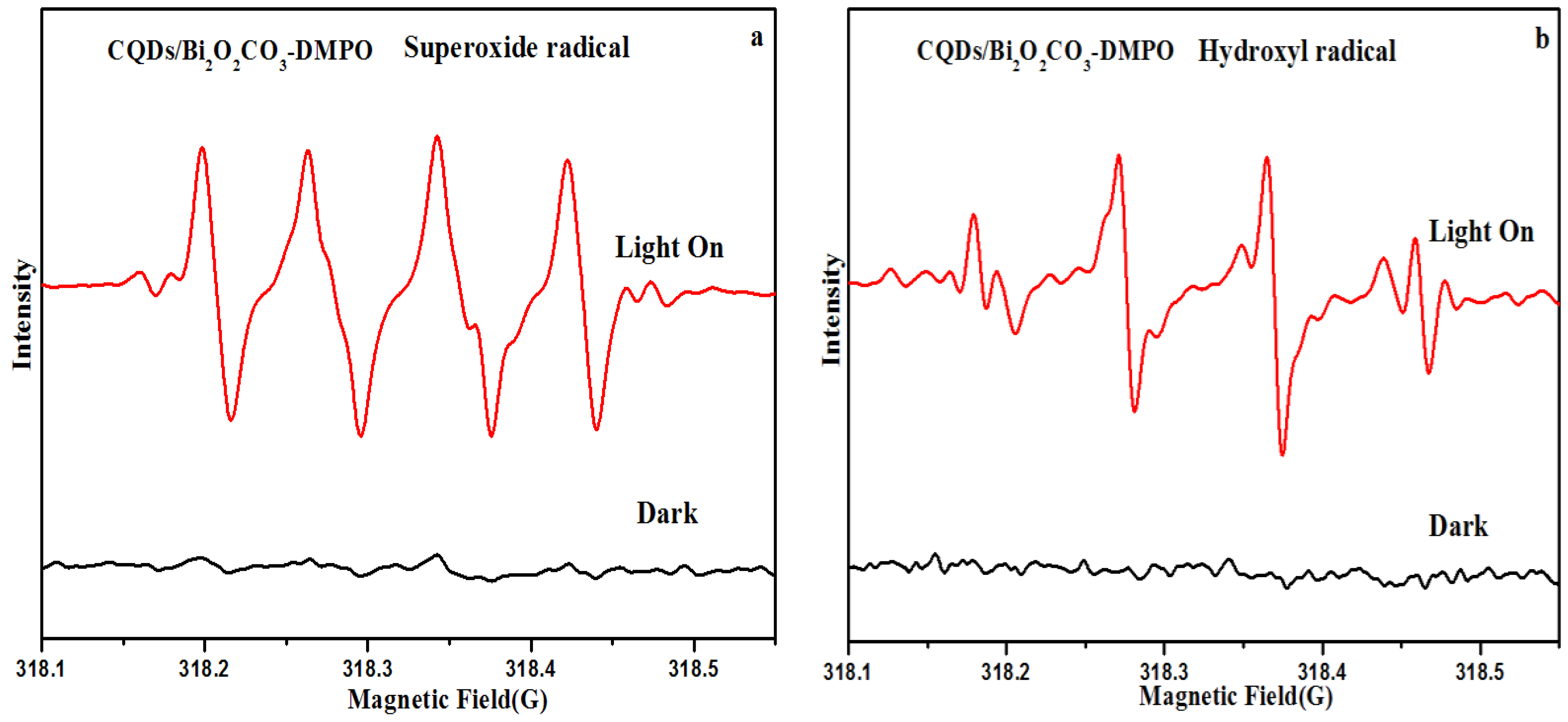
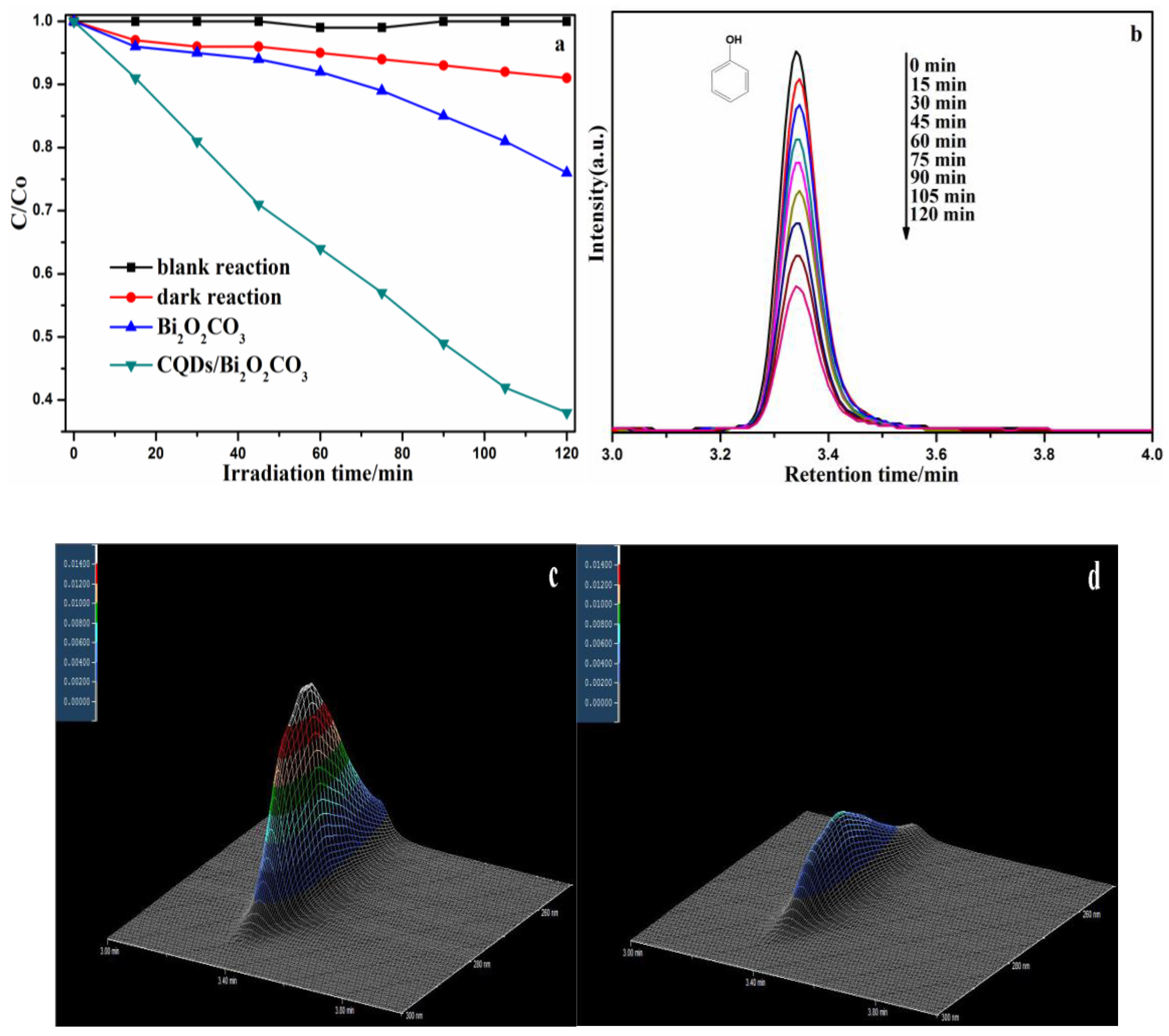
3.2. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Que, Q.H.; Xing, Y.L.; He, Z.L.; Yang, Y.W.; Yin, X.T.; Que, W.X. Bi2O3/Carbon quantum dots heterostructured photocatalysts with enhanced photocatalytic activity. Mater. Lett. 2017, 209, 220–223. [Google Scholar] [CrossRef]
- Kian, M.L.; Chin, W.L.; Koh, S.N.; Joon, C.J. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar]
- Yogendra, K.M.; Rainer, A. ZnO tetrapod materials for functional applications. Mater. Today 2018. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhang, L.S.; Chen, Z.G.; Hu, J.Q.; Li, S.J.; Wang, Z.H.; Liu, J.S.; Wang, X.C. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.M.; Sun, J.; Xing, S.M.; Liu, D.J.; Zhang, G.J.; Bai, L.J.; Jiang, B.L. Enhanced Raman scattering and photocatalytic activity of TiO2 films with embedded Ag nanoparticles deposited by magnetron sputtering. J. Alloys Compd. 2016, 679, 88–93. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.Y.; Ma, Z.B.; Ren, Z.F.; Fan, H.J.; Yang, X.M. Elementary photocatalytic chemistry on TiO2 surfaces. Chem. Soc. Rev. 2016, 45, 3701–3730. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.X.; Han, S.C.; Liu, H.; Yu, P.P.; Fang, X.S. Hierarchical MoS2 nanosheet@TiO2 nanotube array composites with enhanced photocatalytic and photocurrent performances. Small 2016, 12, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, R.R.; Ji, H.M.; Sun, T.; Zhao, J.; Chen, N.N.; Chen, J.; Guo, X.F.; Hou, W.H.; Ding, W.P. S-doped mesoporous nanocomposite of HTiNbO5 nanosheets and TiO2 nanoparticles with enhanced visible light photocatalytic activity. Phys. Chem. Chem. Phys. 2016, 18, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.P.; Xu, L.J.; Liu, C.L.; Wang, Y. Magnetic composite ZnFe2O4/SrFe12O19: Preparation, characterization, and photocatalytic activity under visible light. Appl. Surf. Sci. 2013, 273, 684–691. [Google Scholar] [CrossRef]
- García-Pérez, U.M.; Sepúlveda-Guzmán, S.; Martínezde la Cruz, A. Nanostructured BiVO4 photocatalysts synthesized via a polymer-assisted coprecipitation method and their photocatalytic properties under visible-light irradiation. Solid State Sci. 2012, 14, 293–298. [Google Scholar] [CrossRef]
- Zhu, Z.F.; Zhang, L.; Li, J.Q.; Du, J.; Zhang, Y.B.; Zhou, J.Q. Synthesis and photocatalytic behavior of BiVO4 with decahedral structure. Ceram. Int. 2013, 39, 7461–7465. [Google Scholar] [CrossRef]
- Dong, S.Y.; Feng, J.L.; Li, Y.K.; Hu, L.M.; Liu, M.L.; Wang, Y.F.; Pi, Y.Q.; Sun, J.Y.; Sun, J.H. Shape-controlled synthesis of BiVO4 hierarchical structures with unique natural-sunlight-driven photocatalytic activity. Appl. Catal. B 2014, 152–153, 413–424. [Google Scholar] [CrossRef]
- Zhuo, Y.Q.; Huang, J.F.; Cao, L.Y.; Ouyang, H.B.; Wu, J.P. Photocatalytic activity of snow-like Bi2WO6 microcrystalline for decomposition of Rhodamine B under natural sunlight irradiation. Mater. Lett. 2013, 90, 107–110. [Google Scholar] [CrossRef]
- Dumrongrojthanath, P.; Thongtem, T.; Phuruangrat, A.; Thongtem, S. Hydrothermal synthesis of Bi2WO6 hierarchical flowers with their photonic and photocatalytic properties. Superlattices Microstruct. 2013, 54, 71–77. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wang, H.L.; Chen, Z.G.; Wong, P.K.; Liu, J.S. Bi2WO6 micro/nanostructures: Synthesis modifications and visible-light-driven photocatalytic applications. Appl. Catal. B 2011, 106, 1–13. [Google Scholar]
- Hu, J.L.; Fan, W.J.; Ye, W.Q.; Huang, C.J.; Qiu, X.Q. Insights into the photosensitivity activity of BiOCl under visible light irradiation. Appl. Catal. B 2014, 158–159, 182–189. [Google Scholar] [CrossRef]
- Chai, B.; Zhou, H.; Zhang, F.; Liao, X.; Ren, M.X. Visible light photocatalytic performance of hierarchical BiOBr microspheres synthesized via a reactable ionic liquid. Mater. Sci. Semicond. Process. 2014, 23, 151–158. [Google Scholar] [CrossRef]
- Li, T.T.; Luo, S.L.; Yang, L.X. Three-dimensional hierarchical Ag/AgI/BiOI microspheres with high visible-light photocatalytic activity. Mater. Lett. 2013, 109, 247–252. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Tian, H.; Li, N.; Chen, M.D.; Teng, F. Controllable growth of novel BiPO4 dendrites by an innovative approach and high energy facets-dependent photocatalytic activity. CrystEngComm 2014, 16, 8334–8339. [Google Scholar] [CrossRef]
- Lin, X.; Liu, D.; Guo, X.Y.; Sun, N.; Zhao, S.; Chang, L.M.; Zhai, H.J.; Wang, Q.W. Fabrication and efficient visible light-induced photocatalytic activity of Bi2MoO6/BiPO4 composite. J Phys. Chem. Solids 2015, 76, 170–176. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Zhang, Q.; Cao, J.J.; Huang, R.J.; Ho, W.K.; Lee, S.C. In situ fabrication of a-Bi2O3/BiO,2CO3 nanoplate heterojunctions with tunable optical property and photocatalytic activity. Sci. Rep. 2016, 6, 23435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Duan, F.; Chen, M.Q.; Xie, Y. Synthetic Bi2O2CO3 nanostructures: Novel photocatalyst with controlled special surface exposed. J. Mol. Catal. A Chem. 2010, 317, 34–40. [Google Scholar] [CrossRef]
- Yang, L.L.; Han, Q.F.; Zhu, J.W.; Wang, X. Synthesis of egg-tart shaped Bi2O2CO3 hierarchical nanostructures from single precursor and its photocatalytic performance. Mater. Lett. 2015, 138, 235–237. [Google Scholar] [CrossRef]
- Cai, G.Y.; Xu, L.L.; Wei, B.; Che, J.X.; Gao, H.; Sun, W.J. Facile synthesis of β-Bi2O3/Bi2O2CO3 nanocomposite with high visible-light photocatalytic activity. Mater. Lett. 2014, 120, 1–4. [Google Scholar] [CrossRef]
- Chen, L.; Yin, S.F.; Luo, S.L.; Huang, R.; Zhang, Q.; Hong, T.; Au, P.C.T. Bi2O2CO3/BiOI Photocatalysts with Heterojunctions Highly Efficient for Visible-Light Treatment of Dye-Containing Wastewater. Ind. Eng. Chem. Res. 2012, 51, 6760–6768. [Google Scholar] [CrossRef]
- Jin, L.; Zhu, G.Q.; Hojamberdiev, M.; Luo, X.C.; Tan, C.W.; Peng, J.H.; Wei, X.M.; Li, J.P.; Liu, P. A plasmonic AgAgBr/Bi2O2CO3 composite photocatalyst with enhanced visible-light photocatalytic activity. Ind. Eng. Chem. Res. 2014, 53, 13718–13727. [Google Scholar] [CrossRef]
- Wang, W.J.; Cheng, H.F.; Huang, B.B.; Lin, X.J.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. Synthesis of Bi2O2CO3/Bi2S3 hierarchical microspheres with heterojunctions and their enhanced visible light-driven photocatalytic degradation of dye pollutants. J Colloid. Interface Sci. 2013, 402, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.H.; Zhu, Y.H.; Yang, X.L.; Li, C.Z. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Kang, Z.H.; Liu, Y.; Lee, S.T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, H.; Huang, H.; Liu, R.; Han, Y.; Liu, Y.; Tong, C.; Kang, Z.H. Carbon quantum dots enhance the photocatalytic performance of BiVO4 with different exposed facets. Dalton Trans. 2013, 42, 6285–6289. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.; Yang, X.; Lee, S.T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Chaudhary, N.; Srivastava, R.; Sharma, G.D.; Bhardwaj, R.; Chand, S. Luminscent Graphene Quantum Dots for Organic Photovoltaic Devices. J. Am. Chem. Soc. 2011, 133, 9960–9963. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Liu, R.H.; Lian, S.Y.; Liu, Y.; Huang, H.; Kang, Z.H. Near-infrared light controlled photocatalytic activity of carbon quantum dots for highly selective oxidation reaction. Nanoscale 2013, 5, 3289–3297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Ming, H.; Lian, S.Y.; Huang, H.; Li, H.T.; Zhang, L.L.; Liu, Y.; Kang, Z.H.; Lee, S.T. Fe2O3/carbon quantum dots complex photocatalysts and their enhanced photocatalytic activity under visible light. Dalton Trans. 2011, 40, 10822–10825. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, H.C.; Huang, H.; Liu, Y.; Li, H.T.; Ming, H.; Kang, Z.H. ZnO/carbon quantum dots nanocomposites: One-step fabrication and superior photocatalytic ability for toxic gas degradation under visible light at room temperature. New J. Chem. 2012, 36, 1031–1035. [Google Scholar] [CrossRef]
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.M.; Yu, H.; Wang, F.; Kang, Z.H. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalton Trans. 2012, 41, 9526–9531. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Huang, H.W.; Guo, Y.X.; He, Y.; Zhang, Y.H. A g-C3N4/Bi2O2CO3 composite with high visible-light-driven photocatalytic activity for rhodamine B degradation. Appl. Surf. Sci. 2014, 322, 249–254. [Google Scholar] [CrossRef]
- Huang, H.W.; Wang, S.B.; Tian, N.; Hang, Y.H. One-step hydrothermal preparation strategy for layered BiIO4/Bi2WO6 heterojunctions with enhanced visible light photocatalytic activities. RSC Adv. 2014, 4, 5561–5567. [Google Scholar] [CrossRef]
- Huang, H.W.; Li, X.W.; Wang, J.J.; Dong, F.; Chu, P.K.; Zhang, T.R.; Zhang, Y.H. Anionic Group Self-Doping as a Promising Strategy: Band-Gap Engineering and Multi-Functional Applications of High-Performance CO32− -Doped Bi2O2CO3. ACS Catal. 2015, 5, 4094–4103. [Google Scholar] [CrossRef]
- Huang, H.W.; Yao, J.Y.; Lin, Z.S.; Wang, X.Y.; He, R.; Yao, W.J.; Zhai, N.X.; Chen, C.T. NaSr3Be3B3O9F4: A promising deep-ultraviolet nonlinear optical material resulting from the cooperative alignment of the [Be3B3O12F]10− anionic group. Angew. Chem. Int. Ed. 2011, 50, 9141–9144. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Y.H.; Liu, L.; Hu, J.S.; Cui, W.Q. Reduced graphene oxide wrapped Bi2WO6 hybrid with ultrafast charge separation and improved photoelectrocatalytic performance. Appl. Surf. Sci. 2017, 392, 51–60. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.G.; Ho, W.K.; Jiang, Z.T.; Zhang, L.Z. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Tobon-Zapata, G.E.; Etcheverry, S.B.; Baran, E.J. Vibrational spectrum of bismuth subcarbonate. J. Mater. Sci. Lett. 1997, 16, 656–657. [Google Scholar] [CrossRef]
- Li, J.Z.; Wang, N.Y.; Tran, T.T.; Huang, C.A.; Chen, L.; Yuan, L.J.; Zhou, L.P.; Shen, R.; Cai, Q.Y. Electrogenerated chemiluminescence detection of trace level pentachlorophenol using carbon quantum dots. Analyst 2013, 138, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sharma, S.; Kansal, S.K. Synthesis of ZnS/CQDs nanocomposite and its application as a photocatalyst for the degradation of an anionic dye. Superlattice Microstruct. 2016, 98, 86–95. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.L.; Zhang, M.Y.; Guo, Z.C.; Mu, J.B.; Zhang, Z.Y.; Zhang, X.; Liu, Y.C. Bi2MoO6 ultrathin nanosheets on ZnTiO3 nanofibers: A 3D open hierarchical heterostructures synergistic system with enhanced visible-light-driven photocatalytic activity. J. Hazard. Mater. 2012, 217–218, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.F.; Yue, D.T.; Tian, Z.Y.; Reng, M.; Zhu, Y.; Kan, M.; Zhang, T.Y.; Zhao, Y.X. Carbon quantum dots decorated Bi2WO6 nanocomposite with enhanced photocatalytic oxidation activity for VOCs. Appl. Catal. B 2016, 193, 16–21. [Google Scholar] [CrossRef]
- Yu, B.Y.; Kwak, S.Y. Carbon quantum dots embedded with mesoporous hematite nanospheres as efficient visible light-active photocatalysts. J. Mater. Chem. 2012, 22, 8345–8353. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Zhao, Z.Y.; Dong, F.; Dong, X.A.; Zhou, Y. N-Doped Bi2O2CO3/Graphene Quantum Dot Composite Photocatalyst: Enhanced Visible-Light Photocatalytic NO Oxidation and In Situ DRIFTS Studies. J. Phys. Chem. C 2017, 121, 12168–12177. [Google Scholar] [CrossRef]
- Yu, X.J.; Liu, J.J.; Yu, Y.C.; Zuo, S.L.; Li, B.S. Preparation and visible light photocatalytic activity of carbon quantum dots/TiO2 nanosheet composites. Carbon 2014, 68, 718–724. [Google Scholar] [CrossRef]
- Donat, F.; Corbel, S.; Alem, H.; Pontvianne, S.; Balan, L.; Medjahdi, G.; Schneider, R. ZnO nanoparticles sensitized by CuInZnxS2+x quantum dots as highly efficient solar light driven photocatalysts. Beilstein J. Nanotechnol. 2017, 8, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Candal, R.J.; Zeltner, W.A.; Anderson, M.A. Effects of pH and Applied Potential on Photocurrent and Oxidation Rate of Saline Solutions of Formic Acid in a Photoelectrocatalytic Reactor. Environ. Sci. Technol. 2000, 34, 3443–3451. [Google Scholar] [CrossRef]
- Duo, F.F.; Wang, Y.W.; Fan, C.M.; Zhang, X.C.; Wang, Y.F. Enhanced visible light photocatalytic activity and stability of CQDs/BiOBr composites: The upconversion effect of CQDs. J Alloys Compd. 2016, 685, 34–41. [Google Scholar] [CrossRef]
- Hasan, M.R.; Hamid, S.B.A.; Basirun, W.J.; Chowdhury, Z.Z.; Kandjani, A.E.; Bhargava, S.K. Ga doped RGO–TiO2 composite on an ITO surface electrode for investigation of photoelectrocatalytic activity under visible light irradiation. New J. Chem. 2015, 39, 369–376. [Google Scholar] [CrossRef]
- Liang, Y.H.; Lin, S.L.; Liu, L.; Hu, J.S.; Cui, W.Q. Oil-in-water self-assembled Ag@AgCl QDs sensitized Bi2WO6: Enhanced photocatalytic degradation under visible light irradiation. Appl. Catal. B 2015, 164, 192–203. [Google Scholar] [CrossRef]
- Cong, Y.Q.; Ji, Y.; Ge, Y.H.; Jin, H.; Zhang, Y.; Wang, Q. Fabrication of 3D Bi2O3-BiOI heterojunction by a simple dipping method: Highly enhanced visible-light photoelectrocatalytic activity. Chem. Eng. J. 2017, 307, 572–582. [Google Scholar] [CrossRef]
- Fang, S.; Xia, Y.; Lv, K.L.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalyticactivity of g-C3N4. Appl. Catal. B 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Hu, C.; Lan, Y.Q.; Qu, J.H.; Hu, X.X.; Wang, A.M. Ag/AgBr/TiO2 Visible Light Photocatalyst for Destruction of Azodyes and Bacteria. J. Phys. Chem. B 2006, 110, 4066–4072. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Lin, S.; Li, X.; Li, H.; Zhang, T.; Cui, W. Enhanced Photocatalytic Activity toward Organic Pollutants Degradation and Mechanism Insight of Novel CQDs/Bi2O2CO3 Composite. Nanomaterials 2018, 8, 330. https://doi.org/10.3390/nano8050330
Zhang Z, Lin S, Li X, Li H, Zhang T, Cui W. Enhanced Photocatalytic Activity toward Organic Pollutants Degradation and Mechanism Insight of Novel CQDs/Bi2O2CO3 Composite. Nanomaterials. 2018; 8(5):330. https://doi.org/10.3390/nano8050330
Chicago/Turabian StyleZhang, Zisheng, Shuanglong Lin, Xingang Li, Hong Li, Tong Zhang, and Wenquan Cui. 2018. "Enhanced Photocatalytic Activity toward Organic Pollutants Degradation and Mechanism Insight of Novel CQDs/Bi2O2CO3 Composite" Nanomaterials 8, no. 5: 330. https://doi.org/10.3390/nano8050330
APA StyleZhang, Z., Lin, S., Li, X., Li, H., Zhang, T., & Cui, W. (2018). Enhanced Photocatalytic Activity toward Organic Pollutants Degradation and Mechanism Insight of Novel CQDs/Bi2O2CO3 Composite. Nanomaterials, 8(5), 330. https://doi.org/10.3390/nano8050330