Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease
Abstract
1. Introduction
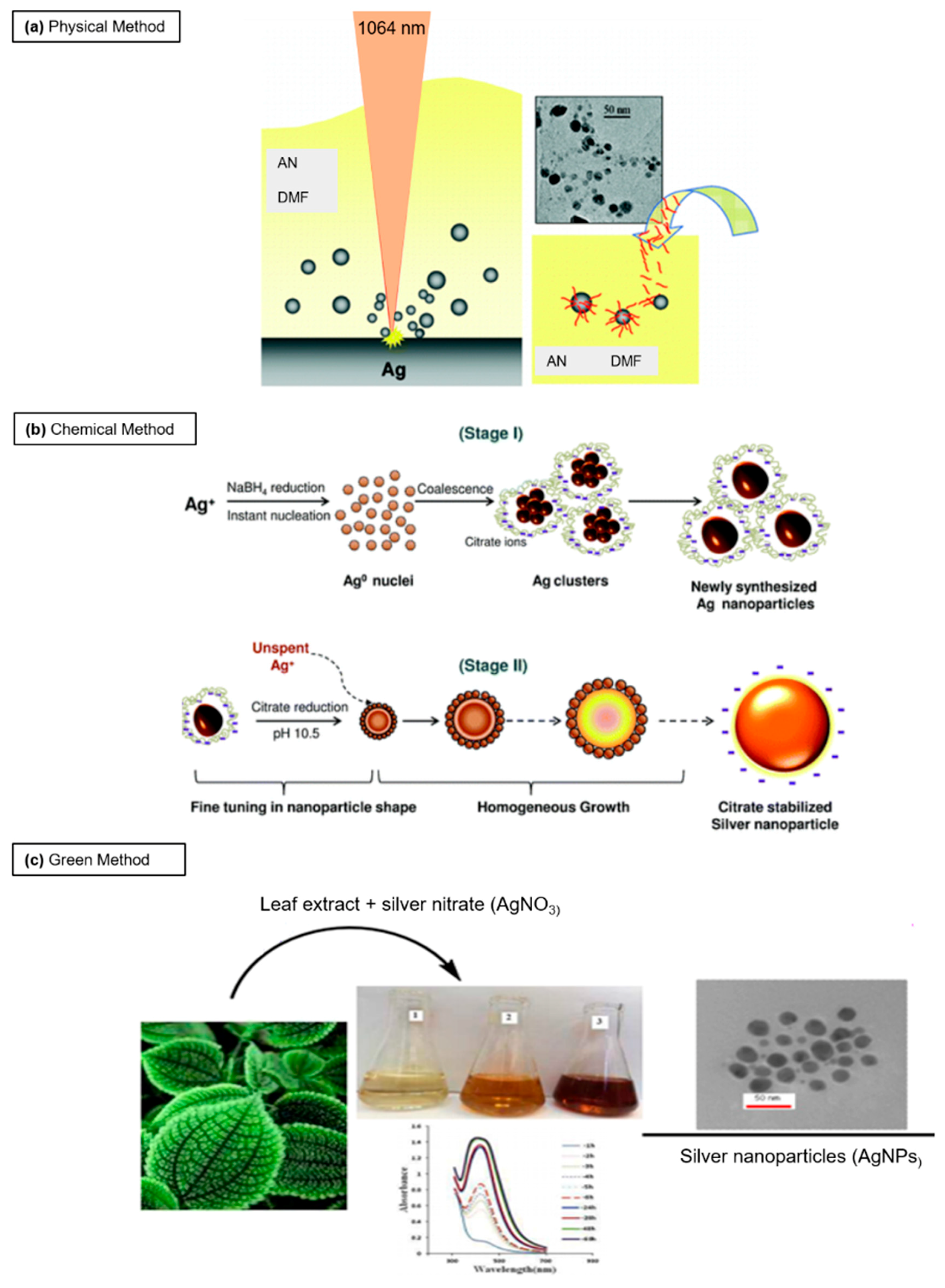
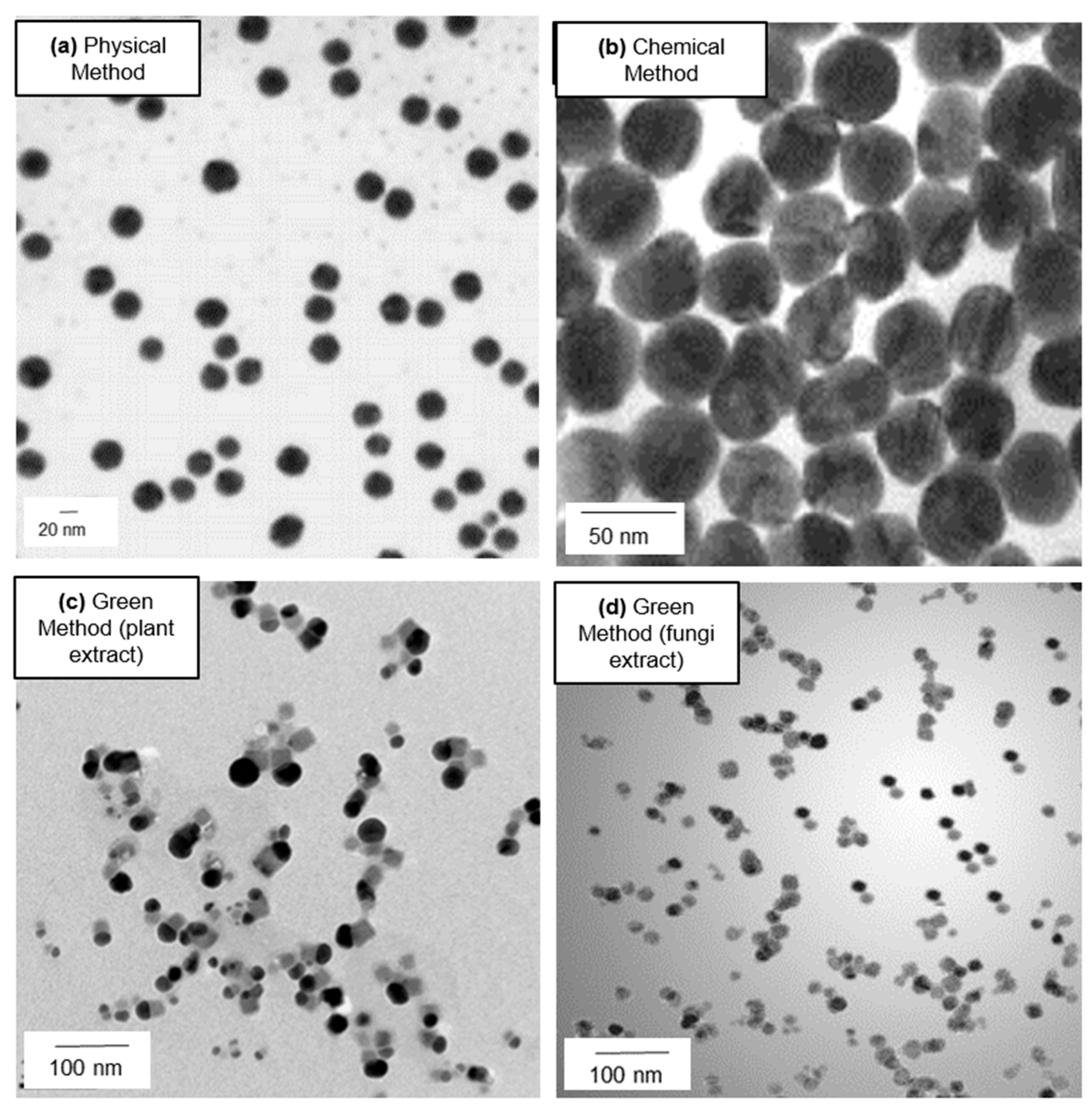
2. Principal Routes of Ag NPs Synthesis
2.1. Physical Synthesis
2.2. Chemical Approach
2.3. Green Synthesis
- (a)
- microorganisms (fungi, yeasts, bacteria, and actinomycetes),
- (b)
- plants and plant extract,
- (c)
- membranes, viruses’ DNA, and diatoms.
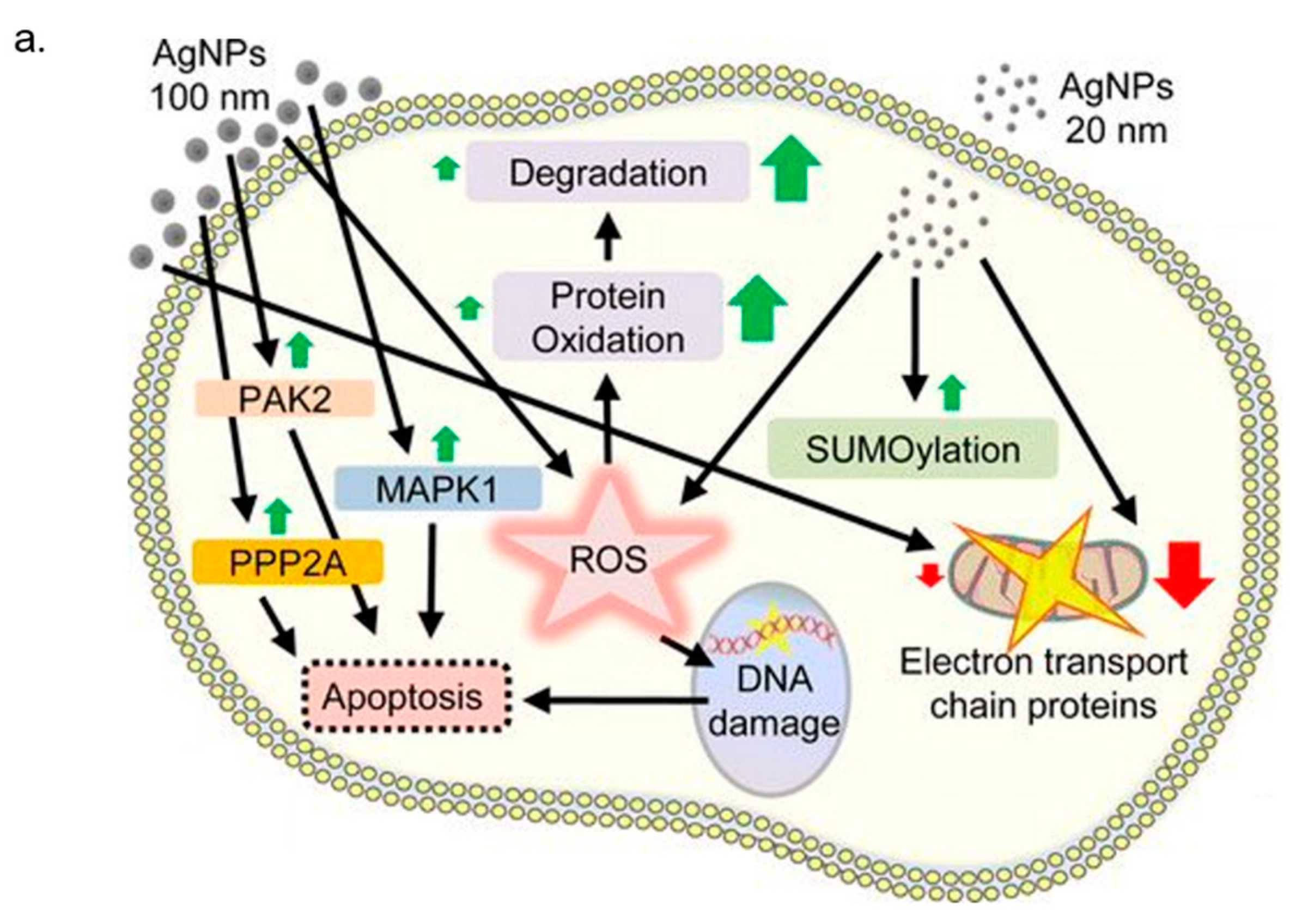
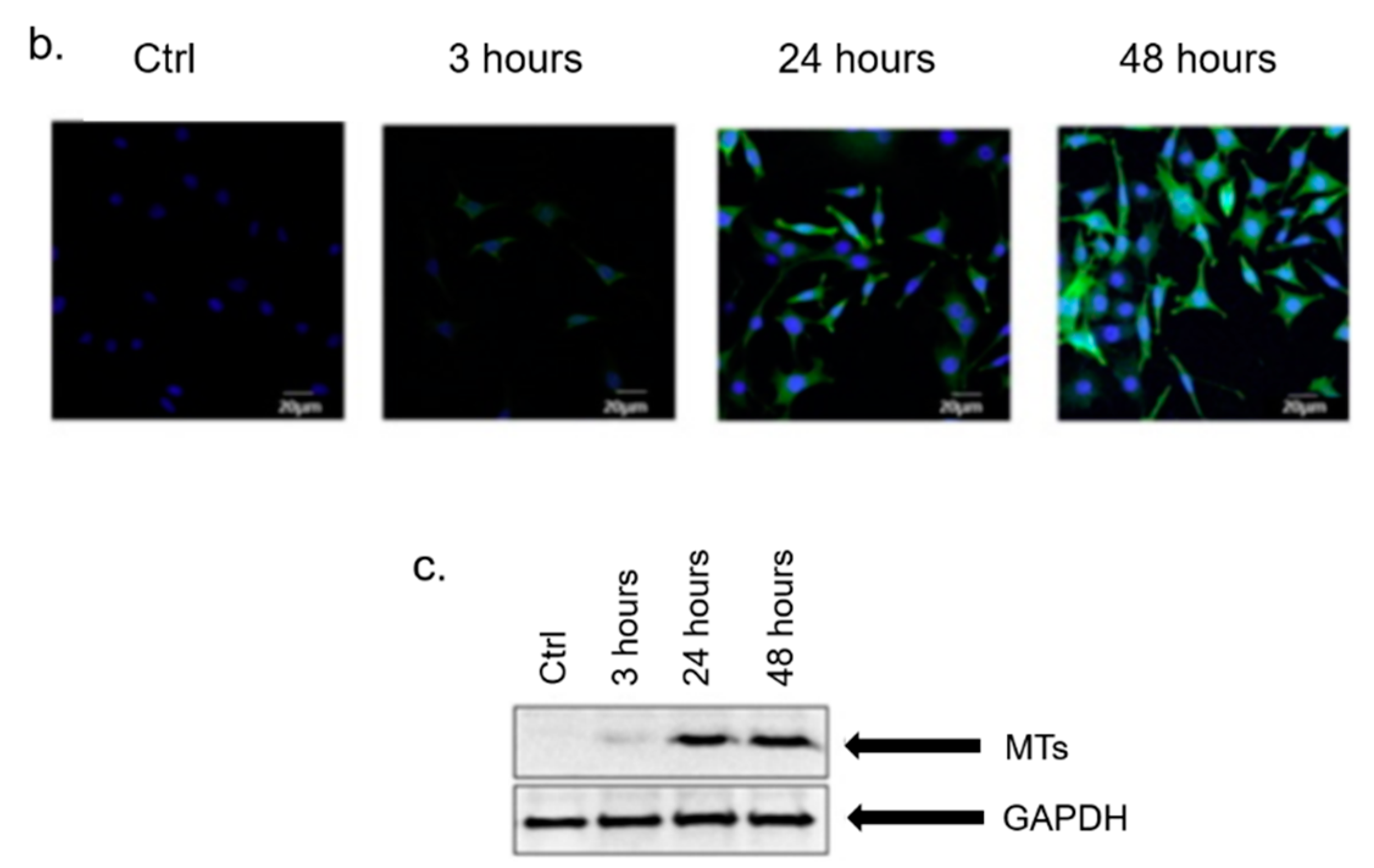
3. In Vitro Cytotoxicity Mechanism Induced by Ag NPs
4. AgNPs and Theranostics
5. Conclusions
Conflicts of Interest
References
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (Ag NPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Zhang, Q.; Carmichael, P.L.; Boekelheide, K.; Andersen, M.E. Toxicity testing in the 21 century: Defining new risk assessment approaches based on perturbation of intracellular toxicity pathways. PLoS ONE 2011, 6, e20887. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Jurj, A.; Braicu, C.; Pop, L.A.; Tomuleasa, C.; Gherman, C.D.; Berindan-Neagoe, I. The new era of nanotechnology, an alternative to change cancer treatment. Drug Des. Dev. Ther. 2017, 11, 2871–2890. [Google Scholar] [CrossRef] [PubMed]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine--challenge and perspectives. Angew. Chem. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fan, C.Q.; Dong, H.; Wang, S.M.; Yang, X.C.; Yang, S.M. Current applications and future prospects of nanomaterials in tumor therapy. Int. J. Nanomed. 2017, 12, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Doria, G.; Baptista, P. Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012, 2012, 751075. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic nanoparticles in cancer therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Rogach, A.L.; Jackel, F.; Klar, T.A.; Feldmann, J. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv. Mater. 2010, 22, 1805–1825. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; El-Sayed, M.A. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernandez, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N. Nanomedicine: Nanocarriers shape up for long life. Nat. Nanotechnol. 2007, 2, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Day, E.S.; Morton, J.G.; West, J.L. Nanoparticles for thermal cancer therapy. J. Biomech. Eng. 2009, 131, 074001. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Rinaldi, R. Toxicity assessment in the nanoparticle era. Adv. Exp. Med. Biol. 2018, 1048, 1–19. [Google Scholar] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Gurav, A.S.; Kodas, T.T.; Wang, L.-M.; Kauppinen, E.I.; Joutsensaari, J. Generation of nanometer-size fullerene particles via vapor condensation. Chem. Phys. Lett. 1994, 218, 304–308. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of nanoparticles by laser ablation: A review. KONA Powder Part. J 2017, 80–90. [Google Scholar] [CrossRef]
- Pyatenko, A.; Shimokawa, K.; Yamaguchi, M.; Nishimura, O.; Suzuki, M. Synthesis of silver nanoparticles by laser ablation in pure water. Appl. Phys. A 2004, 79, 803–806. [Google Scholar] [CrossRef]
- Tsuji, T.; Iryo, K.; Watanabe, N.; Tsuji, M. Preparation of silver nanoparticles by laser ablation in solution: Influence of laser wavelength on particle size. Appl. Surf. Sci. 2002, 202, 80–85. [Google Scholar] [CrossRef]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Free silver nanoparticles synthesized by laser ablation in organic solvents and their easy functionalization. Langmuir ACS J. Surf. Colloids 2007, 23, 6766–6770. [Google Scholar] [CrossRef] [PubMed]
- Balboul, B.A. Thermal decomposition study of erbium oxalate hexahydrate. Thermochim. Acta 2000, 351, 55–60. [Google Scholar] [CrossRef]
- Lee, D.K.; Kang, Y.S. Preparation and characterization of magnetic nanoparticles by γ-irradiation. Mater. Sci. Eng. C 2004, 24, 107–111. [Google Scholar] [CrossRef]
- Navaladian, S.; Viswanathan, B.; Viswanath, R.; Varadarajan, T. Thermal decomposition as route for silver nanoparticles. Nanoscale Res. Lett. 2006, 2, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Pluym, T.; Powell, Q.; Gurav, A.; Ward, T.; Kodas, T.; Wang, L.; Glicksman, H. Solid silver particle production by spray pyrolysis. J. Aerosol Sci. 1993, 24, 383–392. [Google Scholar] [CrossRef]
- Pingali, K.C.; Rockstraw, D.A.; Deng, S. Silver nanoparticles from ultrasonic spray pyrolysis of aqueous silver nitrate. Aerosol Sci. Technol. 2005, 39, 1010–1014. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, A.K.; Sharma, V. Synthesis of carbon nanotubes by Arc discharge and chemical vapor deposition method with analysis of its morphology, dispersion and functionalization characteristics. Cogent Eng. 2015, 2, 1094017. [Google Scholar] [CrossRef]
- Tien, D.-C.; Tseng, K.-H.; Liao, C.-Y.; Huang, J.-C.; Tsung, T.-T. Discovery of ionic silver in silver nanoparticle suspension fabricated by Arc discharge method. J. Alloys Compd. 2008, 463, 408–411. [Google Scholar] [CrossRef]
- Siegel, J.; Kvítek, O.; Ulbrich, P.; Kolská, Z.; Slepička, P.; Švorčík, V. Progressive approach for metal nanoparticle synthesis. Mater. Lett. 2012, 89, 47–50. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-F.; Zhang, H. Aggregation kinetics of nanosilver in different water conditions. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 035006. [Google Scholar] [CrossRef]
- Dang, T.M.D.; Le, T.T.T.; Fribourg-Blanc, E.; Dang, M.C. Influence of surfactant on the preparation of silver nanoparticles by polyol method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 035004. [Google Scholar] [CrossRef]
- Dadosh, T.; Sperling, J.; Bryant, G.W.; Breslow, R.; Shegai, T.; Dyshel, M.; Haran, G.; Bar-Joseph, I. Plasmonic control of the shape of the raman spectrum of a single molecule in a silver nanoparticle dimer. ACS Nano 2009, 3, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; De Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. Negligible particle-specific toxicity mechanism of silver nanoparticles: The role of Ag+ ion release in the cytosol. Nanomedicine Nanotechnol. Biol. Med. 2015, 11, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, H.; Huang, W.; Zhou, Y.; Yan, D. Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J. Colloid Interface Sci. 2008, 325, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Suriati, G.; Mariatti, M.; Azizan, A. Synthesis of silver nanoparticles by chemical reduction method: Effect of reducing agent and surfactant concentration. Int. J. Autom. Mech. Eng. 2014, 10, 1920. [Google Scholar] [CrossRef]
- Martinez-Castanon, G.; Nino-Martinez, N.; Martinez-Gutierrez, F.; Martinez-Mendoza, J.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Guzmán, M.G.; Dille, J.; Godet, S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int. J. Chem. Biomol. Eng. 2009, 2, 104–111. [Google Scholar]
- Pinto, V.V.; Ferreira, M.J.; Silva, R.; Santos, H.A.; Silva, F.; Pereira, C.M. Long time effect on the stability of silver nanoparticles in aqueous medium: Effect of the synthesis and storage conditions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 19–25. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.N.J.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Solanki, J.N.; Murthy, Z.V.P. Controlled size silver nanoparticles synthesis with water-in-oil microemulsion method: A topical review. Ind. Eng. Chem. Res. 2011, 50, 12311–12323. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, X.; Chen, J. Synthesis of nanosilver colloidal particles in water/oil microemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 22–28. [Google Scholar] [CrossRef]
- Pileni, M.; Taleb, A.; Petit, C. Silver metal nanosized particles: Control of particle size, self assemblies in 2d and 3d superlattices and optical properties. J. Dispers. Sci. Technol. 1998, 19, 185–206. [Google Scholar] [CrossRef]
- Chen, D.-H.; Wu, S.-H. Synthesis of nickel nanoparticles in water-in-oil microemulsions. Chem. Mater. 2000, 12, 1354–1360. [Google Scholar] [CrossRef]
- Zhanjiang, Z.; Jinpei, L. Synthesis and characterization of silver nanoparticles by a sonochemical method. Rare Met. Mater. Eng. 2012, 41, 1700–1705. [Google Scholar] [CrossRef]
- Okitsu, K.; Yue, A.; Tanabe, S.; Matsumoto, H.; Yobiko, Y. Formation of colloidal gold nanoparticles in an ultrasonic field: Control of rate of gold (iii) reduction and size of formed gold particles. Langmuir ACS J. Surf. Colloids 2001, 17, 7717–7720. [Google Scholar] [CrossRef]
- Zhu, J.; Palchik, O.; Chen, S.; Gedanken, A. Microwave assisted preparation of CdSe, PbSe, and Cu2−xSe nanoparticles. J. Phys. Chem. B 2000, 104, 7344–7347. [Google Scholar] [CrossRef]
- Gutierrez, M.; Henglein, A.; Dohrmann, J.K. Hydrogen atom reactions in the sonolysis of aqueous solutions. J. Phys. Chem. 1987, 91, 6687–6690. [Google Scholar] [CrossRef]
- Wani, I.A.; Ganguly, A.; Ahmed, J.; Ahmad, T. Silver nanoparticles: Ultrasonic wave assisted synthesis, optical characterization and surface area studies. Mater. Lett. 2011, 65, 520–522. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Zhai, S.-R.; Shao, X.; Zhou, D.; Zhai, B.; An, Q.-D. Sol-gel synthesis of nanosilver embedded hybrid materials using combined organosilica precursors. J. Sol-Gel Sci. Technol. 2012, 62, 281–286. [Google Scholar] [CrossRef]
- Lkhagvajav, N.; Yasa, I.; Celik, E.; Koizhaiganova, M.; Sari, O. Antimicrobial activity of colloidal silver nanoparticles prepared by sol-gel method. Dig. J. Nanomater. Biostruct. 2011, 6, 149–154. [Google Scholar]
- Reetz, M.T.; Helbig, W. Size-selective synthesis of nanostructured transition metal clusters. J. Am. Chem. Soc. 1994, 116, 7401–7402. [Google Scholar] [CrossRef]
- Ma, H.; Yin, B.; Wang, S.; Jiao, Y.; Pan, W.; Huang, S.; Chen, S.; Meng, F. Synthesis of silver and gold nanoparticles by a novel electrochemical method. ChemPhysChem 2004, 5, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, L.; Blanco, M.; Lopez-Quintela, M. Electrochemical synthesis of silver nanoparticles. J. Phys. Chem. B 2000, 104, 9683–9688. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, S.; Tyagi, R.; Surampalli, R. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 2011, 82, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from staphylococcus aureus and its antimicrobial activity against mrsa and mrse. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, C.; Rajesh, R.; Kaviarasan, T.; Muthukumar, K.; Kavitake, D.; Shetty, P.H. Synthesis of silver nanoparticles using bacterial exopolysaccharide and its application for degradation of azo-dyes. Biotechnol. Rep. 2017, 15, 33–40. [Google Scholar] [CrossRef] [PubMed]
- El-Shanshoury, A.E.-R.R.; ElSilk, S.E.; Ebeid, M.E. Extracellular biosynthesis of silver nanoparticles using escherichia coli ATCC 8739, bacillus subtilis atcc 6633, and streptococcus thermophilus esh1 and their antimicrobial activities. ISRN Nanotechnol. 2011, 2011, 385480. [Google Scholar] [CrossRef]
- Mokhtari, N.; Daneshpajouh, S.; Seyedbagheri, S.; Atashdehghan, R.; Abdi, K.; Sarkar, S.; Minaian, S.; Shahverdi, H.R.; Shahverdi, A.R. Biological synthesis of very small silver nanoparticles by culture supernatant of klebsiella pneumonia: The effects of visible-light irradiation and the liquid mixing process. Mater. Res. Bull. 2009, 44, 1415–1421. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Kottaisamy, M.; BarathManiKanth, S.; Kartikeyan, B.; Gurunathan, S. Biosynthesis of silver and gold nanoparticles using brevibacterium casei. Colloids Surf. B Biointerfaces 2010, 77, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.-K.; Zhang, W.; Liu, Y.-Y.; Lin, Z.-Y.; Yao, B.-X.; Weng, S.-Z.; Zeng, J.-L. Characterization of adsorption and reduction of noble metal ions by bacteria. Chem. J. Chin. Univ. 1999, 20, 1452–1454. [Google Scholar]
- Samadi, N.; Golkaran, D.; Eslamifar, A.; Jamalifar, H.; Fazeli, M.R.; Mohseni, F.A. Intra/extracellular biosynthesis of silver nanoparticles by an autochthonous strain of proteus mirabilis isolated fromphotographic waste. J. Biomed. Nanotechnol. 2009, 5, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (i) nitrate complex. Langmuir ACS J. Surf. Colloids 2007, 23, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Minaeian, S.; Shahverdi, A.; Nouhi, A.A.; Shahverdi, H.R. Extracellular biosynthesis of silver nanoparticles by somebacteria. J. Sci. I. U. A. (JSIAU) 2008, 17, 66. [Google Scholar]
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain mky3. Nanotechnology 2002, 14, 95. [Google Scholar] [CrossRef]
- Bhangale, H.; Sarode, K.; Patil, A.; Patil, D. Microbial Synthesis of Silver Nanoparticles Using Aspergillus Flavus and Their Characterization; Techno-Societal 2016, International Conference on Advanced Technologies for Societal Applications; Springer: Berlin, Germany, 2016; pp. 463–470. [Google Scholar]
- Vigneshwaran, N.; Ashtaputre, N.; Varadarajan, P.; Nachane, R.; Paralikar, K.; Balasubramanya, R. Biological synthesis of silver nanoparticles using the fungus Aspergillus Flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Kathiresan, K.; Manivannan, S.; Nabeel, M.; Dhivya, B. Studies on silver nanoparticles synthesized by a marine fungus, penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf. B Biointerfaces 2009, 71, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, K.; Alikunhi, N.M.; Pathmanaban, S.; Nabikhan, A.; Kandasamy, S. Analysis of antimicrobial silver nanoparticles synthesized by coastal strains of Escherichia Coli and aspergillus niger. Can. J. Microbiol. 2010, 56, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.; Bonde, P.; Ingle, A.; Marcato, P.; Duran, N.; Rai, M. Exploitation of Aspergillus Niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- De Souza, A.O.; Rodrigues, A.G. Biosynthesis of silver nanoparticles by fungi. Fungal Biomol. Sources Appl. Recent Dev. 2015, 115–135. [Google Scholar] [CrossRef]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 2011, 13, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Raudabaugh, D.B.; Tzolov, M.B.; Calabrese, J.P.; Overton, B.E. Synthesis of silver nanoparticles by a bryophilous rhizoctonia species. Nanomater. Nanotechnol. 2013, 3, 2. [Google Scholar] [CrossRef]
- Devika, R.; Elumalai, S.; Manikandan, E.; Eswaramoorthy, D. Biosynthesis of silver nanoparticles using the fungus Pleurotus Ostreatus and their antibacterial activity. Open Access Sci. Rep. 2012, 1, 557. [Google Scholar]
- Kulkarni, N.; Muddapur, U. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 2014, 2014, 510246. [Google Scholar] [CrossRef]
- Kumar, D.A.; Palanichamy, V.; Roopan, S.M. Green synthesis of silver nanoparticles using alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 127, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, S.; Bhatt, D.; Sreedhar, B. Green synthesis of silver nanoparticles using coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C 2016, 58, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of silver nanoparticles from tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B Biointerfaces 2012, 96, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Jagan, E.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.; Mohan, N. Synthesis of silver nanoparticles using acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Veeraputhiran, V. Bio-catalytic synthesis of silver nanoparticles. Int. J. ChemTech Res. 2013, 5, 255–2562. [Google Scholar]
- Khalil, M.M.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Rashid, Z.; Moadi, T.; Ghahremanzadeh, R. Green synthesis and characterization of silver nanoparticles using ferula latisecta leaf extract and their application as a catalyst for the safe and simple one-pot preparation of spirooxindoles in water. New J. Chem. 2016, 40, 3343–3349. [Google Scholar] [CrossRef]
- Roy, N.; Gaur, A.; Jain, A.; Bhattacharya, S.; Rani, V. Green synthesis of silver nanoparticles: An approach to overcome toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar]
- Chen, Y.-H.; Yeh, C.-S. Laser ablation method: Use of surfactants to form the dispersed ag nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2002, 197, 133–139. [Google Scholar] [CrossRef]
- Qin, Y.; Ji, X.; Jing, J.; Liu, H.; Wu, H.; Yang, W. Size control over spherical silver nanoparticles by ascorbic acid reduction. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 172–176. [Google Scholar] [CrossRef]
- Kannan, R.; Stirk, W.; Van Staden, J. Synthesis of silver nanoparticles using the seaweed Codium Capitatum P.C. Silva (Chlorophyceae). S. Afr. J. Bot. 2013, 86, 1–4. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of silver nanoparticles on human cells: Effect of particle size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Hsin, Y.H.; Chen, C.F.; Huang, S.; Shih, T.S.; Lai, P.S.; Chueh, P.J. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett. 2008, 179, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In Vitro toxicity of nanoparticles in BRL 3a rat liver cells. Toxicology In Vitro 2005, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Verano-Braga, T.; Miethling-Graff, R.; Wojdyla, K.; Rogowska-Wrzesinska, A.; Brewer, J.R.; Erdmann, H.; Kjeldsen, F. Insights into the cellular response triggered by silver nanoparticles using quantitative proteomics. ACS Nano 2014, 8, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, a549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.H.; Park, K.; Yi, J.; Ryu, D.Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicology In Vitro 2009, 23, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Avalos, A.; Haza, A.I.; Mateo, D.; Morales, P. Interactions of manufactured silver nanoparticles of different sizes with normal human dermal fibroblasts. Int. Wound J. 2016, 13, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Irving, E.S.; Hayashi, Y.; Sutherland, D.S.; Thorsen, K.; Autrup, H.; Beer, C. Global gene expression profiling of human lung epithelial cells after exposure to nanosilver. Toxicol. Sci. 2012, 130, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.J.; Choi, J. P38 mapk activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in jurkat t cells. Environ. Sci. Technol. 2010, 44, 8337–8342. [Google Scholar] [CrossRef] [PubMed]
- Nishanth, R.P.; Jyotsna, R.G.; Schlager, J.J.; Hussain, S.M.; Reddanna, P. Inflammatory responses of raw 264.7 macrophages upon exposure to nanoparticles: Role of ROS-NFkB signaling pathway. Nanotoxicology 2011, 5, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.; Sethu, S.; Lim, H.K.; Balaji, G.; Valiyaveettil, S.; Hande, M.P. Differential regulation of intracellular factors mediating cell cycle, DNA repair and inflammation following exposure to silver nanoparticles in human cells. Genome Integr. 2012, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Chichova, M.; Shkodrova, M.; Vasileva, P.; Kirilova, K.; Doncheva-Stoimenova, D. Influence of silver nanoparticles on the activity of rat liver mitochondrial atpase. J. Nanopart. Res. 2014, 16, 1–14. [Google Scholar] [CrossRef]
- Lee, D.Y.; Li, K.C. Molecular theranostics: A primer for the imaging professional. AJR Am. J. Roentgenol. 2011, 197, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S. Theranostics: Are we there yet? Mol. Pharm. 2013, 10, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Theranostic nanoshells: From probe design to imaging and treatment of cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.J.; Yang, V.C.; David, A.E. Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011, 29, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Mani, P.; Sharma, N.R.; Krishnan, A.; Kumar, V.V.; Reddy, B.S.; Chaudhuri, A.; Roy, R.P.; Sarkar, D.P. Histidylated lipid-modified sendai viral envelopes mediate enhanced membrane fusion and potentiate targeted gene delivery. J. Biol. Chem. 2005, 280, 35399–35409. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mishra, P.K.; Talegaonkar, S.; Vaidya, B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today 2015, 20, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Gogoi, S.K.; Chattopadhyay, A.; Ghosh, S.S. Implications of silver nanoparticle induced cell apoptosis for in vitro gene therapy. Nanotechnology 2008, 19, 075104. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.L.; Sharp, B. Nano-particle labelling of nucleic acids for enhanced detection by inductively-coupled plasma mass spectrometry (ICP-MS). Chem. Commun. 2007, 4537–4539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whaley, S.R.; English, D.S.; Hu, E.L.; Barbara, P.F.; Belcher, A.M. Selection of peptides with semiconductor binding specificity for directed nanocrystal assembly. Nature 2000, 405, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Yang, L.; Yang, H.Y.; Wang, K.; Yao, W.G.; Jiang, K.; Huang, X.L.; Zheng, Z. Antineoplastic activities of protein-conjugated silver sulfide nano-crystals with different shapes. J. Inorg. Biochem. 2010, 104, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl. Mater. Interfaces 2011, 3, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Shi, H.; Zhu, W.; Gui, Q.; Xu, Y.; Meng, J.; Guo, X.; Gong, Z.; Chen, H. Silver nanoparticles enhance the sensitivity of temozolomide on human glioma cells. Oncotarget 2017, 8, 7533–7539. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, E.; Naddaka, M.; Uboldi, C.; Loudos, G.; Fragogeorgi, E.; Molinari, V.; Pucci, A.; Tsotakos, T.; Psimadas, D.; Ponti, J.; et al. Targeted delivery of silver nanoparticles and alisertib: In vitro and in vivo synergistic effect against glioblastoma. Nanomedicine 2014, 9, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.G.; Fernandez-Baldo, M.A.; Fernandez, J.G.; Serrano, M.J.; Sanz, M.I.; Diaz-Mochon, J.J.; Lorente, J.A.; Raba, J. Study of antitumor activity in breast cell lines using silver nanoparticles produced by yeast. Int. J. Nanomed. 2015, 10, 2021–2031. [Google Scholar]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. BioMed Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by bacillus tequilensis and calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomed. 2015, 10, 4203–4222. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Park, J.H.; Cho, S.-G.; Lee, K.J.; Kim, J.-H. Green synthesis of anisotropic silver nanoparticles and its potential cytotoxicity in human breast cancer cells (MCF-7). J. Ind. Eng. Chem. 2013, 19, 1600–1605. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.K.; Han, J.W.; Zhang, X.F.; Park, J.H.; Kim, J.H. Multidimensional effects of biologically synthesized silver nanoparticles in helicobacter pylori, helicobacter felis, and human lung (l132) and lung carcinoma a549 cells. Nanoscale Res. Lett. 2015, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Zuberek, M.; Wojciechowska, D.; Krzyzanowski, D.; Meczynska-Wielgosz, S.; Kruszewski, M.; Grzelak, A. Glucose availability determines silver nanoparticles toxicity in HEPG2. J. Nanobiotechnol. 2015, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Clement, M.V.; Ramalingam, J.; Long, L.H. Hydrogen peroxide. Ubiquitous in cell culture and in vivo? IUBMB Life 2000, 50, 251–257. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, Q.; Yu, S.; Cai, D.; Wang, Q.; Zhang, X.; Huang, W.Q. Mechanism on atrial natriuretic peptide receptor in anti-anxiety with acupuncture based on its tranquilizing effect. Zhongguo Zhen Jiu 2015, 35, 101–104. [Google Scholar] [PubMed]
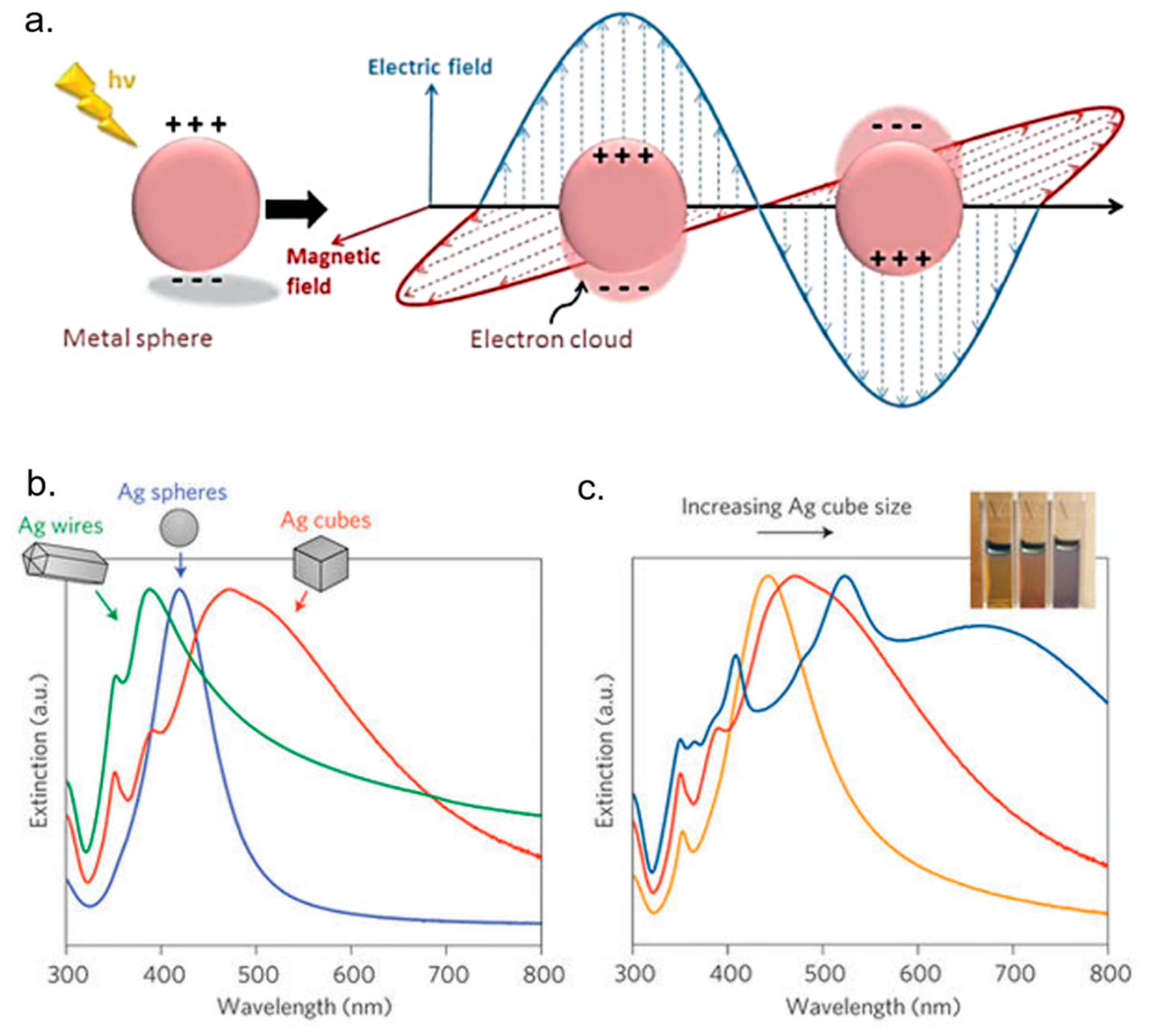
- Hao, E.; Schatz, G.C. Electromagnetic fields around silver nanoparticles and dimers. J. Chem. Phys. 2004, 120, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering by a Sphere; Wiley Online Library: New York, NY, USA, 1983. [Google Scholar]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Science & Business Media: New York, NY, USA, 2013; Volume 25. [Google Scholar]
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Hecht, B.; Bielefeldt, H.; Novotny, L.; Inouye, Y.; Pohl, D. Local excitation, scattering, and interference of surface plasmons. Phys. Rev. Lett. 1996, 77, 1889. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Schasfoort, R.B. Handbook of Surface Plasmon Resonance; Royal Society of Chemistry: Cambridge, UK, 2017. [Google Scholar]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: Effect of shape, size, structure, and assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef] [PubMed]
- Evanoff, D.D.; Chumanov, G. Synthesis and optical properties of silver nanoparticles and arrays. ChemPhysChem 2005, 6, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Peiris, S.; McMurtrie, J.; Zhu, H.-Y. Metal nanoparticle photocatalysts: Emerging processes for green organic synthesis. Catal. Sci. Technol. 2016, 6, 320–338. [Google Scholar] [CrossRef]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef] [PubMed]
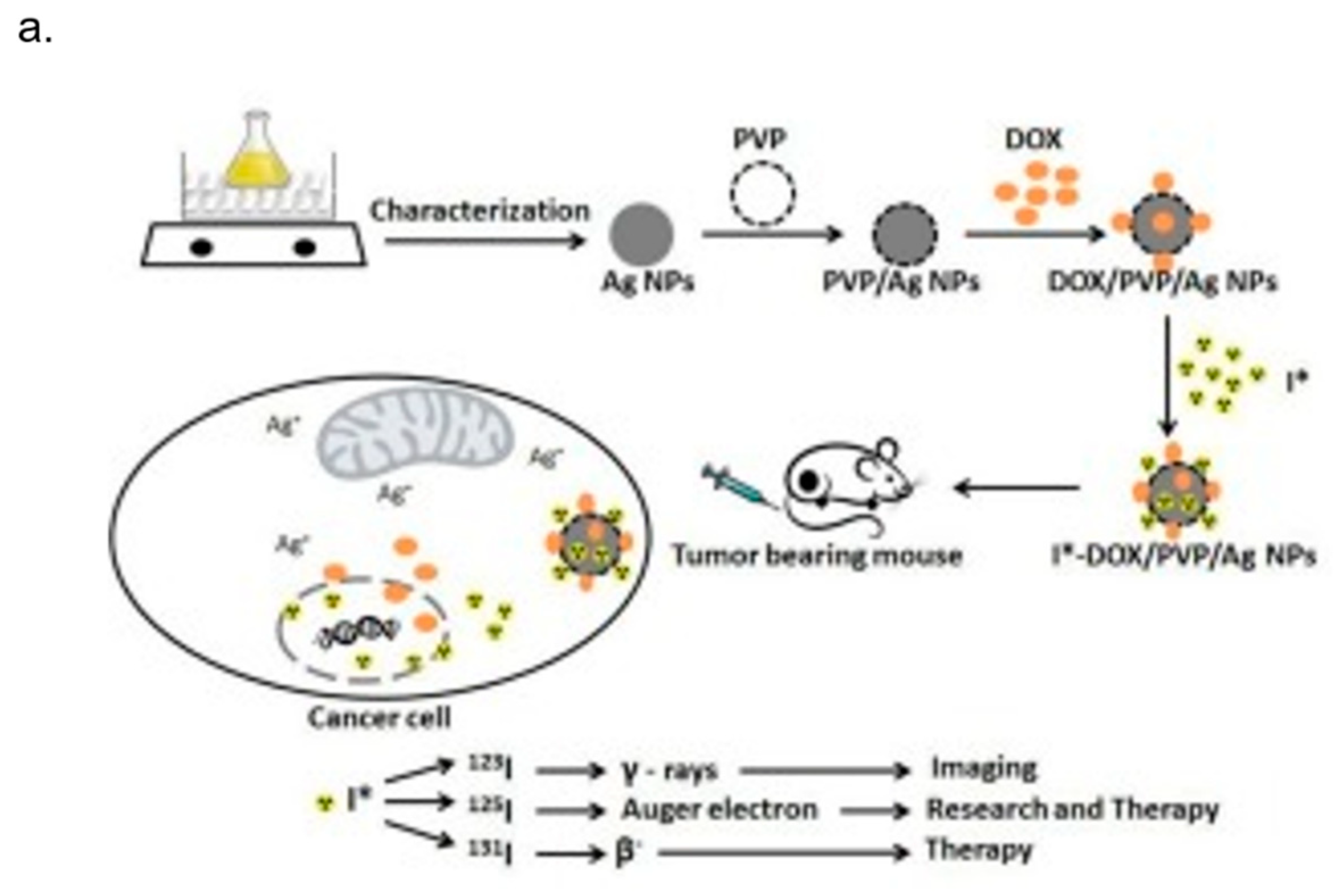
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef] [PubMed]
- Farrag, N.S.; El-Sabagh, H.A.; Al-mahallawi, A.M.; Amin, A.M.; AbdEl-Bary, A.; Mamdouh, W. Comparative study on radiolabeling and biodistribution of core-shell silver/polymeric nanoparticles-based theranostics for tumor targeting. Int. J. Pharm. 2017, 529, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.C.; Potara, M.; Gabudean, A.M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011, 311, 131–140. [Google Scholar] [CrossRef] [PubMed]
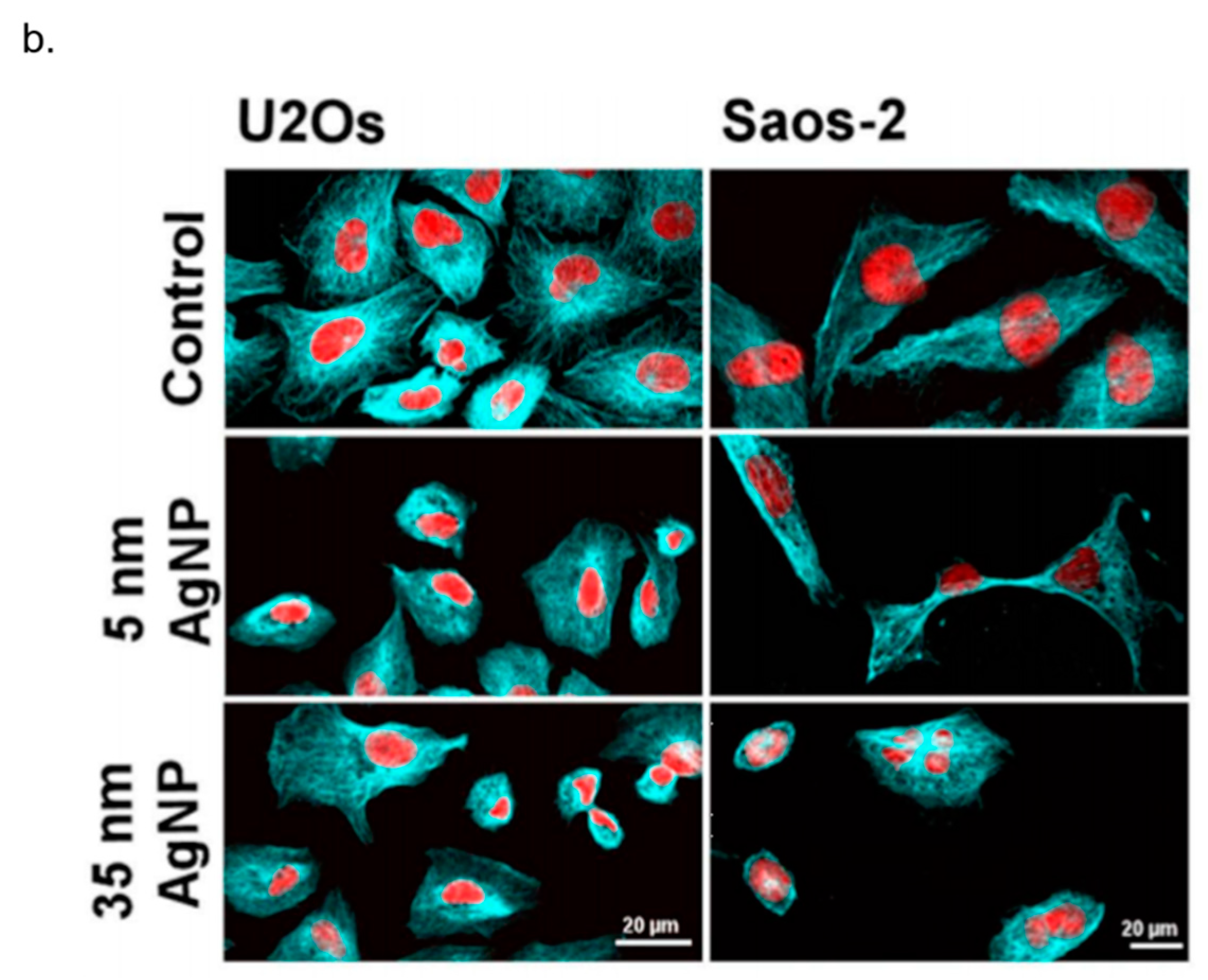
- Kovács, D.; Igaz, N.; Keskeny, C.; Bélteky, P.; Tóth, T.; Gáspár, R.; Madarász, D.; Rázga, Z.; Kónya, Z.; Boros, I.M. Silver nanoparticles defeat p53-positive and p53-negative osteosarcoma cells by triggering mitochondrial stress and apoptosis. Sci. Rep. 2016, 6, 27902. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Braydich-Stolle, L.K.; Schlager, J.J.; Dai, L.; Hussain, S.M. Can silver nanoparticles be useful as potential biological labels? Nanotechnology 2008, 19, 235104. [Google Scholar] [CrossRef] [PubMed]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepulveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Rahman, M.; Wang, J.; Patterson, T.; Saini, U.; Robinson, B.; Newport, G.; Murdock, R.; Schlager, J.; Hussain, S.; Ali, S. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Ji, J.H.; Park, J.D.; Yoon, J.U.; Kim, D.S.; Jeon, K.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H. Subchronic inhalation toxicity of silver nanoparticles. Toxicol. Sci. 2008, 108, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Van Aerle, R.; Lange, A.; Moorhouse, A.; Paszkiewicz, K.; Ball, K.; Johnston, B.D.; de-Bastos, E.; Booth, T.; Tyler, C.R.; Santos, E.M. Molecular mechanisms of toxicity of silver nanoparticles in zebrafish embryos. Environ. Sci. Technol. 2013, 47, 8005–8014. [Google Scholar] [CrossRef] [PubMed]
- Munger, M.A.; Radwanski, P.; Hadlock, G.C.; Stoddard, G.; Shaaban, A.; Falconer, J.; Grainger, D.W.; Deering-Rice, C.E. In Vivo human time-exposure study of orally dosed commercial silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease. Nanomaterials 2018, 8, 319. https://doi.org/10.3390/nano8050319
De Matteis V, Cascione M, Toma CC, Leporatti S. Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease. Nanomaterials. 2018; 8(5):319. https://doi.org/10.3390/nano8050319
Chicago/Turabian StyleDe Matteis, Valeria, Mariafrancesca Cascione, Chiara Cristina Toma, and Stefano Leporatti. 2018. "Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease" Nanomaterials 8, no. 5: 319. https://doi.org/10.3390/nano8050319
APA StyleDe Matteis, V., Cascione, M., Toma, C. C., & Leporatti, S. (2018). Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease. Nanomaterials, 8(5), 319. https://doi.org/10.3390/nano8050319







