Barrier Properties of Layered-Silicate Reinforced Ethylenepropylenediene Monomer/Chloroprene Rubber Nanorubbers
Abstract
:1. Introduction
2. Results and Discussion
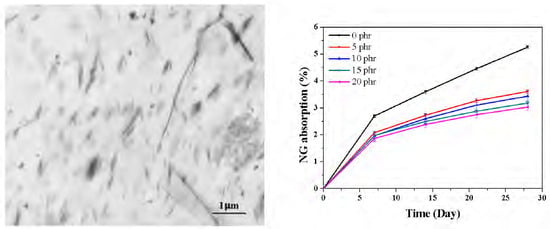
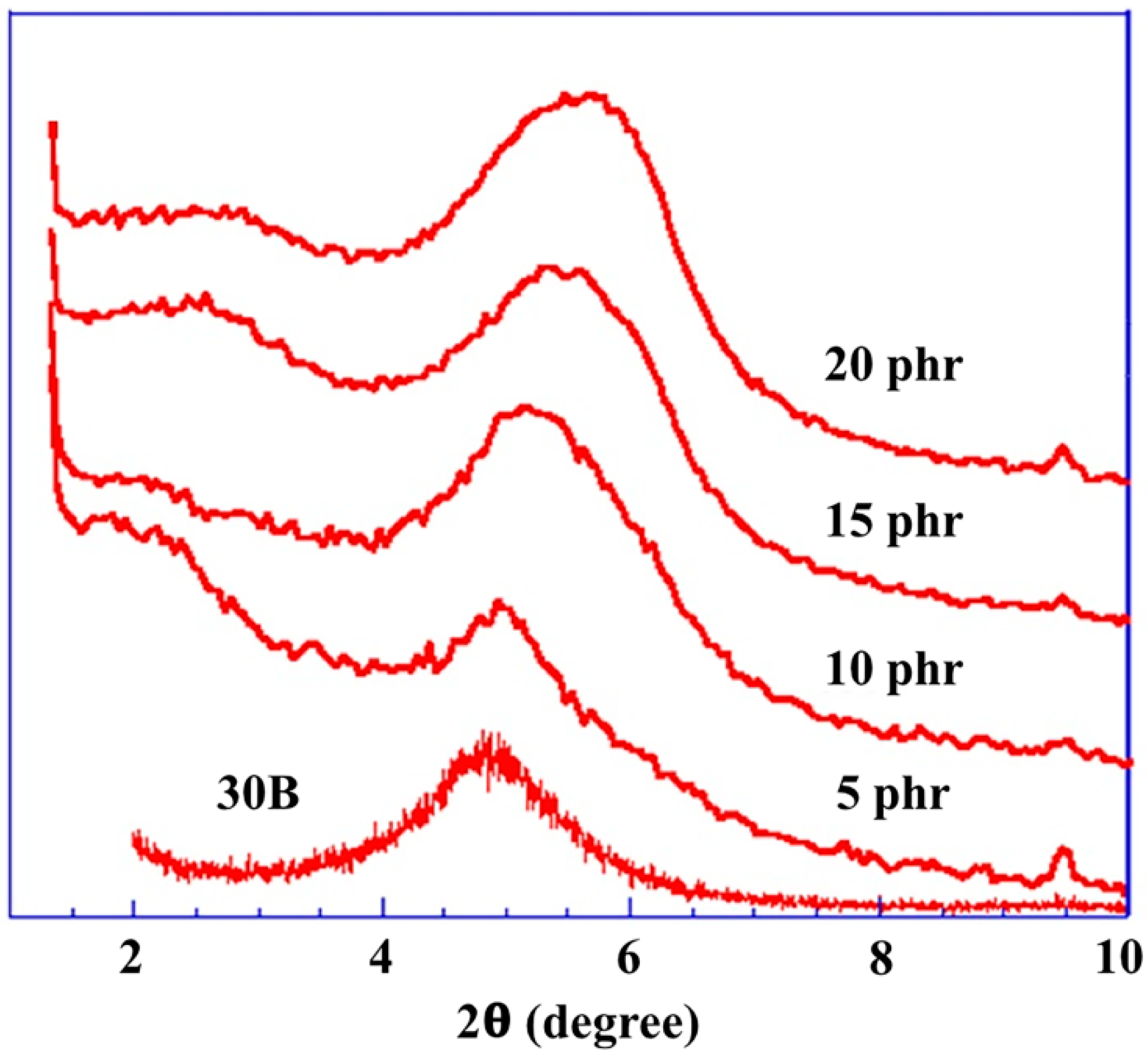
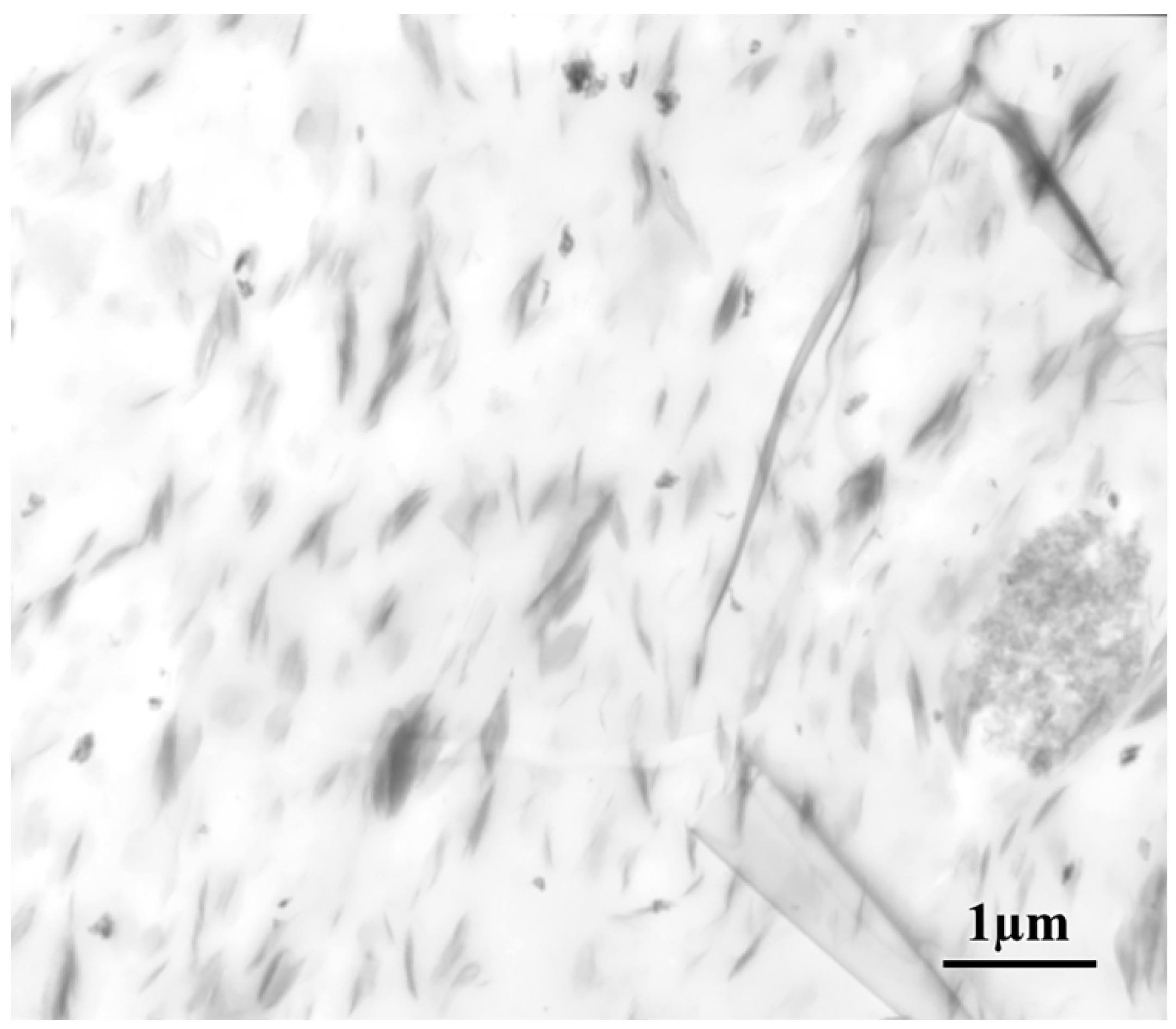
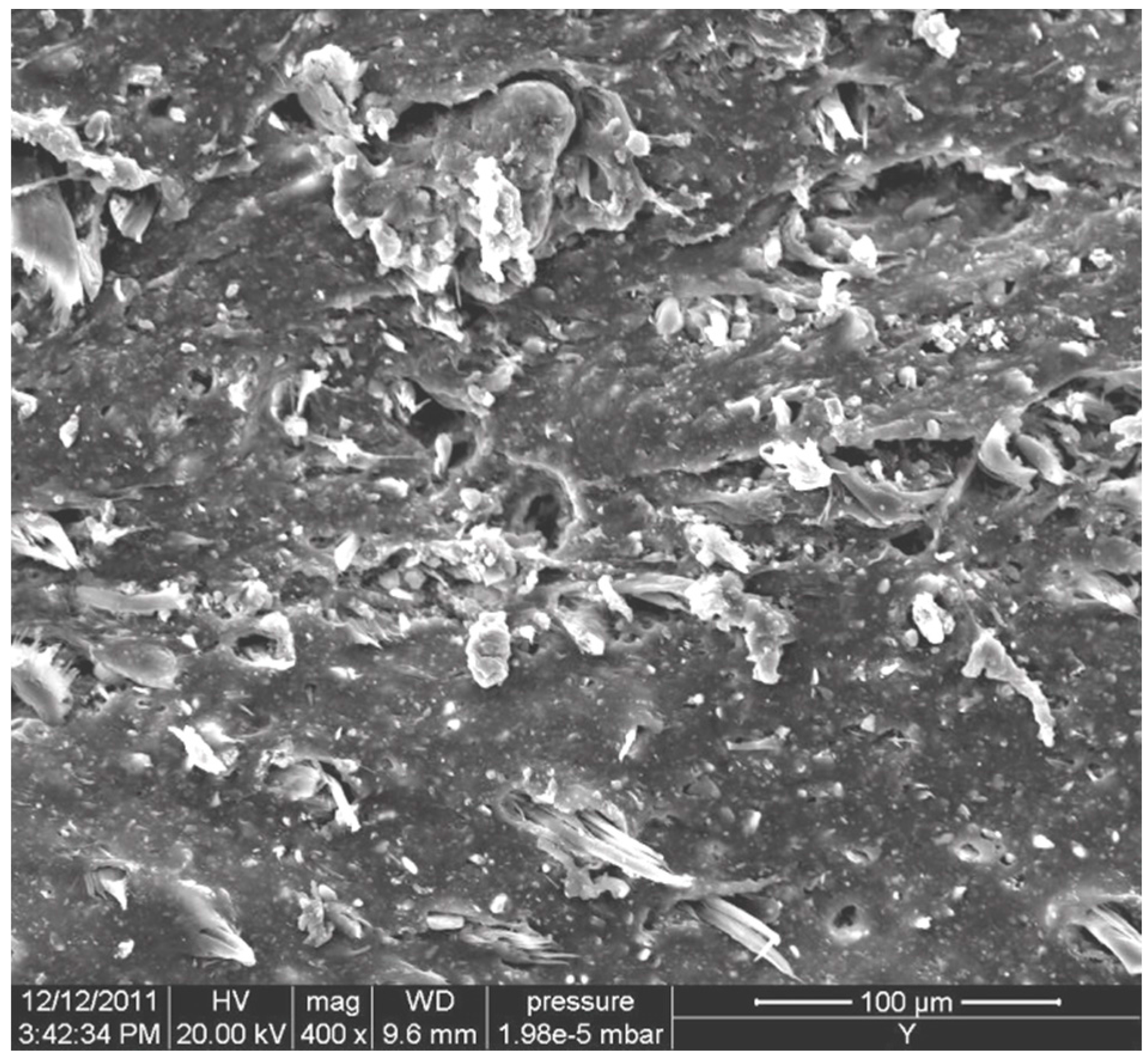
2.1. Morphology
2.2. Mechanical Properties
2.3. Crosslinking Density
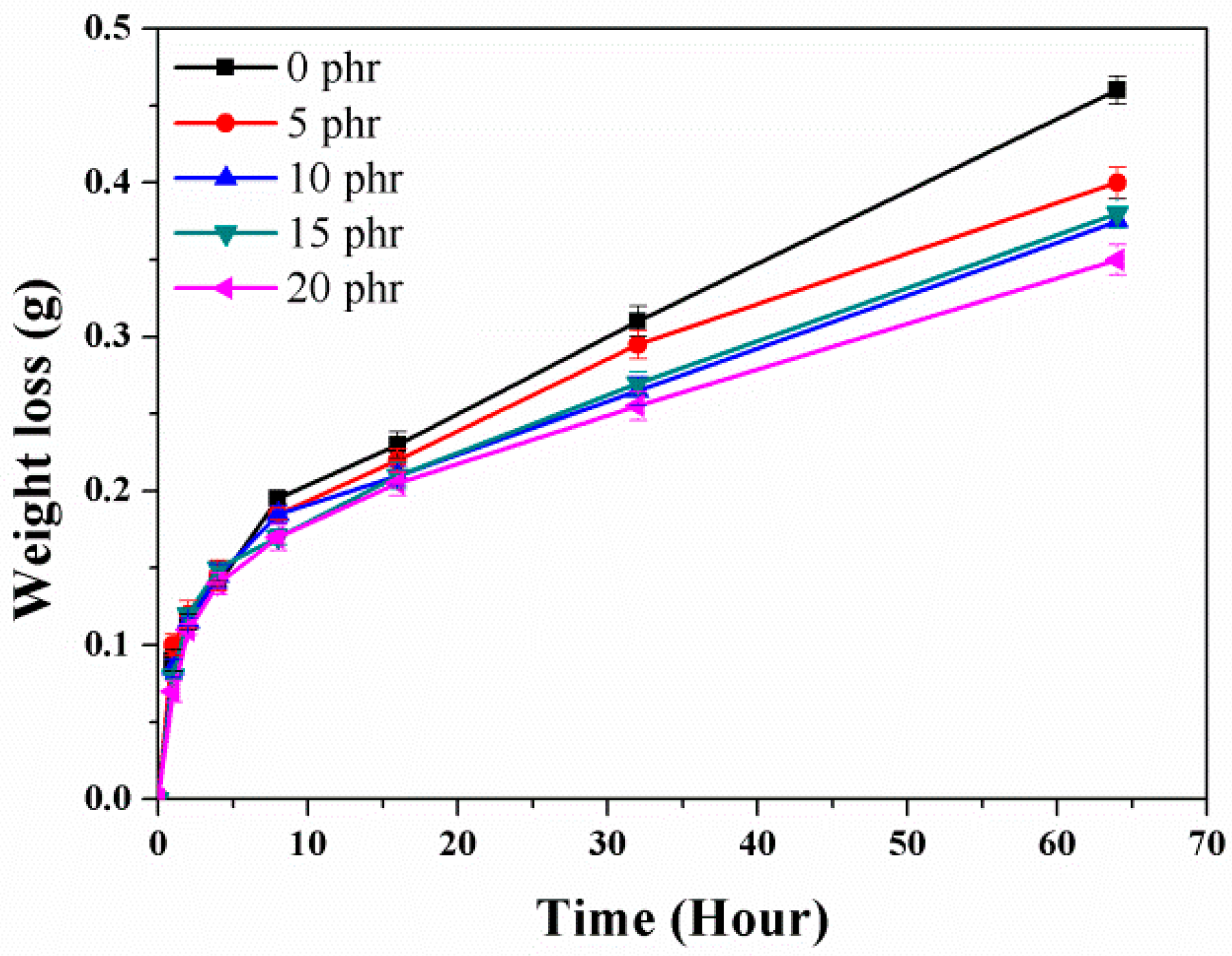
2.4. Barrier Properties
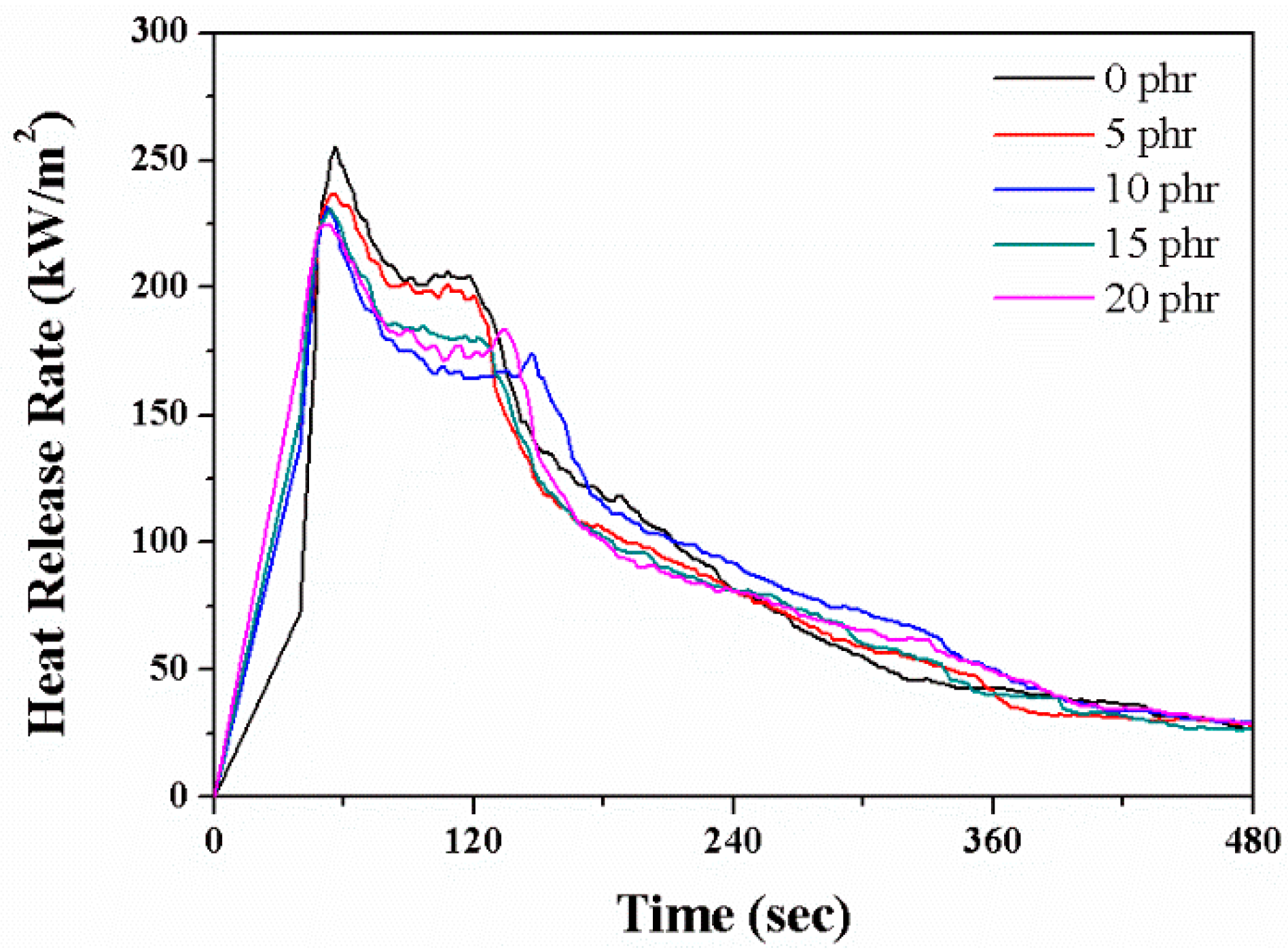
2.5. Flammability
3. Materials and Methods
3.1. Materials Preparation
3.2. The Blending Process
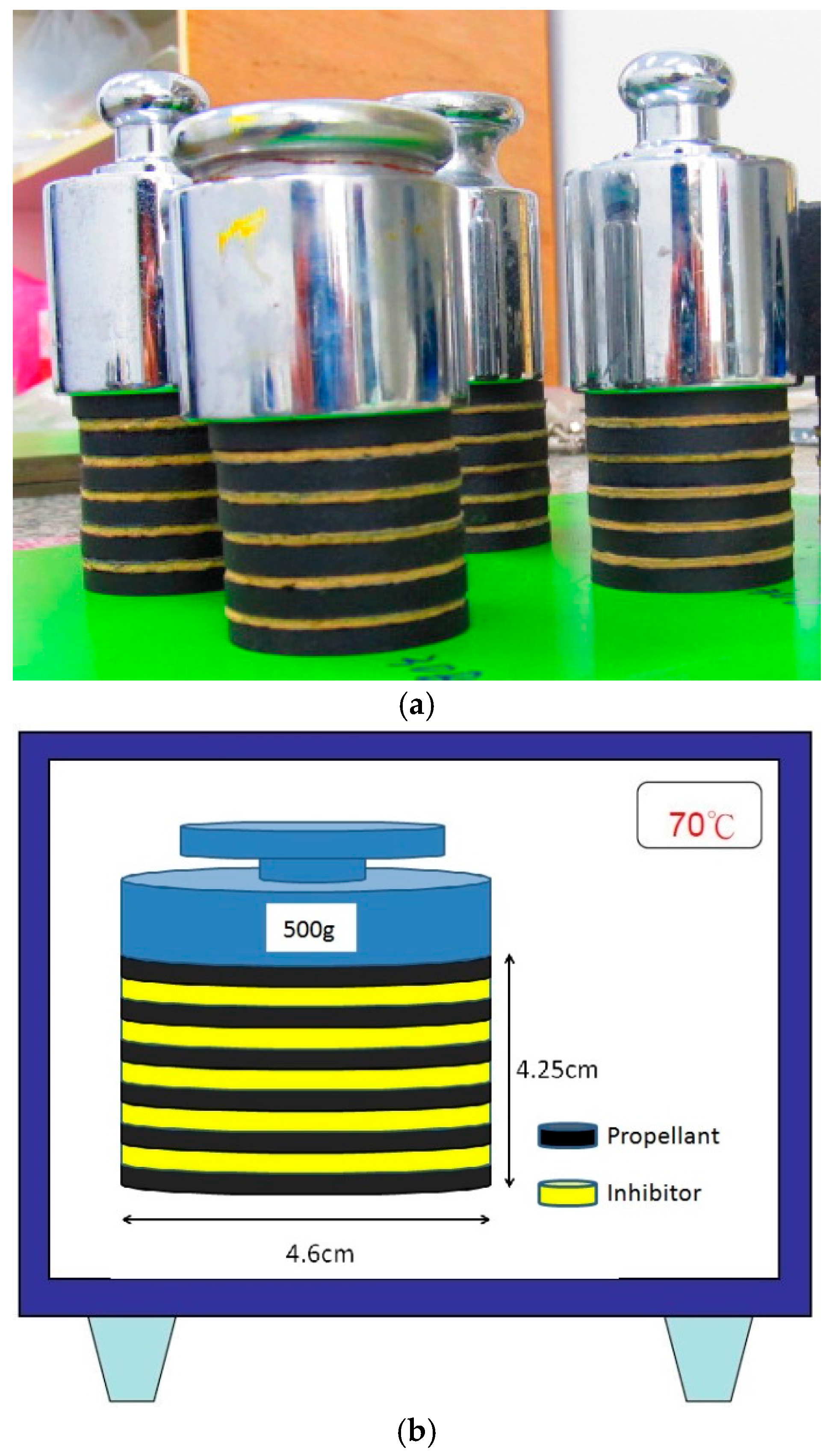
3.3. Sample Preparation and Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robin, E.C.; Bahman, S.; Gary, R. Interface resistance in thermal insulation materials with rough surfaces. Energy Build. 2017, 144, 346–357. [Google Scholar]
- Maurizio, N.; Marco, R.; Josè, K.; Luigi, T. Effect of Wollastonite on the ablation resistance of EPDM based elastomeric heat shielding materials for solid rocket motors. Polym. Degrad. Stab. 2016, 130, 47–57. [Google Scholar]
- Brock, C.; Cynthia, A.C.; Christopher, B. Insulation materials. Compr. Energy Syst. 2018, 2, 760–795. [Google Scholar]
- Li, J.; Xi, K.; Lv, X.; Li, Q.; Wang, S.-X. Characteristics and formation mechanism of compact/porous structures in char layers of EPDM insulation materials. Carbon 2018, 127, 498–509. [Google Scholar] [CrossRef]
- Du, B. Thermal conductivity and dielectric properties of silicone rubber nanocomposites. Prog. Rubber Nanocompos. 2017, 495–522. [Google Scholar] [CrossRef]
- Chen, J.; Huang, W.; Jiang, S.-B.; Li, X.-Y.; An, Y.; Li, C.; Gao, X.-L.; Chen, H.-B. Flame-retardant EPDM compounds containing phenanthrene to enhance radiation resistance. Radia Phys. Chem. 2017, 130, 400–405. [Google Scholar] [CrossRef]
- Maurizio, N.; Marco, R.; Debora, P.; Josè, K.; Luigi, T. EPDM based heat shielding materials for solid rocket motors: A comparative study of different fibrous reinforcements. Polym. Degrad. Stab. 2013, 98, 2131–2139. [Google Scholar]
- Vojislav, J.; Suzana, S.-J.; Jaroslava, B.-S.; Milena, M.-C. Composites based on carbon black reinforced NBR/EPDM rubber blends. Compos. Part B Eng. 2013, 45, 333–340. [Google Scholar]
- Maurizio, N.; Ivan, P.; Marco, R.; Josè, K.; Luigi, T. Ablation modeling of state of the art EPDM based elastomeric heat shielding materials for solid rocket motors. Comput. Mater. Sci. 2016, 111, 460–480. [Google Scholar]
- Ehsani, M.; Borsi, H.; Gockenbach, E.; Morshedian, J.; Bakhshandeh, G.R. An investigation of dynamic mechanical, thermal and electrical properties of housing materials for outdoor polymeric insulators. Eur. Polym. J. 2004, 40, 2495–2503. [Google Scholar] [CrossRef]
- Lei, Y.-J.; He, J.-P.; Zhao, Q.; Liu, T. A nitrile functionalized graphene filled ethylene propylene diene terpolymer rubber composites with improved heat resistance. Compos. Part B Eng. 2018, 134, 81–90. [Google Scholar] [CrossRef]
- Su, J.; Zhang, J. Improvement of mechanical and dielectrical properties of ethylene propylene diene monomer (EPDM)/barium titanate (BaTiO3) by layered mica and graphite flakes. Compos. Part B Eng. 2017, 112, 148–157. [Google Scholar] [CrossRef]
- Sangita, S.; Guchhait, P.K.; Bandyopadhyay, G.G.; Chaki, T.K. Development of polyimide–nanosilica filled EPDM based light rocket motor insulator compound: Influence of polyimide–nanosilica loading on thermal, ablation and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2013, 44, 8–15. [Google Scholar]
- Ning, N.-Y.; Ma, Q.; Zhang, Y.-Q.; Zhang, L.; Wu, H.-G.; Tian, M. Enhanced thermo-oxidative aging resistance of EPDM at high temperature by using synergistic antioxidants. Polym. Degrad. Stab. 2014, 102, 1–8. [Google Scholar] [CrossRef]
- Amit, D.; Francis, R.C.; Udo, W.; Gert, H. Nanocomposites based on chloroprene rubber: Effect of chemical nature and organic modification of nanoclay on the vulcanizate properties. Eur. Polym. J. 2008, 44, 3456–3465. [Google Scholar]
- Pal, K.; Panwar, V.; Bahadur, J. Rubber blend nanocomposites. In Progress in Rubber Nanocomposites, 1st ed.; Thomas, S., Maria, H.J., Eds.; Elsevier: Kidlington, UK, 2017; pp. 319–348. ISBN 978-0-08-100428-9. [Google Scholar]
- Khalil, A. An investigation on chloroprene-compatibilized acrylonitrile butadiene rubber/high density polyethylene blends. J. Adv. Res. 2015, 6, 811–817. [Google Scholar]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Qian, G.; Mark, D.W.; Babatunde, A.O. Quantification of layered silicate dispersion in polymer nanocomposites. Polymer 2014, 55, 4216–4225. [Google Scholar]
- Omid, Z.; Mojtaba, A.; Saeid, N.; Karthik, C.P.; Minoo, N. A technical review on epoxy-clay nanocomposites: Structure, properties and their applications in fiber reinforced composites. Compos. Part B Eng. 2018, 135, 1–24. [Google Scholar]
- Karolina, G.; Roland, K.; Andrzej, R.; Artur, S.; Stanislaw, G. Gas barrier, thermal, mechanical and rheological properties of highly aligned graphene-LDPE nanocomposites. Polymers 2017, 9, 294. [Google Scholar]
- Mahadev, B.; Alagirusamy, R.; Apurba, D. Flame retardant polymer composites. Fibers Polym. 2015, 16, 705–717. [Google Scholar]
- Nah, C.; Kader, M.A. Barrier Properties of Rubber Nanocomposites. In Rubber Nanocomposites: Preparation, Properties and Applications, 1st ed.; Sabu, T., Ranimol, S., Eds.; John Wiley & Sons: Singapore, 2010; pp. 499–526. ISBN 978-0-47-0823453. [Google Scholar]
- Gatos, K.G.; Karger-Kocsis, J. Rubber/clay nanocomposites: Preparation, properties and application. In Rubber Nanocomposites: Preparation, Properties and Applications, 1st ed.; Sabu, T., Ranimol, S., Eds.; John Wiley & Sons: Singapore, 2010; pp. 169–195. ISBN 978-0-47-0823453. [Google Scholar]
- Chow, W.S.; Ishak, Z.A. Polyamide blend-based nanocomposites: A review. eXPRESS Polym. Lett. 2015, 9, 211–232. [Google Scholar] [CrossRef]
- Kenneth, E.J.; James, D.B. Aramid Polymer and Powder Filler Reinforced Elastomeric Composition for Use as a Rocket Motor Insulation. U.S. Patent 4,492,779, 8 January 1985. [Google Scholar]
- Zahraalsadat, E.M.; Mohammad, I.; Mohammad, A. Kinetics of dextran crosslinking by epichlorohydrin: A rheometry and equilibrium swelling study. Carbohydr. Polym. 2013, 92, 1792–1798. [Google Scholar]
- Asish, M.; Das, C.K. Influence of modified graphite flakes on the physical, thermo-mechanical and barrier properties of butyl rubber. J. Alloys Compd. 2017, 699, 38–46. [Google Scholar]
- Zhang, Y.-M.; Liu, Q.-F.; Zhang, S.-L.; Zhang, Y.-D.; Cheng, H.-F. Gas barrier properties and mechanism of kaolin/styrene–butadiene rubber nanocomposites. Appl. Clay Sci. 2015, 111, 37–43. [Google Scholar] [CrossRef]
- Mithun, B.; Subharanjan, B.; Anil, K.B. Permeation characteristics and modeling of barrier properties of multifunctional rubber nanocomposites. Polymer 2011, 52, 1562–1576. [Google Scholar]
- Zhang, Y.-D.; Liu, Q.-F.; Zhang, Q.; Lu, Y.-P. Gas barrier properties of natural rubber/kaolin composites prepared by melt blending. Appl. Clay Sci. 2010, 50, 255–259. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.-J.; Wang, S.-Q.; Li, Y.-T.; Zhou, Y.-L. Highly ordered structured montmorillonite/brominated butyl rubber nanocomposites: Dramatic enhancement of the gas barrier properties by an external magnetic field. J. Membr. Sci. 2017, 546, 22–30. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Jo, J.O.; Datta, S.; Kim, J.K. Effects of variation of oil and zinc oxide type on the gas barrier and mechanical properties of chlorobutyl rubber/epoxidised natural rubber blends. Mater. Des. 2013, 49, 922–928. [Google Scholar] [CrossRef]
- Takagi, H.; Liu, K.; Osugi, R.; Nakagaito, A.N.; Yang, Z. Heat barrier properties of green composites. J. Biobased Mater. Bioenergy 2012, 6, 470–474. [Google Scholar] [CrossRef]
- Chow, W.S.; Teoh, E.L.; Karger-Kocsis, J. Flame retarded poly(lactic acid): A review. eXPRESS Polym. Lett. 2018, 12, 396–417. [Google Scholar] [CrossRef]


| Layered silicate (phr) | 0 | 5 | 10 | 15 | 20 |
| Hardness | 87 | 90 | 90 | 88 | 91 |
| Tensile strength (MD), MPa | 10.3 ± 0.2 | 11.7 ± 0.5 | 11.0 ± 0.6 | 11.7 ± 0.5 | 11.7 ± 0.4 |
| Elongation (MD), % | 31.1 ± 2.4 | 24.1 ± 2.8 | 25.1 ± 4.9 | 26.7 ± 2.4 | 21.0 ± 2.6 |
| Tensile strength (TD), MPa | 6.9 ± 0.1 | 7.9 ± 0.4 | 10.2 ± 0.6 | 7.0 ± 0.2 | 8.7 ± 0.2 |
| Elongation (TD), % | 60.5 ± 22.2 | 50.5 ± 21.2 | 47.2 ± 4.4 | 54.0 ± 14.8 | 52.4 ± 4.3 |
| Crosslinking density × 10−2 (mol/cm3) | 3.35 | 3.41 | 3.49 | 3.56 | 3.70 |
| Relative permeability | 1 | 0.77 | 0.72 | 0.75 | 0.63 |
| Layered Silicate(phr) | avgHRR (kW/m2) | pHRR (kW/m2) |
|---|---|---|
| 0 | 181.7 | 255.4 |
| 5 | 174.7 | 236.9 |
| 10 | 171.0 | 232.2 |
| 15 | 168.2 | 231.4 |
| 20 | 170.2 | 224.8 |
| Function | Ingredient | Composition in Phr |
|---|---|---|
| Rubber | EPDM Neoprene | 75 25 |
| Filler | Organosilicate Barium sulfate Magnesium oxide Diatomaceous Silica | 0–20 (various) 7.5 3 15 20 |
| Flammability resistant materials | Kevlar fibers Decabromethylether Antimony trioxide | 10 5 7.5 |
| Processing aids | Zinc oxide Stearic acid Wax dispersant Phosphate ester silane Dispersant | 5 1 1 4 1.5 1.5 |
| Antioxidants | Antioxidant, RD Naguard, 445 Antioxidant, RQ01 Microwax | 1.5 1.5 3 0.5 |
| Curing agents | Peroxide, F40KE Cure Co-agent, SR-350 Accelerator | 6 2 2 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.M.; Hsieh, W.Y.; Cheng, K.B.; Lai, C.-C.; Lee, K.C. Barrier Properties of Layered-Silicate Reinforced Ethylenepropylenediene Monomer/Chloroprene Rubber Nanorubbers. Nanomaterials 2018, 8, 314. https://doi.org/10.3390/nano8050314
Wu CM, Hsieh WY, Cheng KB, Lai C-C, Lee KC. Barrier Properties of Layered-Silicate Reinforced Ethylenepropylenediene Monomer/Chloroprene Rubber Nanorubbers. Nanomaterials. 2018; 8(5):314. https://doi.org/10.3390/nano8050314
Chicago/Turabian StyleWu, Chang Mou, Wen Yen Hsieh, Kuo Bin Cheng, Chiu-Chun Lai, and Kuei Chi Lee. 2018. "Barrier Properties of Layered-Silicate Reinforced Ethylenepropylenediene Monomer/Chloroprene Rubber Nanorubbers" Nanomaterials 8, no. 5: 314. https://doi.org/10.3390/nano8050314
APA StyleWu, C. M., Hsieh, W. Y., Cheng, K. B., Lai, C.-C., & Lee, K. C. (2018). Barrier Properties of Layered-Silicate Reinforced Ethylenepropylenediene Monomer/Chloroprene Rubber Nanorubbers. Nanomaterials, 8(5), 314. https://doi.org/10.3390/nano8050314