Sol-Gel Processing of MgF2 Antireflective Coatings
Abstract
1. Introduction
2. Synthetic Approaches
2.1. Fluorine Salts
2.2. Trifluoroacetic Acid (TFA)
2.3. Aqueous Hydrofluoric Acid
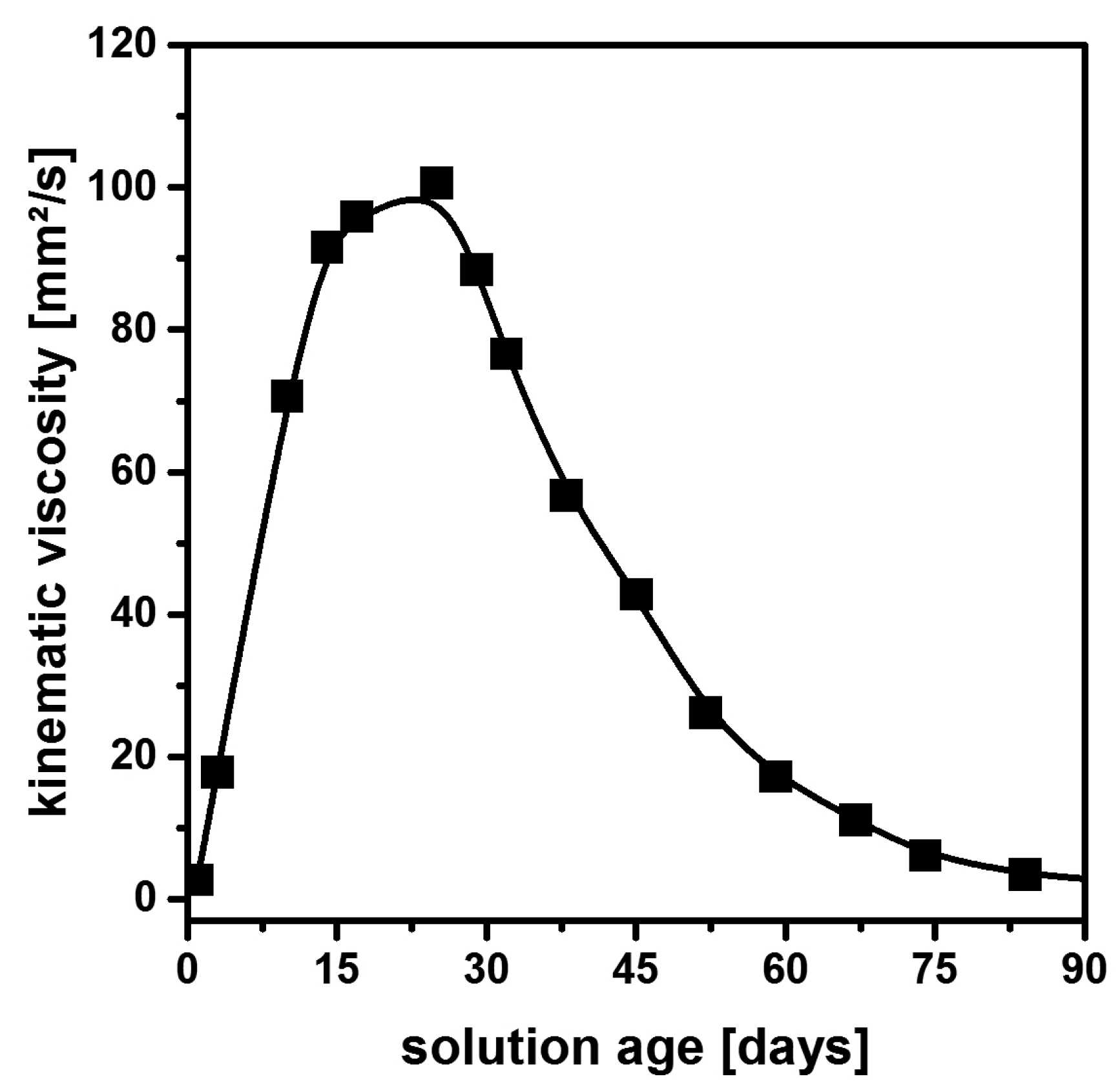
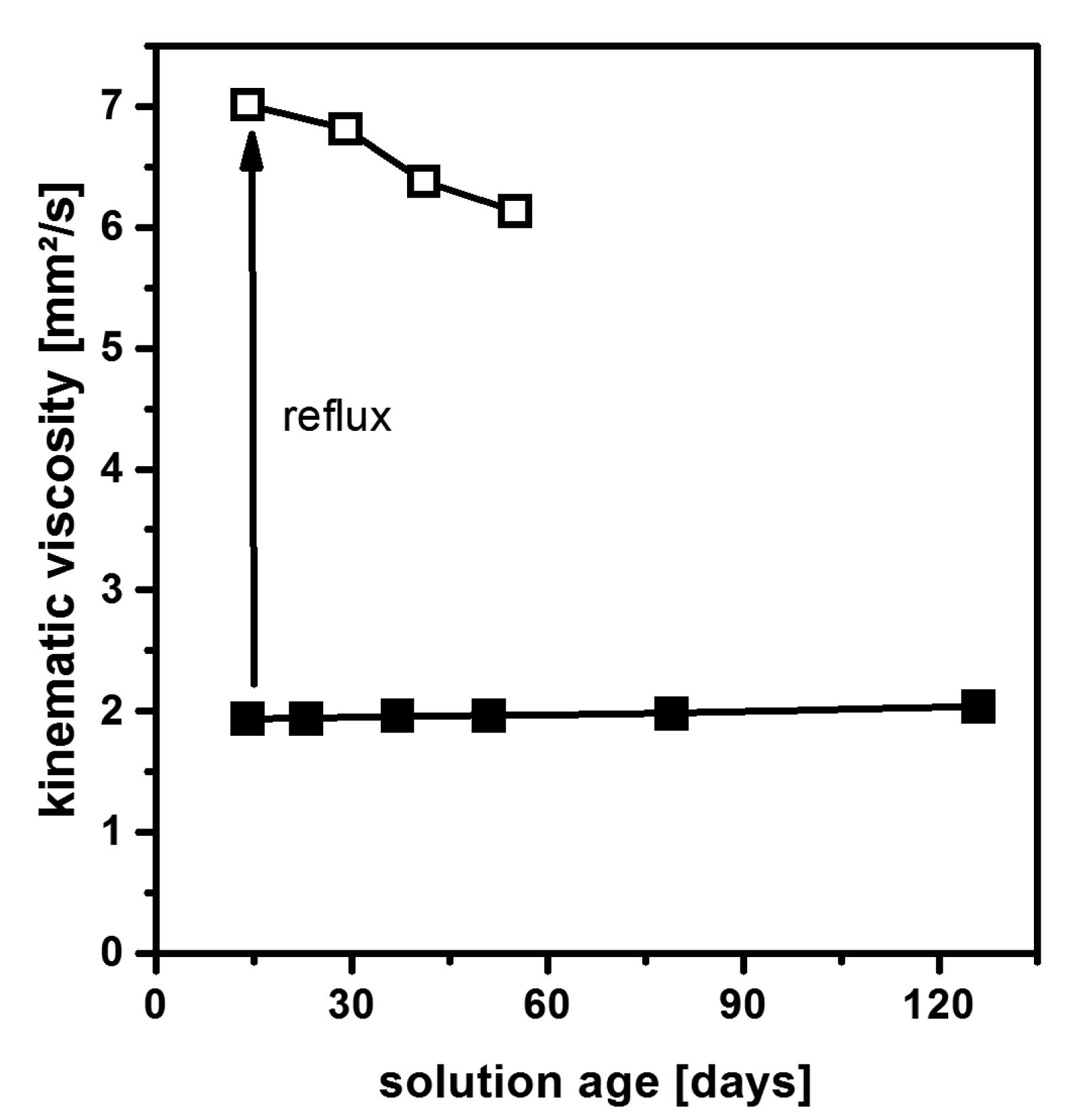
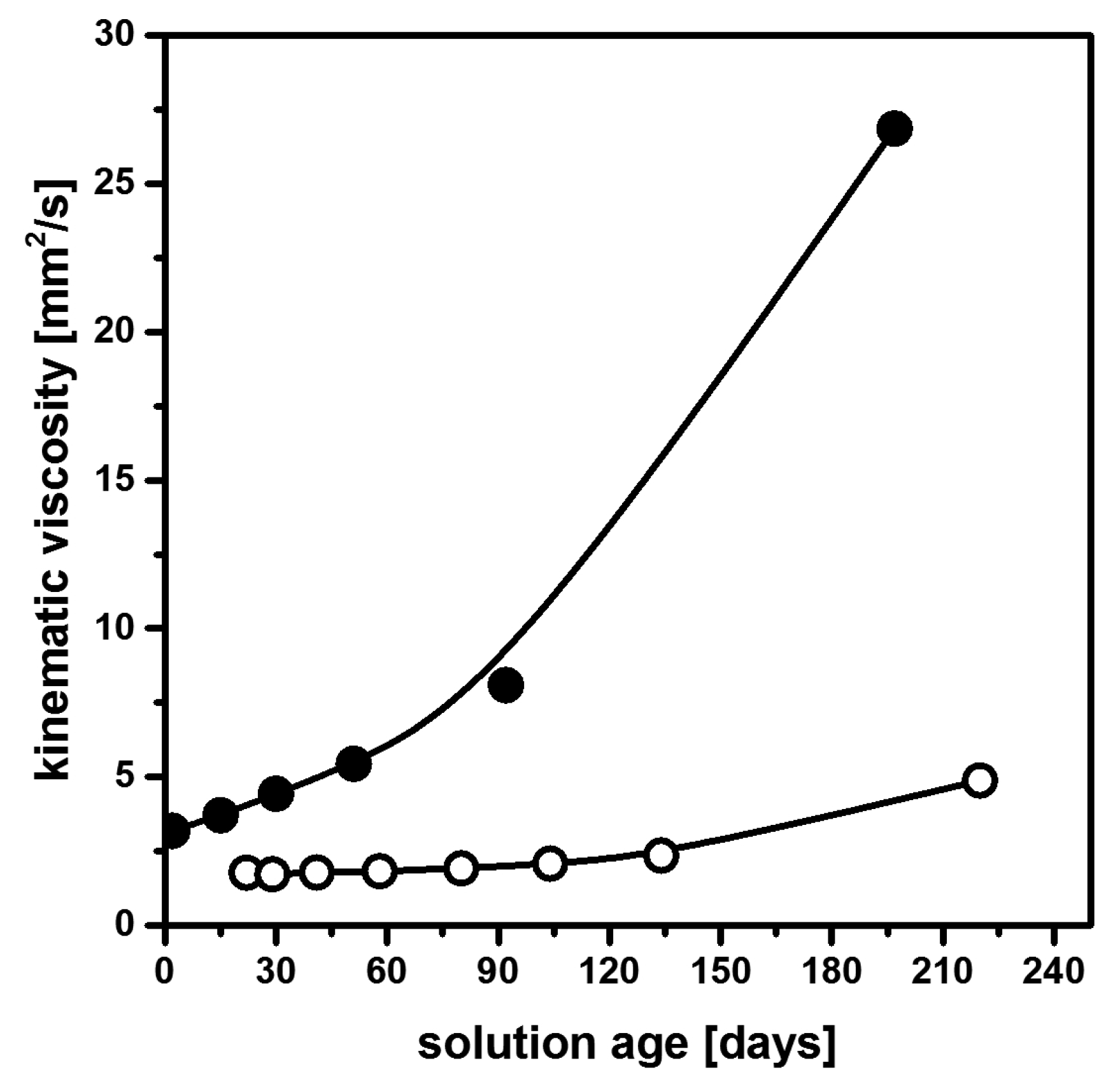
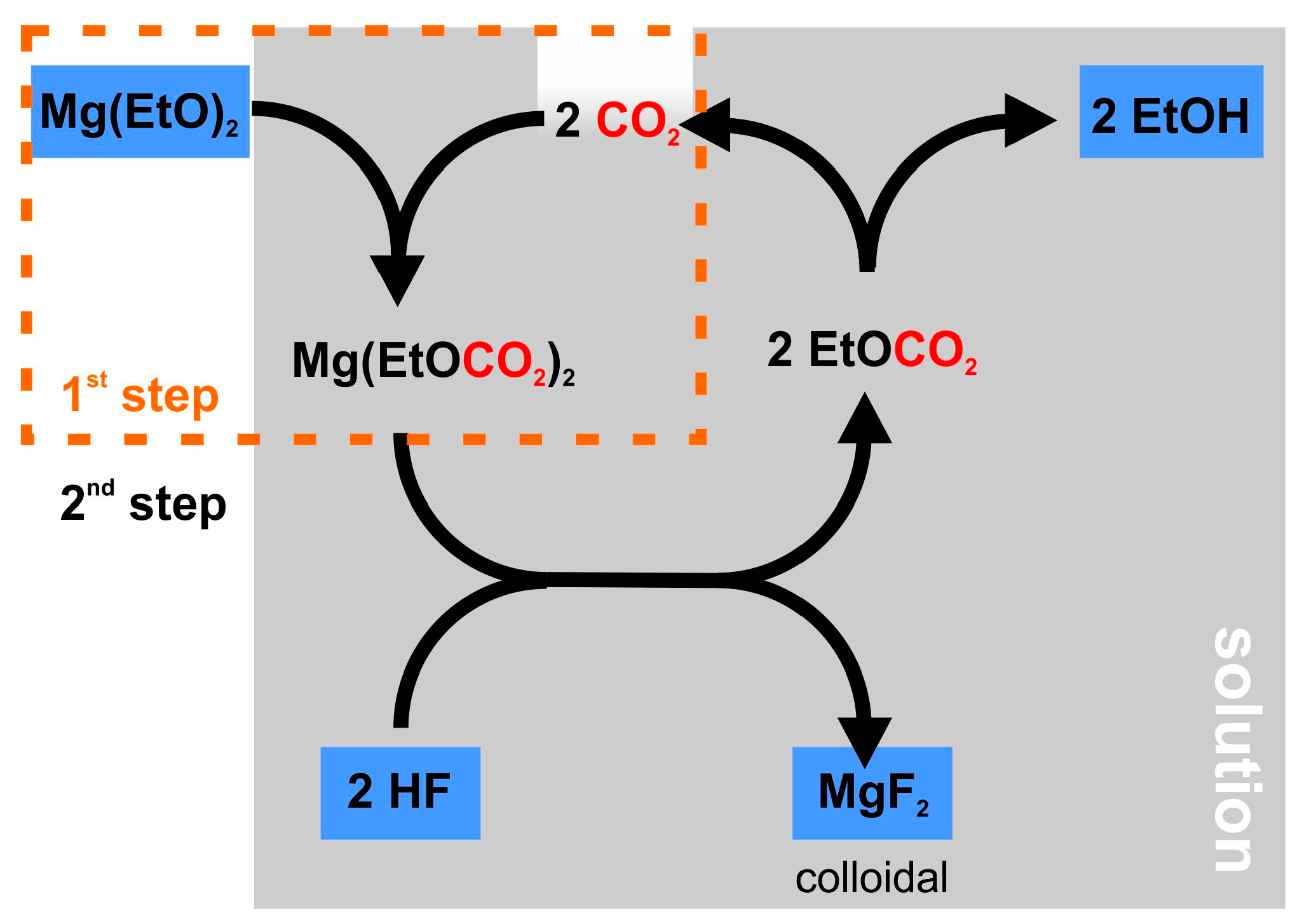
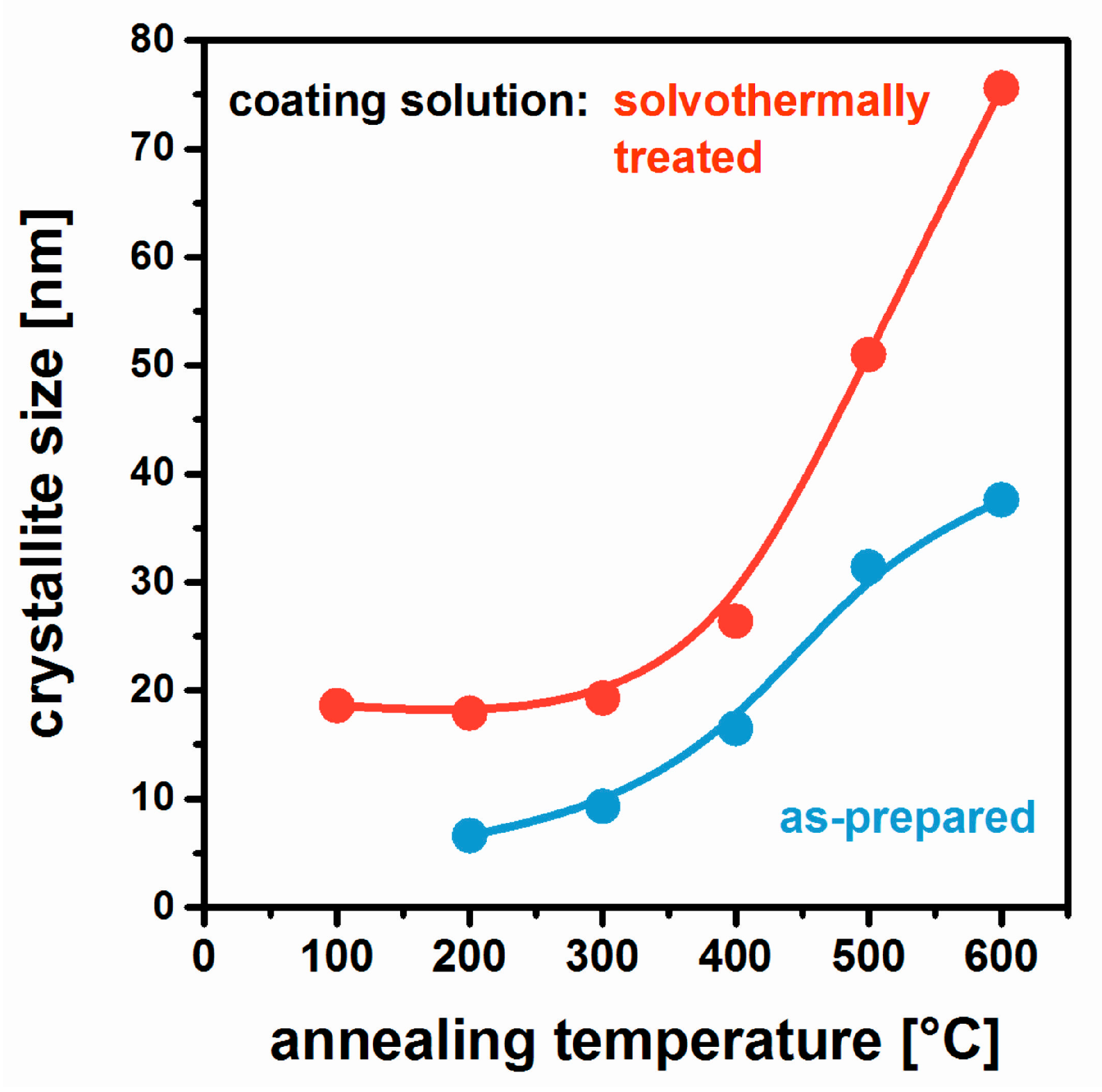
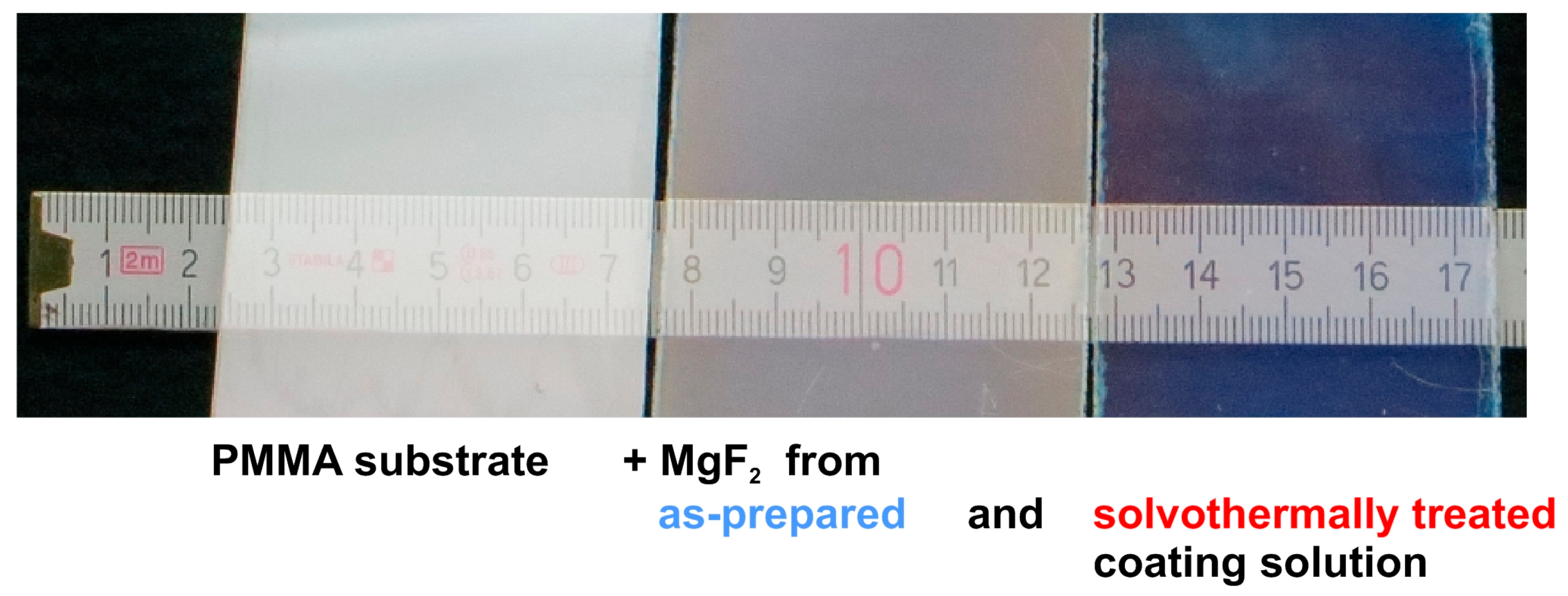
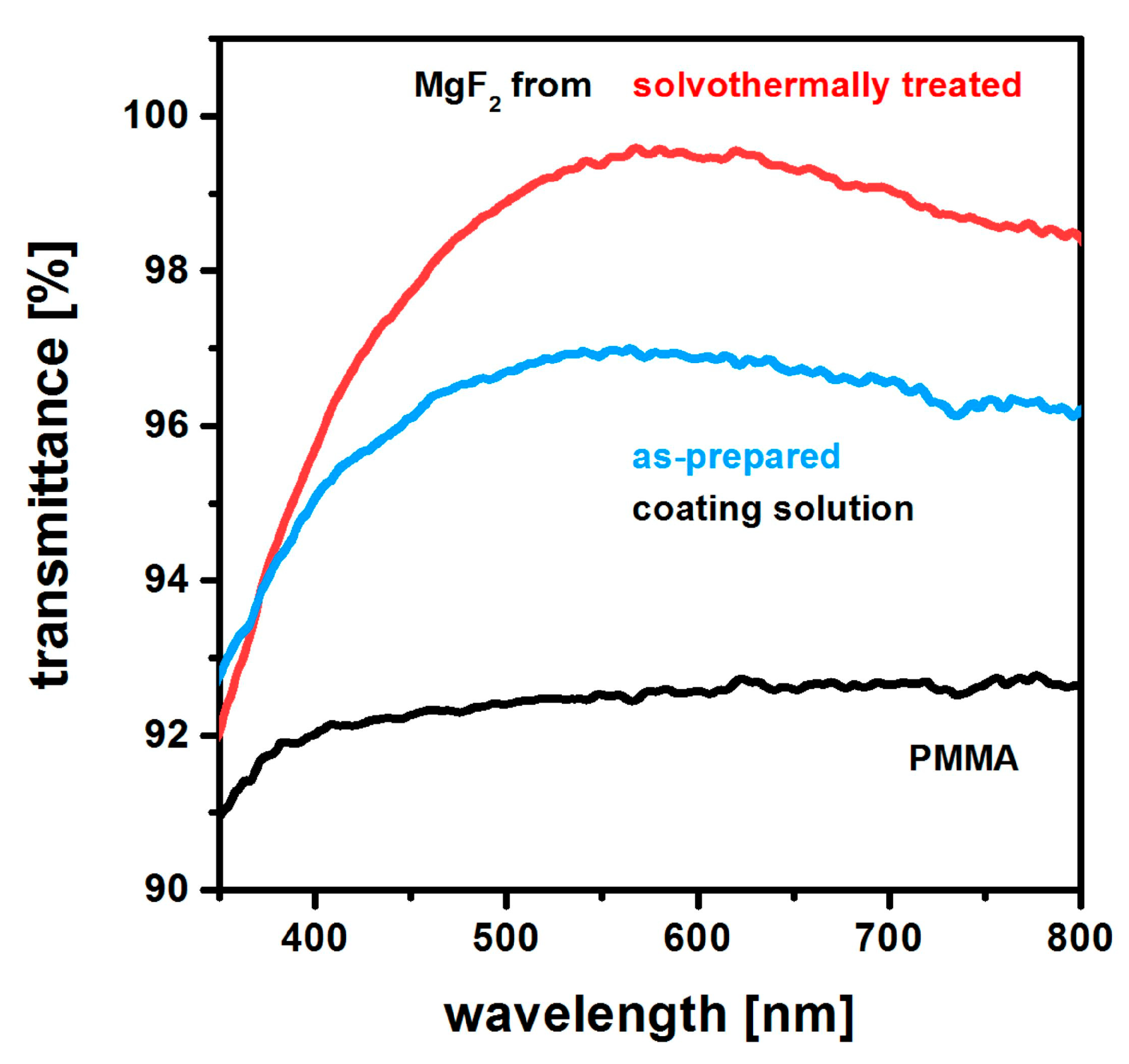
2.4. Non-Aqueous Hydrofluoric Acid
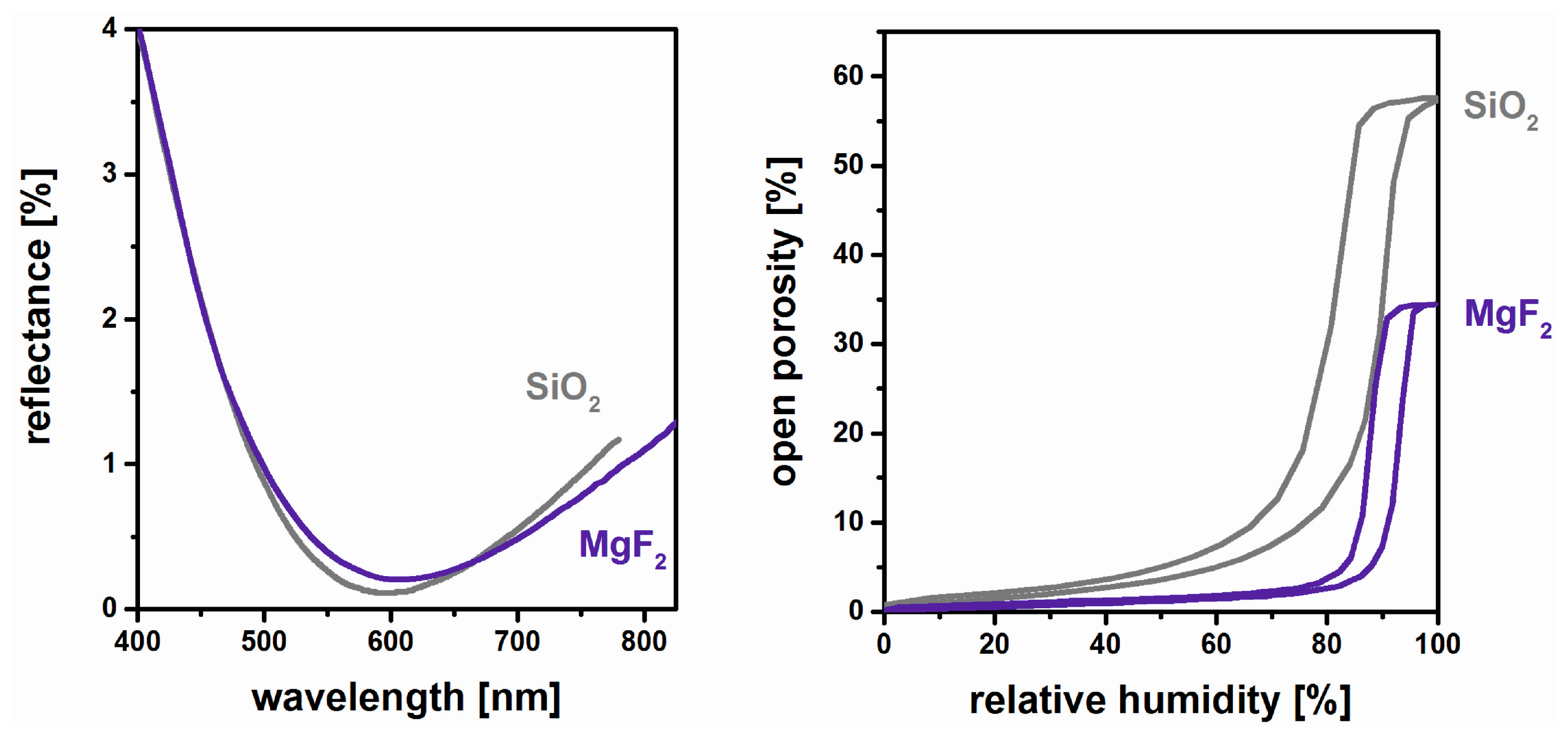
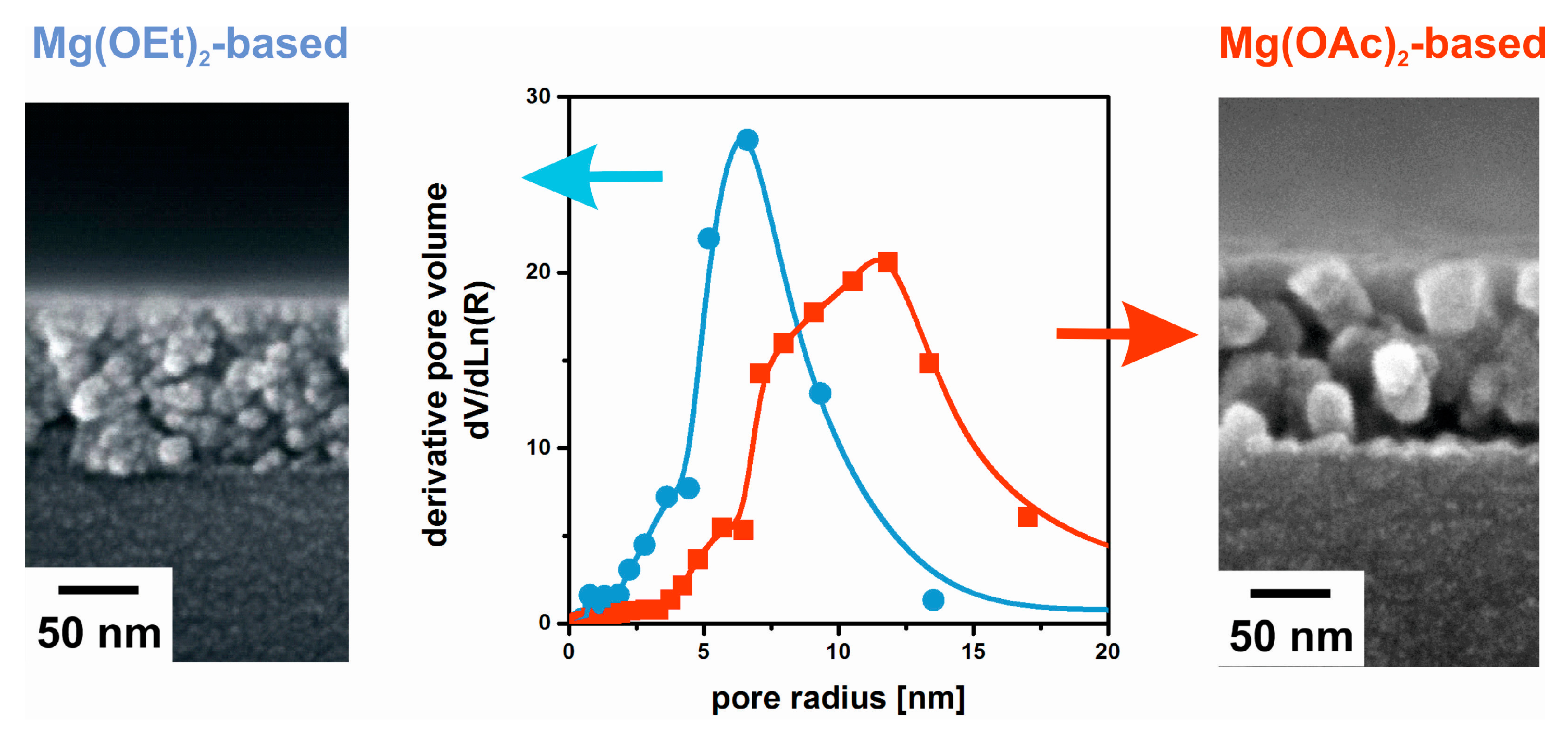
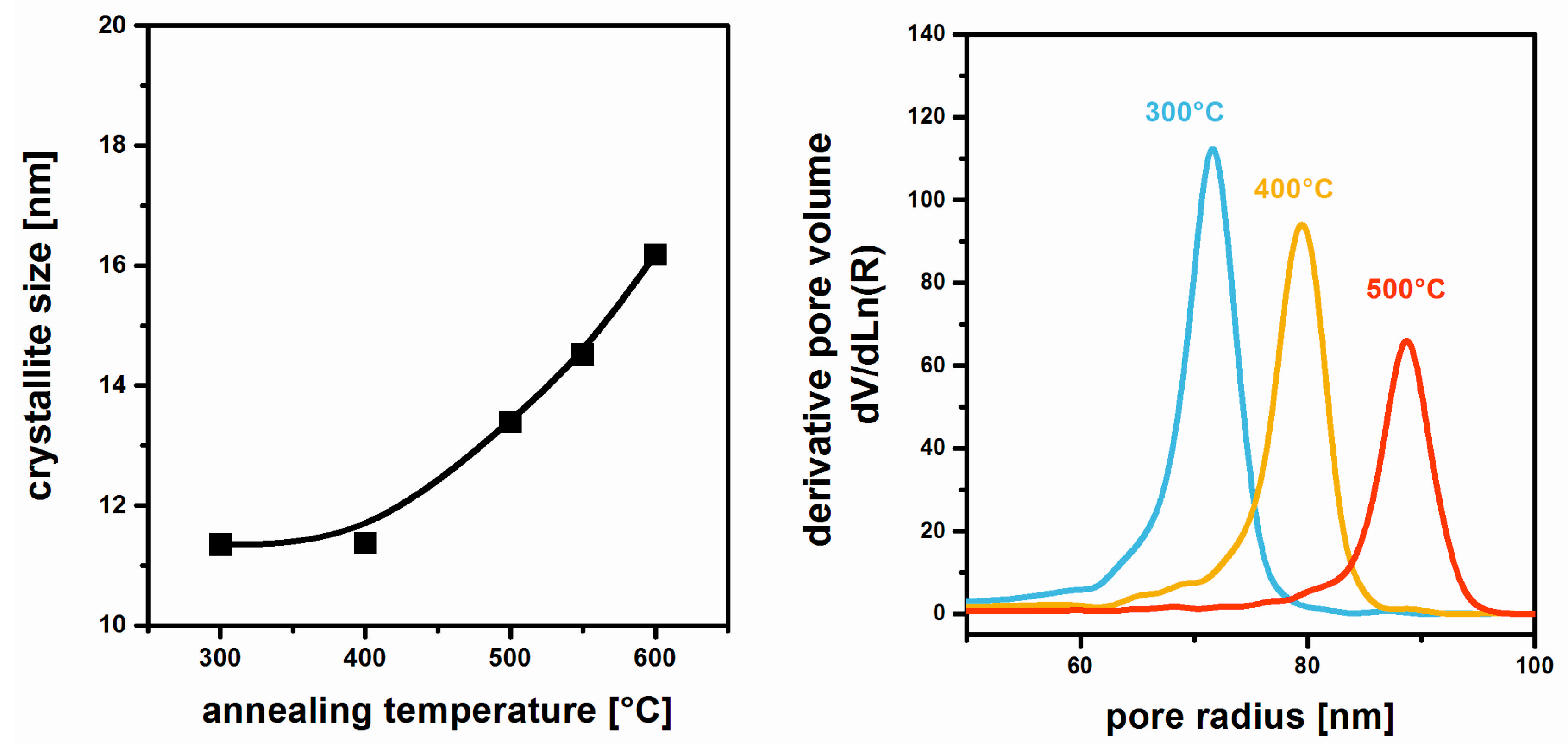
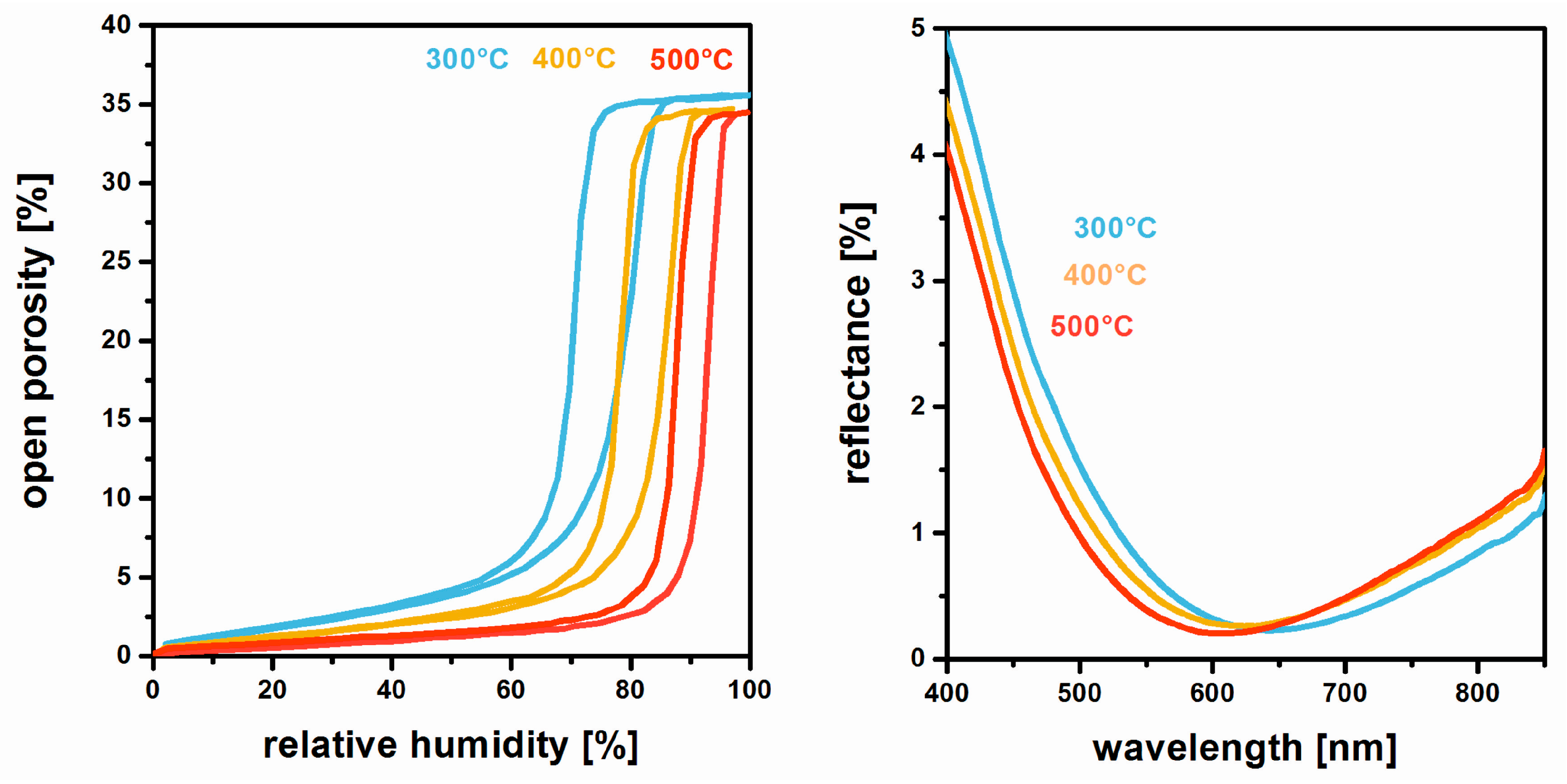
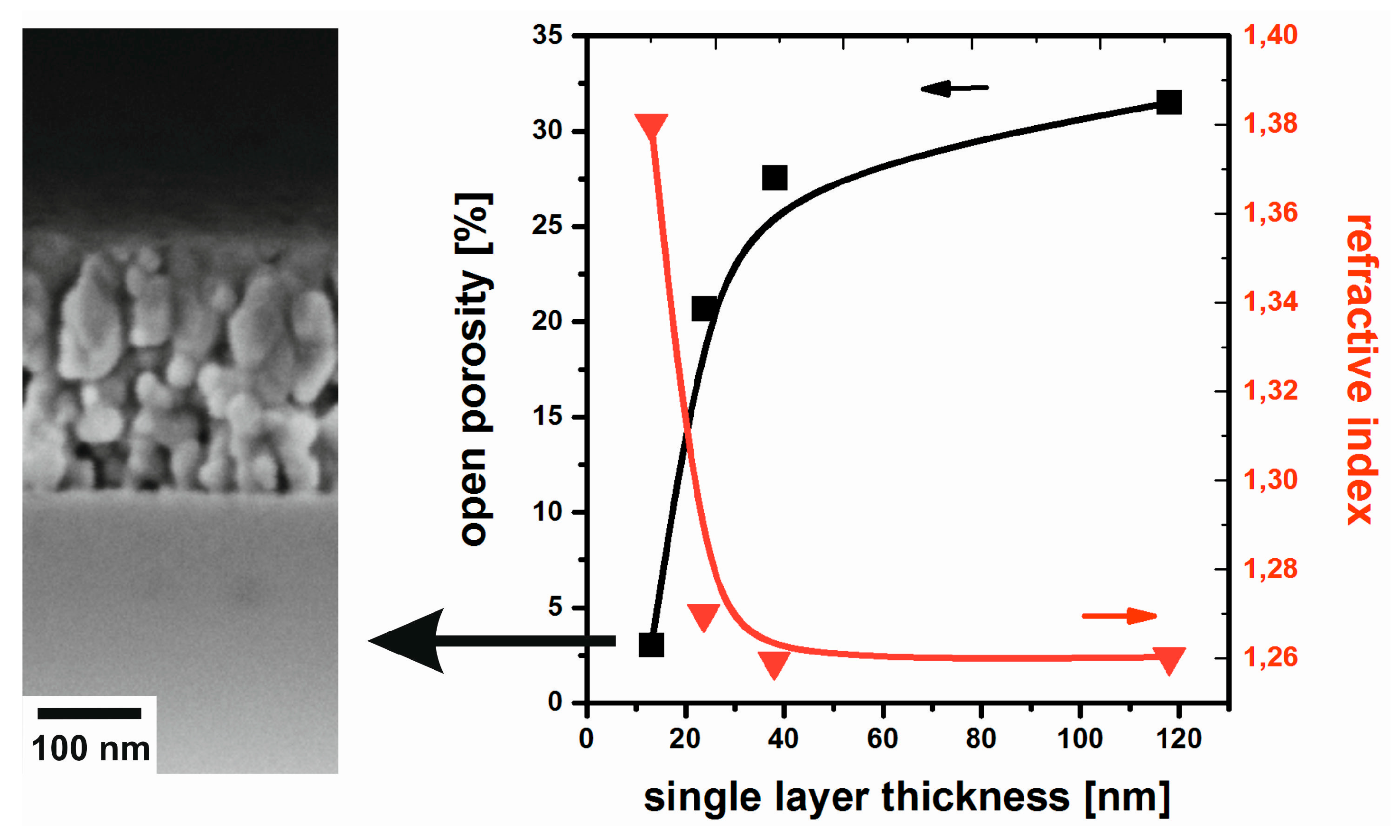
3. Film Microstructure and Optical Performance
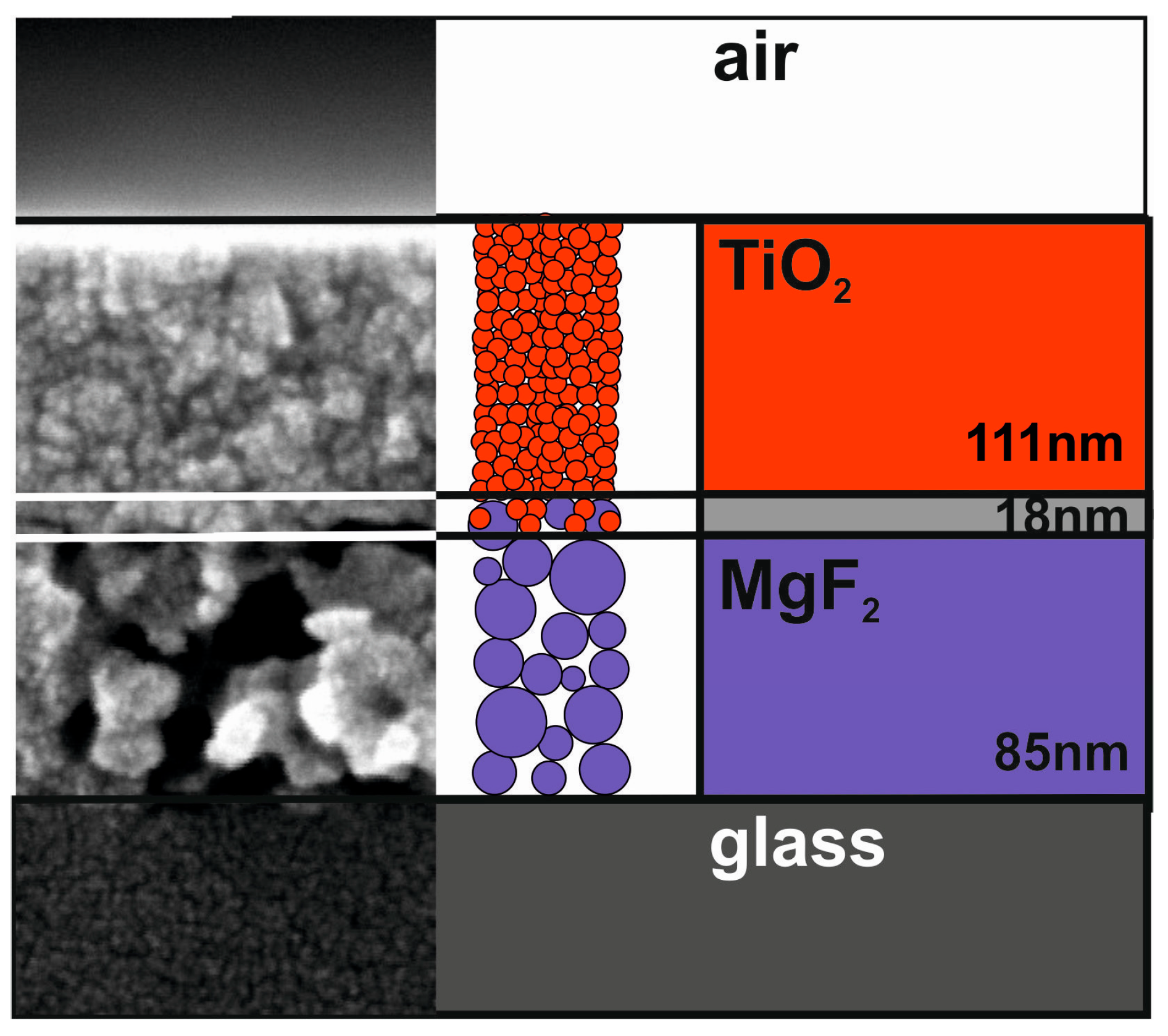
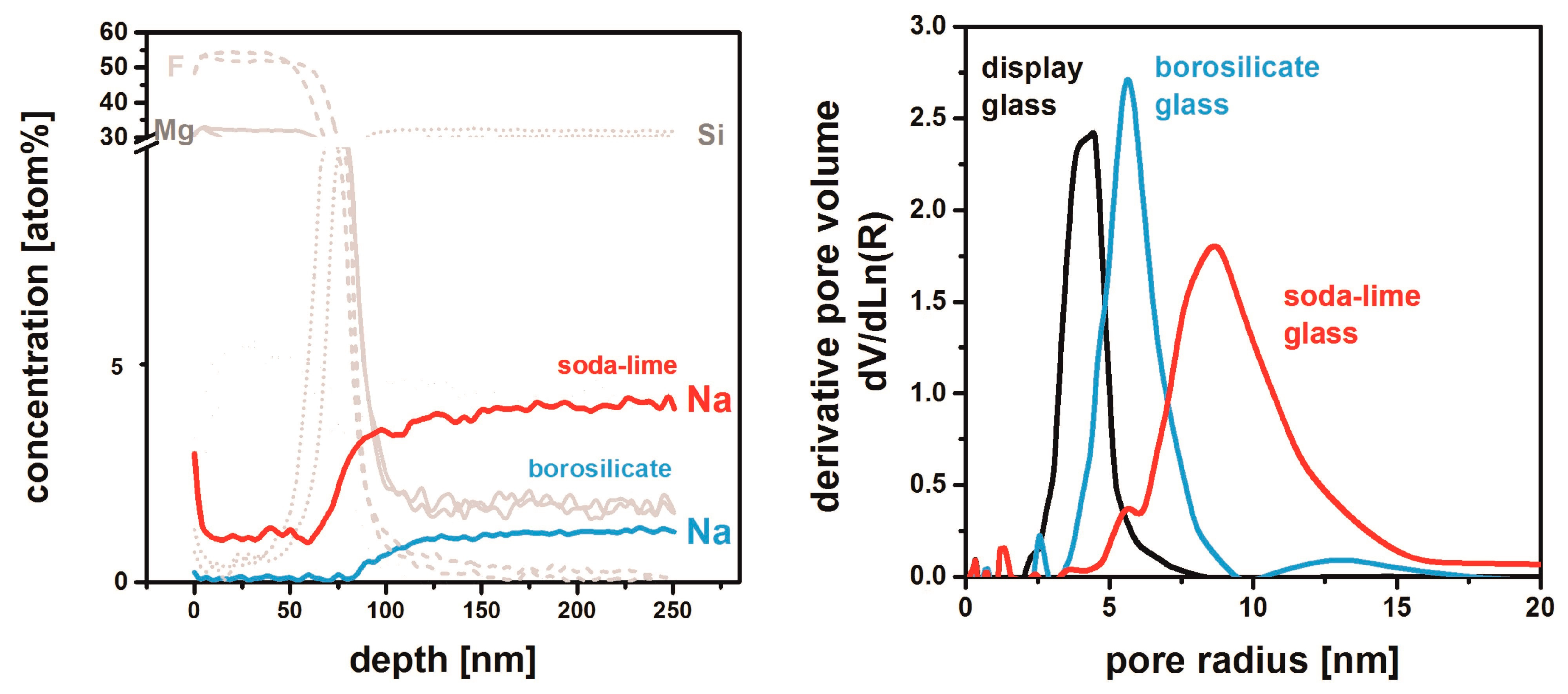
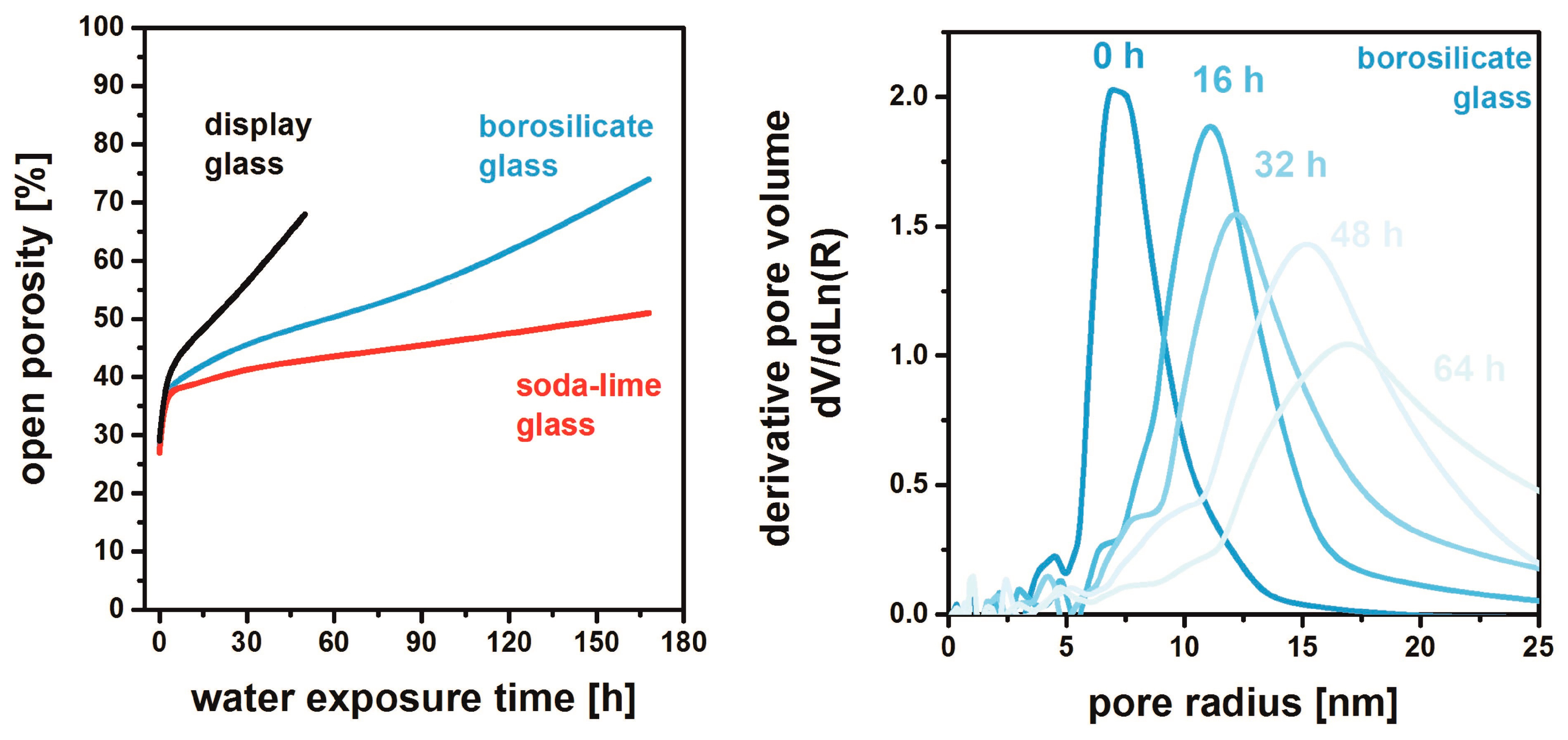
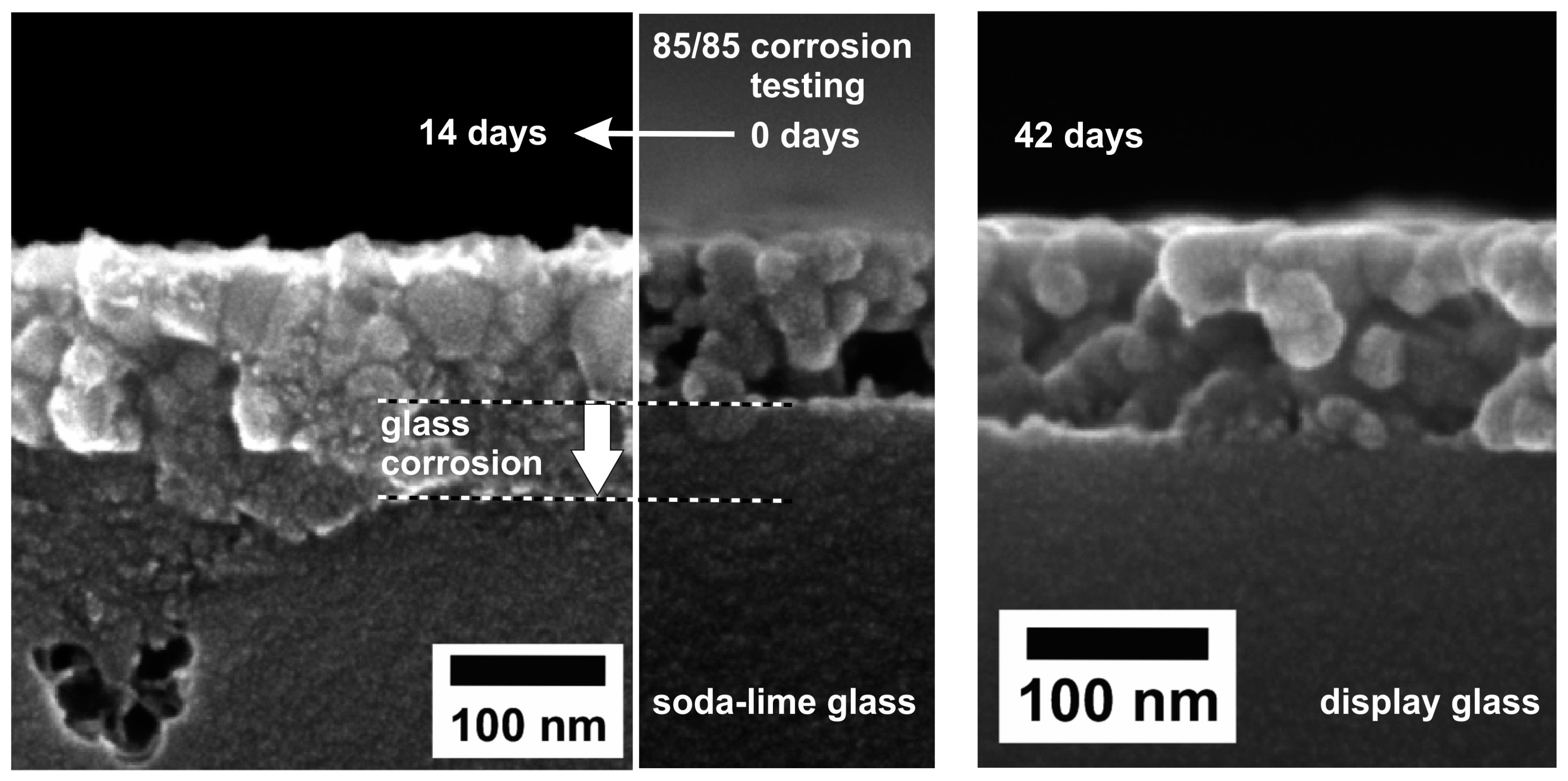
4. MgF2 Film Interaction with Substrate
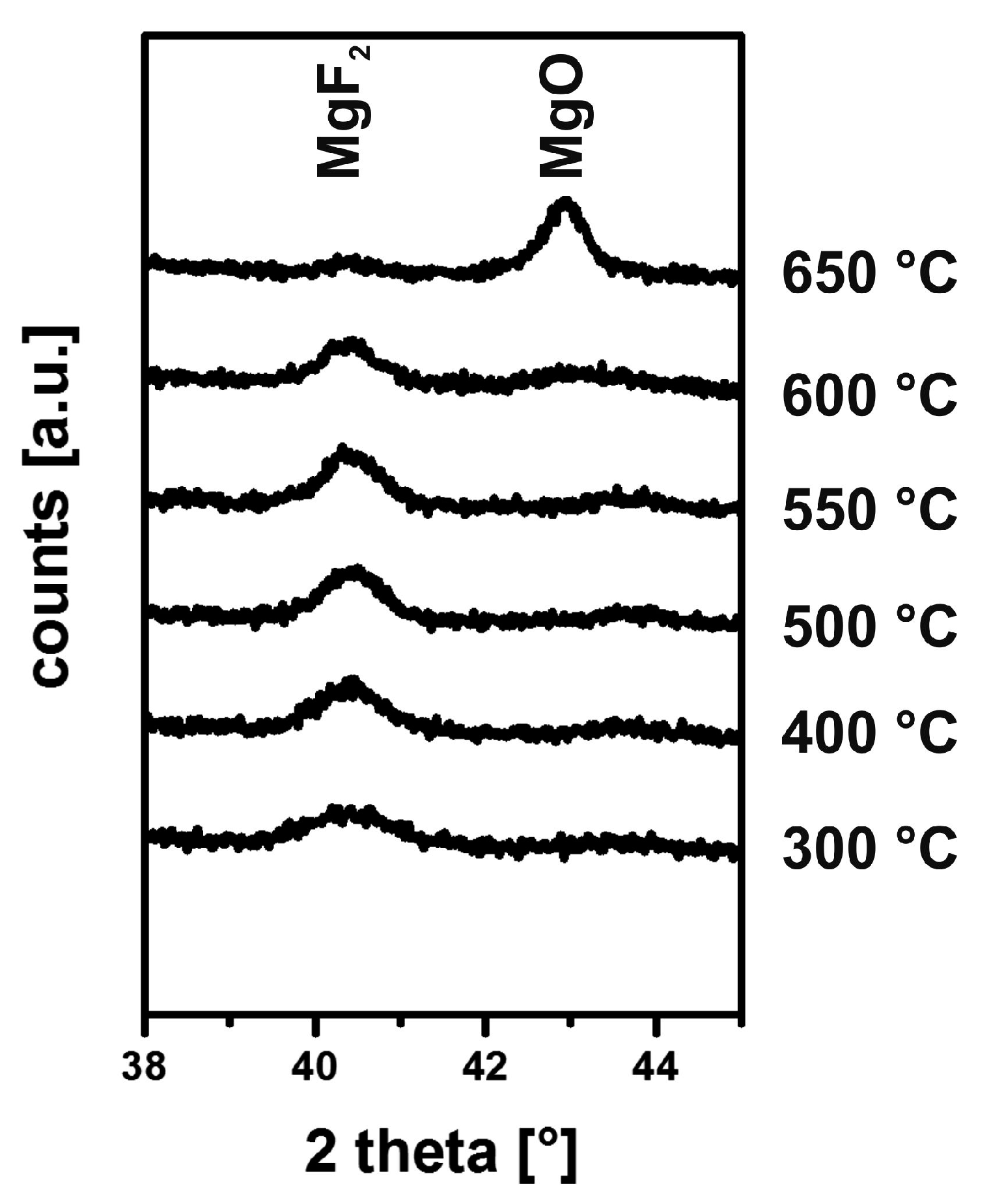
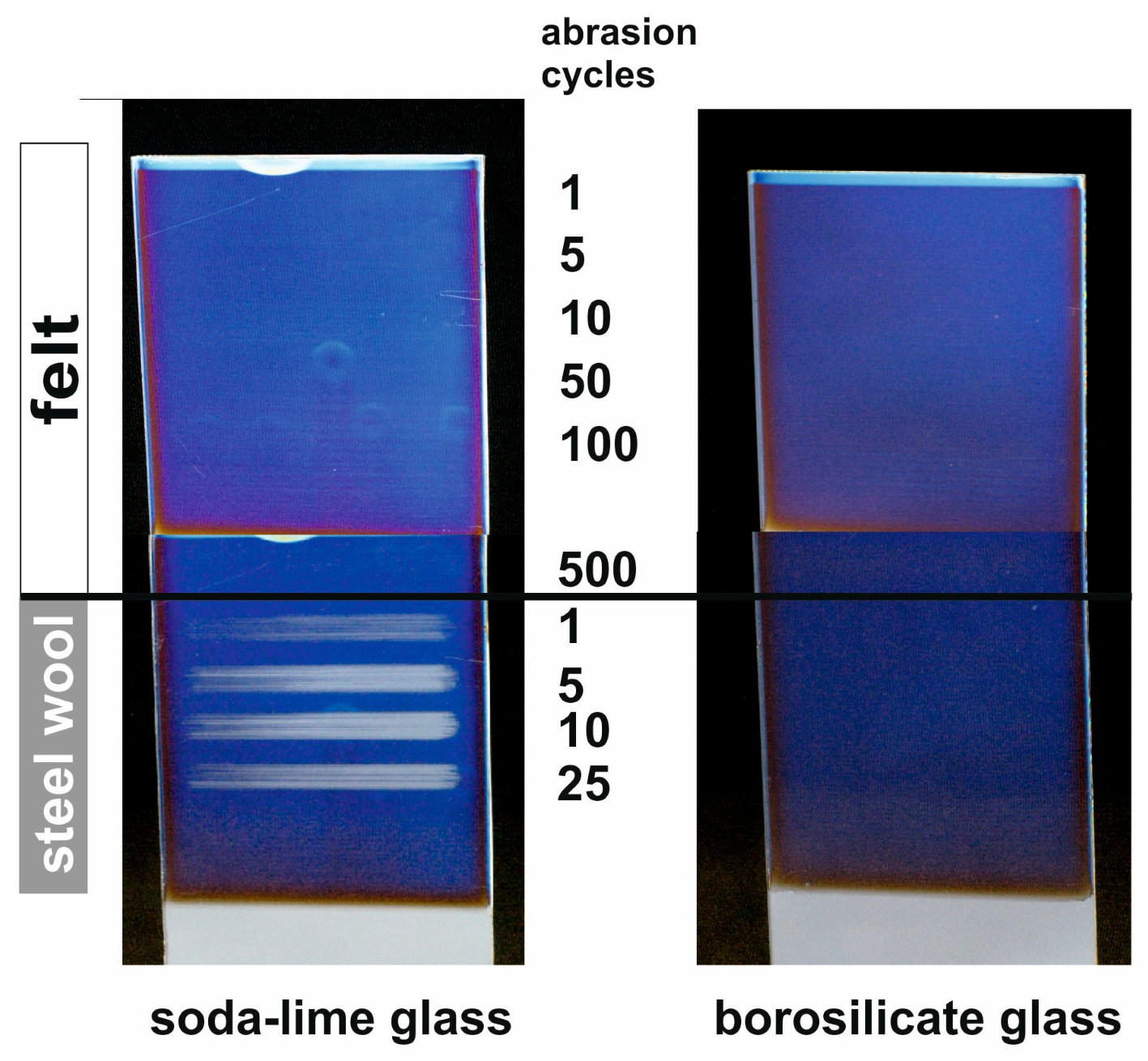
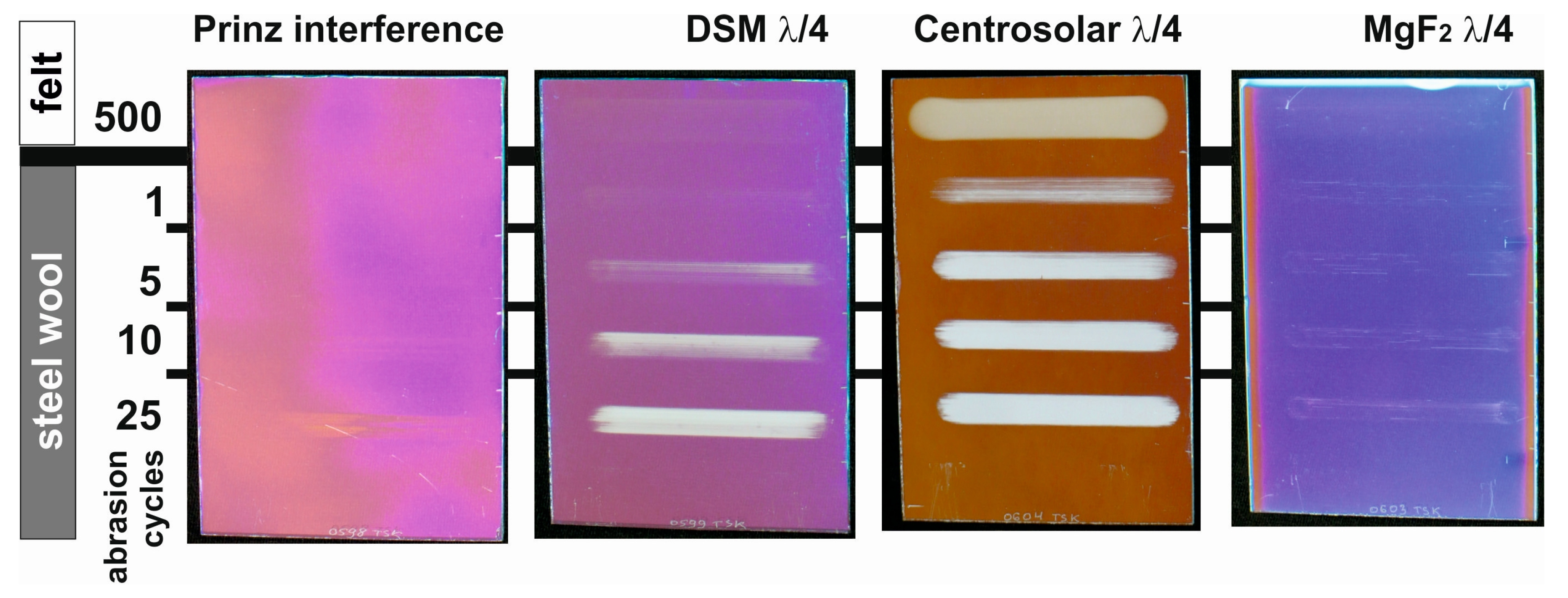
5. Chemical and Mechanical Film Stability
6. Coating of Polymer Substrates
7. Conclusions and Outlook
Conflicts of Interest
References
- Löbmann, P. Antireflective Coatings and Optical Filters. In Chemical Solution Deposition of Functional Oxide Thin Films; Schneller, T., Waser, R., Kosec, M., Payne, D., Eds.; Springer Vienna: Vienna, Austria, 2013; pp. 707–724. [Google Scholar]
- Buskens, P.; Burghoorn, M.; Mourad, M.C.D.; Vroon, Z. Antireflective coatings for glass and transparent polymers. Langmuir 2016, 32, 6781–6793. [Google Scholar] [CrossRef] [PubMed]
- Glaubitt, W.; Löbmann, P. Antireflective coatings prepared by sol–gel processing: Principles and applications. J. Eur. Ceram. Soc. 2012, 32, 2995–2999. [Google Scholar] [CrossRef]
- Zhang, Y.C. Preparation and characterization of porous silica antireflective thin film on glass substrates by chemical etching. Adv. Mater. Res. 2014, 860–863, 903–906. [Google Scholar] [CrossRef]
- Cai, J.; Qi, L. Recent advances in antireflective surfaces based on nanostructure arrays. Mater. Horiz. 2015, 2, 37–53. [Google Scholar] [CrossRef]
- Schneller, T. Chemical Solution Deposition of Functional Oxide Thin Films; Waser, R., Kosec, M., Payne, D., Eds.; Springer Vienna: Vienna, Austria, 2013. [Google Scholar]
- Schottner, G. Hybrid sol−gel-derived polymers: Applications of multifunctional materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Heermann, J.; Löbmann, P. Coating and structuring of glass surfaces by sol-gel and embossing techniques. Proc. Int. Congr. Glass 2001, 2, 2–3. [Google Scholar]
- Gombert, A.; Glaubitt, W.; Rose, K.; Dreibholz, J.; Zanke, C.; Bläsi, B.; Heinzel, A.; Horbelt, W.; Sporn, D.; Döll, W.; et al. Glazing with very high solar transmittance. Sol. Energy 1998, 62, 177–188. [Google Scholar] [CrossRef]
- Li, T.; He, J. Mechanically robust, humidity-resistant, thermally stable high performance antireflective thin films with reinforcing silicon phosphate centers. Sol. Energy Mater. Sol. Cells 2017, 170, 95–101. [Google Scholar] [CrossRef]
- Sevonkaev, I.; Matijević, E. Formation of magnesium fluoride particles of different morphologies. Langmuir 2009, 25, 10534–10539. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.D.; Boissiere, C.; Nicole, L.; Grosso, D.; Sanchez, C. Thermally induced porosity in CSD MgF2 -based optical coatings: An easy method to tune the refractive index. Chem. Mater. 2008, 20, 5550–5556. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Iskandar, F.; Ogi, T.; Okuyama, K. Nanometer to submicrometer magnesium fluoride particles with controllable morphology. Langmuir 2010, 26, 12260–12266. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Ogi, T.; Okuyama, K. Control of the shell structural properties and cavity diameter of hollow magnesium fluoride particles. ACS Appl. Mater. Interface 2014, 6, 4418–4427. [Google Scholar] [CrossRef] [PubMed]
- Karthik, D.; Pendse, S.; Sakthivel, S.; Ramasamy, E.; Joshi, S.-V. High performance broad band antireflective coatings using a facile synthesis of ink-bottle mesoporous MgF2 nanoparticles for solar applications. Sol. Energy Mater. Sol. Cells 2017, 159, 204–211. [Google Scholar] [CrossRef]
- Fujihara, S. Sol-gel processing of fluoride and oxyfluoride materials. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar]
- Fujihara, S.; Tada, M.; Kimura, T. Controlling factors for the conversion of trifluoroacetate sols into thin metal fluoride coatings. J. Sol-Gel Sci. Technol. 2000, 19, 311–314. [Google Scholar] [CrossRef]
- Fujihara, S.; Tokumo, K. Chemical processing for inorganic fluoride and oxyfluoride materials having optical functions. J. Fluor. Chem. 2009, 130, 1106–1110. [Google Scholar] [CrossRef]
- Fujihara, S.; Naito, H.; Tada, M.; Kimura, T. Sol-gel preparation and optical properties of MgF2 thin films containing metal and semiconductor nanoparticles. Scr. Mater. 2001, 44, 2031–2034. [Google Scholar] [CrossRef]
- Bas, S.; Chatterjee, U.; Soucek, M.D. Synthesis of amphiphilic triblock copolymers for the formation of magnesium fluoride (MgF2) nanoparticles. J. Appl. Polym. Sci. 2012, 126, 998–1007. [Google Scholar] [CrossRef]
- Raut, H.K.; Dinachali, S.S.; Ansah-Antwi, K.K.; Ganesh, V.A.; Ramakrishna, S. Fabrication of highly uniform and porous MgF2 anti-reflective coatings by polymer-based sol-gel processing on large-area glass substrates. Nanotechnology 2013, 24, 505201–505208. [Google Scholar] [CrossRef] [PubMed]
- Hody-Le Caër, V.; De Chambrier, E.; Mertin, S.; Joly, M.; Schaer, M.; Scartezzini, J.-L.; Schüler, A. Optical and morphological characterisation of low refractive index materials for coatings on solar collector glazing. Renew. Energy 2013, 53, 27–34. [Google Scholar] [CrossRef]
- Chi, F.; Yan, L.; Lv, H.; Yan, H.; Yuan, X.; Jiang, B. Sol-gel preparation of ultralow refractive index: Magnesium fluoride optical films for broadband antireflective coatings. ASP Nanosci. Nanotechnol. Lett. 2012, 4, 441–444. [Google Scholar] [CrossRef]
- Chi, F.; Zhang, Q.; Zhang, L.; Wei, G.; Wang, L.; Yi, F. Nanostructured magnesium fluoride antireflective films with ultra-high laser induced damage thresholds. Mater. Lett. 2015, 150, 28–30. [Google Scholar] [CrossRef]
- Murata, T.; Ishizawa, H.; Motoyama, I.; Tanaka, A. Investigations of MgF2 optical thin films prepared from autoclaved sol. J. Sol-Gel Sci. Technol. 2004, 32, 161–165. [Google Scholar] [CrossRef]
- Ishizawa, H.; Niisaka, S.; Murata, T.; Tanaka, A. Preparation of MgF2-SiO2 thin films with a low refractive index by a solgel process. Appl. Opt. 2008, 47, C200–C205. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Hieda, J.; Saito, N.; Takai, O. Wettability of MgF2 porous nanoparticle layers covered with fluoroalkylsilane self-assembled monolayer. J. Ceram. Soc. Jpn. 2011, 119, 591–594. [Google Scholar] [CrossRef][Green Version]
- Murata, T.; Hieda, J.; Saito, N.; Takai, O. Wettability characterization of transparent MgF2 nanoparticlecoatings with SiO2 binder covered with fluoroalkylsilaneself-assembled monolayers. J. Sol-Gel Sci. Technol. 2011, 60, 125–130. [Google Scholar] [CrossRef]
- Chi, F.; Wei, G.; Zhang, Q.; Sun, X.; Zhang, L.; Lu, X.; Wang, L.; Yi, F.; Gao, X. Antireflective coatings with adjustable transmittance and high laser-induced damage threshold prepared by deposition of magnesium fluoride nanoparticles. Appl. Surf. Sci. 2015, 356, 593–598. [Google Scholar] [CrossRef]
- Ding, R.; Cui, X.; Zhang, C.; Zhang, C.; Xu, Y. Tri-wavelength broadband antireflective coating built from refractive index controlled MgF2 films. J. Mater. Chem. C 2015, 3, 3219–3224. [Google Scholar] [CrossRef]
- Reddy, K.C.S.; Karthik, D.; Bhanupriya, D.; Ganesh, K.; Ramakrishna, M.; Sakthivel, S. Broad band antireflective coatings using novel in-situ synthesis of hollow MgF2 nanoparticles. Sol. Energy Mater. Sol. Cells 2018, 176, 259–265. [Google Scholar] [CrossRef]
- Ji, Z.; Bao, L.; Wang, H.; Chen, R. Preparation of super-hydrophobic antireflective films by rod-like MgF2 and SiO2 mixed sol. Mater. Lett. 2017, 207, 21–24. [Google Scholar] [CrossRef]
- Lellouche, J.; Friedman, A.; Lellouche, J.-P.; Gedanken, A.; Banin, E. Improved antibacterial and antibiofilm activity of magnesium fluoride nanoparticles obtained by water-based ultrasound chemistry. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Eshed, M.; Lellouche, J.; Banin, E.; Gedanken, A. MgF2 nanoparticle-coated teeth inhibit Streptococcus mutans biofilm formation on a tooth model. J. Mater. Chem. B 2013, 1, 3985. [Google Scholar] [CrossRef]
- Natan, M.; Edin, F.; Perkas, N.; Yacobi, G.; Perelshtein, I.; Segal, E. Two are better than one: Combining ZnO and MgF2 nanoparticles reduces streptococcus pneumoniaeand staphylococcus aureus biofilm formationon cochlear implants. Adv. Funct. Mater. 2016, 26, 2473–2481. [Google Scholar] [CrossRef]
- Kemnitz, E.; Noack, J. The non-aqueous fluorolytic sol–gel synthesis of nanoscaled metal fluorides. Dalton Trans. 2015, 44, 19411–19431. [Google Scholar] [CrossRef] [PubMed]
- Kemnitz, E. Fluorolytic sol-gel processes. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing AG: Cham, Switzerland, 2016. [Google Scholar]
- Krüger, H.; Kemnitz, E.; Hertwig, A.; Beck, U. Transparent MgF2-films by sol-gel coating: Synthesis and optical properties. Thin Solid Films 2008, 516, 4175–4177. [Google Scholar] [CrossRef]
- Wuttke, S.; Coman, S.M.; Scholz, G.; Kirmse, H.; Vimont, A.; Daturi, M.; Schroeder, S.L.M.; Kemnitz, E. Novel sol-gel synthesis of acidic MgF(2−x)(OH)x materials. Chemistry 2008, 14, 11488–11499. [Google Scholar] [CrossRef] [PubMed]
- Noack, J.; Emmerling, F.; Kirmsec, H.; Kemnitz, E. Sols of nanosized magnesium fluoride: formation and stabilisation of nanoparticles. J. Mater. Chem. 2011, 21, 15015–15021. [Google Scholar] [CrossRef]
- Scheurell, K.; Kemnitz, E.; Garcia-Juan, P.; Eicher, J.; Lintner, B.; Hegmann, J.; Jahn, R.; Hofmann, T.; Löbmann, P. Porous MgF2 antireflective λ/4 films prepared by sol-gel processing: Comparison of synthesis approaches. J. Sol-Gel Sci. Technol. 2015, 76, 82–89. [Google Scholar]
- Noack, J.; Scheurell, K.; Kemnitz, E.; Garcia-Juan, P.; Rau, H.; Lacroix, M.; Eicher, J.; Lintner, B.; Sontheimer, T.; Hofmann, T.; et al. MgF2 antireflective coatings by sol–gel processing: Film preparation and thermal densification. J. Mater. Chem. 2012, 22, 18535–18541. [Google Scholar] [CrossRef]
- Scheurell, K.; Noack, J.; König, R.; Hegmann, J.; Jahn, R.; Hofmann, T.; Löbmann, P.; Lintner, B.; Garcia-Juan, P.; Eicher, J.; et al. Optimisation of a sol-gel synthesis route for the preparation of MgF2 particles for a large scale coating process. Dalton Trans. 2015, 44, 19501–19508. [Google Scholar] [CrossRef] [PubMed]
- Krahl, T.; Broßke, D.; Scheurell, K.; Lintner, B.; Kemnitz, E. Novel aspects in the chemistry of the non-aqueous fluorolytic sol-gel synthesis of nanoscaled homodisperse MgF2 sols for antireflective coatings. J. Mater. Chem. C 2016, 4. [Google Scholar] [CrossRef]
- Löbmann, P. Characterization of sol-gel thin films by ellipsometric porosimetry. J. Sol-Gel Sci. Technol. 2017, 84, 2–15. [Google Scholar] [CrossRef]
- Wang, C.; Meinhardt, J.; Löbmann, P. Growth mechanism of Nb-doped TiO2 sol-gel multilayer films characterized by SEM and focus/defocus TEM. J. Sol-Gel Sci. Technol. 2010, 53, 148–153. [Google Scholar] [CrossRef]
- Jahn, R.; Löbmann, P. Microstructure and performance of AZO thin films prepared by sol-gel processing. J. Sol-Gel Sci. Technol. 2013, 66, 120–125. [Google Scholar] [CrossRef]
- Hegmann, J.; Löbmann, P. Sol-gel preparation of TiO2 and MgF2 multilayers. J. Sol-Gel Sci. Technol. 2013, 67, 436–441. [Google Scholar] [CrossRef]
- Krüger, H.; Hertwig, A.; Beck, U.; Kemnitz, E. Low temperature sol-gel metal oxide and fluoride layer stacks for optical applications. Thin Solid Films 2010, 518, 6080–6086. [Google Scholar] [CrossRef]
- Bittner, A.; Schmitt, A.; Jahn, R.; Löbmann, P. Characterization of stacked sol–gel films: Comparison of results derived from scanning electron microscopy, UV–vis spectroscopy and ellipsometric porosimetry. Thin Solid Films 2012, 520, 1880–1884. [Google Scholar] [CrossRef]
- Hegmann, J.; Jahn, R.; Löbmann, P. Solubility of porous MgF2 films in water: influence of glass substrates. J. Sol-Gel Sci. Technol. 2017, 82, 40–44. [Google Scholar] [CrossRef]
- Löbmann, P. Antireflective coatings by sol-gel processing: Commercial products and future perspectives. J. Sol-Gel Sci. Technol. 2017, 83, 291–295. [Google Scholar] [CrossRef]
- Jahn, R.; Löbmann, P. MgF2 films prepared from solvothermally treated precursor solutions. J. Sol-Gel Sci. Technol. 2018. [Google Scholar] [CrossRef]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löbmann, P. Sol-Gel Processing of MgF2 Antireflective Coatings. Nanomaterials 2018, 8, 295. https://doi.org/10.3390/nano8050295
Löbmann P. Sol-Gel Processing of MgF2 Antireflective Coatings. Nanomaterials. 2018; 8(5):295. https://doi.org/10.3390/nano8050295
Chicago/Turabian StyleLöbmann, Peer. 2018. "Sol-Gel Processing of MgF2 Antireflective Coatings" Nanomaterials 8, no. 5: 295. https://doi.org/10.3390/nano8050295
APA StyleLöbmann, P. (2018). Sol-Gel Processing of MgF2 Antireflective Coatings. Nanomaterials, 8(5), 295. https://doi.org/10.3390/nano8050295



