In Situ Formation of AgCo Stabilized on Graphitic Carbon Nitride and Concomitant Hydrolysis of Ammonia Borane to Hydrogen
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Preparation of AgxCo1−x/g-C3N4
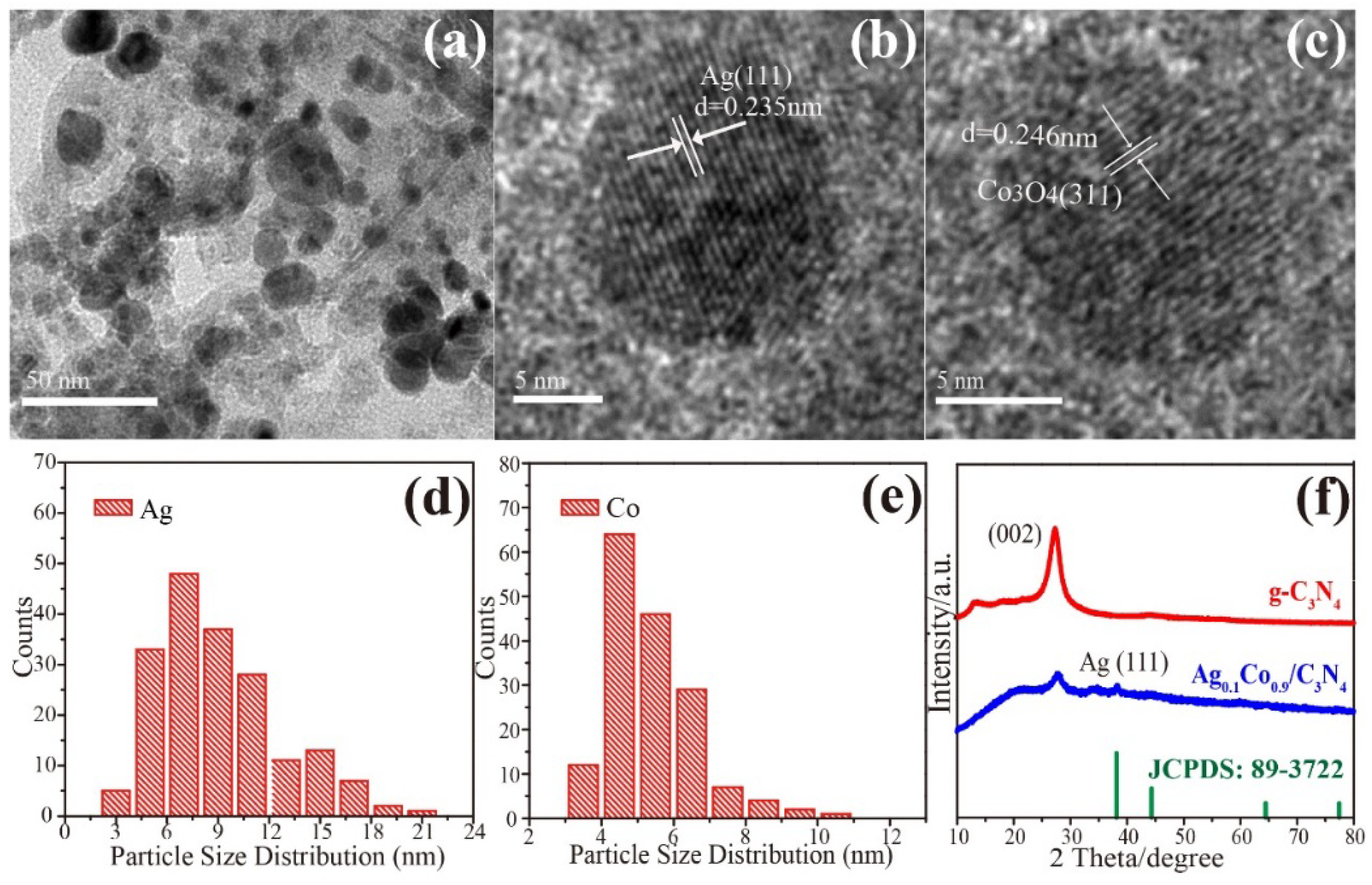
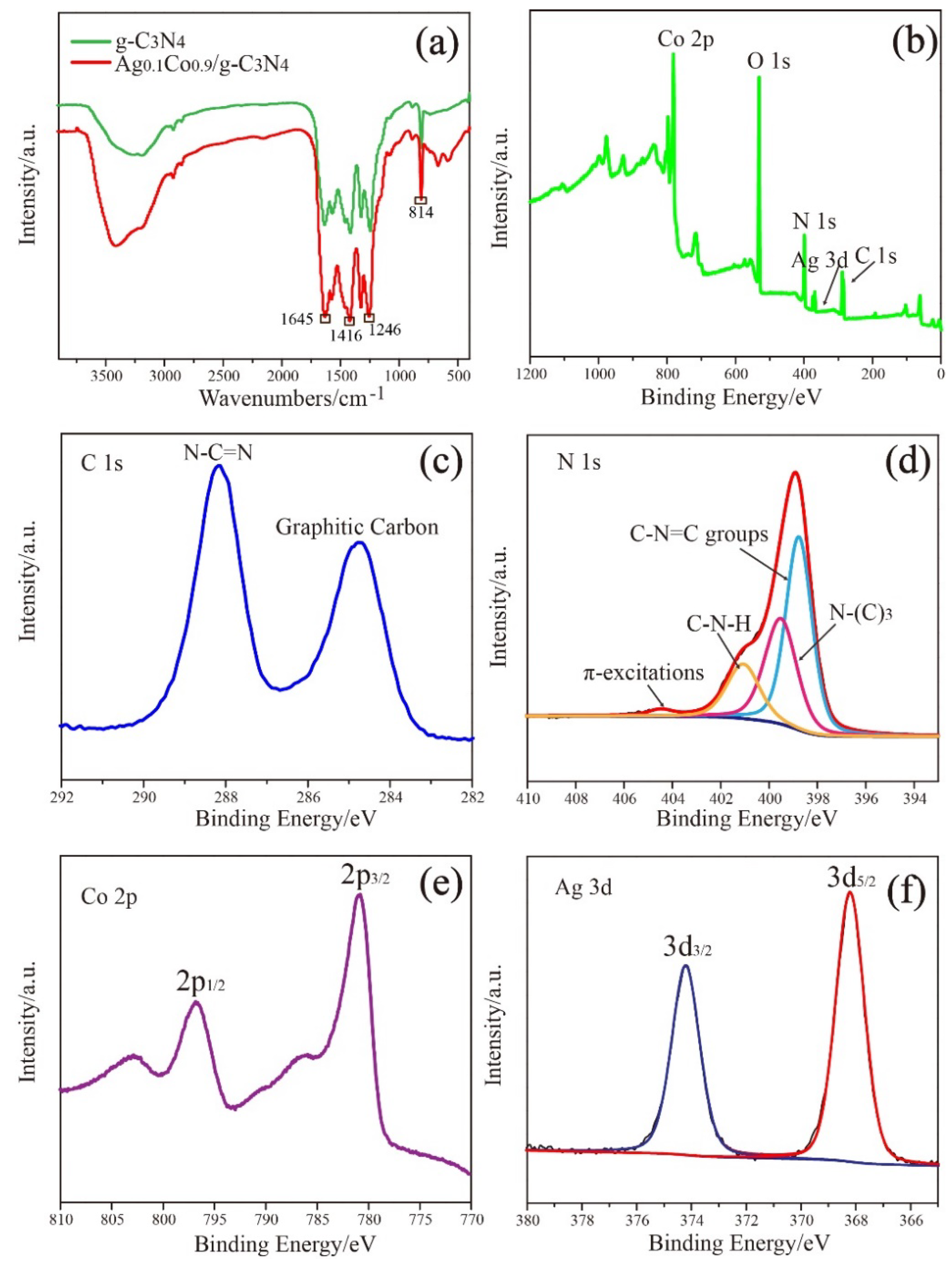
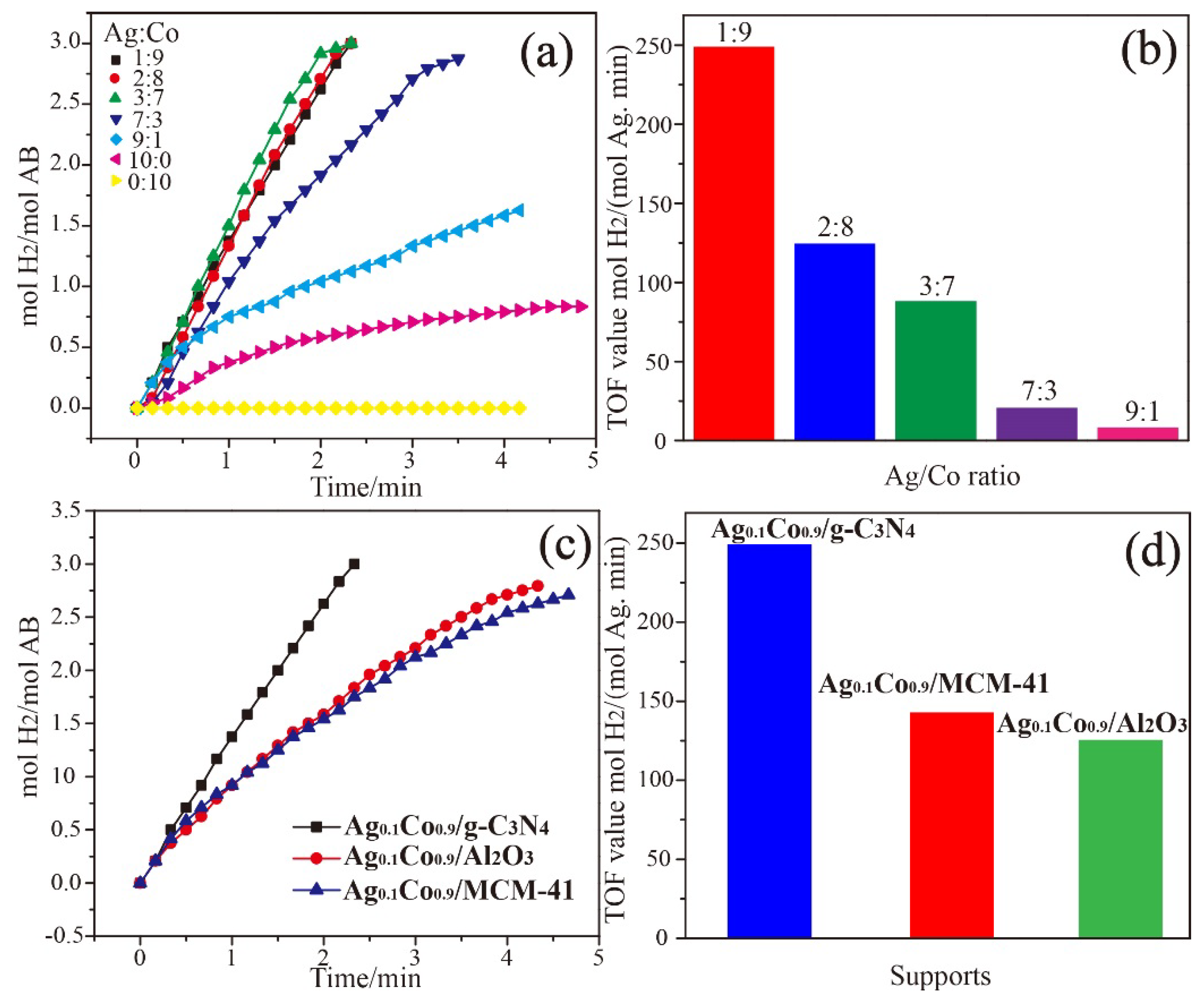
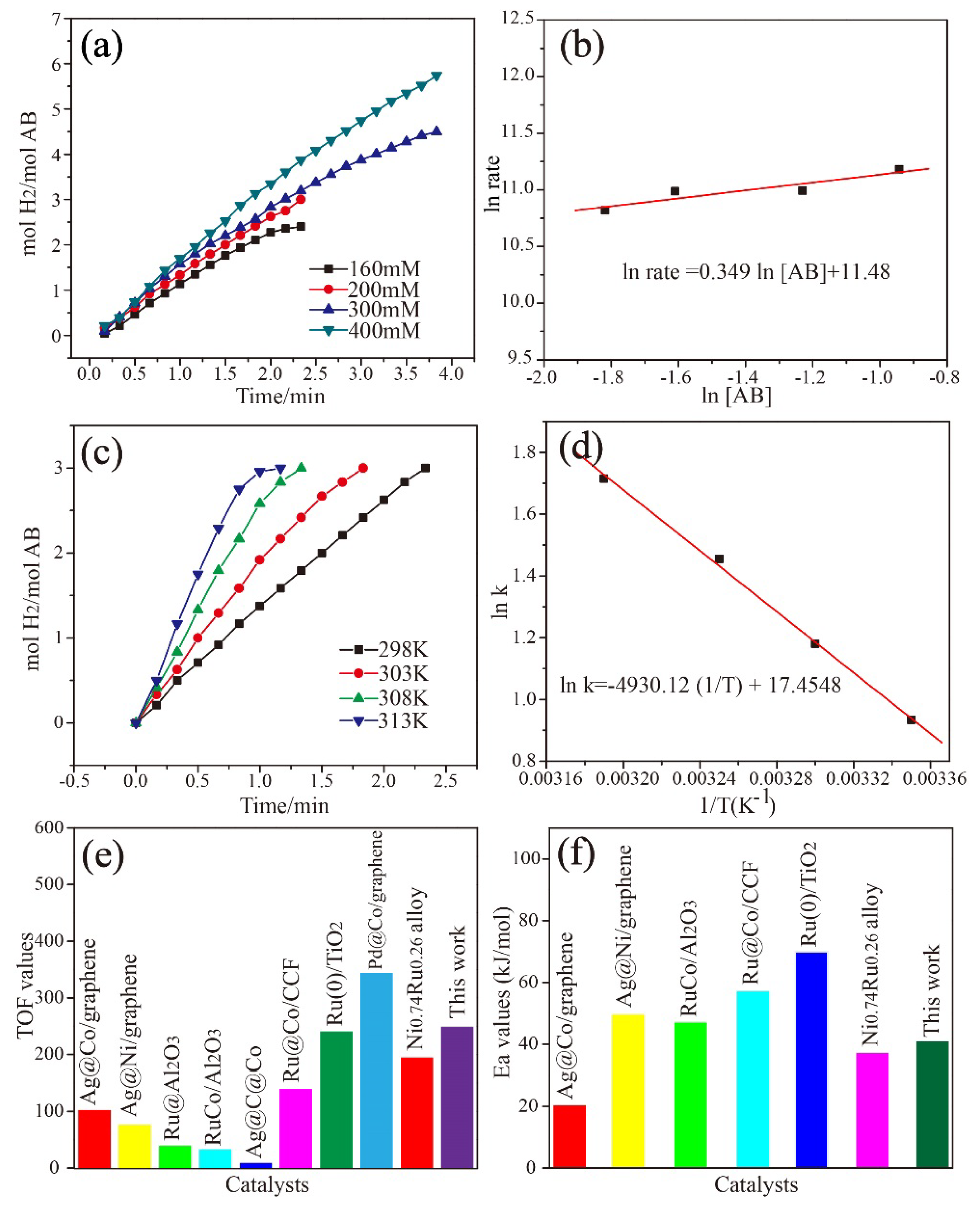
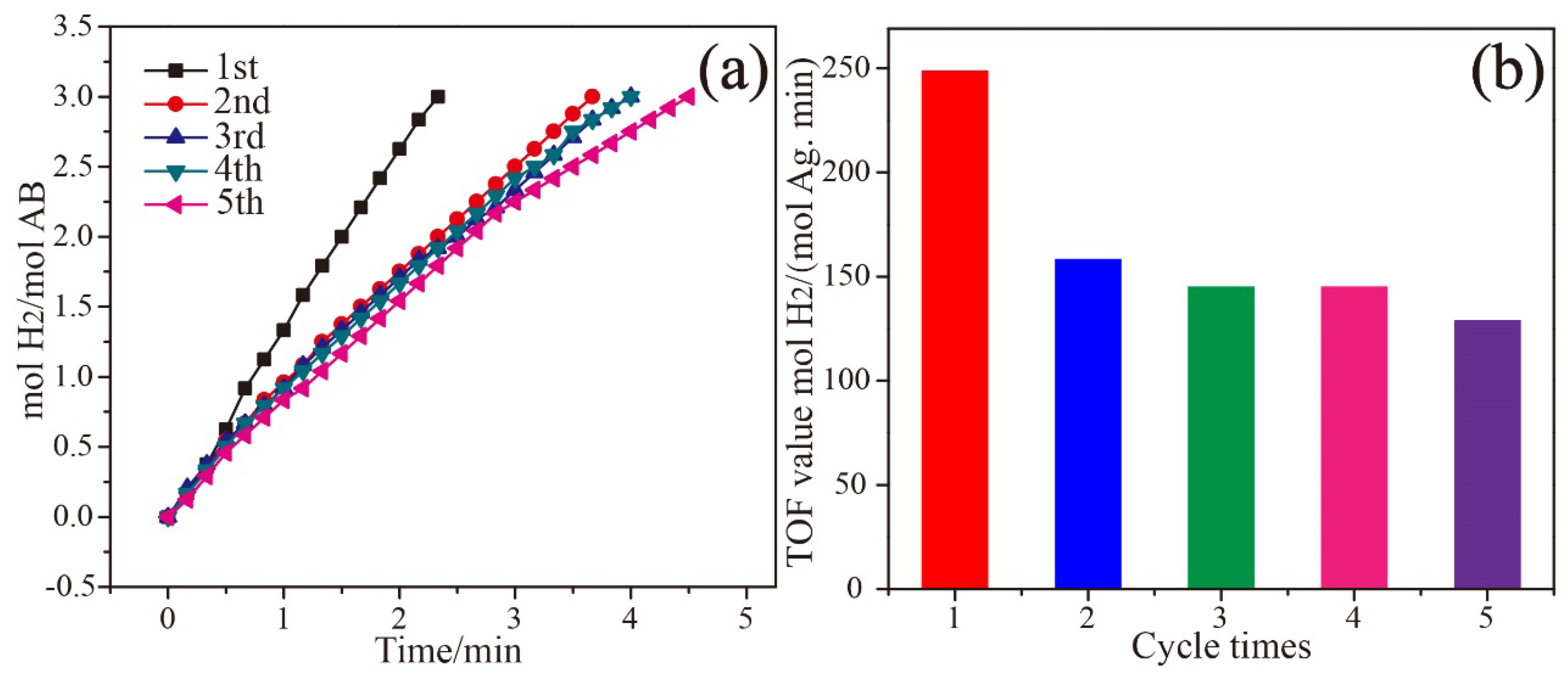
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hu, M.; Wang, H.; Wang, Y.; Zhang, Y.; Wu, J.; Xu, B.; Gao, D.; Bi, J.; Fana, G. Alumina nanofiber-stabilized ruthenium nanoparticles: Highly efficient catalytic materials for hydrogen evolution from ammonia borane hydrolysis. Int. J. Hydrogen Energy 2017, 42, 24142–24149. [Google Scholar] [CrossRef]
- Nunes, H.X.; Ferreira, M.J.F.; Rangel, C.M.; Pinto, A.M.F.R. Hydrogen generation and storage by aqueous sodium borohydride (NaBH4) hydrolysis for small portable fuel cells (H2-PEMFC). Int. J. Hydrogen Energy 2016, 41, 15426–15432. [Google Scholar] [CrossRef]
- Li, X.; Fan, G.; Zeng, C. Synthesis of ruthenium nanoparticles deposited on graphene-like transition metal carbide as an effective catalyst for the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2014, 39, 14927–14934. [Google Scholar] [CrossRef]
- Ai, L.; Liu, X.; Jiang, J. Synthesis of loofah sponge carbon supported bimetallic silver–cobalt nanoparticles with enhanced catalytic activity towards hydrogen generation from sodium borohydride hydrolysis. J. Alloy. Compd. 2015, 625, 164–170. [Google Scholar] [CrossRef]
- Li, X.; Zeng, C.; Fan, G. Magnetic RuCo nanoparticles supported on two-dimensional titanium carbide as highly active catalysts for the hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2015, 40, 9217–9224. [Google Scholar] [CrossRef]
- Li, X.; Zeng, C.; Fan, G. Ultrafast hydrogen generation from the hydrolysis of ammonia borane catalyzed by highly efficient bimetallic RuNi nanoparticles stabilized on Ti3C2X2 (X=OH and/or F). Int. J. Hydrogen Energy 2015, 40, 3883–3891. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X.; Zhang, Y.; Chen, L.; Wu, J.; Gao, D.; Bi, J.; Fana, G. Ruthenium nanoparticles supported on TiO2 (B) nanotubes: Effective catalysts in hydrogen evolution from the hydrolysis of ammonia borane. J. Alloy. Compd. 2017, 708, 270–277. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Tsumori, N.; Xu, Q. Sodium hydroxide-assisted growth of uniform Pd nanoparticles on nanoporous carbon MSC-30 for efficient and complete dehydrogenation of formic acid under ambient conditions. Chem. Sci. 2014, 5, 195–199. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Ma, Y.; Zhang, H.; Gao, J.; Nie, Y.; Sun, X. Aqueous solution synthesis of Pt-M (M = Fe, Co. Ni) bimetallic nanoparticles and their catalysis for the hydrolytic dehydrogenation of ammonia borane. ACS Appl. Mater. Interfaces 2014, 6, 12429–12435. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, F.; Liang, J.; Tao, Z.; Chen, J. PtxNi1−x nanoparticles as catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2009, 34, 8785–8791. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia-borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Akbayrak, S.; Tanyıldızı, S.; Morkan, İ.; Özkar, S. Ruthenium(0) nanoparticles supported on nanotitania as highly active and reusable catalyst in hydrogen generation from the hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2014, 39, 9628–9637. [Google Scholar] [CrossRef]
- Chen, G.; Desinan, S.; Rosei, R.; Rosei, F.; Ma, D. Synthesis of Ni-Ru alloy nanoparticles and their high catalytic activity in dehydrogenation of ammonia borane. Chem. Eur. J. 2012, 18, 7925–7930. [Google Scholar] [CrossRef] [PubMed]
- Rachiero, G.P.; Demirci, U.B.; Miele, P. Bimetallic RuCo and RuCu catalysts supported on γ-Al2O3. A comparative study of their activity in hydrolysis of ammonia-borane. Int. J. Hydrogen Energy 2011, 36, 7051–7065. [Google Scholar] [CrossRef]
- Metin, Ö.; Şahin, Ş.; Özkar, S. Water-soluble poly(4-styrenesulfonic acid-co-maleic acid) stabilized ruthenium(0) and palladium(0) nanoclusters as highly active catalysts in hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrogen Energy 2009, 34, 6304–6313. [Google Scholar] [CrossRef]
- Wang, J.; Qin, Y.-L.; Liu, X.; Zhang, X.-B. In situ synthesis of magnetically recyclable graphene-supported Pd@Co. core–shell nanoparticles as efficient catalysts for hydrolytic dehydrogenation of ammonia borane. J. Mater. Chem. 2012, 22, 12468–12470. [Google Scholar] [CrossRef]
- Yang, L.; Luo, W.; Cheng, G. Graphene-supported Ag-based core-shell nanoparticles for hydrogen generation in hydrolysis of ammonia borane and methylamine borane. ACS Appl. Mater. Interfaces 2013, 5, 8231–8240. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Q.L.; Xu, Q. Highly active AuCo alloy nanoparticles encapsulated in the pores of metal-organic frameworks for hydrolytic dehydrogenation of ammonia borane. Chem. Commun. 2014, 50, 5899–5901. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-T.; Cai, Y.-Y.; Ge, J.-M.; Zhang, Y.-N.; Gong, L.-H.; Li, X.-H.; Wang, K.X.; Ren, Q.Z.; Su, J.; Chen, J.S. Multifunctional Au–Co@CN nanocatalyst for highly efficient hydrolysis of ammonia borane. ACS Catal. 2014, 5, 388–392. [Google Scholar] [CrossRef]
- Ming, M.; Ren, Y.R.; Hu, M.; Zhang, Y.; Sun, T.; Ma, Y.L.; Li, X.J.; Jiang, W.D.; Gao, D.J.; Bi, J.; et al. Promoted effect of alkalization on the catalytic performance of Rh/alk-Ti3C2X2(X=O, F) for the hydrodechlorination of chlorophenols in base-free aqueous medium. Appl. Catal. B 2017, 210, 462–469. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent advances of graphitic carbon nitride-based structures and applications in catalyst, sensing, imaging, and LEDs. Nano Micro Lett. 2017, 9, 47–68. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Chen, J.; Liu, J.; Liang, J.; Du, A.; Zhang, W.; Zhu, Z.; Smith, S.C.; Jaroniec, M.; et al. Nanoporous graphitic-C3N4@carbon metal-free electrocatalysts for highly efficient oxygen reduction. J. Am. Chem. Soc. 2011, 133, 20116–20119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J. Graphene supported Co-g-C3N4 as a novel metal–macrocyclic electrocatalyst for the oxygen reduction reaction in fuel cells. Langmuir 2013, 29, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, S.; Yu, J.-S. Novel ordered nanoporous graphitic C3N4 as a support for Pt–Ru anode catalyst in direct methanol fuel cell. J. Mater. Chem. 2007, 17, 1656–1659. [Google Scholar] [CrossRef]
- Samanta, S.; Martha, S.; Parida, K. Facile Synthesis of Au/g-C3N4 Nanocomposites: An inorganic/organic hybrid plasmonic photocatalyst with enhanced hydrogen gas evolution under visible-light irradiation. ChemCatChem 2014, 6, 1453–1462. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cazorla-Amorós, D.; Linares-Solano, A.; Find, J.; Wild, U.; Schlögl, R. Structural characterization of N-containing activated carbon fibers prepared from a low softening point petroleum pitch and a melamine resin. Carbon 2014, 40, 597–608. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Kundu, S.; Xia, W.; Busser, W.; Becker, M.; Schmidt, D.A.; Havenith, M.; Muhler, M. The formation of nitrogen-containing functional groups on carbon nanotube surfaces: a quantitative XPS and TPD study. Phys. Chem. Chem. Phys. 2010, 12, 4351–4359. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yang, L.; Cao, N.; Du, C.; Dai, H.; Luo, W.; Cheng, G. In situ facile synthesis of bimetallic CoNi catalyst supported on graphene for hydrolytic dehydrogenation of amine borane. Int. J. Hydrogen Energy 2014, 39, 3371–3380. [Google Scholar] [CrossRef]
- Qiu, F.; Li, L.; Liu, G.; Wang, Y.; Wang, Y.; An, C.; Xu, Y.; Xu, C.; Wang, Y.; Jiao, L.; et al. In situ synthesized Fe–Co/C nano-alloys as catalysts for the hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2013, 38, 3241–3249. [Google Scholar] [CrossRef]
- Sun, B.; Wen, M.; Wu, Q.; Peng, J. Oriented growth and assembly of Ag@C@Co. pentagonalprism nanocables and their highly sctive selected catalysis along the edges for dehydrogenation. Adv. Funct. Mater. 2012, 22, 2860–2866. [Google Scholar] [CrossRef]
- Can, H.; Metin, Ö. A facile synthesis of nearly monodisperse ruthenium nanoparticles and their catalysis in the hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. Appl. Catal. B 2012, 125, 304–310. [Google Scholar] [CrossRef]
- Yang, J.; Cui, Z.; Ma, J.; Dong, Z. Ru coated Co. nanoparticles decorated on cotton derived carbon fibers as a highly efficient and magnetically recyclable catalyst for hydrogen generation from ammonia borane. Int. J. Hydrogen Energy 2018, 43, 1355–1364. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Xu, C.; Ming, M.; Yang, Y.; Xu, B.; Wang, Y.; Zhang, Y.; Wu, J.; Fan, G. In Situ Formation of AgCo Stabilized on Graphitic Carbon Nitride and Concomitant Hydrolysis of Ammonia Borane to Hydrogen. Nanomaterials 2018, 8, 280. https://doi.org/10.3390/nano8050280
Wang Q, Xu C, Ming M, Yang Y, Xu B, Wang Y, Zhang Y, Wu J, Fan G. In Situ Formation of AgCo Stabilized on Graphitic Carbon Nitride and Concomitant Hydrolysis of Ammonia Borane to Hydrogen. Nanomaterials. 2018; 8(5):280. https://doi.org/10.3390/nano8050280
Chicago/Turabian StyleWang, Qi, Caili Xu, Mei Ming, Yingchun Yang, Bin Xu, Yi Wang, Yun Zhang, Jie Wu, and Guangyin Fan. 2018. "In Situ Formation of AgCo Stabilized on Graphitic Carbon Nitride and Concomitant Hydrolysis of Ammonia Borane to Hydrogen" Nanomaterials 8, no. 5: 280. https://doi.org/10.3390/nano8050280
APA StyleWang, Q., Xu, C., Ming, M., Yang, Y., Xu, B., Wang, Y., Zhang, Y., Wu, J., & Fan, G. (2018). In Situ Formation of AgCo Stabilized on Graphitic Carbon Nitride and Concomitant Hydrolysis of Ammonia Borane to Hydrogen. Nanomaterials, 8(5), 280. https://doi.org/10.3390/nano8050280



