Comparison Study on the Adsorption Capacity of Rhodamine B, Congo Red, and Orange II on Fe-MOFs
Abstract
1. Introduction
2. Experimental
2.1. Synthesis and Characterization of Experimental Materials
2.2. Removal of Organic Dyes
3. Results and Discussion
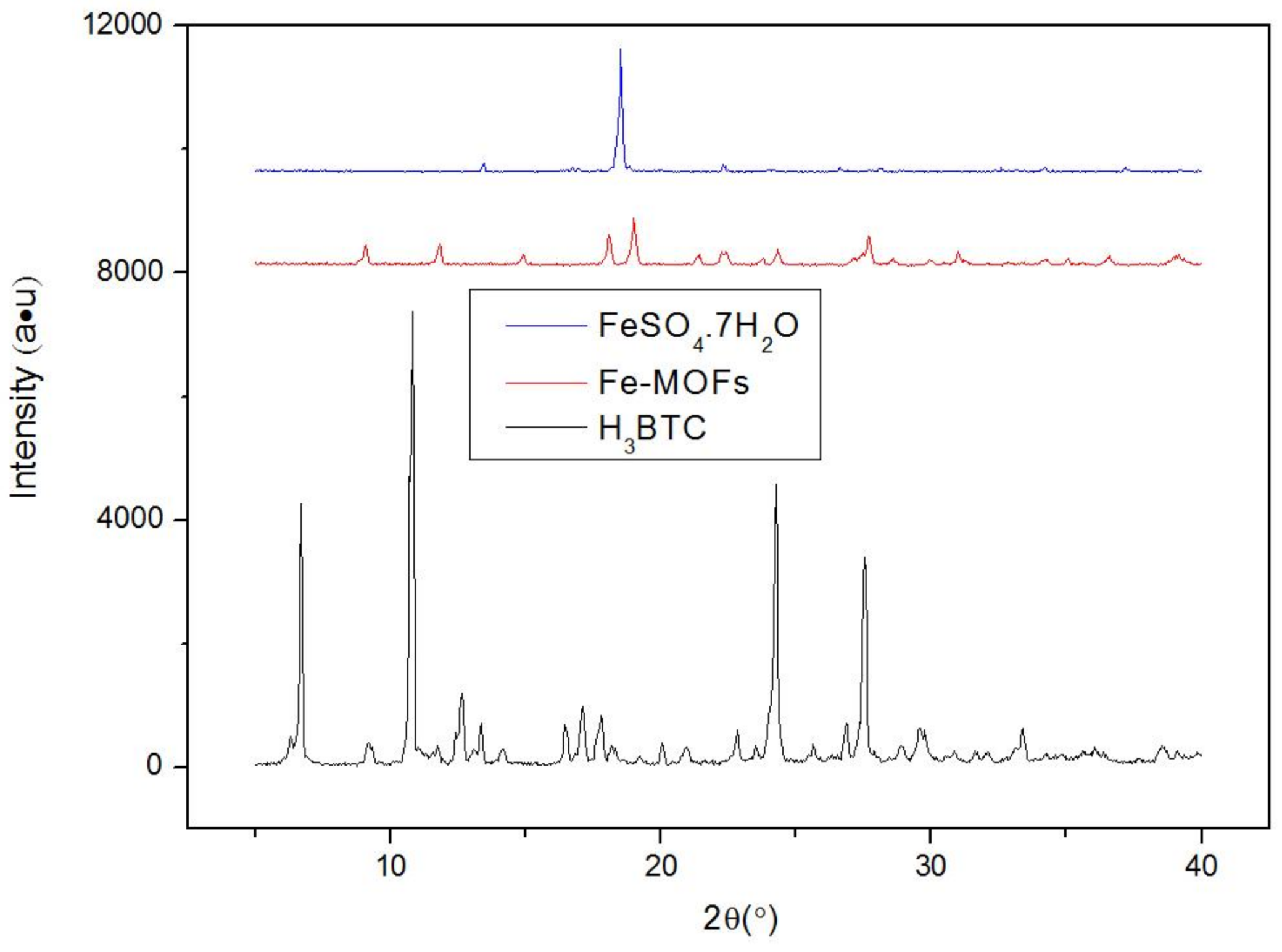
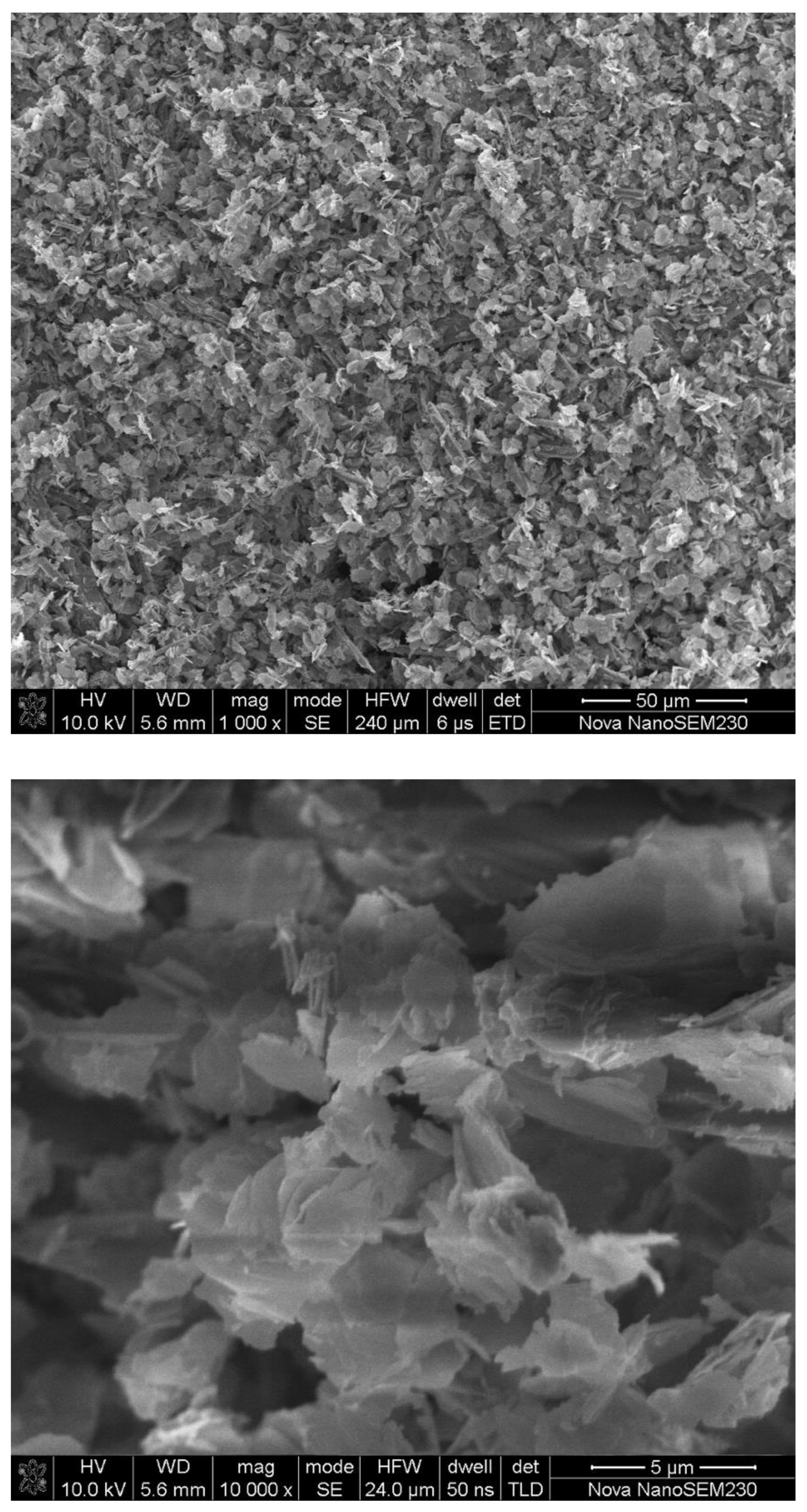
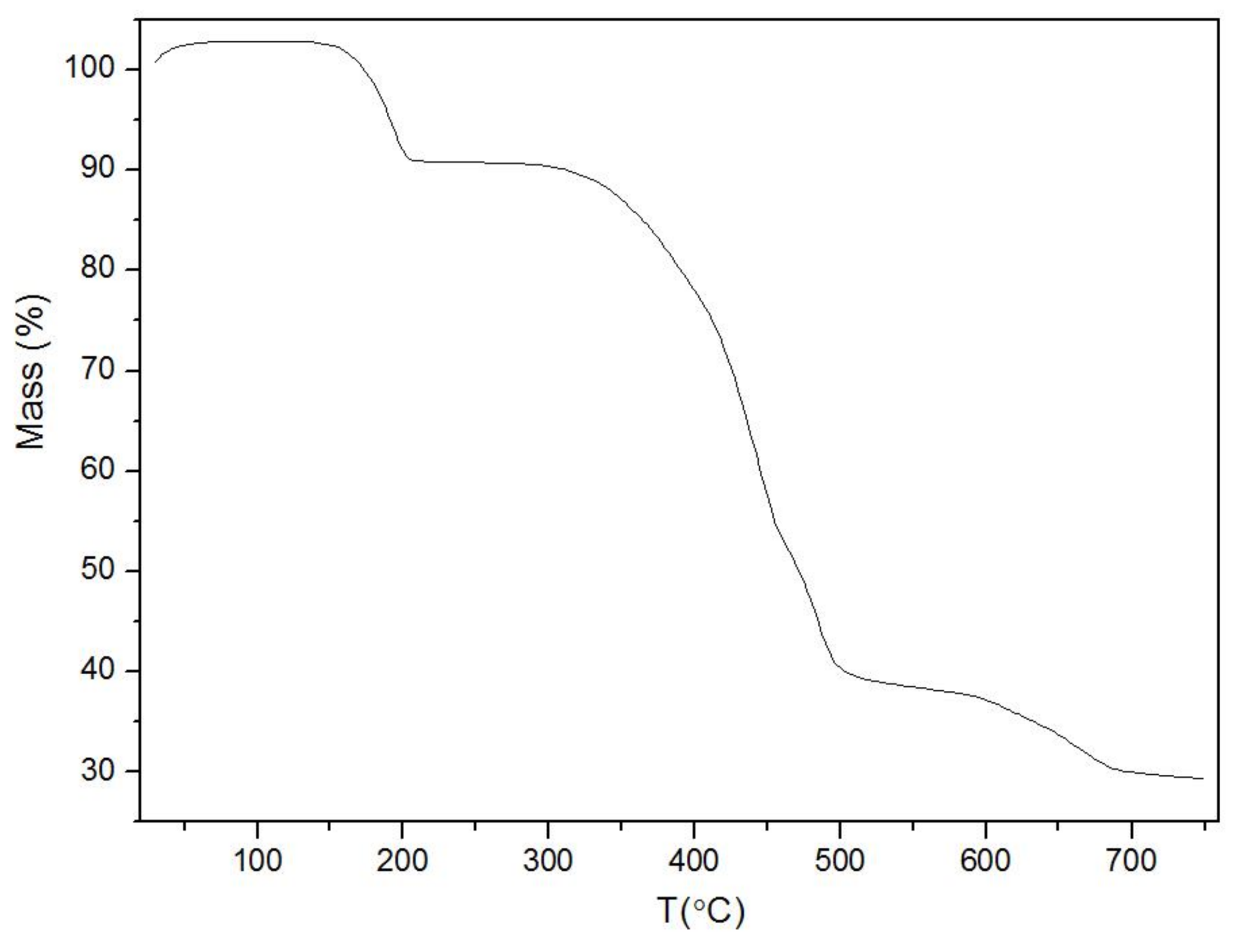
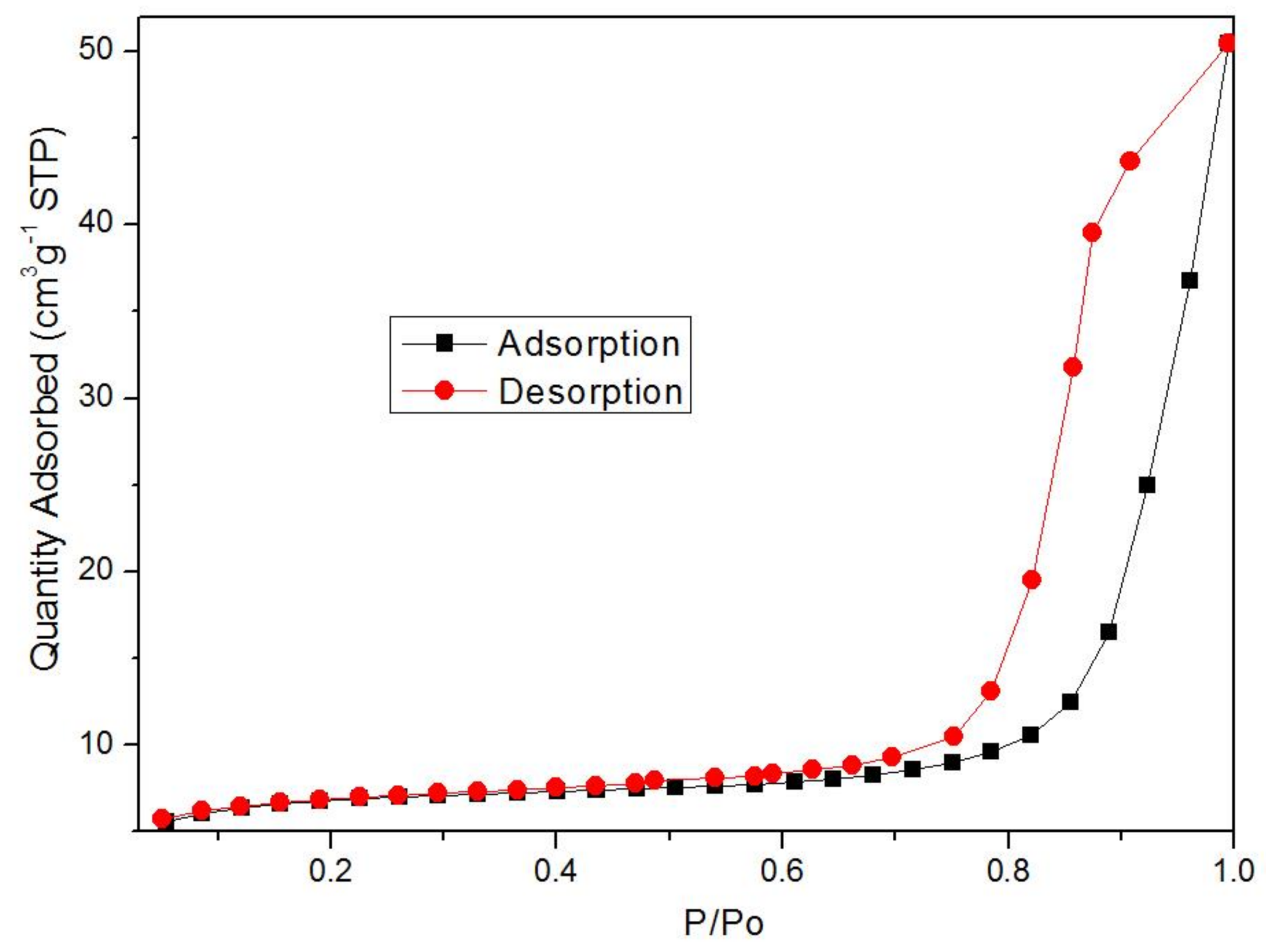
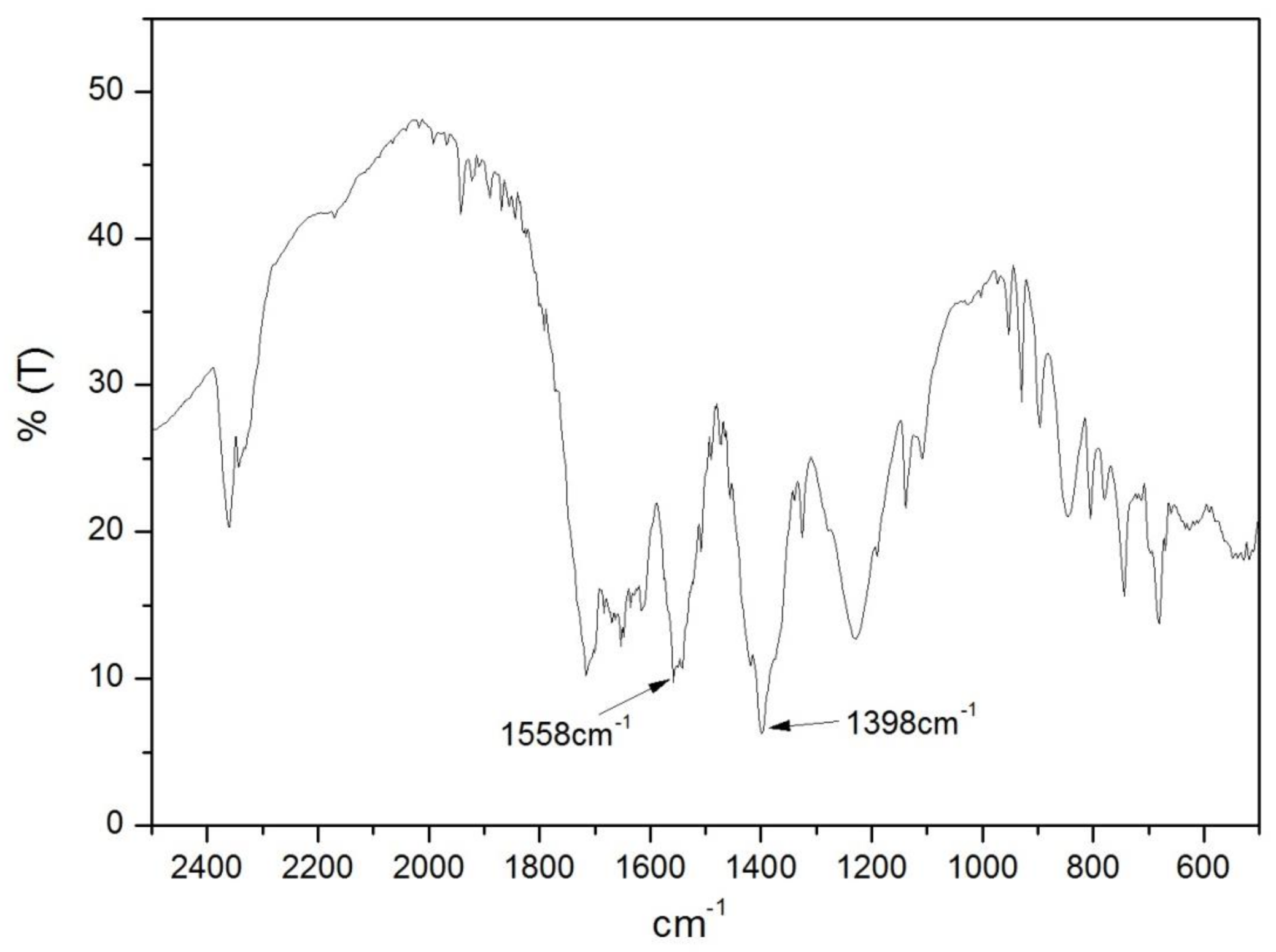
3.1. Synthesis and Characterization of Fe-MOFs
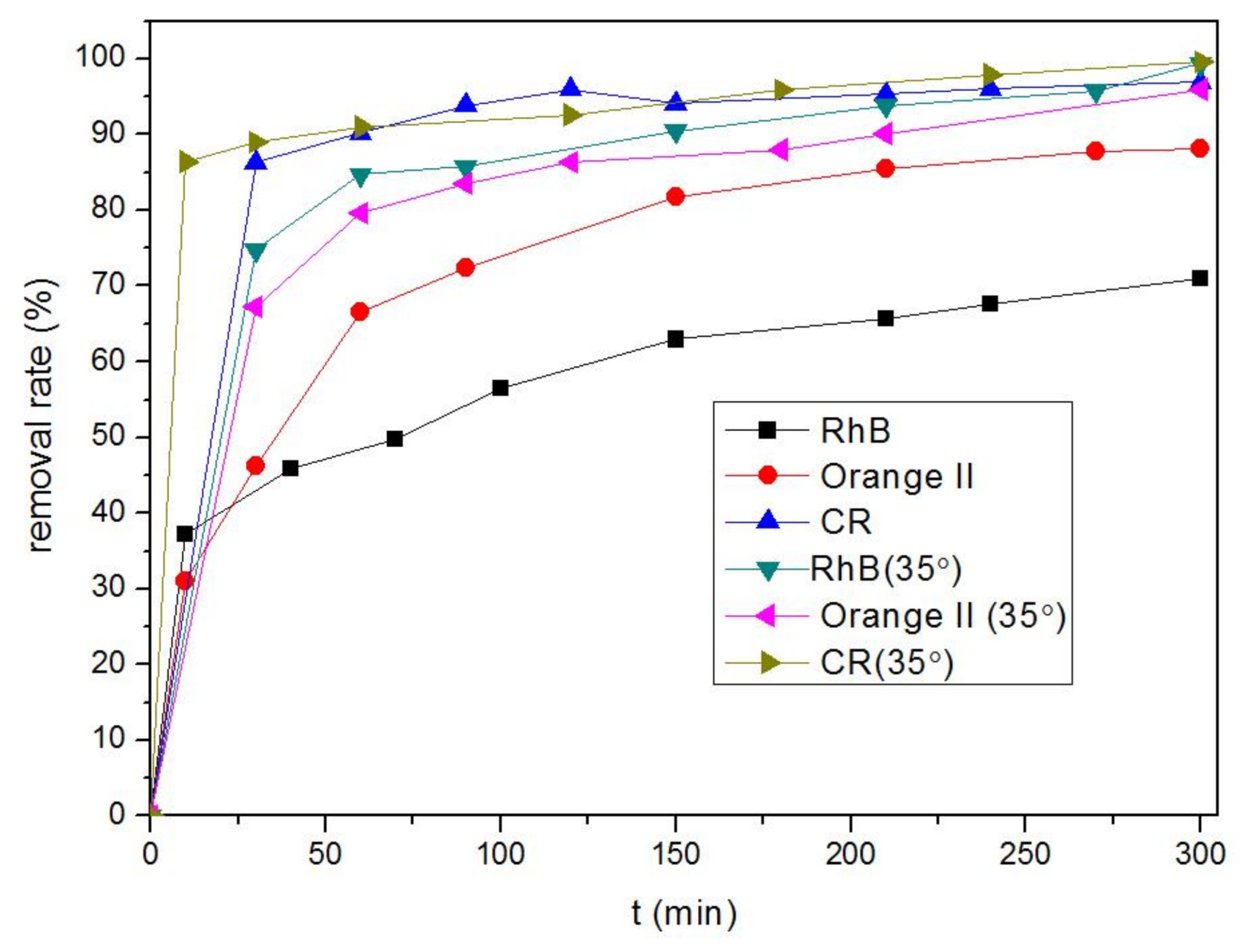
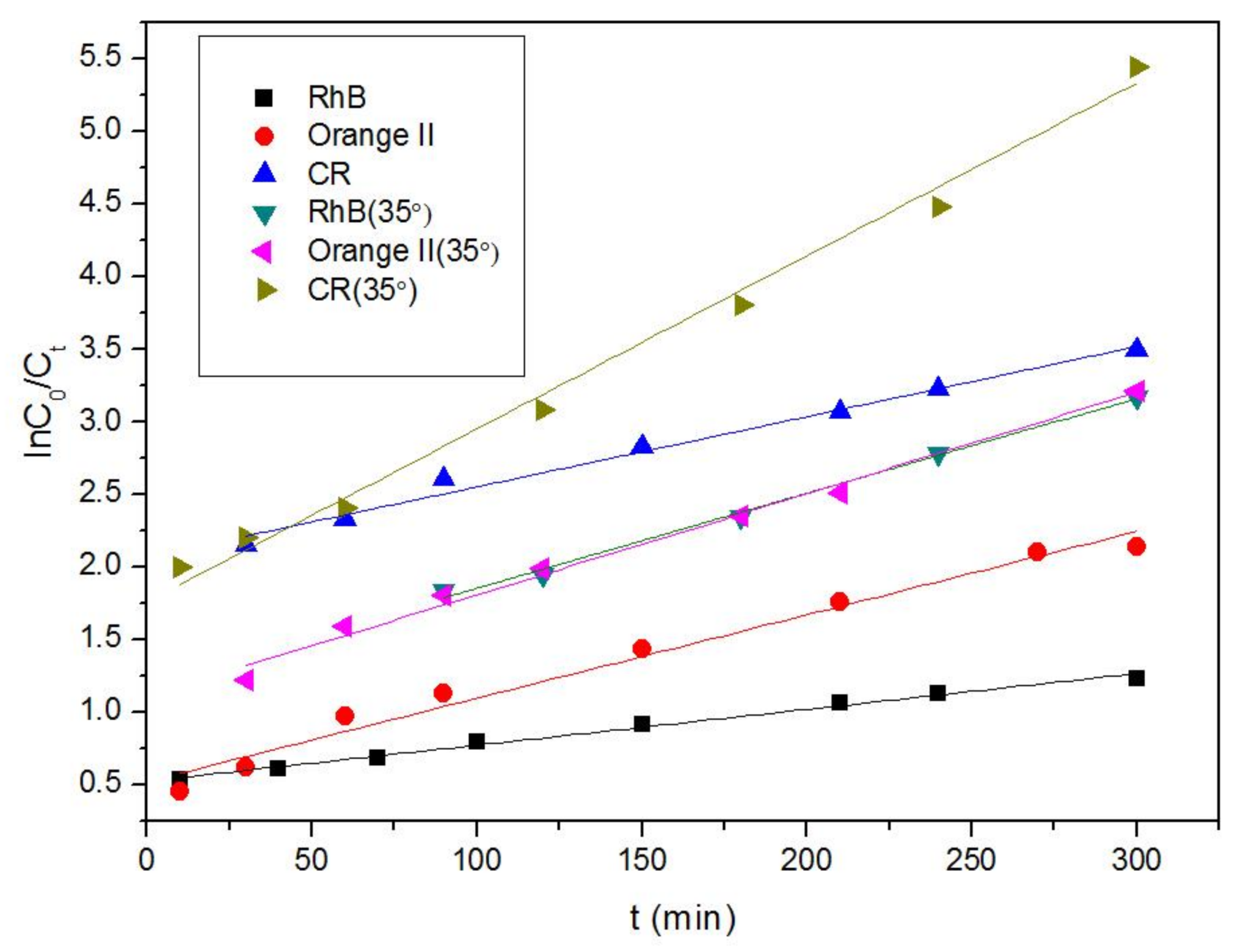
3.2. Removal of Organic Dyes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bao, C.; Fang, C.-L. Water Resources Flows Related to Urbanization in China, Challenges and Perspectives for Water Management and Urban Development. Water. Resour. Manag. 2012, 26, 531–552. [Google Scholar] [CrossRef]
- Maleki, A.; Hayati, B.; Naghizade, M.; Joo, H.S.W. Adsorption of hexavalent chromium by metal organic frameworks from aqueous solution. J. Ind. Eng. Chem. 2015, 28, 211–216. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Finizio, A.; Azimonti, G.; Villa, S. Occurrence of pesticides in surface water bodies, a critical analysis of the Italian national pesticide survey programs. J. Environ. Monit. 2011, 13, 49–57. [Google Scholar] [CrossRef] [PubMed]
- He, M.-C.; Sun, Y.; Li, X.R.; Yang, Z.F. Distribution patterns of nitrobenzenes and polychlorinated biphenyls in water, suspended particulate matter and sediment from mid- and down-stream of the Yellow River (China). Chemosphere 2006, 65, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents—A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, J.; Zhang, C.; Yue, Q.; Li, Y.; Li, C. Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 2010, 252, 149–156. [Google Scholar] [CrossRef]
- Mittal, A.; Malviya, A.; Kaur, D.; Mittal, J.; Kurup, L. Studies on the adsorption kinetics and isotherms for the removal and recovery of Methyl Orange from wastewaters using waste materials. J. Hazard. Mater. 2007, 148, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Sanny, V.; Nasir Baig, R.B.; Mallikarjuna, N.N.; Christophe, L.; Rajender, S.V. Sustainable pathway to furanics from biomass via heterogeneous organo-catalysis. Green Chem. 2017, 19, 164–168. [Google Scholar]
- Tadele, K.; Verma, S.; Michael, A.G.; Rajender, S.V. A sustainable approach to empower the bio-based future: Upgrading of biomass via process intensification. Green Chem. 2017, 19, 1624–1627. [Google Scholar] [CrossRef]
- Tadele, K.; Verma, S.; Nadagouda, M.N.; Gonzalez, M.A.; Varma, R.S. A rapid flow strategy for the oxidative cyanation of secondary and tertiary amines via C-H activation. Sci. Rep. 2017, 7, 16311. [Google Scholar] [CrossRef] [PubMed]
- Sanny, V.; Nadagouda, M.N.; Varma, R.S. Porous nitrogen-enriched carbonaceous material from marine waste: Chitosan-derived carbon nitride catalyst for aerial oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid. Sci. Rep. 2017, 7, 13596. [Google Scholar]
- Sanny, V.; Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Fixation of carbon dioxide into dimethyl carbonate over titanium-based zeolitic thiophene-benzimidazolate framework. Sci. Rep. 2017, 7, 655. [Google Scholar]
- Kumar, S.; Verma, S.; Shawat, E.; Nessim, G.D.; Jain, S.L. Amino-functionalized carbon nanofibres as an efficient metal free catalyst for the synthesis of quinazoline-2,4(1H,3H)-diones from CO2 and 2-aminobenzonitriles. RSC Adv. 2015, 5, 24670–24674. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, S.; Jain, S.L. Base-free direct formylation of aromatic iodides using CO2 as C1 source catalyzed by palladium nanoparticles grafted onto amino-functionalized nanostarch. Tetrahedron Lett. 2015, 56, 2430–2433. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents, A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, D.; Zhong, C.; Li, J.-R. Development of Computational Methodologies for Metal–Organic Frameworks and Their Application in Gas Separations. Chem. Rev. 2013, 113, 8261–8323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Du, D.-Y.; Qin, J.-S.; Li, S.-L.; Su, Z.-M.; Lan, Y.-Q. Recent advances in porous polyoxometalate-based metal-organic framework materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Goestena, M.G.; Juan-Alcañiz, J.; Ramos-Fernandez, E.; Gupta, V.K.B.S.S.; Stavitski, E.; van Bekkum, H.; Gascon, J.; Kapteijn, F. Sulfation of metal–organic frameworks, Opportunities for acid catalysis and proton conductivity. J. Catal. 2011, 281, 177–187. [Google Scholar] [CrossRef]
- Britt, D.; Lee, C.; Uribe-Romo, F.J.; Furukawa, H.; Yaghi, O.M. Ring-opening reactions within porous metal-organic frameworks. Inorg. Chem. 2010, 49, 6387–6389. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Garcia, H. Catalysis by metal nanoparticles embedded on metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 5262–5284. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Yanai, N.; Kitagawa, S. Polymerization reactions in porous coordination polymers. Chem. Soc. Rev. 2009, 38, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Jun, J.W.; Jeong, J.H.; Jhung, S.H. Remarkable adsorptive performance of a metal-organic framework, vanadium-benzenedicarboxylate (MIL-47), for benzothiophene. Chem. Commun. 2011, 47, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-organic frameworks for separation. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Van de Voorde, B.; Bueken, B.; Denayer, J.; De Vos, D. Adsorptive separation on metal-organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–778. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2012, 244–245, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Khan, N.A.; Jhung, S.H. Graphite oxide/metal-organic framework (MIL-101), remarkable performance in the adsorptive denitrogenation of model fuels. Inorg. Chem. 2013, 52, 14155–14161. [Google Scholar] [CrossRef] [PubMed]
- Cyshosz, K.A.; Wong-Foy, A.G.; Matzger, A.J. Liquid phase adsorption by microporous coordination polymers, removal of organosulfur compounds. J. Am. Chem. Soc. 2008, 130, 6938–6939. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Jhung, S.H. Effect of central metal ions of analogous metal-organic frameworks on the adsorptive removal of benzothiophene from a model fuel. J. Hazard. Mater. 2013, 260, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.K.; Hundal, G.I.; Jang, T.; Hwang, Y.K.; Jun, C.H.; Chang, J.S. Microwave synthesis of hybrid inorganic–organic materials including porous Cu3(BTC)2 from Cu(II)-trimesate mixture. Microporous Mesoporous Mater. 2009, 119, 331–337. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H.L.; Groy, T.L. Construction of Porous Solids from Hydrogen-Bonded Metal Complexes of 1,3,5-Benzenetricarboxylic Acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Schlesinger, M.; Schulze, S.; Hietschold, M.; Mehring, M. Evaluation of synthetic methods for microporous metal–organic frameworks exemplified by the competitive formation of [Cu2(BTC)3(H2O)3] and [Cu2(BTC)(OH)(H2O)]. Microporous Mesoporous Mater. 2010, 132, 121–127. [Google Scholar] [CrossRef]
- Yuan, W.B.; Friscic, T.; Apperley, D.; James, S.L. High Reactivity of Metal–Organic Frameworks under Grinding Conditions, Parallels with Organic Molecular Materials. Angew. Chem. Int. Ed. 2010, 49, 3916–3919. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Q.; Qiu, L.-G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.-Y.; Jiang, X. Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure, An efficient and environmentally friendly method. Mater. Lett. 2009, 63, 78–80. [Google Scholar] [CrossRef]
- Chen, D.; Ai, S.Y.; Liang, Z.; Wei, F.H. Preparation and photocatalytic properties of zinc oxide nanoparticles by microwave-assisted ball milling. Ceram. Int. 2016, 42, 3692–3696. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, D.; Wei, F. Synthesis of graphene oxide/metal-organic frameworks hybrid materials for enhanced removal of Methylene blue in acidic and alkaline solutions. J. Chem. Technol. Biotechnol. 2018, 93, 698–709. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.Z.; Kang, Z.T. A low temperature synthesis of MnFe2O4 nanocrystals by microwave-assisted ball-milling. Chem. Eng. J. 2013, 215, 235–239. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.Z.; Chen, B.Y.; Kaqng, Z. Coupling Effect of Microwave and Mechanical Forces during the Synthesis of Ferrite Nanoparticles by Microwave-Assisted Ball Milling. Ind. Eng. Chem. Res. 2013, 52, 14179–14184. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Kang, Z.T.; Chen, D. Synthesis and microwave absorbing properties of Mn-Zn nanoferrite produced by microwave assisted ball milling. J. Mater. Sci. Mater. Electron. 2014, 25, 4246–4251. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Kang, Z.T.; Chen, D. Process of synthesizing high sayuration magnetization Ni0.5Zn0.5Fe2O4 by microwave-assisted ball milling. Mater. Lett. 2014, 133, 259–261. [Google Scholar] [CrossRef]
- Wei, F.H.; Chen, D.; Liang, Z.; Zhao, S.Q.; Luo, Y. Synthesis and characterization of metal–organic frameworks fabricated by microwave-assisted ball milling for adsorptive removal of Congo red from aqueous solutions. RSC Adv. 2017, 7, 46520–46528. [Google Scholar] [CrossRef]
- Wei, F.H.; Chen, D.; Liang, Z.; Zhao, S.Q.; Luo, Y. Preparation of Fe-MOFs by microwave-assisted ball milling for reducing Cr(VI) in wastewater. Dalton Trans. 2017, 46, 16525–16531. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Wang, Y.; Li, M.; Wu, Q.; Wang, C.; Wang, Z. Synthesis ofmagnetic nanoporous carbon from metal-organic framework for the fast removal of organic dye from aqueous solution. J. Magn. Magn. Mater. 2016, 407, 24–30. [Google Scholar] [CrossRef]
- Chen, G.; Li, L.; Tao, C.Y. Effects of microwave heating on microstructures and structure properties of the manganese ore. J. Alloys Compd. 2016, 657, 515–518. [Google Scholar] [CrossRef]
- He, A.X.; Chen, G.; Chen, J.; Peng, J.H.; Srinivasakannan, C.; Ruan, R.S. A novel method of synthesis and investigation on transformation of synthetic rutile powders from Panzhihua sulphate titanium slag using microwave heating. Powder Technol. 2018, 323, 115–119. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Xu, Q.-Q.; Fan, H.-J.; Li, Y.-T.; Christensen, K.E.; Ren, T.-Z. Zn (Ce,Mn)-MOFs with (3,4,5)-connected 3-D topology network and test of photocatalysis on the reduction of Cr6+ by Zn (Ce)-MOFs. Polyhedron 2015, 92, 60–67. [Google Scholar] [CrossRef]
- Shi, L.; Hu, L.; Zheng, J.; Zhang, M.; Xu, J. Adsorptive Removal of Methylene Blue from Aqueous Solution using a Ni-Metal Organic Framework Material. J. Dispers. Sci. Technol. 2016, 37, 1226–1231. [Google Scholar] [CrossRef]
- Hameed, B.H.; Rahman, A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.C.; Swamy, M.M.; Mall, I.D.; Prasad, B.; Mishra, I.M. Adsorptive removal of phenol by bagasse fly ash and activated carbon, equilibrium, kinetics and thermodynamics. Colloid Surf. A 2006, 272, 89–104. [Google Scholar] [CrossRef]
- Din, A.T.M.; Hameed, B.H.; Ahmad, A.L. Batch adsorption of phenol onto physiochemical-activated coconut shell. J. Hazard. Mater. 2009, 161, 1522–1529. [Google Scholar]
- Haque, E.; Khan, N.A.; Talapaneni, S.N.; Vinu, A.; Jegal, J.; Jhung, S.H. Adsorption of phenol on mesoporous carbon CMK-3, effect of textural properties. Bull. Korean Chem. Soc. 2010, 31, 1638–1642. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, Z.-H.; Zhang, J.-M.; Wu, C.-S. The Strengthening Role of the Amino Group in Metal–Organic Framework MIL-53 (Al) for Methylene Blue and Malachite Green Dye Adsorption. J. Chem. Eng. Data 2015, 60, 3414–3422. [Google Scholar] [CrossRef]
- Wu, T.; Cai, X.; Tan, S.; Li, H.; Liu, J.; Yang, W. Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem. Eng. J. 2011, 173, 144–149. [Google Scholar] [CrossRef]
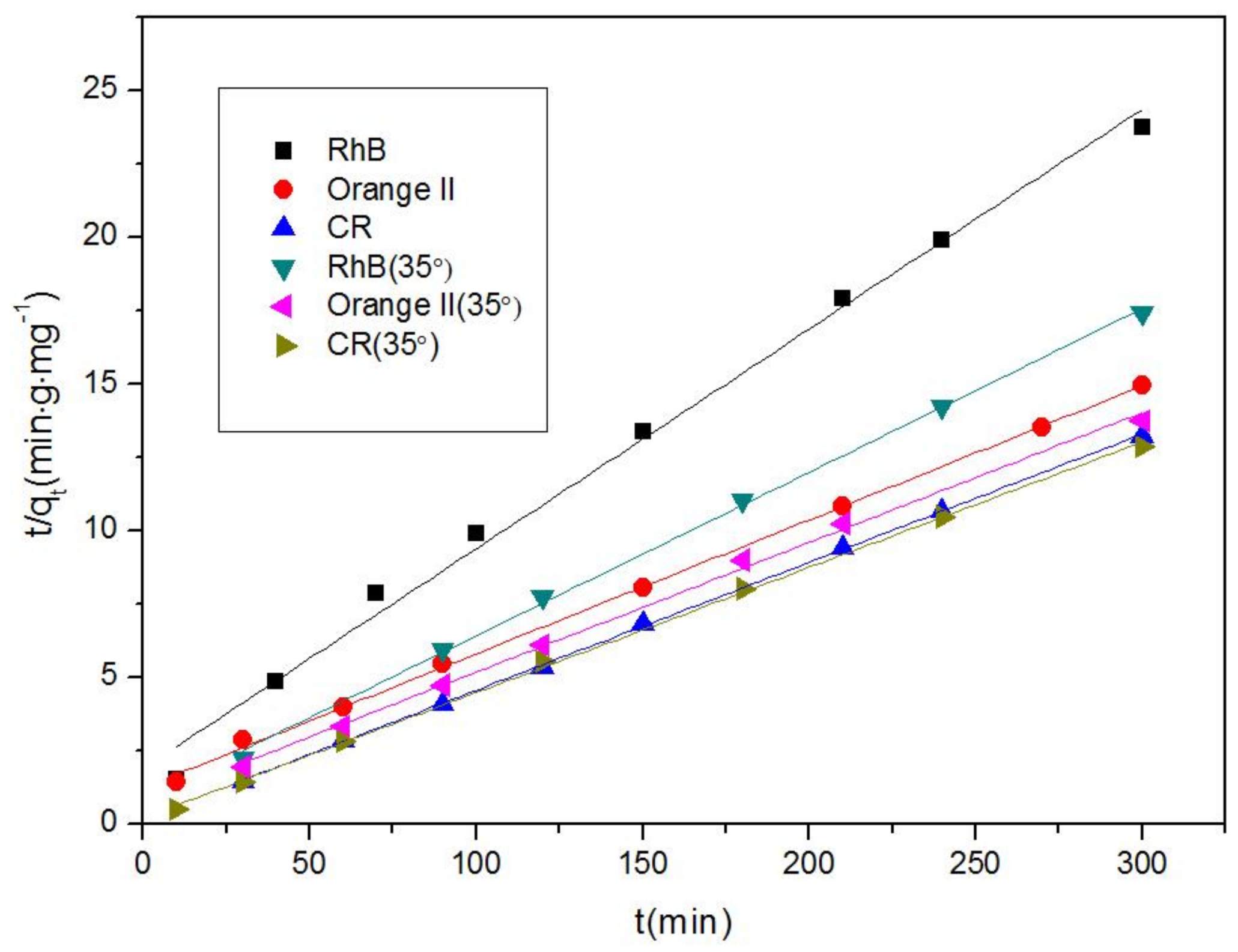
| Dyes | k1 | k2 | R2 | ||
|---|---|---|---|---|---|
| Pseudo-First-Order Kinetic | Pseudo-Second-Order Kinetic | ||||
| 15 °C | RhB | 0.00247 | 0.07488 | 0.99251 | 0.99248 |
| Orange II | 0.00575 | 0.04568 | 0.97634 | 0.99899 | |
| CR | 0.00483 | 0.04368 | 0.98598 | 0.99979 | |
| 35 °C | RhB | 0.00653 | 0.05576 | 0.99513 | 0.99832 |
| Orange II | 0.00698 | 0.04424 | 0.99231 | 0.99746 | |
| CR | 0.0119 | 0.04272 | 0.99242 | 0.99908 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, F.; Chen, D.; Liang, Z.; Zhao, S. Comparison Study on the Adsorption Capacity of Rhodamine B, Congo Red, and Orange II on Fe-MOFs. Nanomaterials 2018, 8, 248. https://doi.org/10.3390/nano8040248
Wei F, Chen D, Liang Z, Zhao S. Comparison Study on the Adsorption Capacity of Rhodamine B, Congo Red, and Orange II on Fe-MOFs. Nanomaterials. 2018; 8(4):248. https://doi.org/10.3390/nano8040248
Chicago/Turabian StyleWei, Fuhua, Ding Chen, Zhao Liang, and Shuaiqi Zhao. 2018. "Comparison Study on the Adsorption Capacity of Rhodamine B, Congo Red, and Orange II on Fe-MOFs" Nanomaterials 8, no. 4: 248. https://doi.org/10.3390/nano8040248
APA StyleWei, F., Chen, D., Liang, Z., & Zhao, S. (2018). Comparison Study on the Adsorption Capacity of Rhodamine B, Congo Red, and Orange II on Fe-MOFs. Nanomaterials, 8(4), 248. https://doi.org/10.3390/nano8040248






