Effect of Storage Conditions on the Long-Term Stability of Bactericidal Effects for Laser Generated Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
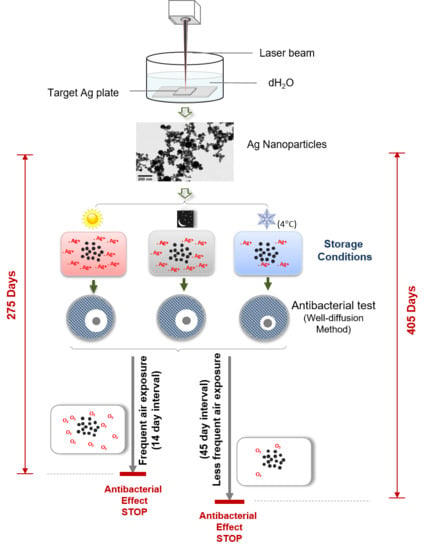
2.1. Nanoparticles Production
2.2. Bacteria Culture and the Determination of the Antibacterial Activities of NPs
2.3. Storage Conditions
2.4. NP’s Morphology and Elemental Composition
2.5. Statistical Analysis
3. Results
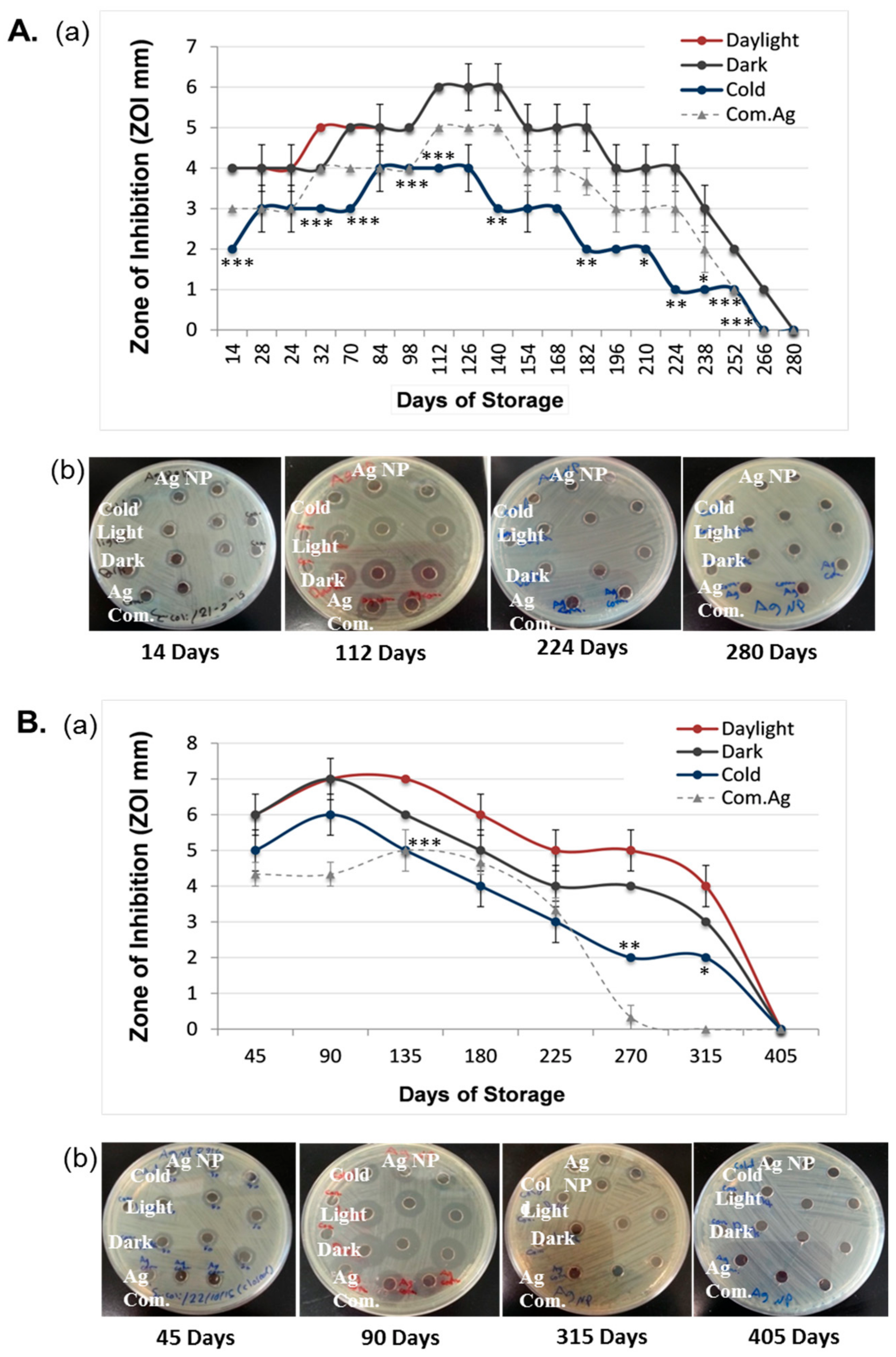
3.1. Duration of the Antibacterial Effects of Laser Generated AgNPs
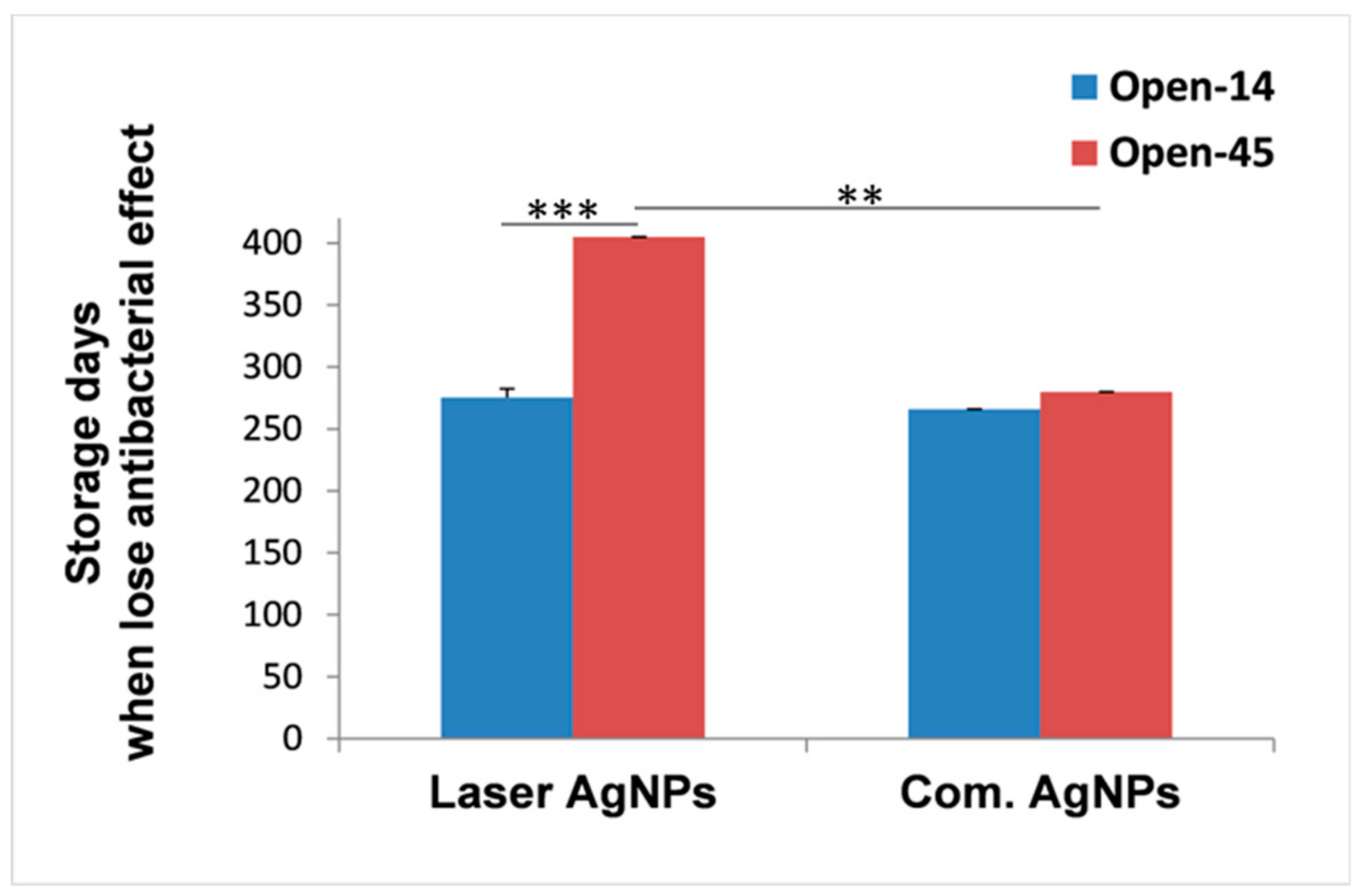
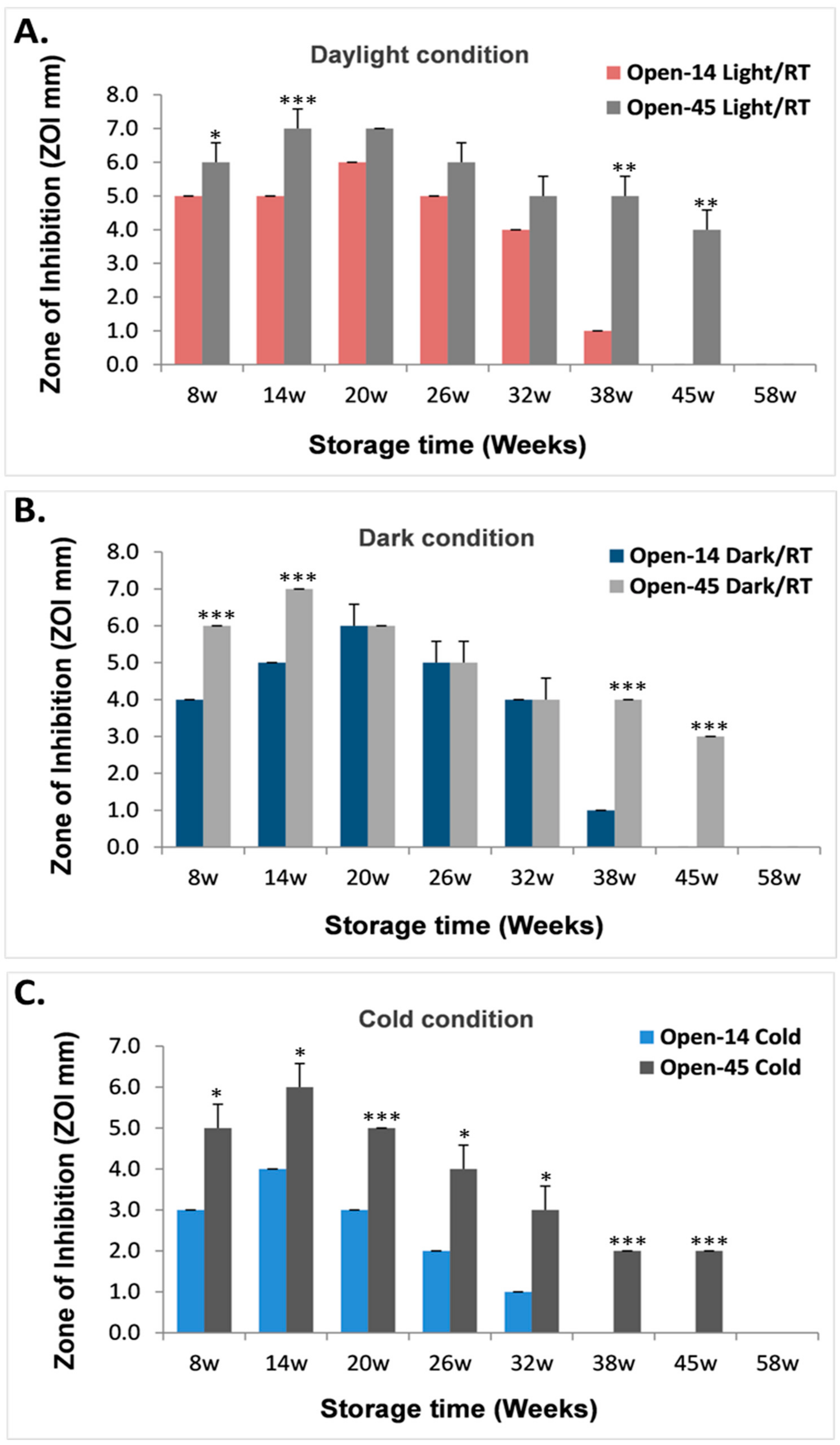
3.2. Frequent Air Exposure on the Antibacterial Effects of Laser Generated AgNPs
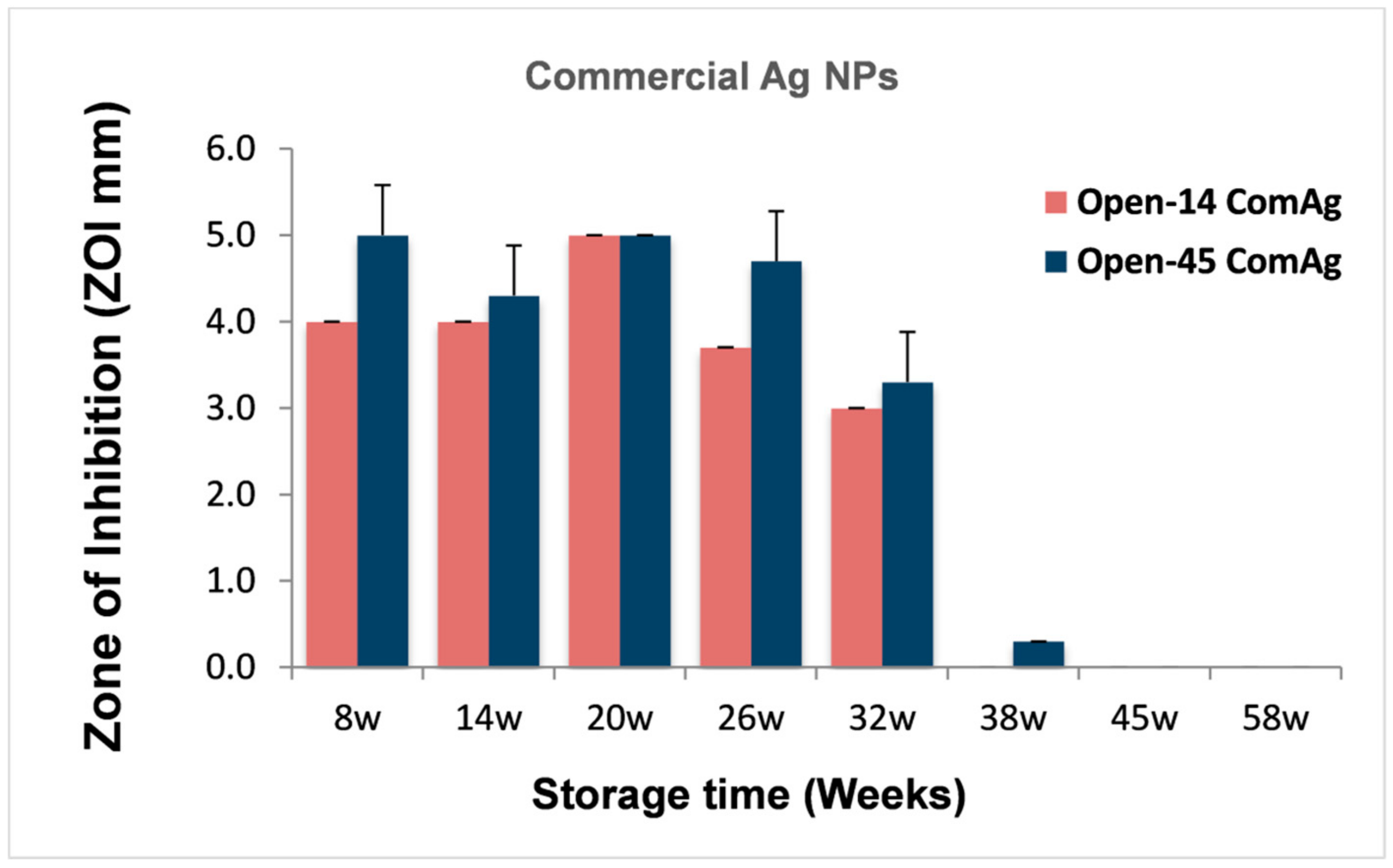
3.3. Comparison of the Antibacterial Duration Between Laser and Chemically Generated AgNPs
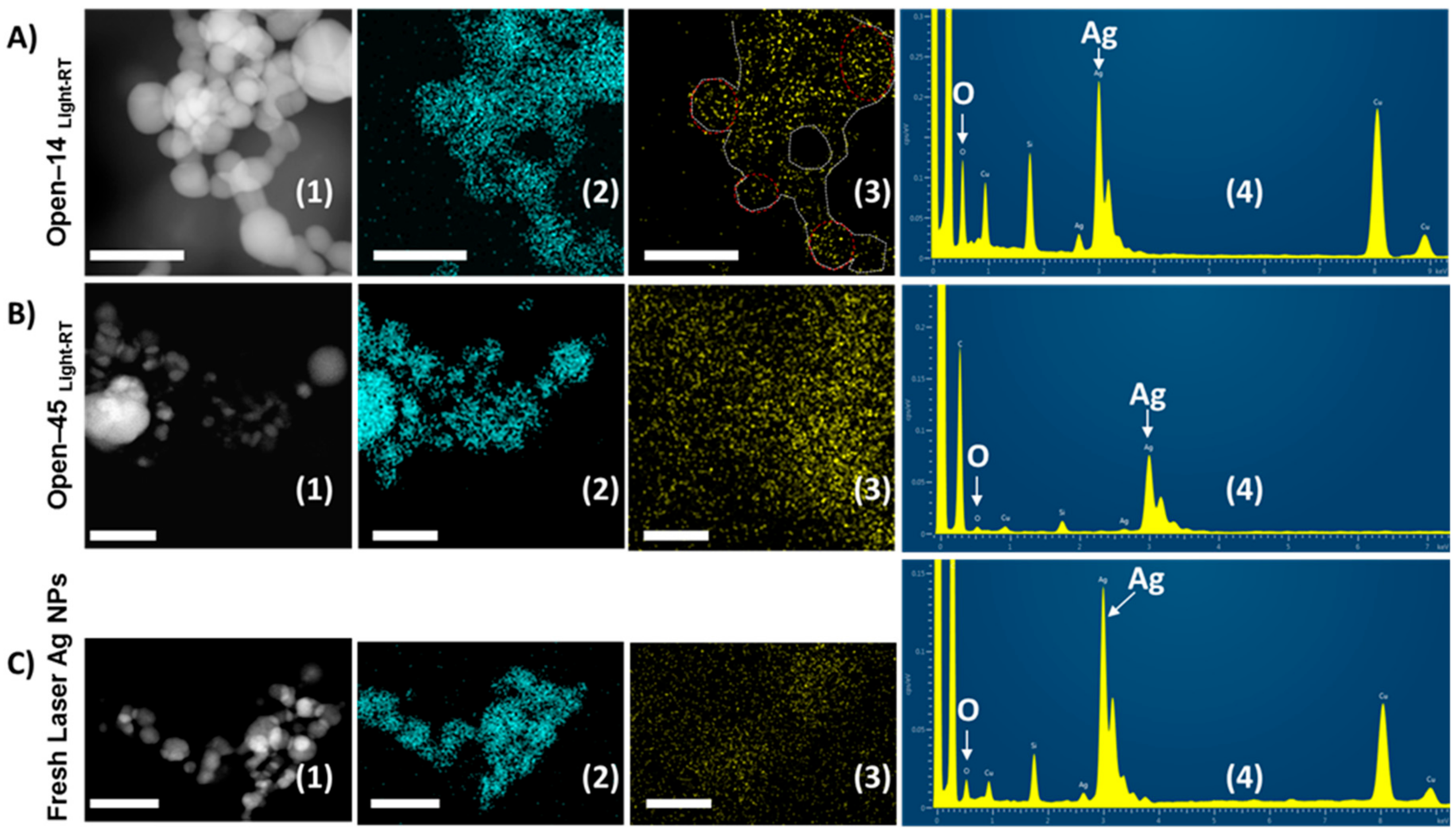
3.4. Frequent Air Exposure Increase Oxidation of Laser Generated AgNPs
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nunez, N.V.; Turrent, L.D.I.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Bogle, K.A.; Dhole, S.D.; Bhoraskar, V.N. Silver nanoparticles: Synthesis and size control by electron irradiation. Nanotechnology 2006, 17, 3204–3208. [Google Scholar] [CrossRef]
- Mahapatra, S.S.; Karak, N. Silver nanoparticle in hyperbranched polyamine: Synthesis, characterization and antibacterial activity. Mater. Chem. Phys. 2008, 112, 1114–1119. [Google Scholar] [CrossRef]
- Yang, G.W.; Li, H.L. Sonochemical synthesis of highly monodispersed and size controllable Ag nanoparticles in ethanol solution. Mater. Lett. 2008, 62, 2189–2191. [Google Scholar] [CrossRef]
- Xiu, Z.-M.; Zhang, Q.-B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.B.; Wu, Y.H.; Zhang, Y.Y.; Fu, F.Y.; Liu, X.D. Durable antibacterial cotton modified by silver nanoparticles and chitosan derivative binder. Fibers Polym. 2016, 17, 1782–1789. [Google Scholar] [CrossRef]
- Pinto, V.V.; Ferreira, M.J.; Silva, R.; Santos, H.A.; Silva, F.; Pereira, C.M. Long time effect on the stability of silver nanoparticles in aqueous medium: Effect of the synthesis and storage conditions. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 364, 19–25. [Google Scholar] [CrossRef]
- Izak-Nau, E.; Huk, A.; Reidy, B.; Uggerud, H.; Vadset, M.; Eiden, S.; Voetz, M.; Himly, M.; Duschl, A.; Dusinska, M.; et al. Impact of storage conditions and storage time on silver nanoparticles' physicochemical properties and implications for their biological effects. RSC Adv. 2015, 5, 84172–84185. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Ong, B.S. Facile synthesis of silver nanoparticles useful for fabrication of high-conductivity elements for printed electronics. J. Am. Chem. Soc. 2005, 127, 3266–3267. [Google Scholar] [CrossRef] [PubMed]
- Henglein, A. Colloidal silver nanoparticles: Photochemical preparation and interaction with O2, CCl4, and some metal ions. Chem. Mater. 1998, 10, 444–450. [Google Scholar] [CrossRef]
- Henglein, A. Physicochemical properties of small metal particles in solution—Microelectrode reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J. Phys. Chem. 1993, 97, 5457–5471. [Google Scholar] [CrossRef]
- Ho, C.M.; Wong, C.K.; Yau, S.K.W.; Lok, C.N.; Che, C.M. Oxidative dissolution of silver nanoparticles by dioxygen: A kinetic and mechanistic study. Chem. Asian J.l 2011, 6, 2506–2511. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L., Jr.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Velgosova, O.; Cizmarova, E.; Malek, J.; Kavulicova, J. Effect of storage conditions on long-term stability of ag nanoparticles formed via green synthesis. Int. J. Miner. Metall. Mater. 2017, 24, 1177–1182. [Google Scholar] [CrossRef]
- Wang, R.; Neoh, K.G.; Kang, E.T.; Tambyah, P.A.; Chiong, E. Antifouling coating with controllable and sustained silver release for long-term inhibition of infection and encrustation in urinary catheters. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Korshed, P.; Li, L.; Liu, Z.; Wang, T. The molecular mechanisms of the antibacterial effect of picosecond laser generated silver nanoparticles and their toxicity to human cells. PLoS ONE 2016, 11, e0160078. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Li, L.; Liu, Z.; Zhong, X.L.; Liu, H.; Wang, T. Generation of silver titania nanoparticles from an ag-ti alloy via picosecond laser ablation and their antibacterial activities. RSC Adv. 2015, 5, 72981–72994. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial activity of nanosilver ions and particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Joutel, A.; Monet, M.; Domenga, V.; Riant, F.; Tournier-Lasserve, E. Pathogenic mutations associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy differently affect jagged1 binding and notch3 activity via the rbp/jk signaling pathway. Am. J. Hum. Genet. 2004, 74, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Odzak, N.; Kistler, D.; Sigg, L. Influence of daylight on the fate of silver and zinc oxide nanoparticles in natural aquatic environments. Environ. Pollut. 2017, 226, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Yin, L.Y.; Lin, S.H.; Wiesner, M.; Bernhardt, E.; Liu, J. Toxicity reduction of polymer-stabilized silver nanoparticles by sunlight. J. Phys. Chem. C 2011, 115, 4425–4432. [Google Scholar] [CrossRef]
- George, S.; Gardner, H.; Seng, E.K.; Chang, H.; Wang, C.; Yu Fang, C.H.; Richards, M.; Valiyaveettil, S.; Chan, W.K. Differential effect of solar light in increasing the toxicity of silver and titanium dioxide nanoparticles to a fish cell line and zebrafish embryos. Environ. Sci. Technol. 2014, 48, 6374–6382. [Google Scholar] [CrossRef] [PubMed]
- Gorham, J.M.; MacCuspie, R.I.; Klein, K.L.; Fairbrother, D.H.; Holbrook, R.D. Uv-induced photochemical transformations of citrate-capped silver nanoparticle suspensions. J. Nanoparticle Res. 2012, 14, 1139. [Google Scholar] [CrossRef]
- Yu, S.J.; Yin, Y.G.; Zhou, X.X.; Dong, L.J.; Liu, J.F. Transformation kinetics of silver nanoparticles and silver ions in aquatic environments revealed by double stable isotope labeling. Environ. Sci. Nano 2016, 3, 883–893. [Google Scholar] [CrossRef]
- Xiu, Z.-M.; Ma, J.; Alvarez, P.J. Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ. Sci. Technol. 2011, 45, 9003–9008. [Google Scholar] [CrossRef] [PubMed]
| Sample ID | Storage Condition | Sampling Frequency |
|---|---|---|
| Open-14Light/RT | Daylight RT | Every 14 days |
| Open-45Light/RT | Daylight RT | Every 45 days |
| Open-14Dark/RT | Dark RT | Every 14 days |
| Open-45Dark/RT | Dark RT | Every 45 days |
| Open-14Cold | Cold (4 °C) | Every 14 days |
| Open-45Cold | Cold (4 °C) | Every 45 days |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korshed, P.; Li, L.; Ngo, D.-T.; Wang, T. Effect of Storage Conditions on the Long-Term Stability of Bactericidal Effects for Laser Generated Silver Nanoparticles. Nanomaterials 2018, 8, 218. https://doi.org/10.3390/nano8040218
Korshed P, Li L, Ngo D-T, Wang T. Effect of Storage Conditions on the Long-Term Stability of Bactericidal Effects for Laser Generated Silver Nanoparticles. Nanomaterials. 2018; 8(4):218. https://doi.org/10.3390/nano8040218
Chicago/Turabian StyleKorshed, Peri, Lin Li, Duc-The Ngo, and Tao Wang. 2018. "Effect of Storage Conditions on the Long-Term Stability of Bactericidal Effects for Laser Generated Silver Nanoparticles" Nanomaterials 8, no. 4: 218. https://doi.org/10.3390/nano8040218
APA StyleKorshed, P., Li, L., Ngo, D.-T., & Wang, T. (2018). Effect of Storage Conditions on the Long-Term Stability of Bactericidal Effects for Laser Generated Silver Nanoparticles. Nanomaterials, 8(4), 218. https://doi.org/10.3390/nano8040218