Ultrafast Synthesis of Ni-MOF in One Minute by Ball Milling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
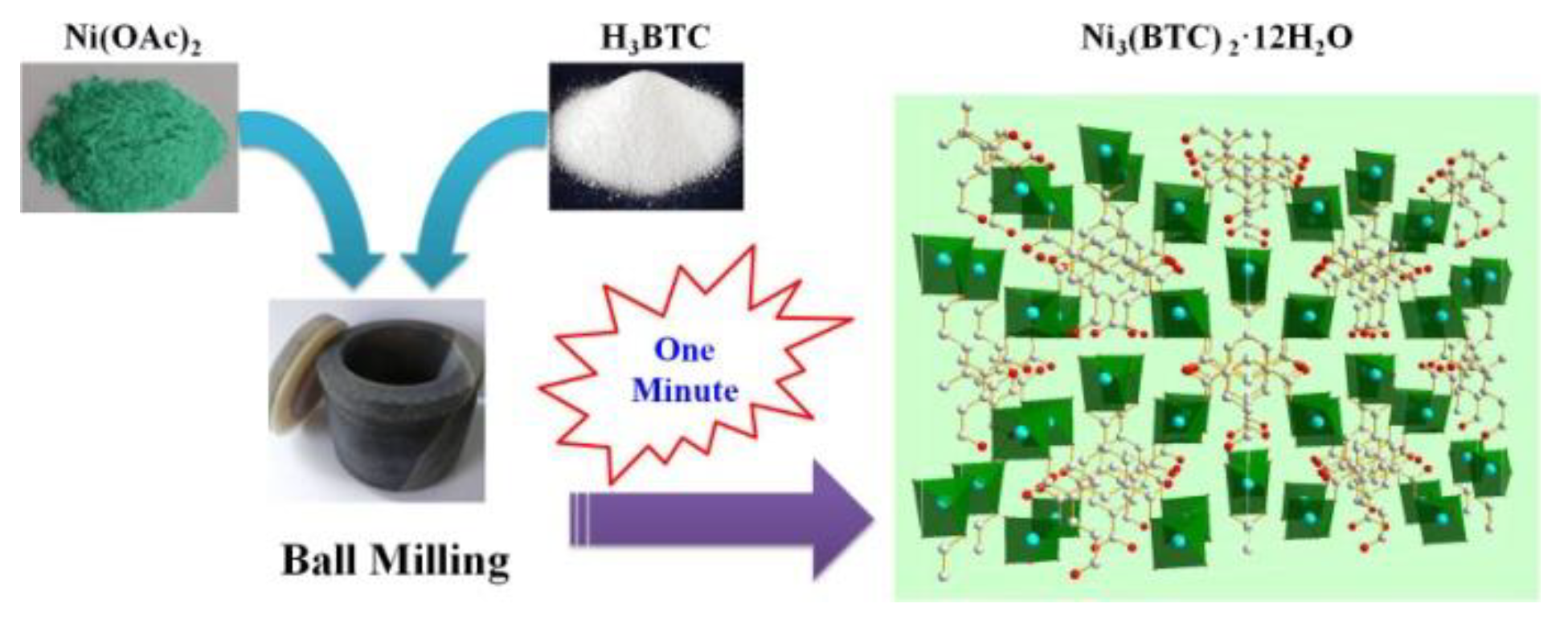
2.2. Synthesis of Ni-MOF
2.3. Characterization
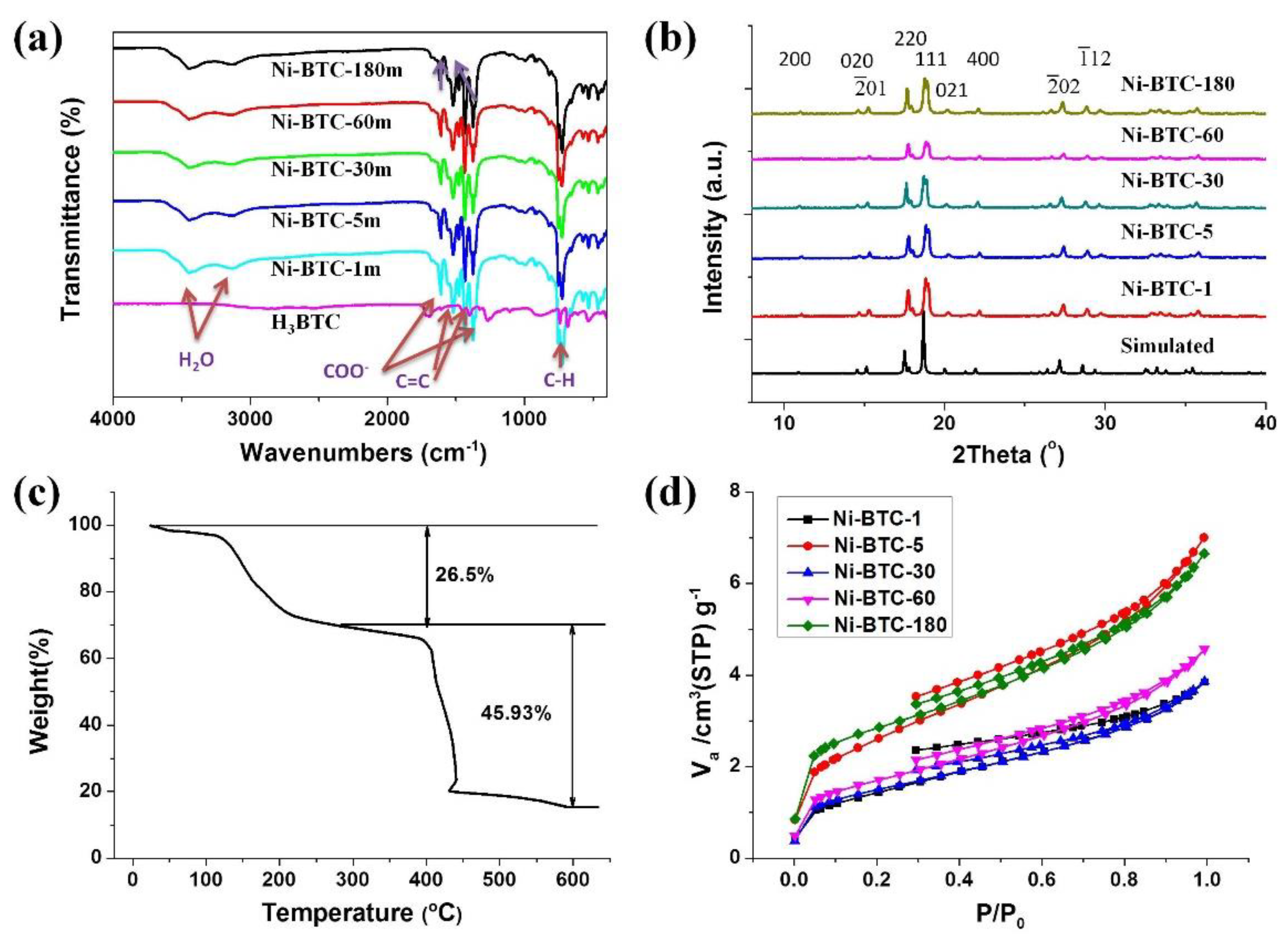
3. Results and Discussion
3.1. Synthesis of Ni-MOF
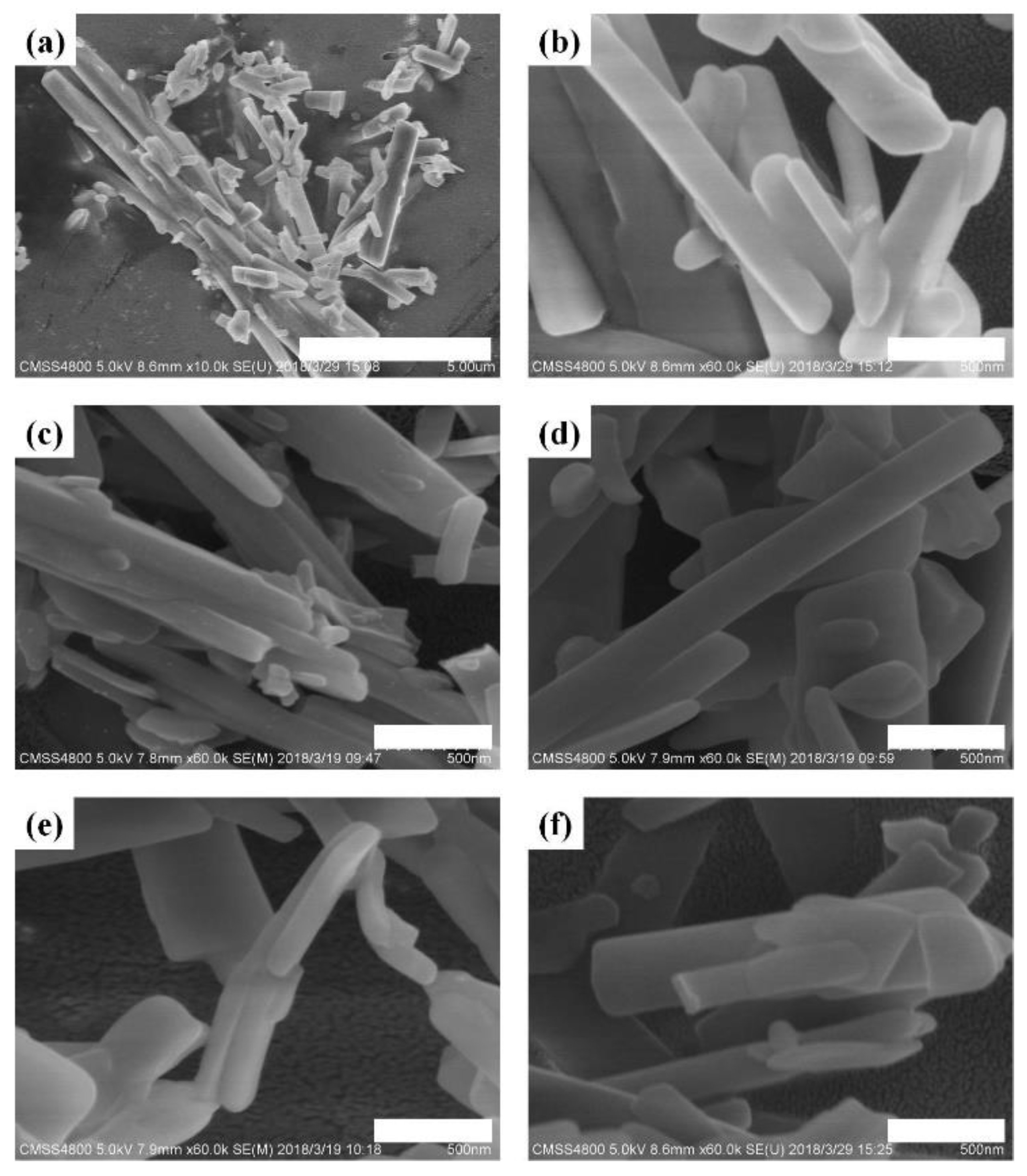
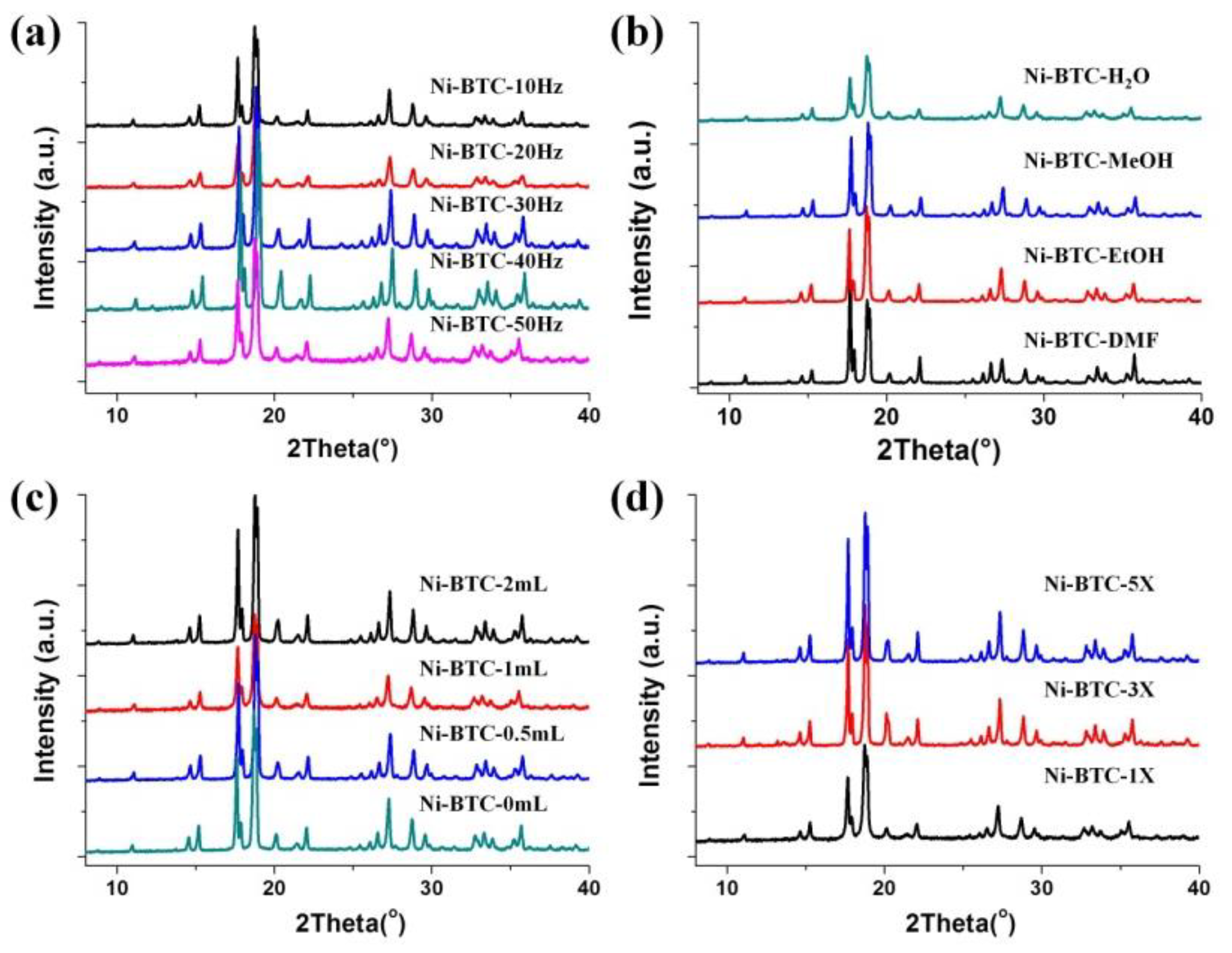
3.1.1. Effect of Grinding Time
3.1.2. Effect of Mechano-Frequency of Grinding
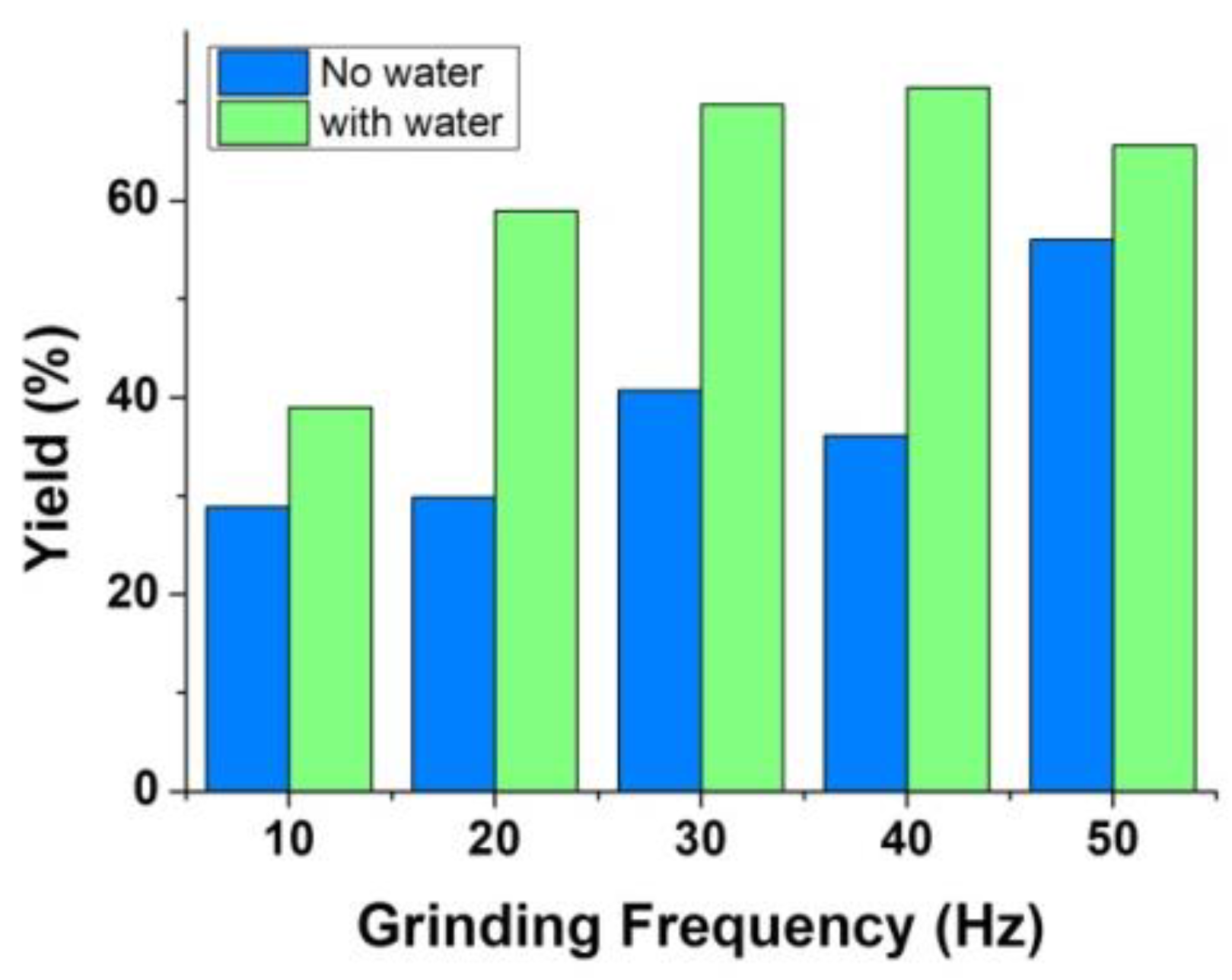
3.1.3. Effect of Auxiliary Liquid
3.1.4. Scalability
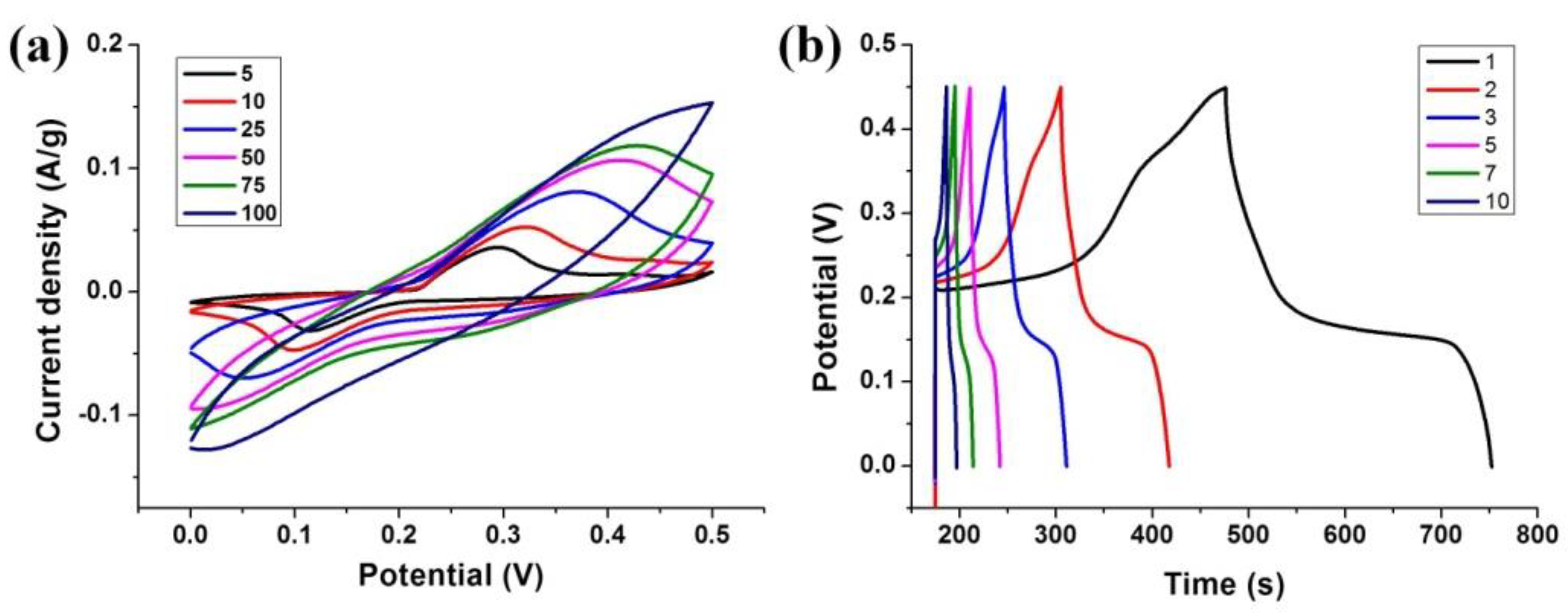
3.2. Electrochemical Performance of Ni-MOF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hendon, C.H.; Rieth, A.J.; Korzyński, M.D.; Dincă, M. Grand challenges and future opportunities for metal–organic frameworks. ACS Central Sci. 2017, 3, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Alezi, D.; Belmabkhout, Y.; Suyetin, M.; Bhatt, P.M.; Weselinski, Ł.J.; Solovyeva, V.; Adil, K.; Spanopoulos, I.; Trikalitis, P.N.; Emwas, A.M. Mof crystal chemistry paving the way to gas storage needs: Aluminum-based soc-mof for CH4, O2, and CO2 storage. J. Am. Chem. Soc. 2015, 137, 13308–13318. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Fan, L.; Sun, D. Recent advances and challenges of metal–organic framework membranes for gas separation. J. Mater. Chem. 2017, 5, 10073–10091. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Liang, J.; Wang, X.-S.; Cao, R. Multifunctional metal–organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tao, C.-A.; Liu, H.; Zou, X.; Zhu, H.; Wang, J. Fabrication of an NH2-MIL-88B photonic film for naked-eye sensing of organic vapors. J. Mater. Chem. A 2014, 2, 14222–14227. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Y.; Liu, S.; Xu, M. The applications of metal−organic frameworks in electrochemical sensors. ChemElectroChem 2018, 5, 6–19. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal–organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Zheng, S.; Li, X.; Yan, B.; Hu, Q.; Xu, Y.; Xiao, X.; Xue, H.; Pang, H. Transition-metal (Fe, Co, Ni) based metal-organic frameworks for electrochemical energy storage. Adv. Energy Mater. 2017, 7, 1602733. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal organic frameworks for energy storage and conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, P.; Zheng, C.; Qiu, H.; Wei, M. Metal–organic frameworks: A new promising class of materials for a high performance supercapacitor electrode. J. Mater. Chem. A 2014, 2, 16640–16644. [Google Scholar] [CrossRef]
- Kang, L.; Sun, S.-X.; Kong, L.-B.; Lang, J.-W.; Luo, Y.-C. Investigating metal-organic framework as a new pseudo-capacitive material for supercapacitors. Chin. Chem. Lett. 2014, 25, 957–961. [Google Scholar] [CrossRef]
- Wang, D.; Ni, W.; Pang, H.; Lu, Q.; Huang, Z.; Zhao, J. Preparation of mesoporous NiO with a bimodal pore size distribution and application in electrochemical capacitors. Electrochim. Acta 2010, 55, 6830–6835. [Google Scholar] [CrossRef]
- Chen, L.; Bai, J.; Wang, C.; Pan, Y.; Scheer, M.; You, X. One-step solid-state thermolysis of a metal–organic framework: A simple and facile route to large-scale of multiwalled carbon nanotubes. Chem. Commun. 2008, 1581–1583. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Dong, Y.; Liu, C.; Wei, W.; Liu, D.; Liu, P. Fabrication of hierarchical porous nickel based metal-organic framework (Ni-MOF) constructed with nanosheets as novel pseudo-capacitive material for asymmetric supercapacitor. J. Colloid Interface Sci. 2018, 518, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.-N.; Liu, Q.; Sun, W.-Y. Room temperature solution-phase synthesis of flower-like nanostructures of [Ni3(BTC)2·12H2O] and their conversion to porous NiO. Chin. Chem. Lett. 2013, 24, 663–667. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A force of synthesis. ACS Central Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T. Supramolecular concepts and new techniques in mechanochemistry: Cocrystals, cages, rotaxanes, open metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 3493–3510. [Google Scholar] [CrossRef] [PubMed]
- Pichon, A.; James, S.L. An array-based study of reactivity under solvent-free mechanochemical conditions—Insights and trends. CrystEngComm 2008, 10, 1839–1847. [Google Scholar] [CrossRef]
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Friščić, T. Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry. Angew. Chem. 2010, 122, 9834–9837. [Google Scholar] [CrossRef]
- Katsenis, A.D.; Puškarić, A.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Užarević, K.; Pham, M.-H.; Do, T.-O.; Kimber, S.A.J.; Lazić, P.; et al. In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework. Nat. Commun. 2015, 6, 6662. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; O’Connor, J.; James, S.L. Mechanochemical synthesis of homo- and hetero-rare-earth(iii) metal–organic frameworks by ball milling. CrystEngComm 2010, 12, 3515–3517. [Google Scholar] [CrossRef]
- Prochowicz, D.; Sokołowski, K.; Justyniak, I.; Kornowicz, A.; Fairen-Jimenez, D.; Friščić, T.; Lewiński, J. A mechanochemical strategy for IRMOF assembly based on pre-designed oxo-zinc precursors. Chem. Commun. 2015, 51, 4032–4035. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, M.; Padella, F.; Ennas, G.; Lai, S.; Bellusci, M.; Rombi, E.; Sini, F.; Pentimalli, M.; Delitala, C.; Scano, A.; et al. Liquid-assisted mechanochemical synthesis of an iron carboxylate metal organic framework and its evaluation in diesel fuel desulfurization. Microp. Mesoporous Mater. 2015, 213, 14–21. [Google Scholar] [CrossRef]
- Julien, P.A.; Užarević, K.; Katsenis, A.D.; Kimber, S.A.J.; Wang, T.; Farha, O.K.; Zhang, Y.; Casaban, J.; Germann, L.S.; Etter, M.; et al. In situ monitoring and mechanism of the mechanochemical formation of a microporous MOF-74 framework. J. Am. Chem. Soc. 2016, 138, 2929–2932. [Google Scholar] [CrossRef] [PubMed]
- Stolar, T.; Batzdorf, L.; Lukin, S.; Žilić, D.; Motillo, C.; Friščić, T.; Emmerling, F.; Halasz, I.; Užarević, K. In situ monitoring of the mechanosynthesis of the archetypal metal–organic framework HKUST-1: Effect of liquid additives on the milling reactivity. Inorg. Chem. 2017, 56, 6599–6608. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, K.; Teat, S.J.; Deibert, B.J.; Yuan, W.; Li, J. A mechanochemical route toward the rational, systematic, and cost-effective green synthesis of strongly luminescent copper iodide based hybrid phosphors. J. Mater. Chem. C 2017, 5, 5962–5969. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Liu, Z.; Sun, X.; Xia, Q.; Li, Z. Liquid-assisted mechanochemical synthesis of copper based MOF-505 for the separation of CO2 over CH4 or N2. Ind. Eng. Chem. Res. 2018, 57, 703–709. [Google Scholar] [CrossRef]
- Užarević, K.; Wang, T.C.; Moon, S.-Y.; Fidelli, A.M.; Hupp, J.T.; Farha, O.K.; Friščić, T. Mechanochemical and solvent-free assembly of zirconium-based metal–organic frameworks. Chem. Commun. 2016, 52, 2133–2136. [Google Scholar] [CrossRef] [PubMed]
- Fidelli, A.M.; Karadeniz, B.; Howarth, A.J.; Huskić, I.; Germann, L.S.; Halasz, I.; Etter, M.; Moon, S.-Y.; Dinnebier, R.E.; Stilinović, V.; et al. Green and rapid mechanosynthesis of high-porosity NU- and UiO-type metal–organic frameworks. Chem. Commun. 2018, 54, 6999–7002. [Google Scholar] [CrossRef] [PubMed]
- Ali-Moussa, H.; Navarro Amador, R.; Martinez, J.; Lamaty, F.; Carboni, M.; Bantreil, X. Synthesis and post-synthetic modification of UiO-67 type metal-organic frameworks by mechanochemistry. Mater. Lett. 2017, 197, 171–174. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H.; Groy, T.L. Construction of porous solids from hydrogen-bonded metal complexes of 1,3,5-benzenetricarboxylic acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- CCDC No. 1274034. Available online: http://www.ccdc.cam.ac.uk/access (accessed on 17 December 2017).
- Duan, C.; Li, F.; Xiao, J.; Liu, Z.; Li, C.; Xi, H. Rapid room-temperature synthesis of hierarchical porous zeolitic imidazolate frameworks with high space-time yield. Sci. China Mater. 2017, 60, 1205–1214. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Lo, W.-S.; Kuo, Y.-W.; Chen, W.-J.; Lin, C.-H.; Shieh, F.-K. Green and rapid synthesis of zirconium metal–organic frameworks via mechanochemistry: UiO-66 analog nanocrystals obtained in one hundred seconds. Chem. Commun. 2017, 53, 5818–5821. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiao, J.; Lv, D.; Huang, T.; Xu, F.; Sun, X.; Xi, H.; Xia, Q.; Li, Z. Highly efficient mechanochemical synthesis of an indium based metal-organic framework with excellent water stability. Chem. Eng. Sci. 2017, 158, 539–544. [Google Scholar] [CrossRef]
- Yang, H.; Orefuwa, S.; Goudy, A. Study of mechanochemical synthesis in the formation of the metal–organic framework Cu3(BTC)2 for hydrogen storage. Microp. Mesoporous Mater. 2011, 143, 37–45. [Google Scholar] [CrossRef]
- Bowmaker, G.A. Solvent-assisted mechanochemistry. Chem. Commun. 2013, 49, 334–348. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Tao, C.-A.; Chen, R.; Wu, L.; Zou, X.; Wang, J. Ultrafast Synthesis of Ni-MOF in One Minute by Ball Milling. Nanomaterials 2018, 8, 1067. https://doi.org/10.3390/nano8121067
Zhang R, Tao C-A, Chen R, Wu L, Zou X, Wang J. Ultrafast Synthesis of Ni-MOF in One Minute by Ball Milling. Nanomaterials. 2018; 8(12):1067. https://doi.org/10.3390/nano8121067
Chicago/Turabian StyleZhang, Ren, Cheng-An Tao, Rui Chen, Lifang Wu, Xiaoxuan Zou, and Jianfang Wang. 2018. "Ultrafast Synthesis of Ni-MOF in One Minute by Ball Milling" Nanomaterials 8, no. 12: 1067. https://doi.org/10.3390/nano8121067
APA StyleZhang, R., Tao, C.-A., Chen, R., Wu, L., Zou, X., & Wang, J. (2018). Ultrafast Synthesis of Ni-MOF in One Minute by Ball Milling. Nanomaterials, 8(12), 1067. https://doi.org/10.3390/nano8121067





