Design of Therapeutic Self-Assembled Monolayers of Thiolated Abiraterone
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Measurements
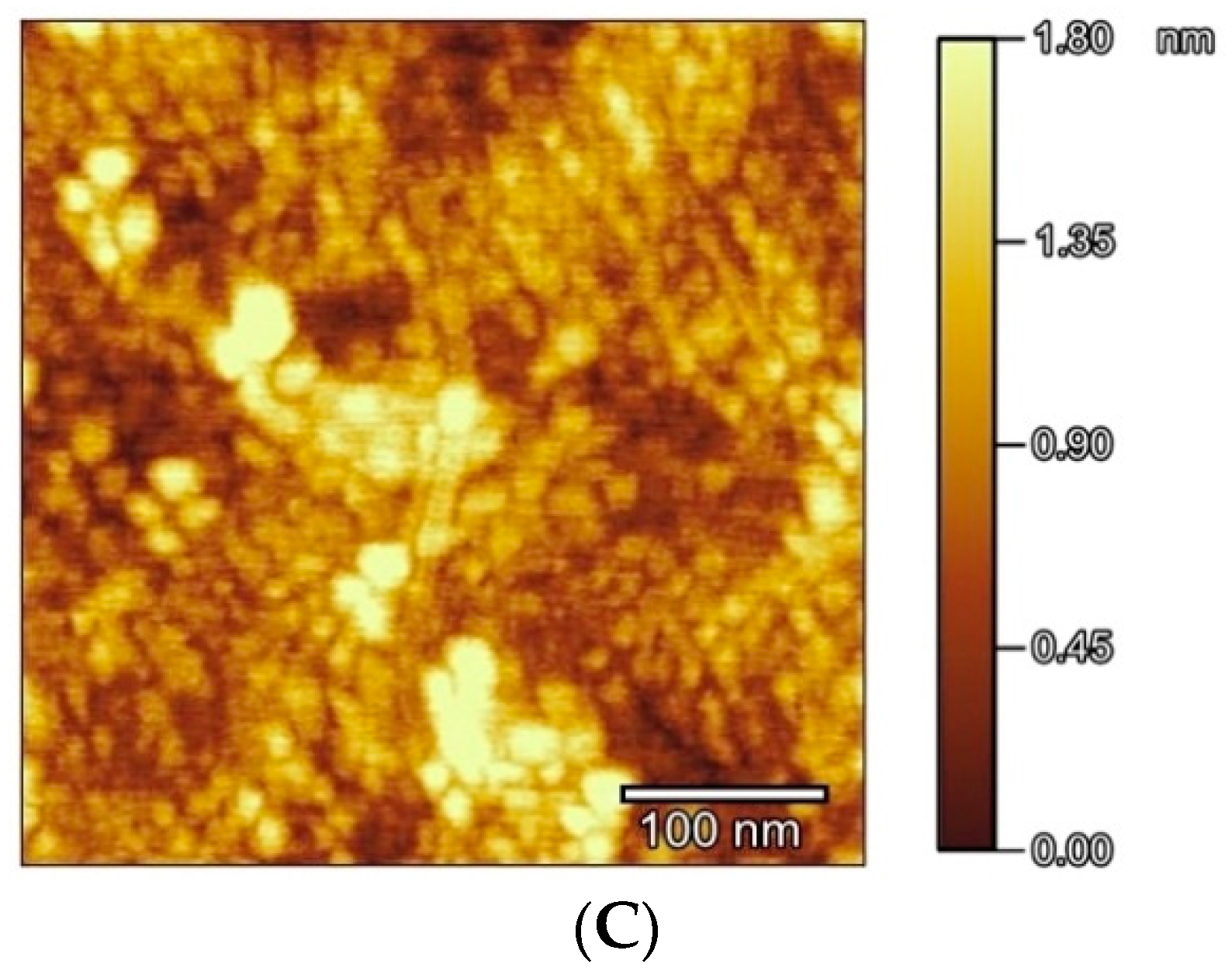
2.3. Atomic Force Microscopy (AFM)
2.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.5. MS Spectrometry
2.6. Chromatography
2.7. Chemistry
3. Results and Discussion
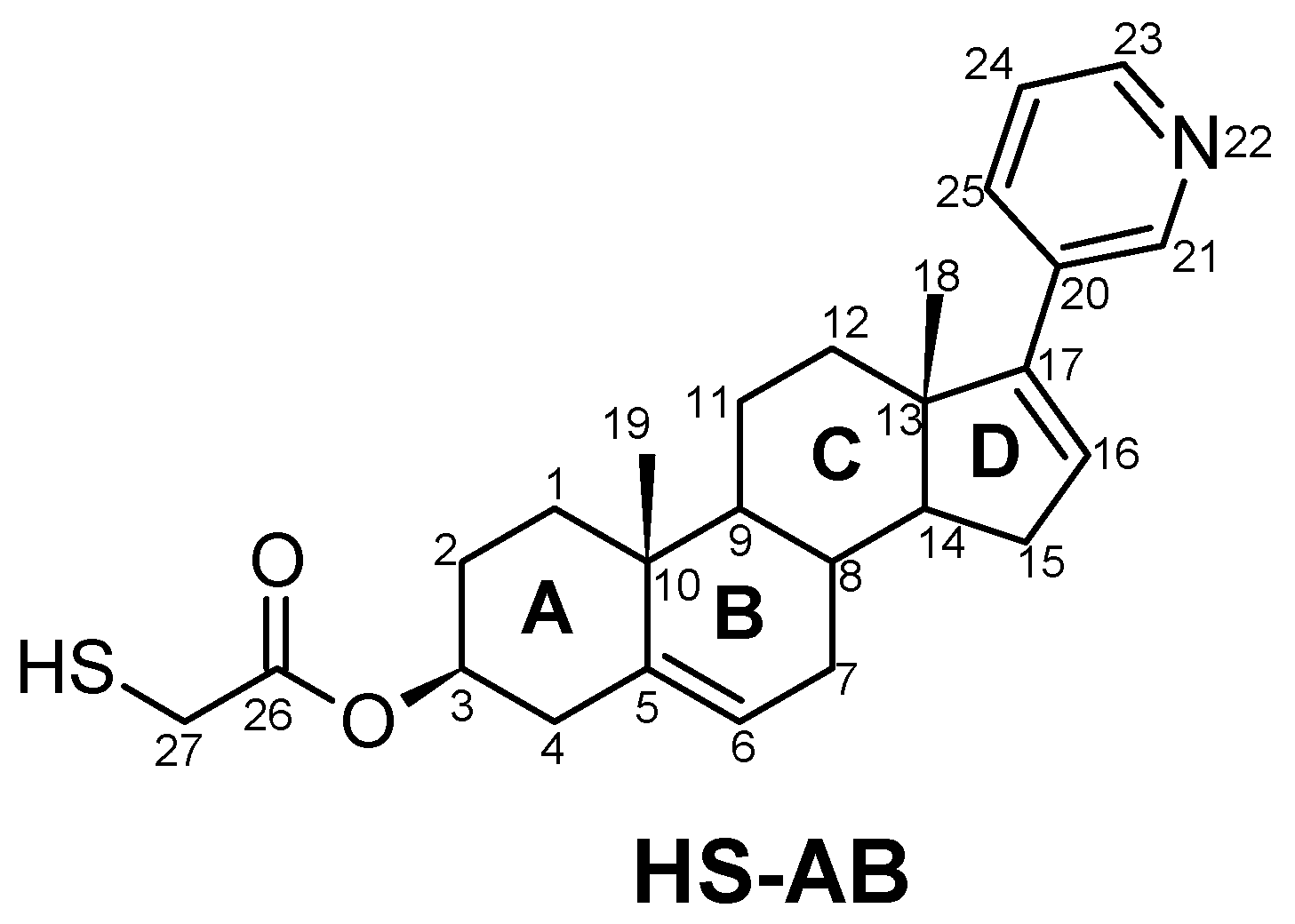
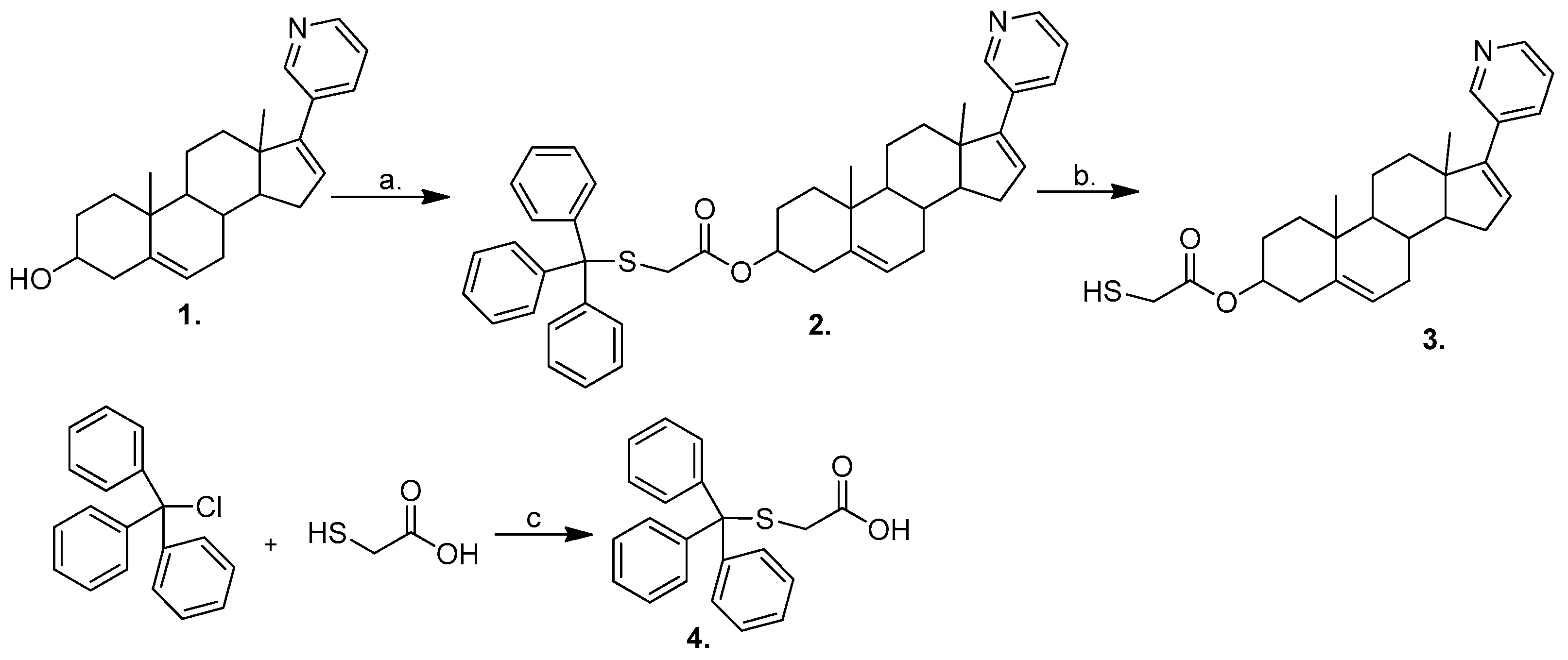
3.1. Chemistry
3.2. Electrochemical and AFM Characteristics of the Gold Electrodes Modified with HS-AB or HS-GA
3.3. Electrochemical Characteristics of Gold Electrodes Modified with HS-AB or HS-GA in the Presence of the Redox Probe Fe(CN)64−
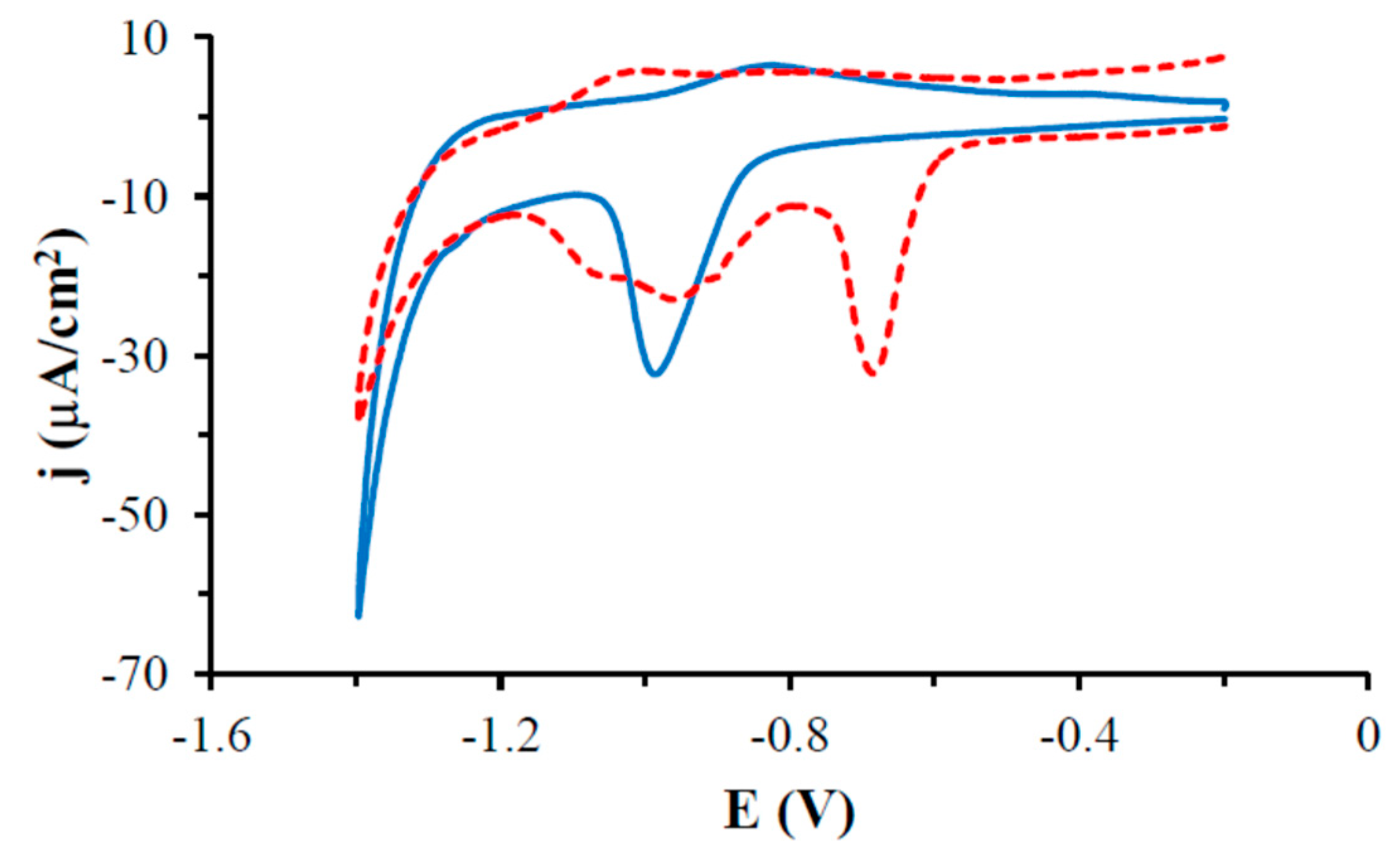
3.4. Electrochemical Characteristics of Gold Electrodes Modified with HS-AB or HS-GA in the NaOH Solution
3.5. Electrochemical Characteristics of Gold Electrodes Modified with HS-AB or HS-GA in the Solution Containing Oxygen
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/132971#section=Drug-and-Medication-Information (accessed on 1 November 2018).
- Stolarczyk, E.U.; Łaszcz, M.; Leś, A.; Kubiszewski, M.; Kuziak, K.; Sidoryk, K.; Stolarczyk, K. Design and molecular modeling of abiraterone-functionalized gold nanoparticles. Nanomaterials 2018, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.M.; Mahapatro, A.; Patel, D.N.; Feldman, M.D.; Ayon, A.A.; Agrawal, C.M. Drug delivery from therapeutic self-assembled monolayers (T-SAMs) on 316L stainless steel. Curr. Top. Med. Chem. 2008, 8, 281–289. [Google Scholar] [CrossRef]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Plancarte, A.; Haro-Poniatowski, E.; Picquart, M.; Morales-Méndez, J.G.; Lara-Cruz, C.; Jiménez-Salazar, J.E.; Damián-Matsumura, P.; Escobar-Alarcon, L.; Batina, N. Development of a nanostructured platform for identifying HER2-heterogeneity of breast cancer cells by surface-enhanced raman scattering. Nanomaterials 2018, 8, 549. [Google Scholar] [CrossRef]
- Mani, G.; Johnson, D.M.; Marton, D.; Feldman, M.D.; Patel, D.; Ayon, A.A.; Agrawal, C.M. Drug delivery from gold and titanium surfaces using self-assembled monolayers. Biomaterials 2008, 29, 4561–4573. [Google Scholar] [CrossRef]
- Ko, E.-B.; Cho, H.-Y.; Kim, T.-H.; Yea, Ch.-H.; Choi, J.-W. Cell chip with a thiolated chitosan self-assembled monolayer to detect the effects of anticancer drugs on breast normal and cancer cells. Colloids Surf. B Biointerfaces 2013, 112, 387–392. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Corthey, G.; Pensa, E.; Cortes, E.; Fonticelli, M.H.; Ibanez, F.; Benitez, G.E.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiolates on metals: A review article on sulfur-metal chemistry and surface structures. RSC Adv. 2014, 4, 27730–27754. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Kriebel, J.K.K.; Love, J.C. Molecular engineering of surfaces using self-assembled monolayers. Sci. Prog. 2005, 88, 17–48. [Google Scholar] [CrossRef]
- Stolarczyk, K.; Bilewicz, R. Electron transport through alkanethiolate films decorated with monolayer protected gold clusters. Electrochim. Acta 2006, 51, 2358–2365. [Google Scholar] [CrossRef]
- Finklea, H.O. Electroanalytical Chemistry; Bard, A.J., Rubinstein, I., Eds.; Marcel Dekker: New York, NY, USA, 1996; Volume 19, pp. 109–335. [Google Scholar]
- Stolarczyk, K.; Bilewicz, R.; Skwierawska, A.; Biernat, J.F. Functionalization of electrode surfaces with monolayers of azocompounds and gold clusters. J. Incl. Phenom. 2004, 49, 173–179. [Google Scholar] [CrossRef]
- Ding, R.; He, Y.; Xu, J.; Liu, H.; Wang, X.; Feng, M.; Qi, C.; Zhang, J.; Peng, C. Preparation and bioevaluation of 99mTc nitrido radiopharmaceuticals with pyrazolo[1,5-a]pyrimidine as tumor imaging agents. Med. Chem. Res. 2012, 21, 523–530. [Google Scholar] [CrossRef]
- Parsons, R. The electrical double layer at the solid-liquid interface. Croat. Chem. Acta 1980, 53, 133–146. [Google Scholar] [CrossRef]
- Yang, L.; Wei, W.; Xie, J.; Tao, H.; Yang, P. Electrochemical studies of derivatized thiol self-assembled monolayers on gold electrode in the presence of surfactants. Anal. Sci. 2005, 21, 679–684. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bandyopadhyay, S.; Dey, A. Tuning the apparent formal potential of covalently attached ferrocene using SAM bearing ionizable COOH groups. Electrochim. Acta 2013, 108, 624–633. [Google Scholar] [CrossRef]
- Porter, M.D.; Bright, T.B.; Allara, D.L.; Chidsey, C.E.D. Spontaneously organized molecular assemblies. 4. Structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry. J. Am. Chem. Soc. 1987, 109, 3559–3568. [Google Scholar] [CrossRef]
- Miller, C.; Cuendet, P.; Gratzel, M. Adsorbed ω-hydroxy thiol monolayers on gold electrodes: Evidence for electron tunneling to redox species in solution. J. Phys. Chem. 1991, 95, 877–886. [Google Scholar] [CrossRef]
- Chidsey, C.E.D.; Loiacono, D.N. Chemical functionality in self-assembled monolayers: Structural and electrochemical properties. Langmuir 1990, 6, 682–691. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, G. Construction of ionic liquid self-assembled monolayer on gold electrode for hemoglobin modifying and peroxide biosensing. Int. J. Electrochem. Sci. 2012, 7, 12907–12921. [Google Scholar]
- Gupta, R.K.; Suresh, K.A. AFM studies on Langmuir-Blodgett films of cholesterol. Eur. Phys. J. 2004, E14, 35–42. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, C.; Xu, G.; Sun, R. Effects of concentration and surface pressure on MBP interaction with cholesterol in Langmuir Films. Hindawi Scanning 2017, 1542156. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Nava, R.D.; Martin-Mirones, J.M.; Vazquez-Martınez, E.A.; Roca, J.A.; Ruiz-Garcıa, J. Direct observations of phase changes in Langmuir films of cholesterol. Revista Mexicana de Física 2006, 52, 32–40. [Google Scholar]
- Chen, P.Y.; Nien, P.C.; Ho, K.C. Highly selective dopamine sensor based on an imprinted SAM/mediator gold electrode. Procedia Chem. 2009, 1, 285–288. [Google Scholar] [CrossRef]
- Rahman, M.M. Fabrication of L-lactate biosensor based on redox species mediated lactate oxidase using micro-device. Int. J. Biol. Med. Res. 2010, 1, 9–14. [Google Scholar]
- Kang, J.; Zhuo, L.; Lu, X.; Wang, X. Electrochemical behavior of dopamine at a quercetin-SAM-modified gold electrode and analytical application. J. Solid. State. Electrochem. 2005, 9, 114–120. [Google Scholar] [CrossRef]
- Wang, L.-H.; Zhang, Y.H. Electrochemical oxidation of L-selenomethionine and Se-methylseleno-l-cysteine at a thiol-compound-modified gold electrode: Its application in a flow-through voltammetric sensor. Sensors 2017, 17, 383. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.Y.; Wang, L.; Wang, H.J.; Huang, P.F.; Zhao, Y.Q.; Fan, S.D. Electrochemical behavior and determination of epinephrine at a mercaptoacetic acid self-assembled gold electrode. Microchim. Acta 2007, 156, 321–326. [Google Scholar] [CrossRef]
- Thioglycolic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/mercaptoacetic_acid (accessed on 19 August 2018).
- Xiao, X.; Li, H.; Wang, M.; Zhang, K.; Si, P. Examining the effects of self-assembled monolayers on nanoporous gold based amperometric glucose biosensors. Analyst 2014, 139, 488–494. [Google Scholar] [CrossRef]
- Imabayashi, S.I.; Iida, M.; Hobara, D.; Feng, Z.Q.; Niki, K.; Kakiuchi, T. Reductive desorption of carboxylic-acid-terminated alkanethiol monolayers from Au(111) surfaces. J. Electroanal. Chem. 1997, 428, 33–38. [Google Scholar] [CrossRef]
- Choi, S.; Chae, J. A regenerative biosensing surface in microfluidics using electrochemical desorption of short-chain self-assembled monolayer. Microfluid Nanofluid 2009, 7, 819. [Google Scholar] [CrossRef]
- Schilardi, P.L.; Dip, P.; dos Santos Claro, P.C.; Benítez, G.A.; Fonticelli, M.H.; Azzaroni, O.; Salvarezza, R.C. Electrochemical depositionto self-assembled monolayers: New insights into micro- and nanofabrication. Chem. Eur. J. 2006, 12, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Grzybek, M.; Kubiak, J.; Łach, A.; Przybyło, M.; Sikorski, A.F. A raft-associated species of phosphatidylethanolamine interacts with cholesterol comparably to sphingomyelin. A Langmuir-Blodgett Monolayer study. PLoS ONE 2009, 4, e5053. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Santaella, T.D.; Maldonado-Valderrama, J.; Faraudo, J.; Martín-Molina, A. Specific ion effects in cholesterol monolayers. Materials 2016, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Iglesias, F.J.; Solla-Gullon, J.; Herrero, E.; Feliu, J.M. Electrocatalysis in Fuel Cells; Shao, M., Ed.; Springer: London, UK; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands, 2013; Chapter 16; pp. 483–512. [Google Scholar] [CrossRef]
- Gotti, G.; Evrard, D.; Fajerwerg, K.; Gros, P. Oxygen reduction reaction features in neutral media on glassy carbon electrode functionalized by chemically prepared gold nanoparticles. J. Solid State Electrochem. 2016, 20, 1539–1550. [Google Scholar] [CrossRef]
- Rodriguez, P.; Koper, M.T.M. Electrocatalysis on gold. Phys. Chem. Chem. Phys. 2014, 16, 13583–13594. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.G.; Zajicek, J.; Kamat, P.V. Surface binding properties of etraoctylammonium bromide-capped gold nanoparticles. Langmuir 2002, 18, 3722–3727. [Google Scholar] [CrossRef]
- Leff, D.V.; Brandt, L.; Heath, J.R. Synthesis and characterization of hydrophobic, organically-soluble gold nanocrystals functionalized with primary amines. Langmuir 1996, 12, 4723–4730. [Google Scholar] [CrossRef]
- Selvakannan, P.R.; Mandal, S.; Phadtare, S.; Pasricha, R.; Sastry, M. Capping of gold nanoparticles by the amino acid lysine renders them water-dispersible. Langmuir 2003, 19, 3545–3549. [Google Scholar] [CrossRef]
- Anwar, A.; Khalid, S.; Perveen, S.; Ahmed, S.; Siddiqui, R.; Khan, N.A.; Shah, M.R. Synthesis of 4-(dimethylamino)pyridine propylthioacetate coated gold nanoparticles and their antibacterial and photophysical activity. J. Nanobiotechnol. 2018, 16, 6. [Google Scholar] [CrossRef]
- Đurović, M.D.; Puchta, R.; Bugarcic, Z.D.; Eldik, R. Studies on the reactions of [AuCl4]—with different nucleophiles in aqueous solution. Dalton Trans. 2014, 43, 8620–8632. [Google Scholar] [CrossRef]
- El-Batal, A.I.; ElKenawy, N.M.; Yassin, A.S.; Amin, M.A. Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnol. Rep. 2015, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Ma, X.; Tian, B.; Li, T.; Yu, J.; Dai, S.; Weng, Y.; Hua, Y. Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int. J. Nanomed. 2016, 11, 5931–5944. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanavicius, A.; Ramanaviciene, A. Amperometric glucose biosensor based on electrochemically deposited gold nanoparticles covered by polypyrrole. Electroanalysis 2017, 29, 1267–1277. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Kizling, M.; Dzwonek, M.; Olszewski, B.; Bącal, P.; Tymecki, Ł.; Więckowska, A.; Stolarczyk, K.; Bilewicz, R. Reticulated vitreous carbon as a scaffold for enzymatic fuel cell designing. Biosens. Bioelectron. 2017, 95, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Falk, M.; Ortiz, R.; Matsumura, H.; Bobacka, J.; Ludwig, R.; Bergelin, M.; Gorton, L.; Shleev, S. Mediatorless sugar/oxygen enzymatic fuel cells based on gold nanoparticle-modified electrodes. Biosens. Bioelectron. 2012, 31, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, A.; German, N.; Ramanaviciene, A. Evaluation of electron transfer in an electrochemical system based on immobilized gold nanoparticles and glucose oxidase. J. Electrochem. Soc. 2017, 164, G45–G49. [Google Scholar] [CrossRef]
- Stolarczyk, K.; Pałys, B.; Bilewicz, R. Catalytic properties of 4-hydroxythiophenol protected gold nanoclusters supported on gold electrodes. J. Electroanal. Chem. 2004, 564, 93–98. [Google Scholar] [CrossRef]

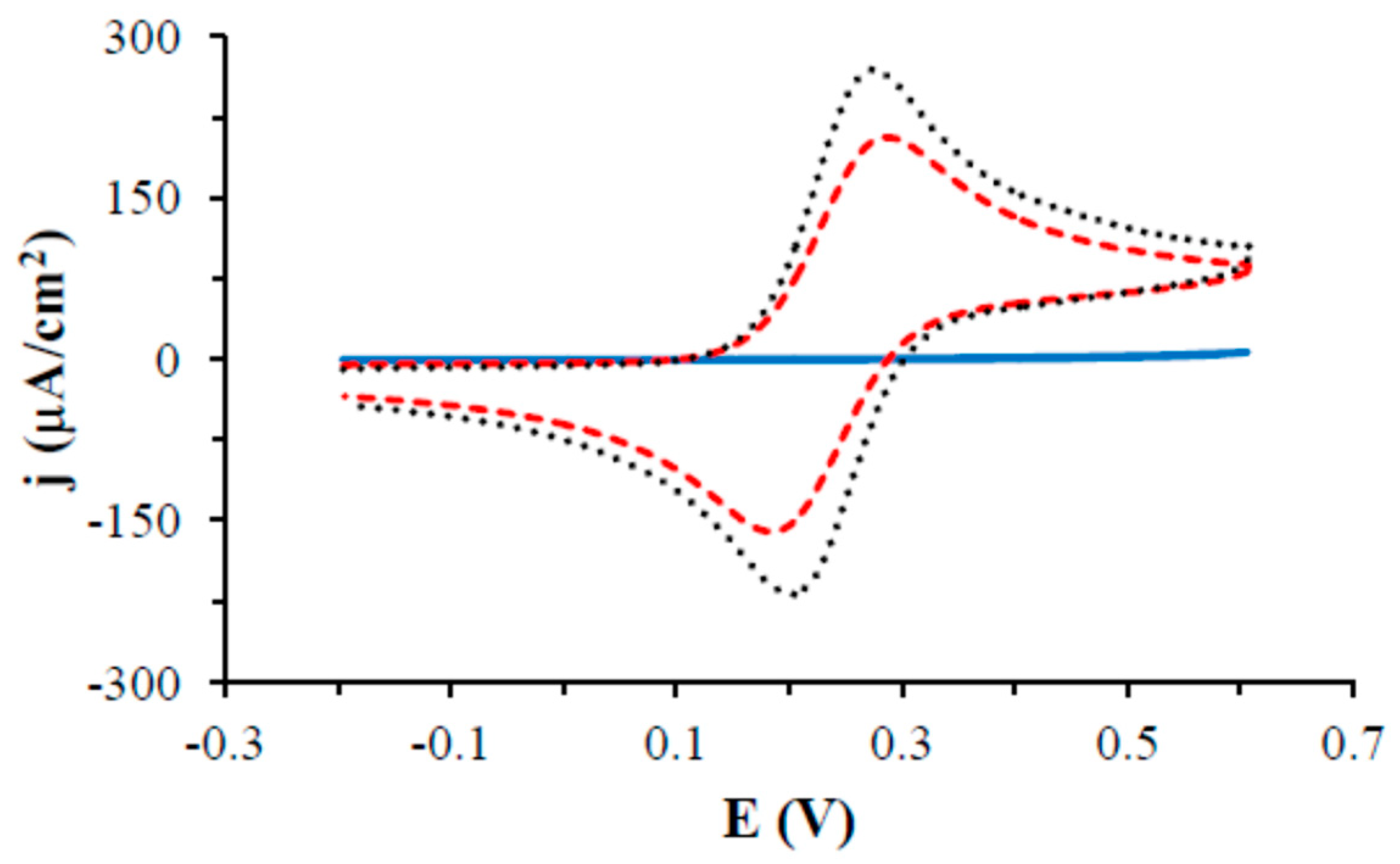

| Electrode | Ep,a [V] | Ip,a [µA] | Ep,c [V] | Ip,c [µA] | Ep,a − Ep,c [V] |
|---|---|---|---|---|---|
| Au HS-GA | 0.281 ± 0.001 | 125.5 ± 0.7 | 0.189 ± 0.003 | −100.5 ± 5.0 | 0.092 ± 0.004 |
| Au | 0.273 ± 0.003 | 163.0 ± 1.0 | 0.203 ±0.001 | −132.7 ± 2.2 | 0.070 ± 0.003 |
| Monolayer | Ed [V] | Q [μC/cm2] | Γ [nmol/cm2] | A [nm2] |
|---|---|---|---|---|
| HS-AB | −0.984 ± 0.008 | 33.39 ± 2.31 | 0.572 ± 0.040 | 0.291 ± 0.019 |
| HS-GA | −0.660 ± 0.023 −0.950 | 45.67 ± 2.73 | 0.782 ± 0.047 | 0.213 ± 0.013 |
| Electrode | E [V] | I [μA/cm2] |
|---|---|---|
| Au | −0.337 ± 0.004 | −164.2 ± 5.8 |
| Au/HS-GA | −0.353 ± 0.007 | −152.1 ± 3.3 |
| Au/HS-AB/AuNp | −0.308 ± 0.009 | −248.8 ± 10.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolarczyk, E.U.; Sidoryk, K.; Cybulski, M.; Kubiszewski, M.; Stolarczyk, K. Design of Therapeutic Self-Assembled Monolayers of Thiolated Abiraterone. Nanomaterials 2018, 8, 1018. https://doi.org/10.3390/nano8121018
Stolarczyk EU, Sidoryk K, Cybulski M, Kubiszewski M, Stolarczyk K. Design of Therapeutic Self-Assembled Monolayers of Thiolated Abiraterone. Nanomaterials. 2018; 8(12):1018. https://doi.org/10.3390/nano8121018
Chicago/Turabian StyleStolarczyk, Elżbieta U., Katarzyna Sidoryk, Marcin Cybulski, Marek Kubiszewski, and Krzysztof Stolarczyk. 2018. "Design of Therapeutic Self-Assembled Monolayers of Thiolated Abiraterone" Nanomaterials 8, no. 12: 1018. https://doi.org/10.3390/nano8121018
APA StyleStolarczyk, E. U., Sidoryk, K., Cybulski, M., Kubiszewski, M., & Stolarczyk, K. (2018). Design of Therapeutic Self-Assembled Monolayers of Thiolated Abiraterone. Nanomaterials, 8(12), 1018. https://doi.org/10.3390/nano8121018







