Construction of Lambda-Cyhalothrin Nano-Delivery System with a High Loading Content and Controlled-Release Property
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the LC Nano-Delivery System
2.3. Characterization
2.4. Determination of Loading and Encapsulation Efficiency
2.5. Controlled Release of LC
2.6. Stability Studies
3. Results and Discussion
3.1. Effects of Process Parameters on Size, Size Distribution, and Pesticide Loading Capacity of LC-Loaded Nanoparticles
3.1.1. Effects of Organic Solvents
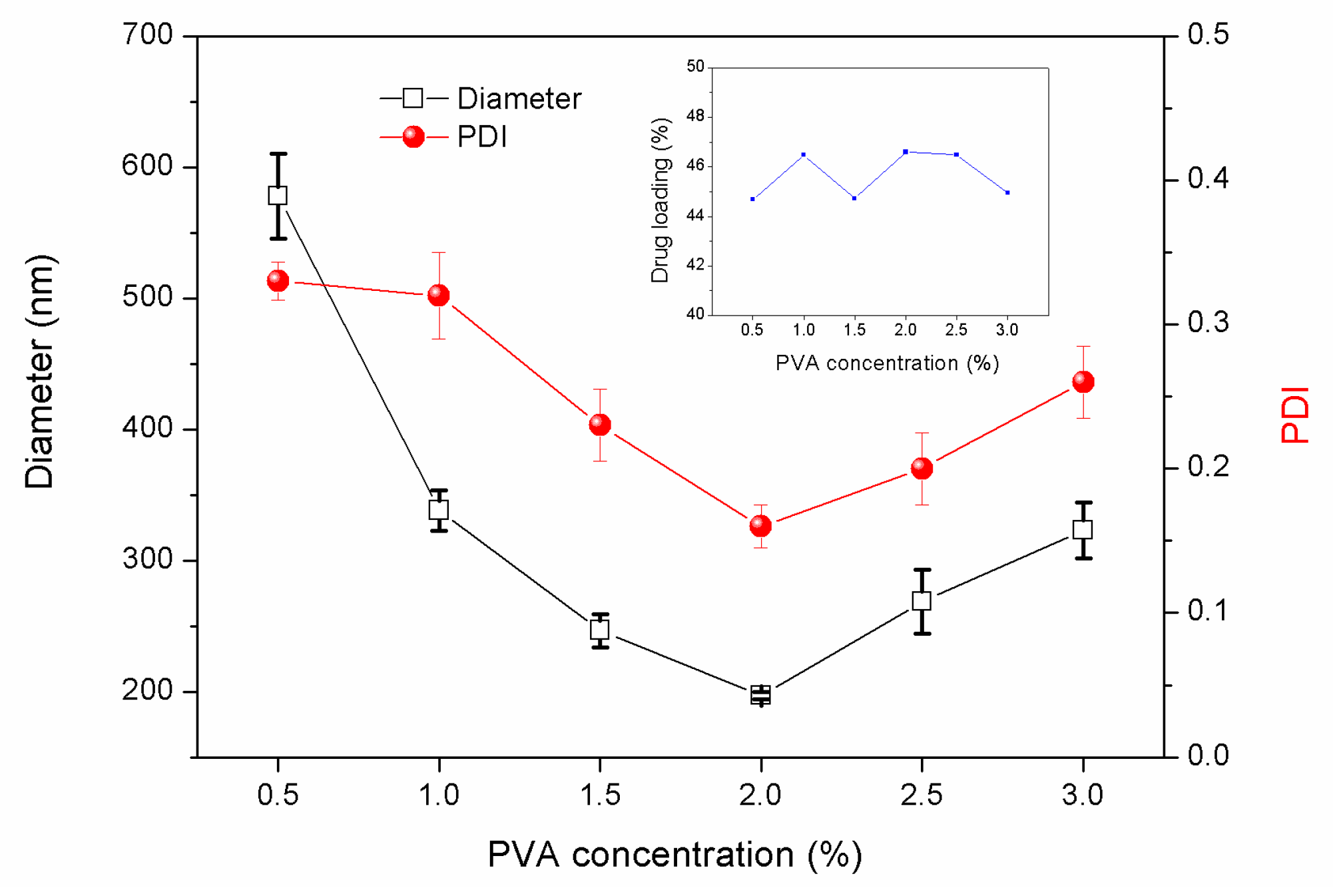
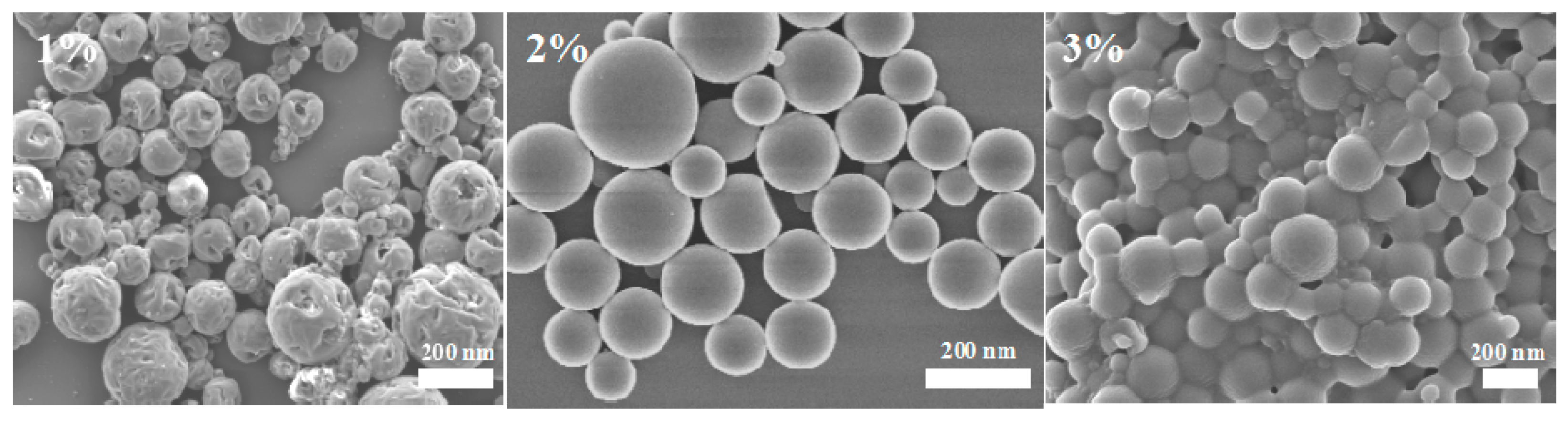
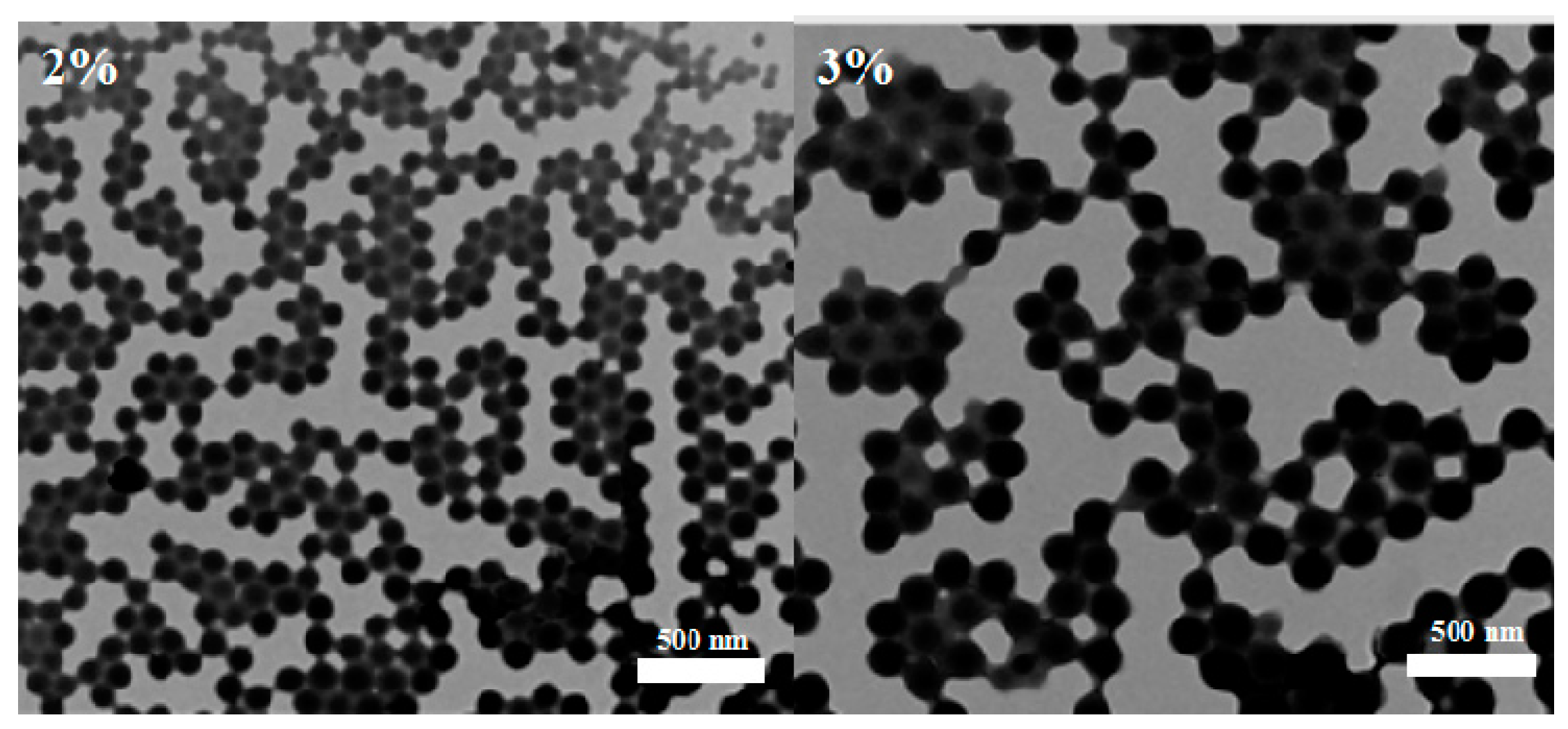
3.1.2. Effects of PVA Concentration in the External Water Phase
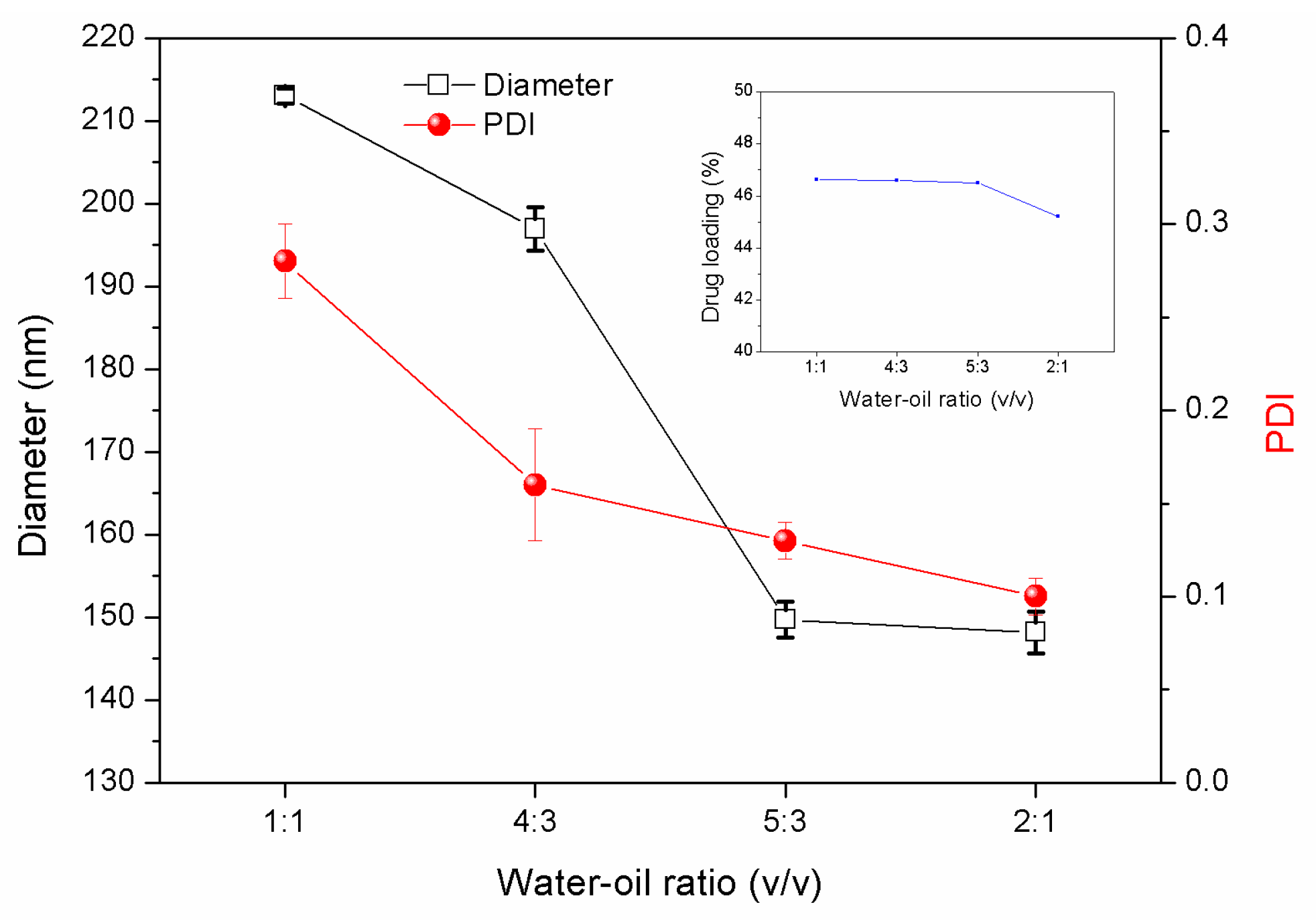
3.1.3. Effects of the Water/Oil Phase Ratio
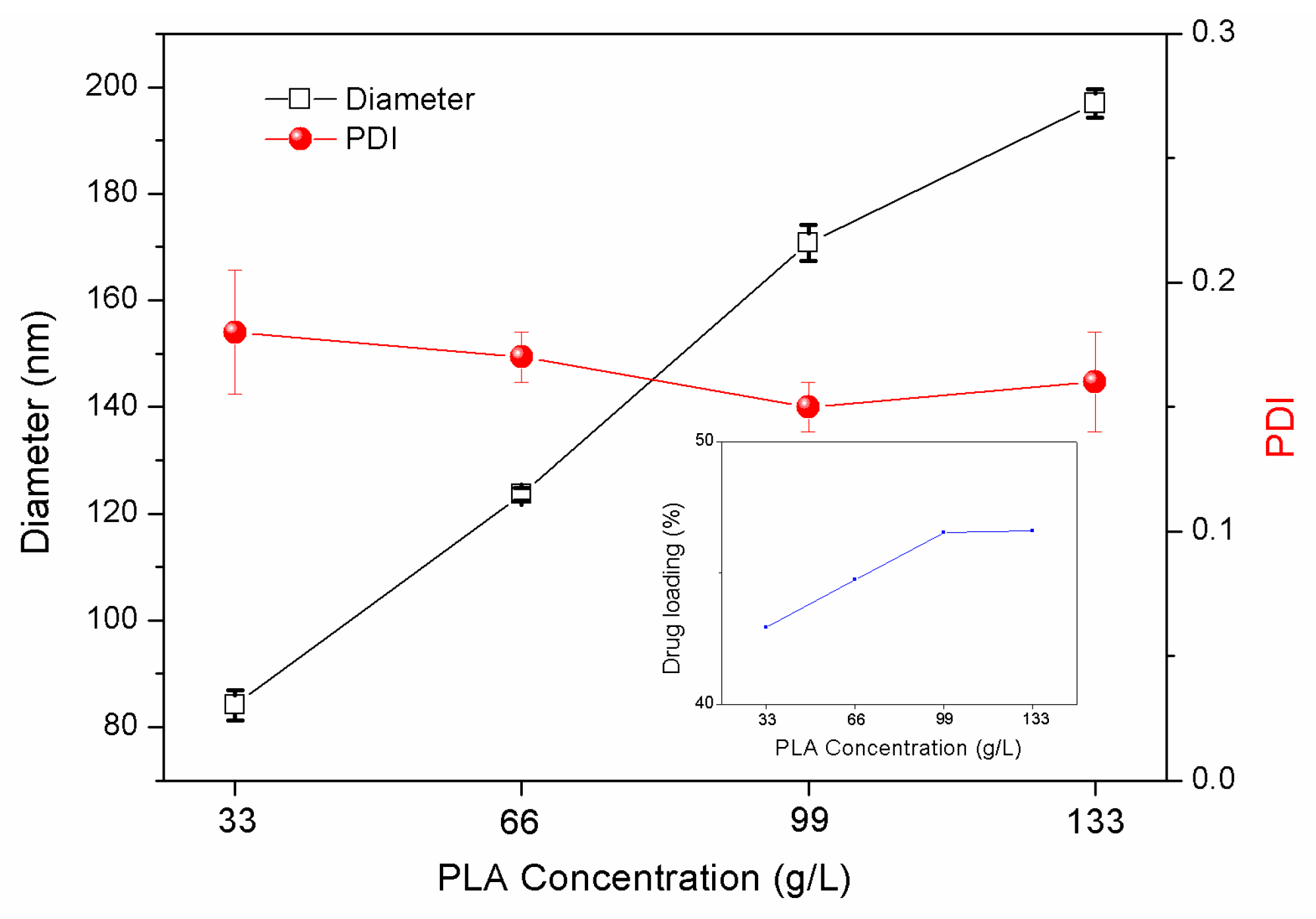
3.1.4. Effects of PLA Concentration
3.2. Preparation of LC-Loaded Nanoparticles with Different Sizes and Pesticide Contents
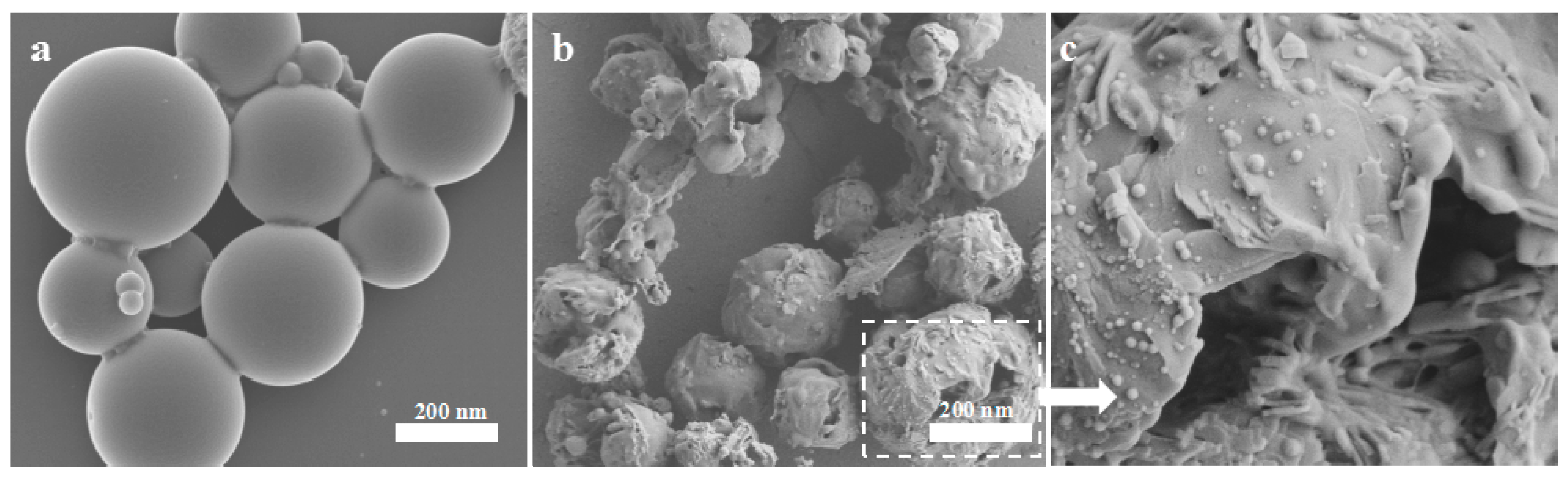
3.2.1. Effects of Shear and Ultrasonic Times
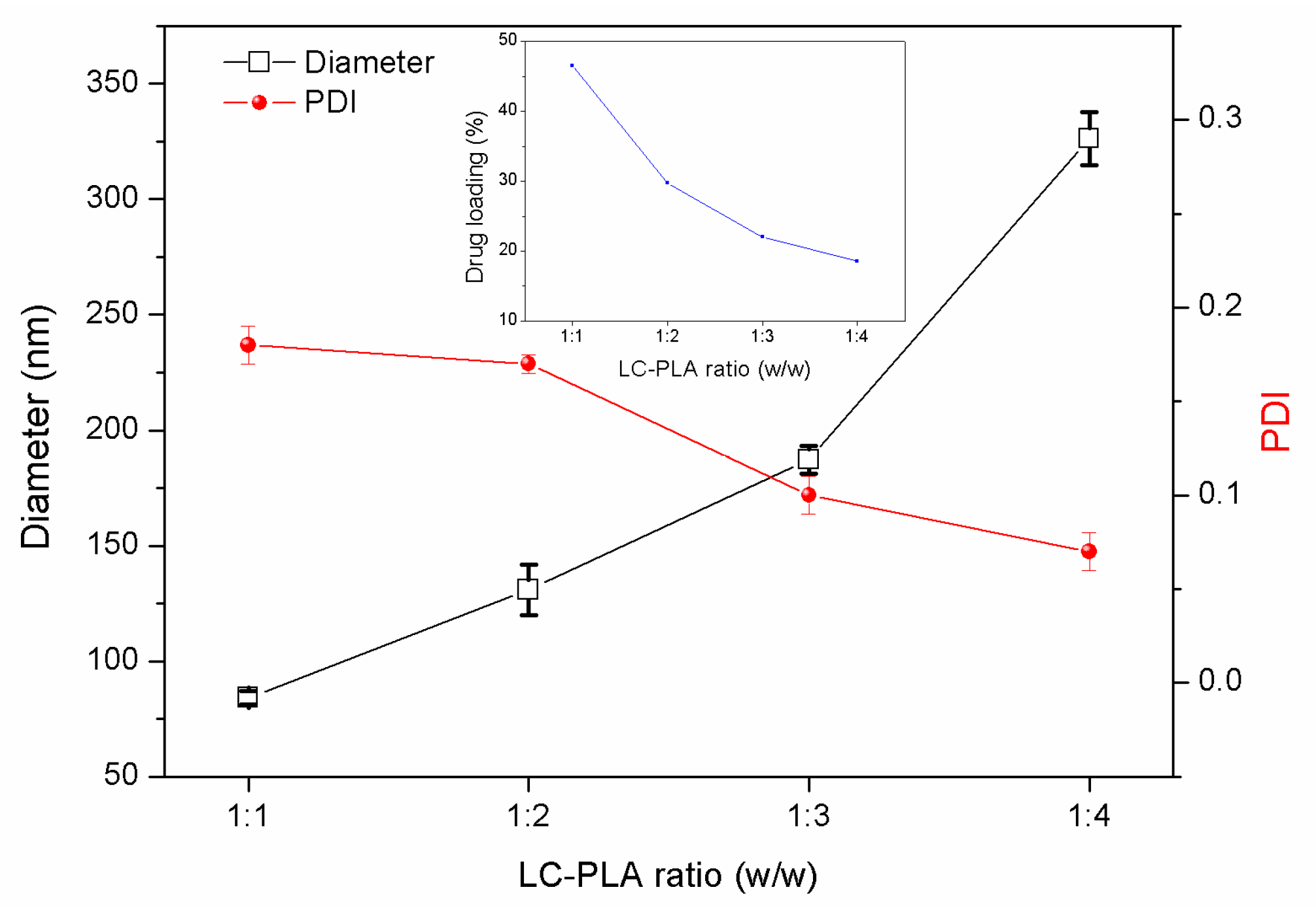
3.2.2. Effects of the PLA/LC Ratio
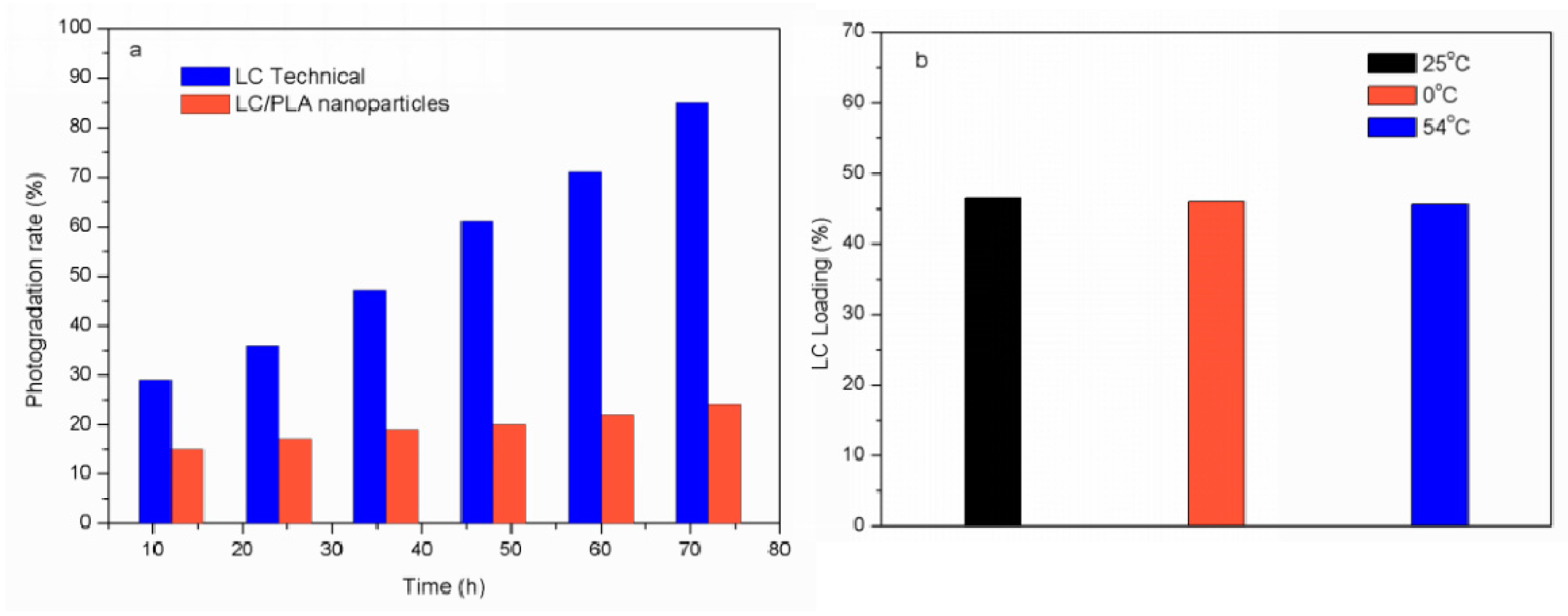
3.3. Controlled Release of LC
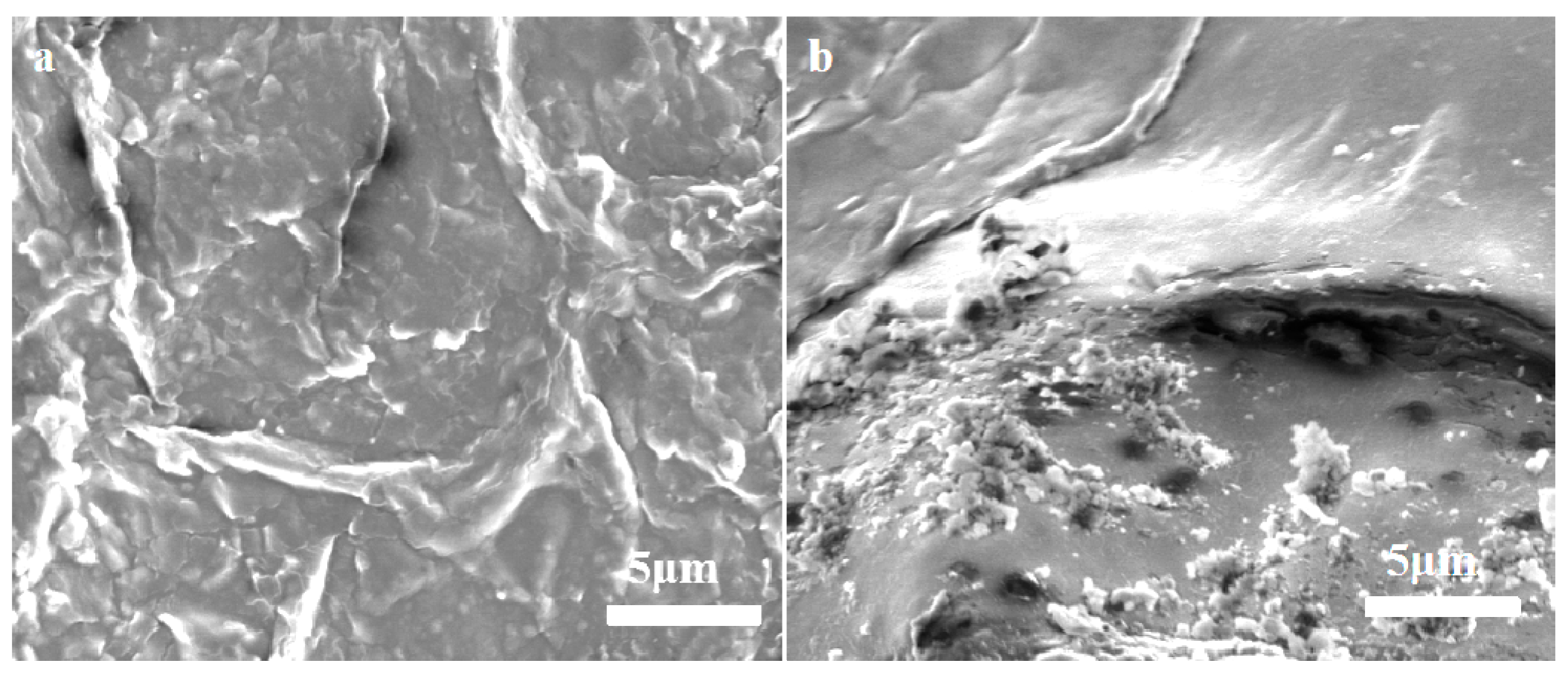
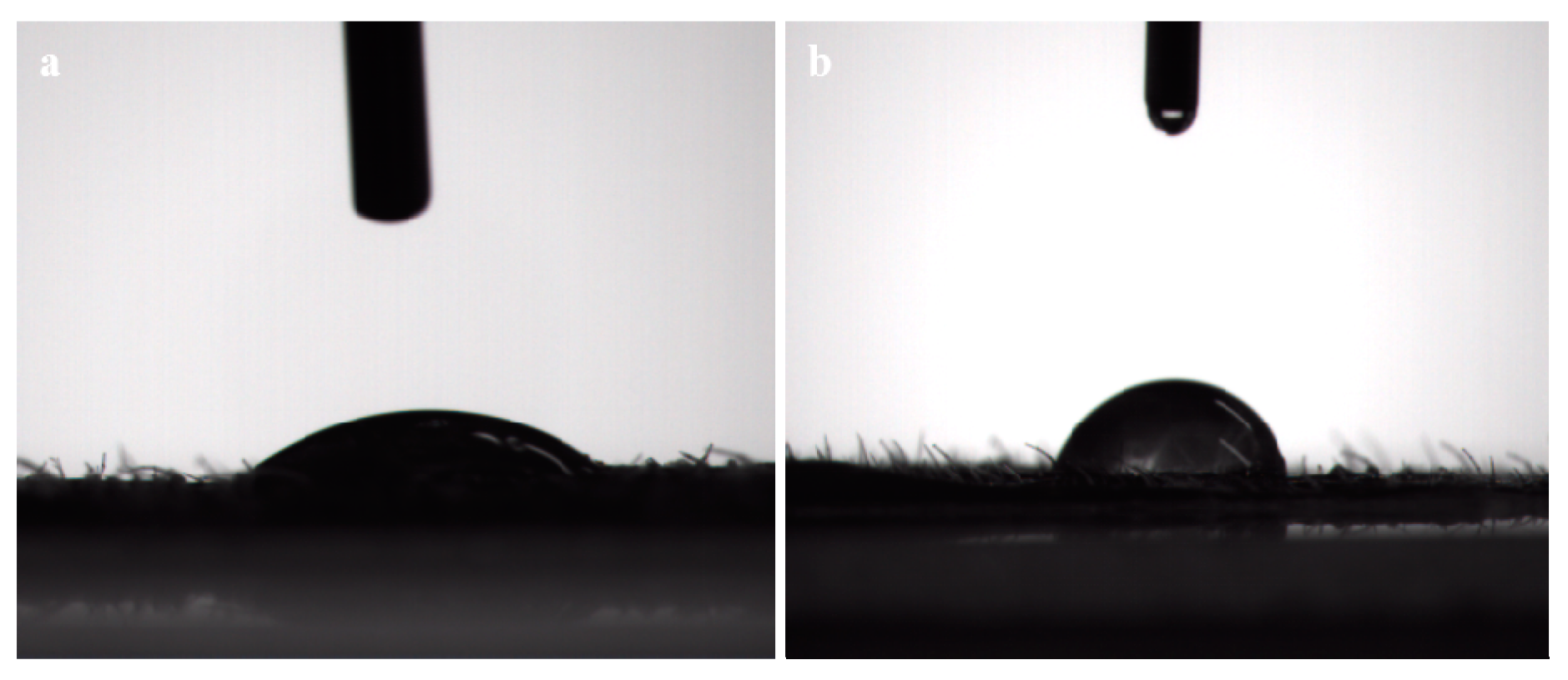
3.4. Foliage Adhesion of the LC/PLA Nano-Delivery System
3.5. Stability Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, X.; Cui, H.X.; Wang, Y.; Sun, C.J.; Cui, B.; Zeng, Z.H. Development strategies and prospects of nano-based smart pesticide formulation. J. Agric. Food Chem. 2018, 66, 6504–6512. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, M.A. FDA’s approach to regulation of products of nanotechnology. Science 2012, 336, 299–300. [Google Scholar] [CrossRef]
- Scott, N.R.; Chen, H.D.; Cui, H.X. Nanotechnology applications and implications of agrochemicals toward sustainable agriculture and food systems. J. Agric. Food Chem. 2018, 66, 6451–6456. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.N.; Chen, F.F.; Shen, Y.; Qian, K.; Wang, Y.; Sun, C.J.; Zhao, X.; Cui, B.; Gao, F.; Zeng, Z.H. Advances in targeted pesticides with environmentally responsive controlled release by nanotechnology. Nanomaterials 2018, 8, 102. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Y.; Zhao, X.; Sun, C.J.; Cui, B.; Gao, F.; Zeng, Z.H.; Cui, H.X. Preparation and physicochemical charateristics of thermo-responsive emamectin benzoate microcapsules. Polymers 2017, 9, 418. [Google Scholar] [CrossRef]
- Yu, M.L.; Yao, J.W.; Liang, J.; Zeng, Z.H.; Cui, B.; Zhao, X.; Sun, C.J.; Wang, Y.; Liu, G.Q.; Cui, H.X. Development of functionalized abamectin poly(lactic acid) nanoparticles with regulatable adhesion to enhance foliar retention. RSC Adv. 2017, 7, 11271–11280. [Google Scholar] [CrossRef]
- Kaziem, A.E.; Gao, Y.H.; Zhang, Y.; Qin, X.Y.; Xiao, Y.N.; Zhang, Y.H.; You, H.; Li, J.H.; He, S. α-amylase triggered carriers based on cyclodextrin anchored hollow mesoporous silica for enhancing insecticidal activity of avermectin against Plutella xylostella. J. Hazard. Mater. 2018, 359, 213–221. [Google Scholar] [CrossRef]
- Zhou, M.S.; Xiong, Z.C.; Yang, D.J.; Pang, Y.X.; Wang, D.P.; Qiu, X.Q. Preparation of slow release nanopesticide microspheres from benzoyl lignin. Holzforschung 2018, 72, 599–607. [Google Scholar] [CrossRef]
- Chen, K.; Yu, G.B.; He, F.R.; Zhou, Q.F.; Xiao, D.C.; Li, J.C.; Feng, Y.H. A pH-responsive emulsion stabilized by alginate-grafted anisotropic silica and its application in the controlled release of λ-Cyhalothrin. Carbohydr. Polym. 2017, 176, 203–213. [Google Scholar] [CrossRef]
- Luo, J.; Jing, T.F.; Zhang, D.X.; Zhang, X.P.; Li, B.X.; Liu, F. Two-stage controlled release system possesses excellent initial and long-term efficacy. Colloids Surf. B Biointerfaces 2018, 169, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Okada, H. One-and three-month release injectable microspheres of the LH-RH superagonist leuprorelin acetate. Adv. Drug Deliv. Rev. 1997, 28, 43–70. [Google Scholar] [CrossRef]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, P.; Sutherland, A.J.; Pal, K. Control of shape and size of poly (lactic acid) microspheres based on surfactant and polymer concentration. Mater. Lett. 2017, 195, 48–51. [Google Scholar] [CrossRef]
- Liu, B.X.; Wang, Y.; Yang, F.; Wang, X.; Shen, H.; Cui, H.X.; Wu, D.C. Construction of a controlled-release delivery system for pesticides using biodegradable PLA-based microcapsules. Colloids Surf. B Biointerfaces 2016, 144, 38–45. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, H.; Li, L.X.; Zhou, X.T.; Li, J.P.; Kan, C.Y. Preparation and properties of lambda-cyhalothrin/polyurethane drug-loaded nanoemulsions. RSC Adv. 2017, 7, 52684–52693. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, A.Q.; Wang, C.X.; Cui, B.; Sun, C.J.; Zhao, X.; Zeng, Z.H.; Shen, Y.; Gao, F.; Liu, G.Q. Synthesis and characterization of emamectin-benzoate slow-release microspheres with different surfactants. Sci. Rep. 2017, 7, 12761. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.K.; Usmani, M.; Ahammad, S.Z.; Saha, S. Unveiling the slow release behavior of hollow particles with prolonged antibacterial activity. J. Mater. Sci. 2018, 53, 5942–5957. [Google Scholar] [CrossRef]
- Chen, X.M.; Liu, Y.Y.; Wang, L.Y.; Liu, Y.; Zhang, W.F.; Fan, B.; Ma, X.W.; Yuan, Q.P.; Ma, G.H.; Su, Z.G. Enhanced humoral and cell-mediated immune responses generated by cationic polymer-coated PLA microspheres with adsorbed HBsAg. Mol. Pharm. 2014, 11, 1772–1784. [Google Scholar] [CrossRef]
- Murakami, H.; Kobayashi, M.; Takeuchi, H.; Kawashima, Y. Further application of a modified spontaneous emulsification solvent diffusion method to various types of PLGA and PLA polymers for preparation of nanoparticles. Powder Technol. 2000, 107, 137–143. [Google Scholar] [CrossRef]
- Falconi, M.; Focaroli, S.; Teti, G.; Salvatore, V.; Durante, S.; Nicolini, B.; Orienti, I. Novel PLA microspheres with hydrophilic and bioadhesive surfaces for the controlled delivery of fenretinide. J. Microencapsul. 2014, 31, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.; Merkle, H.P.; Gander, B. Microencapsulation by solvent extraction/evaporation: Reviewing the state of the art of microsphere preparation process technology. J. Control. Release 2005, 102, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Shelke, N.B.; Rokhade, A.P.; Aminabhavi, T.M. Preparation and evaluation of novel blend microspheres of poly(lactic-co-glycolic) acid and pluronic F68/127 for controlled release of repaglinide. J. Appl. Polym. Sci. 2010, 116, 366–372. [Google Scholar] [CrossRef]
- Schröder, V.; Schubert, H. Production of emulsions Using microporous ceramic membranes. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 103–109. [Google Scholar] [CrossRef]
- Zhang, S.F.; Chen, P.H.; Zhang, F.; Yang, Y.F.; Liu, D.K.; Wu, G. Preparation and physicochemical characteristics of polylactide microspheres of emamectin benzoate by modified solvent evaporation/extraction method. J. Agric. Food Chem. 2013, 61, 12219–12225. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.T.; Ma, G.H.; Qiu, W.; Su, Z.G. W/O/W double emulsion technique using ethyl acetate as organic solvent: Effects of its diffusion rate on the characteristics of microparticles. J. Control. Release 2003, 91, 407–416. [Google Scholar] [CrossRef]
- Li, D.; Liu, B.X.; Yang, F.; Wang, X.; Shen, H.; Wu, D.C. Preparation of uniform starch microcapsules by premix membrane emulsion for controlled release of avermectin. Carbohyd. Polym. 2016, 136, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Massinon, M.; De Cock, N.; Forster, W.A.; Nairn, J.J.; McCue, S.W.; Zabkiewicz, J.A.; Lebeau, F. Spray droplet impaction outcomes for different plant species and spray formulations. Crop Prot. 2017, 99, 65–75. [Google Scholar] [CrossRef]
- He, Y.J.; Zhao, B.B.; Yu, Y.C. Effect, comparison and analysis of pesticide electrostatic spraying and traditional spraying. Bulg. Chem. Commun. 2016, 48, 340–344. [Google Scholar]

| Entry | Shear Time (min) | Ultrasonic Time (min) | Mean Diameter (nm) | PDI | LC Loading Capacity (%) |
|---|---|---|---|---|---|
| 1 | 5 | 10 | 270.33 ± 5.13 | 0.17 ± 0.02 | 45.08 |
| 2 | 10 | 10 | 196.93 ± 2.66 | 0.16 ± 0.015 | 46.6 |
| 3 | 10 | 15 | 190.52 ± 3.74 | 0.10 ± 0.019 | 49.4 |
| 4 | 15 | 10 | 205 ± 1.39 | 0.11 ± 0.023 | 48.21 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Zhu, H.; Cui, J.; Wang, A.; Zhao, X.; Cui, B.; Wang, Y.; Cui, H. Construction of Lambda-Cyhalothrin Nano-Delivery System with a High Loading Content and Controlled-Release Property. Nanomaterials 2018, 8, 1016. https://doi.org/10.3390/nano8121016
Shen Y, Zhu H, Cui J, Wang A, Zhao X, Cui B, Wang Y, Cui H. Construction of Lambda-Cyhalothrin Nano-Delivery System with a High Loading Content and Controlled-Release Property. Nanomaterials. 2018; 8(12):1016. https://doi.org/10.3390/nano8121016
Chicago/Turabian StyleShen, Yue, Huaxin Zhu, Jianxia Cui, Anqi Wang, Xiang Zhao, Bo Cui, Yan Wang, and Haixin Cui. 2018. "Construction of Lambda-Cyhalothrin Nano-Delivery System with a High Loading Content and Controlled-Release Property" Nanomaterials 8, no. 12: 1016. https://doi.org/10.3390/nano8121016
APA StyleShen, Y., Zhu, H., Cui, J., Wang, A., Zhao, X., Cui, B., Wang, Y., & Cui, H. (2018). Construction of Lambda-Cyhalothrin Nano-Delivery System with a High Loading Content and Controlled-Release Property. Nanomaterials, 8(12), 1016. https://doi.org/10.3390/nano8121016







