Novel Weed-Extracted Silver Nanoparticles and Their Antibacterial Appraisal against a Rare Bacterium from River and Sewage Treatment Plan
Abstract
:1. Introduction
2. Results and Discussion
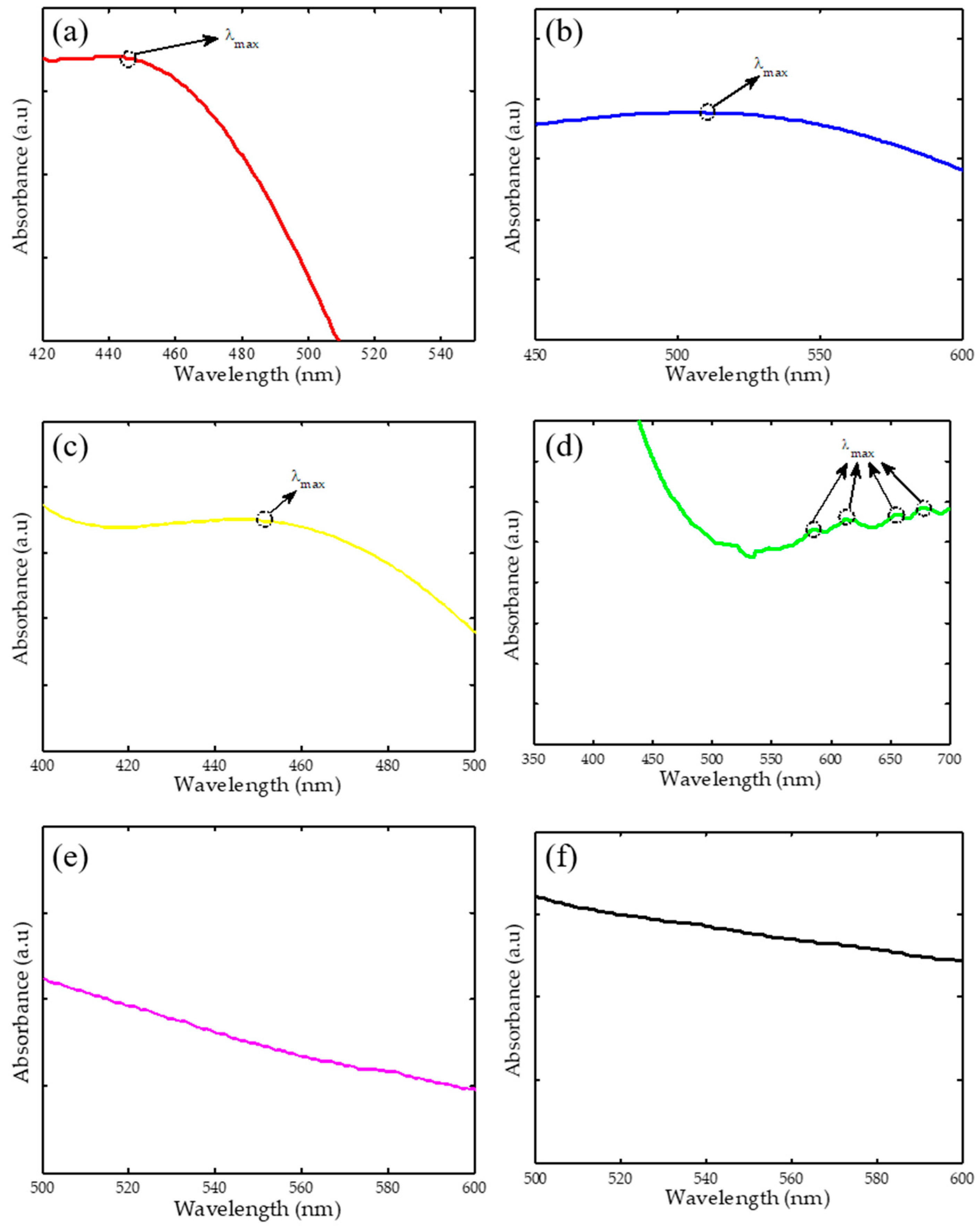
2.1. Ultraviolet-Visible Spectroscopy (UV-Vis)
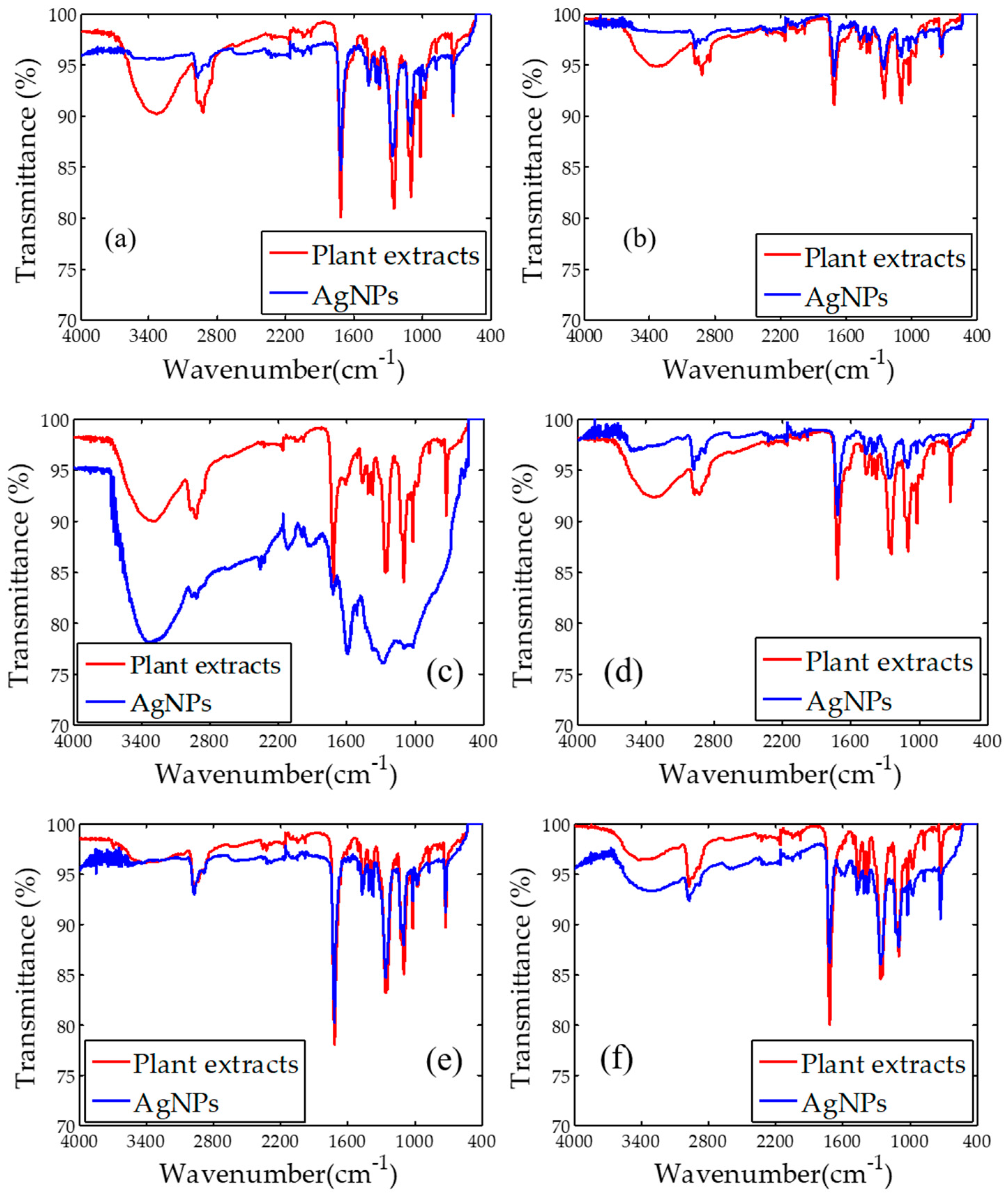
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Field Emission Scanning Electron Microscopy (FESEM)
2.4. Energy-Dispersive X-ray Spectroscopy EDX
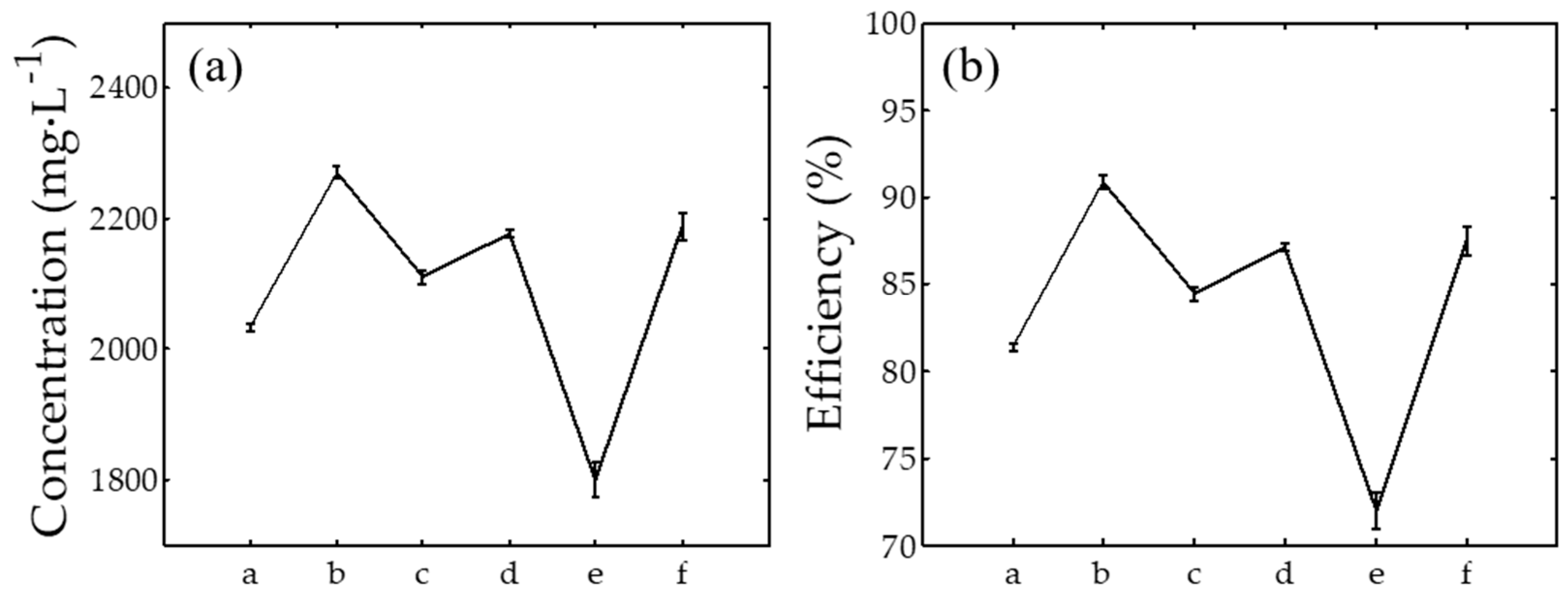
2.5. Inductively Coupled Plasma Atomic Emission Spectroscopy (ICPOES)
2.6. Antibacterial Appraisal
3. Materials and Methods
3.1. Materials
3.2. Procedure of Plant Extraction
3.3. Synthesis of AgNPs
3.4. Water Sampling Location
3.5. Bacteria Identification
3.6. Antibacterial Appraisal
3.7. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Syafiuddin, A.; Salmiati; Salim, M.R.; Kueh, A.B.H.; Hadibarata, T.; Nur, H. A review of silver nanoparticles: Research trends, global consumption, synthesis, properties and future challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Desireddy, A.; Conn, B.E.; Guo, J.; Yoon, B.; Barnett, R.N.; Monahan, B.M.; Kirschbaum, K.; Griffith, W.P.; Whetten, R.L.; Landman, U. Ultrastable silver nanoparticles. Nature 2013, 501, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, Q.; Fan, W.; Qiu, X. Boron nitride encapsulated copper nanoparticles: A facile one-step synthesis and their effect on thermal decomposition of ammonium perchlorate. Sci. Rep. 2015, 5, 16736. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Kuznetsov, A.I.; Miroshnichenko, A.E.; Yu, Y.F.; Luk’yanchuk, B. Directional visible light scattering by silicon nanoparticles. Nat. Commun. 2013, 4, 1527. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Abdelmonem, A.M.; Ali, Z.; Alves, F.; Geiser, M.; Haberl, N.; Hartmann, R.; Hirn, S.; de Aberasturi, D.J.; Kantner, K. In vivo integrity of polymer-coated gold nanoparticles. Nat. Nanotechnol. 2015, 10, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Syafiuddin, A.; Salmiati; Hadibarata, T.; Salim, M.R.; Kueh, A.B.H.; Sari, A.A. A purely green synthesis of silver nanoparticles using Carica papaya, Manihot esculenta and Morinda citrifolia: Synthesis and antibacterial evaluations. Bioprocess Biosyst. Eng. 2017, 40, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Abalkhil, T.A.; Alharbi, S.A.; Salmen, S.H.; Wainwright, M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol. Biotechnol. Equip. 2017, 31, 411–417. [Google Scholar] [CrossRef]
- Mie, R.; Samsudin, M.W.; Din, L.B.; Ahmad, A.; Ibrahim, N.; Adnan, S.N.A. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 2014, 9, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Singh, H.; Wang, C.; Hwang, K.H.; Farh, M.E.-A.; Yang, D.C. Biosynthesis, characterization and antimicrobial applications of silver nanoparticles. Int. J. Nanomed. 2015, 10, 2567–2577. [Google Scholar]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Meng, F.B.; Wang, L.; Xu, H.; Liu, C.C.; Hu, P.C.; Lan, W.; Song, J.X.; Chen, L.S. Biosynthesis of silver nanoparticles using oriental medicinal herb Gynostemma pentaphyllum Makino extract and their antibacterial activity against aquatic pathogen. Mater. Technol. 2016, 31, 181–186. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomedicine 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Dhar, P.; Dhar, D.G.; Rawat, A.K.S.; Srivastava, S. Medicinal chemistry and biological potential of Cyperus rotundus Linn.: An overview to discover elite chemotype(s) for industrial use. Ind. Crops Prod. 2017, 108, 232–247. [Google Scholar] [CrossRef]
- Akomas, S.; Ijioma, S.; Emelike, C. Effect of euphorbia hirta on haematological and biochemical indices in albino rats. Appl. J. Hyg. 2015, 4, 1–5. [Google Scholar]
- Trujillo, E.E.; Latterell, F.; Rossi, A. Colletotrichum gloeosporioides, a possible biological control agent for Clidemia hirta in Hawaiian forests. Plant Dis. 1986, 70, 974–976. [Google Scholar] [CrossRef]
- Catteau, L.; Lautié, E.; Koné, O.; Coppée, M.; Hell, K.; Pomalegni, C.B.; Quetin-Leclercq, J. Degradation of rotenone in yam bean seeds (Pachyrhizus sp.) through food processing. J. Agric. Food Chem. 2013, 61, 11173–11179. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Gnanaraj, C. Eleusine indica L. possesses antioxidant activity and precludes carbon tetrachloride (CCl4)-mediated oxidative hepatic damage in rats. Environ. Health Prev. Med. 2012, 17, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G.; Razon, L.F.; Madulid, D.A.; Agoo, E.M.G.; de Castro, M.E.G. Methyl esters (biodiesel) from Pachyrhizus erosus seed oil. Biofuels 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Kumar, S.; Malhotra, R.; Kumar, D. Antidiabetic and free radicals scavenging potential of Euphorbia hirta flower extract. Indian J. Pharm. Sci. 2010, 72, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ahmed, D.; Gupta, P.S.; Anwar, F.; Mujeeb, M. Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of Melastoma malabathricum Linn. leaves in streptozotocin induced diabetic rats. BMC Complement. Altern. Med. 2013, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- Lopez, T.; Corbin, C.; Falguieres, A.; Doussot, J.; Montguillon, J.; Hagège, D.; Hano, C.; Lainé, É. Secondary metabolite accumulation, antibacterial and antioxidant properties of in vitro propagated Clidemia hirta L. extracts are influenced by the basal culture medium. C. R. Chim. 2016, 19, 1071–1076. [Google Scholar] [CrossRef]
- Morales-Arellano, G.Y.; Chagolla-López, A.; Paredes-López, O.; Barba de la Rosa, A.P. Characterization of yam bean (Pachyrhizus erosus) proteins. J. Agric. Food Chem. 2001, 49, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Susanti, D.; Sirat, H.M.; Ahmad, F.; Ali, R.M.; Aimi, N.; Kitajima, M. Antioxidant and cytotoxic flavonoids from the flowers of Melastoma malabathricum L. Food Chem. 2007, 103, 710–716. [Google Scholar] [CrossRef]
- Singh, A.K.; Talat, M.; Singh, D.; Srivastava, O. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanopart. Res. 2010, 12, 1667–1675. [Google Scholar] [CrossRef]
- Makarov, V.; Love, A.; Sinitsyna, O.; Makarova, S.; Yaminsky, I.; Taliansky, M.; Kalinina, N. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar]
- Ahmad, N.; Sharma, S.; Alam, M.K.; Singh, V.N.; Shamsi, S.F.; Mehta, B.R.; Fatma, A. Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf. B Biointerfaces 2010, 81, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Han, F.S.; Segal, J. Chromobacterium Haemolyticum sp. nov., a strongly haemolytic species. Int. J. Syst. Evol. Microbiol. 2008, 58, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: Kinetic control and catalytic properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Gómez-Graña, S.; Perez-Ameneiro, M.; Vecino, X.; Pastoriza-Santos, I.; Perez-Juste, J.; Cruz, J.; Moldes, A. Biogenic synthesis of metal nanoparticles using a biosurfactant extracted from corn and their antimicrobial properties. Nanomaterials 2017, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Mat Don, M. Biosynthesis and structural characterization of Ag nanoparticles from white rot fungi. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, S.; Bhatt, D.; Sreedhar, B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Zong, R.; Wang, X.; Shi, S.; Zhu, Y. Kinetically controlled seed-mediated growth of narrow dispersed silver nanoparticles up to 120 nm: Secondary nucleation, size focusing and Ostwald ripening. Phys. Chem. Chem. Phys. 2014, 16, 4236–4241. [Google Scholar] [CrossRef] [PubMed]
- Shervani, Z.; Ikushima, Y.; Sato, M.; Kawanami, H.; Hakuta, Y.; Yokoyama, T.; Nagase, T.; Kuneida, H.; Aramaki, K. Morphology and size-controlled synthesis of silver nanoparticles in aqueous surfactant polymer solutions. Colloid Polym. Sci. 2007, 286, 403–410. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Tan, K.S.; Cheong, K.Y. Advances of Ag, Cu and Ag–Cu alloy nanoparticles synthesized via chemical reduction route. J. Nanopart. Res. 2013, 15, 1537. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Xie, A.; Yu, X.; Qiu, L.; Zhang, L.; Zhang, Q. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Kalathil, S.; Lee, J.; Cho, M.H. Electrochemically active biofilm-mediated synthesis of silver nanoparticles in water. Green Chem. 2011, 13, 1482–1485. [Google Scholar] [CrossRef]
- Helmlinger, J.; Heise, M.; Heggen, M.; Ruck, M.; Epple, M. A rapid, high-yield and large-scale synthesis of uniform spherical silver nanoparticles by a microwave-assisted polyol process. RSC Adv. 2015, 5, 92144–92150. [Google Scholar] [CrossRef]
- Yin, H.; Yamamoto, T.; Wada, Y.; Yanagida, S. Large-scale and size-controlled synthesis of silver nanoparticles under microwave irradiation. Mater. Chem. Phys. 2004, 83, 66–70. [Google Scholar] [CrossRef]
- Durán, N.; Menck, C.F.M. Chromobacterium violaceum: A review of pharmacological and industiral perspectives. Crit. Rev. Microbiol. 2001, 27, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [PubMed]
- Hsueh, Y.-H.; Lin, K.-S.; Ke, W.-J.; Hsieh, C.-T.; Chiang, C.-L.; Tzou, D.-Y.; Liu, S.-T. The antimicrobial properties of silver nanoparticles in Bacillus subtilis are mediated by Released Ag+ Ions. PLoS ONE 2015, 10, e0144306. [Google Scholar] [CrossRef] [PubMed]







| Leaf Extract | Biochemical Content | References |
|---|---|---|
| Cyperus rotundus | Essential oils, alkaloids, flavonoids, terpenoids, chromones, phenylpropanoids, phenolic acids and iridoides | [15] |
| Eleusin indica | Flavonoids, terpenoids, phenols, phenolic glycosides, saponins, cyanogenic glycosides, unsaturated lactones, glucosinolates and C-glycosylflavones | [19] |
| Euphorbia hirta | Alkaloids, saponins, terpenoids, flavonoids, tannins phenolic acids and amino acids | [21] |
| Melastoma malabathricum | Naringenin, terpenoids, kaempferol, flavonoids, kaempferol-3-O-d-glucoside and ethyl acetate | [22,25] |
| Clidemia hirta | Flavonoids, terpenoids, phenolics and saponins | [23] |
| Pachyrhizus erosus | Rotenone, cysteine protease, albumins, globulins, prolamins and glutelins. | [18,20,24] |
| Plants Name | Plant Extracts (cm−1) | AgNPs (cm−1) | Possible Compound |
|---|---|---|---|
| Cyperus rotundus | 3363 | - | –OH |
| 2976 | 2976 | –CH | |
| 1715 | 1715 | C=O | |
| 1248 | 1262 | C–O–C | |
| 1099 | 1099 | P–O | |
| 726 | 726 | N–H | |
| Eleusin indica | 3339 | - | –OH |
| 2919 | - | –CH | |
| 1709 | 1709 | C=O | |
| 1250 | 1250 | C–O–C | |
| 1021 | 1021 | P–O | |
| 726 | 721 | N–H | |
| Euphorbia hirta | 3312 | 3340 | –OH |
| 2921 | 2921 | –CH | |
| 1715 | 1715 | C=O | |
| 1100 | 1100 | P–O | |
| 726 | - | N–H | |
| Melastoma malabathricum | 3321 | - | –OH |
| 2925 | 2941 | –CH | |
| 1714 | 1714 | C=O | |
| 1246 | 1250 | C–O–C | |
| 1018 | 1020 | P–O | |
| 724 | 724 | N–H | |
| Clidemia hirta | 2973 | 2973 | –CH |
| 1716 | 1716 | C=O | |
| 1266 | 1266 | C–O–C | |
| 1018 | 1018 | P–O | |
| 726 | 726 | N–H | |
| Pachyrhizus erosus | 2969 | 2969 | –CH |
| 1714 | 1714 | C=O | |
| 1266 | 1266 | C–O–C | |
| 1019 | 1019 | P–O | |
| 725 | 725 | N–H |
| Plant Extracts | Shape | Size (nm) |
|---|---|---|
| Cyperus rotundus | Spherical | 20.5 ± 9.6 |
| Eleusin indica | Spherical | 55.0 ± 24.1 |
| Euphorbia hirta | Irregular | 56.25 ± 21.8 |
| Melastoma malabathricum | Irregular | 108.35 ± 36.3 |
| Clidemia hirta | Irregular | 57.4 ± 24.2 |
| Pachyrhizus erosus | Spherical | 40.6 ± 10.8 |
| Plant | Bacteria | AgNPs (mm) | AgNO3 (mm) | Water (mm) | Plant Extracts (mm) |
|---|---|---|---|---|---|
| Cyperus rotundus | E. coli | 15.00 ± 0.36 | 1.07 ± 0.06 | - | - |
| B. cereus | 10.00 ± 0.26 | 0.93 ± 0.12 | - | - | |
| C. haemolyticum UDIN2 | 8.00 ± 0.26 | 1.00 ± 0.00 | - | - | |
| C. haemolyticum UDIN3 | 10.30 ± 0.23 | 1.19 ± 0.25 | - | - | |
| C. haemolyticum UDIN1 | 9.00 ± 0.25 | 1.00 ± 0.17 | - | - | |
| Eleusin indica | E. coli | 12.30 ± 0.06 | - | - | |
| B. cereus | 9.70 ± 0.30 | - | - | ||
| C. haemolyticum UDIN2 | 8.70 ± 0.06 | - | - | ||
| C. haemolyticum UDIN3 | 9.30 ± 0.15 | - | - | ||
| C. haemolyticum UDIN1 | 12.00 ± 0.17 | - | - | ||
| Euphorbia hirta | E. coli | 12.30 ± 0.50 | - | - | |
| B. cereus | 9.00 ± 0.17 | - | - | ||
| C. haemolyticum UDIN2 | 9.00 ± 0.11 | - | - | ||
| C. haemolyticum UDIN3 | 13.30 ± 0.30 | - | - | ||
| C. haemolyticum UDIN1 | 12.00 ± 0.17 | - | - | ||
| Melastoma malabathricum | E. coli | 1.60 ± 0.10 | - | - | |
| B. cereus | 1.40 ± 0.17 | - | - | ||
| C. haemolyticum UDIN2 | 1.23 ± 0.31 | - | 0.27 ± 0.46 | ||
| C. haemolyticum UDIN3 | 1.20 ± 0.17 | - | 0.40 ± 0.32 | ||
| C. haemolyticum UDIN1 | 1.17 ± 0.50 | - | 0.53 ± 0.47 | ||
| Clidemia hirta | E. coli | 1.60 ± 0.10 | - | - | |
| B. cereus | 1.03 ± 0.06 | - | - | ||
| C. haemolyticum UDIN2 | 1.06 ± 0.06 | - | 0.87 ± 0.1 | ||
| C. haemolyticum UDIN3 | 1.27 ± 0.29 | - | 0.9 ± 0.17 | ||
| C. haemolyticum UDIN1 | 1.33 ± 0.38 | - | 0.8 ± 0.00 | ||
| Pachyrhizus erosus | E. coli | 11.00 ± 0.05 | - | - | |
| B. cereus | 11.00 ± 0.10 | - | - | ||
| C. haemolyticum UDIN2 | 15.00 ± 0.10 | - | - | ||
| C. haemolyticum UDIN3 | 9.00 ± 0.10 | - | - | ||
| C. haemolyticum UDIN1 | 15.00 ± 0.10 | - | - |
| Species | Bacteria Name | Shape | Size (nm) | Inhibition Zone (mm) | References |
|---|---|---|---|---|---|
| Gram-negative | Proteus vulgaris | Cubic | 19 | 8 ± 0.0 | [9] |
| Serratia marcescens | 8 ± 0.8 | ||||
| Salmonella typhi | 8 ± 0.3 | ||||
| Vibrio parahaemolyticus | Spherical | 97 | 25 ± 1.0 | [10] | |
| Salmonella enterica | 12 ± 0.6 | ||||
| Vibrio anguillarum | Spherical and irregular | 7–31 and 7–19 | 11.2 ± 0.5 | [12] | |
| Vibrio alginolyticus | 10.8 ± 1.4 | ||||
| Aeromonas punctata | 10.5 ± 0.7 | ||||
| Vibrio parahaemolyticus | 11.0 ± 1.3 | ||||
| Escherichia coli | Spherical | 9 ± 2 | 9 ± 1.0 | [13] | |
| Pseudomonas aeruginosa | 9 ± 0.5 | ||||
| Gram-positive | Enterococcus faecalis | Spherical | 10–60 | 2.5–6.2 | [8] |
| Staphylococcus epidermidis | Cubic | 19 | 7.5 ± 0.0 | [9] | |
| Methicillin-resistant | 6 | ||||
| Bacillus subtilis | 6 | ||||
| Streptococcus faecalis | 6 | ||||
| Bacillus anthracis | Spherical | 97 | 15 ± 0.8 | [10] | |
| Staphylococcus aureus | Spherical | 20–30 | 7–21 | [11] | |
| Staphylococcus aureus | Spherical | 9 ± 2 | 11 ± 0.6 | [13] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syafiuddin, A.; Salmiati; Hadibarata, T.; Beng Hong Kueh, A.; Razman Salim, M. Novel Weed-Extracted Silver Nanoparticles and Their Antibacterial Appraisal against a Rare Bacterium from River and Sewage Treatment Plan. Nanomaterials 2018, 8, 9. https://doi.org/10.3390/nano8010009
Syafiuddin A, Salmiati, Hadibarata T, Beng Hong Kueh A, Razman Salim M. Novel Weed-Extracted Silver Nanoparticles and Their Antibacterial Appraisal against a Rare Bacterium from River and Sewage Treatment Plan. Nanomaterials. 2018; 8(1):9. https://doi.org/10.3390/nano8010009
Chicago/Turabian StyleSyafiuddin, Achmad, Salmiati, Tony Hadibarata, Ahmad Beng Hong Kueh, and Mohd Razman Salim. 2018. "Novel Weed-Extracted Silver Nanoparticles and Their Antibacterial Appraisal against a Rare Bacterium from River and Sewage Treatment Plan" Nanomaterials 8, no. 1: 9. https://doi.org/10.3390/nano8010009
APA StyleSyafiuddin, A., Salmiati, Hadibarata, T., Beng Hong Kueh, A., & Razman Salim, M. (2018). Novel Weed-Extracted Silver Nanoparticles and Their Antibacterial Appraisal against a Rare Bacterium from River and Sewage Treatment Plan. Nanomaterials, 8(1), 9. https://doi.org/10.3390/nano8010009