Prevention of Synaptic Alterations and Neurotoxic Effects of PAMAM Dendrimers by Surface Functionalization
Abstract
:1. Introduction
2. Results and Discussion
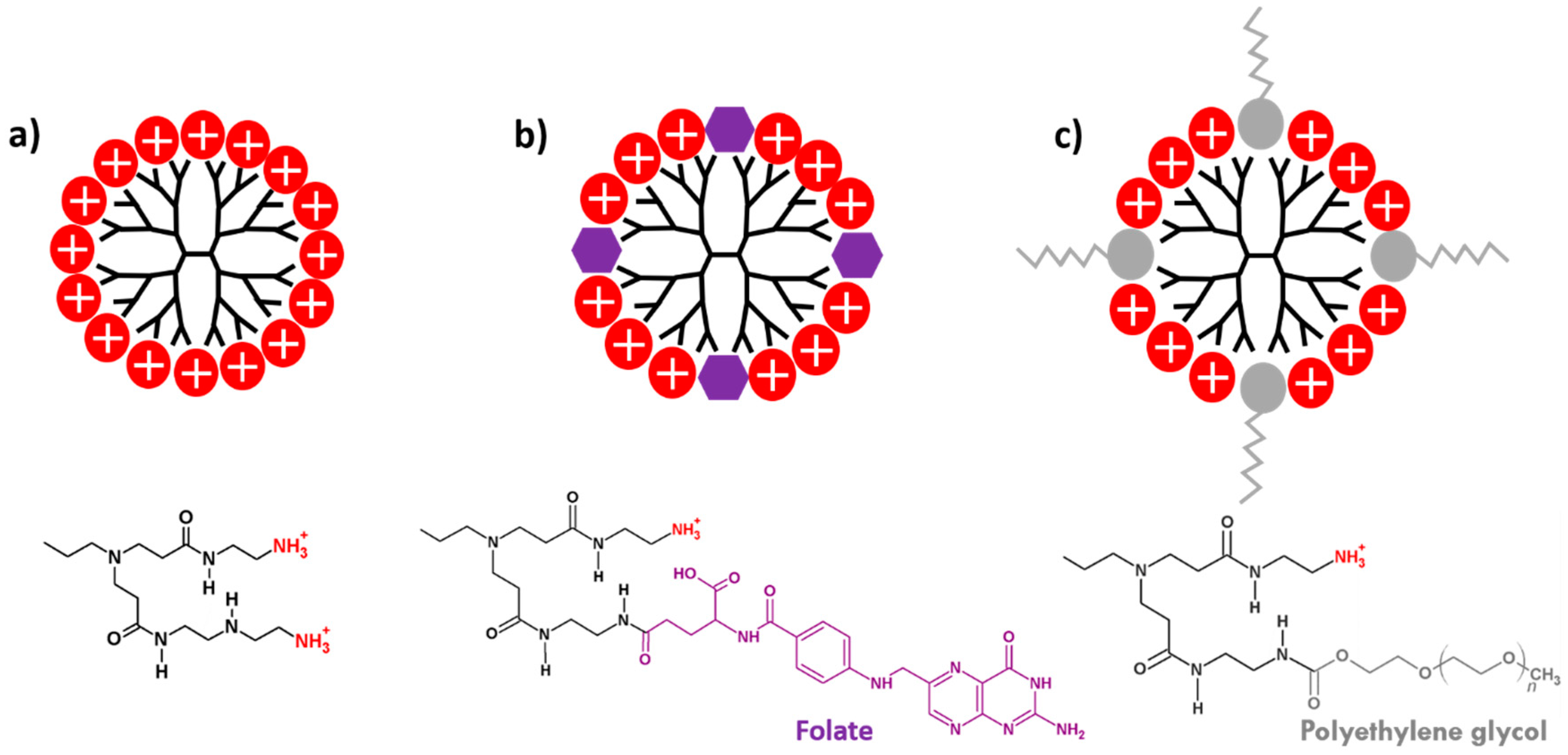
2.1. PAMAM Dendrimers
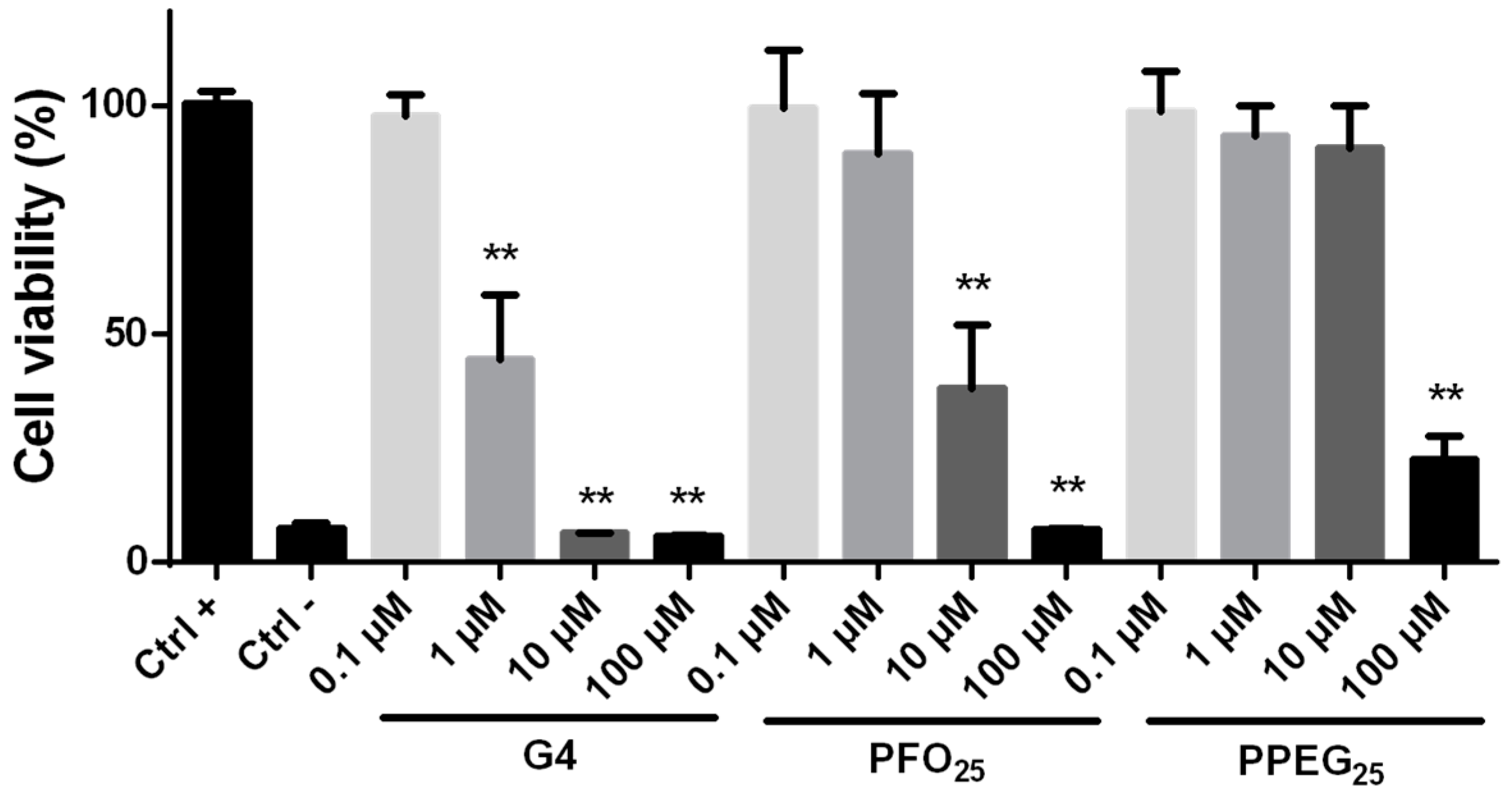
2.2. Cytotoxicity Evaluation
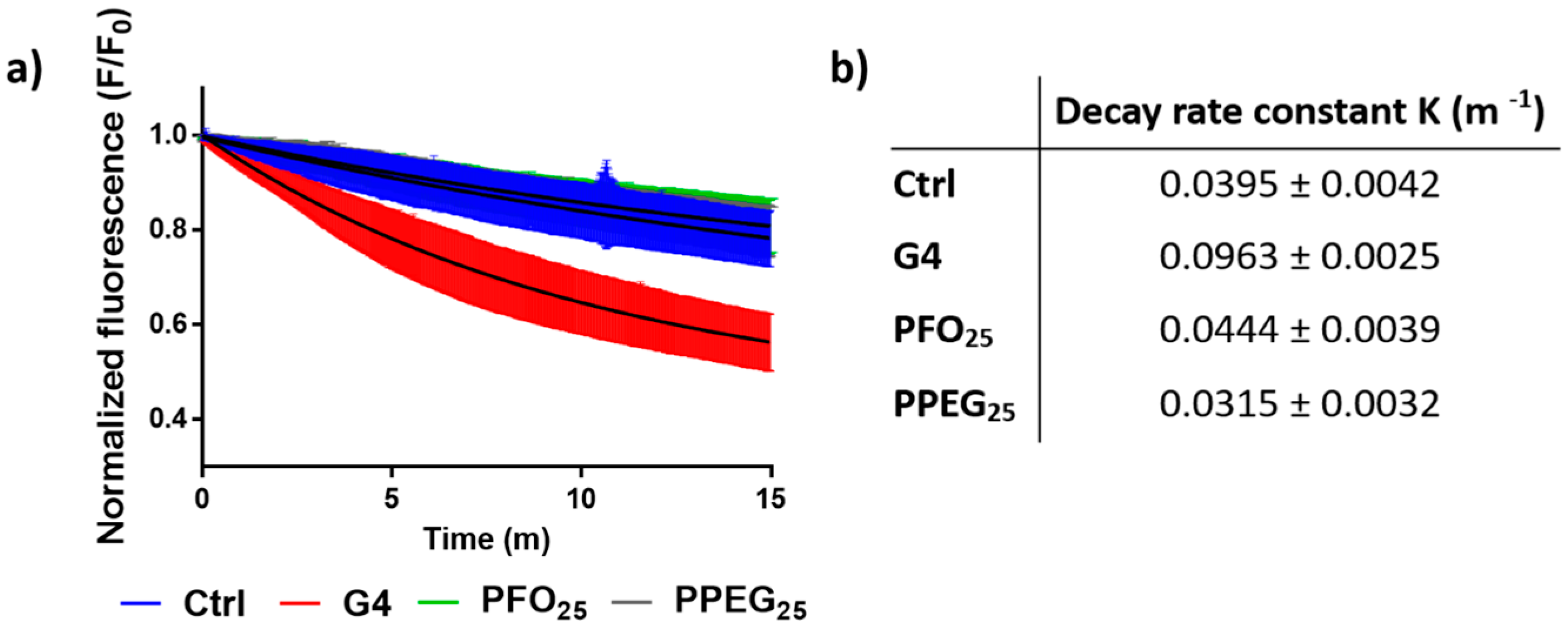
2.3. Membrane Permeability Effects
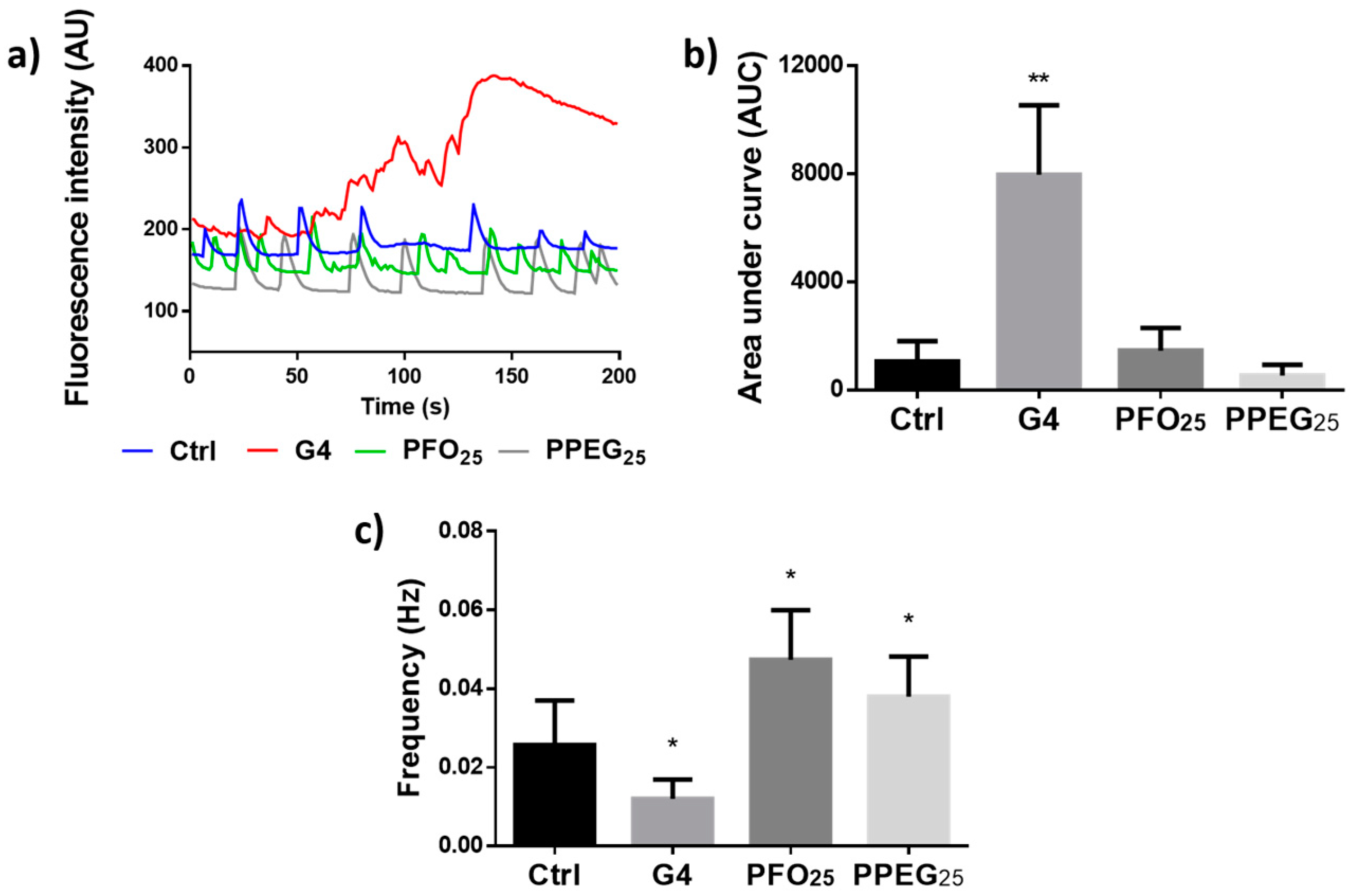
2.4. Analysis of Intracellular Ca2+ Transients
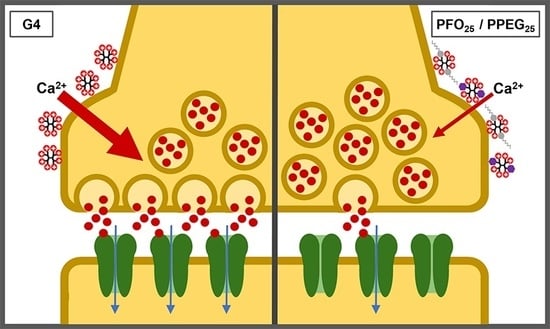
2.5. Synaptic Vesicle Release Study
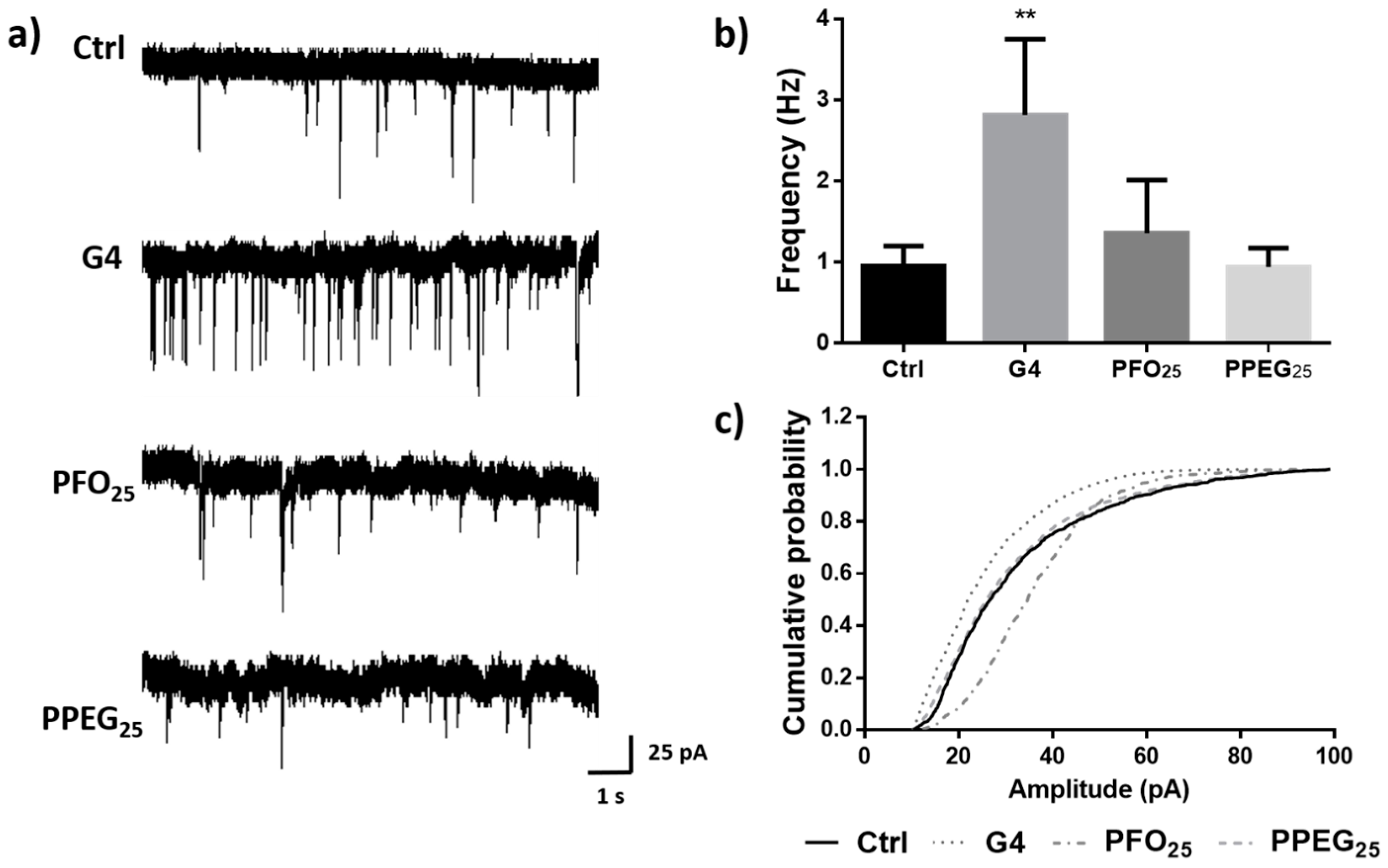
2.6. Effects on Synaptic Activity
3. Materials and Methods
3.1. Materials
3.2. Cell Cultures
3.3. Functionalization of PAMAM Dendrimers
3.4. Cytotoxicity Evaluation
3.5. Analysis of Intracellular Ca2+ Transients
3.6. Electrophysiology
3.7. Synaptic Vesicle Release Study
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jain, N.K.; Gupta, U. Application of dendrimer—Drug complexation in the enhancement of drug solubility and bioavailability. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, F.C.; Guerra, J.; Posadas, I.; Ceña, V. Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm. Res. 2011, 28, 1843–1858. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Ficker, M.; Christensen, J.B.; Trohopoulos, P.N.; Moghimi, S.M. Dendrimers in Medicine: Therapeutic Concepts and Pharmaceutical Challenges. Bioconjug. Chem. 2015, 26, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Tietjen, G.T.; Saucier-Sawyer, J.K.; Saltzman, W.M. A holistic approach to targeting disease with polymeric nanoparticles. Nat. Rev. Drug Discov. 2015, 14, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Manchun, S.; Dass, C.R.; Sriamornsak, P. Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci. 2012, 90, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, W.; He, Z. Dendrimers as carriers for siRNA delivery and gene silencing: A review. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Bielinska, A.U.; Mecke, A.; Keszler, B.; Beals, J.L.; Shi, X.; Balogh, L.; Orr, B.G.; Baker, J.R.; Banaszak Holl, M.M. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: Hole formation and the relation to transport. Bioconjug. Chem. 2004, 15, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Leroueil, P.R.; Berry, S.A.; Duthie, K.; Han, G.; Rotello, V.M.; Mcnerny, D.Q.; Baker, J.R.; Orr, B.G.; Holl, M.M.B. Wide Varieties of Cationic Nanoparticles Induce Defects in Supported Lipid Bilayers. Nano Lett. 2008, 8, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Ciolkowski, M.; Wróbel, D.; Petersen, J.F.; Ficker, M.; Christensen, J.B.; Bryszewska, M.; Klajnert, B. Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups reveals negligible toxicity against three rodent cell-lines. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Byrne, H.J. Polyamidoamine dendrimer nanoparticle cytotoxicity, oxidative stress, caspase activation and inflammatory response: Experimental observation and numerical simulation. Nanomedicine 2013, 9, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiong, W.; Wan, J.; Sun, X.; Xu, H.; Yang, X. The decrease of PAMAM dendrimer-induced cytotoxicity by PEGylation via attenuation of oxidative stress. Nanotechnology 2009, 20, 105103. [Google Scholar] [CrossRef] [PubMed]
- Enciso, A.E.; Neun, B.; Rodriguez, J.; Ranjan, A.P.; Dobrovolskaia, M.A.; Simanek, E.E. Nanoparticle effects on human platelets in vitro: A comparison between PAMAM and triazine dendrimers. Molecules 2016, 21, 428. [Google Scholar] [CrossRef] [PubMed]
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; Mckeown, N.B.; Emanuele, A.D. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int. J. Pharm. 2003, 252, 263–266. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface modification of PAMAM dendrimer improves its biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Del Burgo, L.S.; Hernández, R.M.; Orive, G.; Pedraz, J.L. Nanotherapeutic approaches for brain cancer management. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chem. Neurosci. 2014, 5, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood—Brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, S.R.; Silva, B.L.; Oliveira, J.M.; Mano, J.F.; Sousa, N.; Salgado, A.J.; Reis, R.L. Multifunctionalized CMCht/PAMAM Dendrimer Nanoparticles Modulate the Cellular Uptake by Astrocytes and Oligodendrocytes in Primary Cultures of Glial Cells. Macromol. Biosci. 2012, 12, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Albertazzi, L.; Gherardini, L.; Brondi, M.; Sato, S.S.; Bifone, A.; Pizzorusso, T.; Ratto, G.M.; Bardi, G. In Vivo Distribution and Toxicity of PAMAM Dendrimers in the Central Nervous System Depend on Their Surface Chemistry. Mol. Pharm. 2013, 10, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Vásquez, P.; Díaz, C.; Nova, D.; Alderete, J.; Guzmán, L. Mechanism of PAMAM Dendrimers Internalization in Hippocampal Neurons. Mol. Pharm. 2016, 13, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-Based Postnatal Therapy for Neuroinflammation and Cerebral Palsy in a Rabbit Model. Sci. Transl. Med. 2012, 4, 130ra46. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ma, H.; Guo, Y.; Liu, S.; Kuang, Y.; Shao, K.; Li, J.; Liu, Y.; Han, L.; Huang, S.; et al. Angiopep-conjugated nanoparticles for targeted long-term gene therapy of parkinson’s disease. Pharm. Res. 2013, 30, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, Y.; Huang, R.; Li, J.; Huang, S.; Kuang, Y.; Han, L.; Jiang, C. Gene and doxorubicin co-delivery system for targeting therapy of glioma. Biomaterials 2012, 33, 4907–4916. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Guzman, L. Dendrimer nanocarriers drug action: Perspective for neuronal pharmacology. Neural Regen. Res. 2015, 10, 1029–1031. [Google Scholar] [PubMed]
- Wang, S.; Li, Y.; Fan, J.; Wang, Z.; Zeng, X.; Sun, Y.; Song, P.; Ju, D. The role of autophagy in the neurotoxicity of cationic PAMAM dendrimers. Biomaterials 2014, 35, 7588–7597. [Google Scholar] [CrossRef] [PubMed]
- Nyitrai, G.; Keszthelyi, T.; Bota, A.; Simon, A.; Toke, O.; Horvath, G.; Pal, I.; Kardos, J.; Heja, L. Sodium selective ion channel formation in living cell membranes by polyamidoamine dendrimer. Biochim. Biophys. Acta 2013, 1828, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Nyitrai, G.; Héja, L.; Jablonkai, I.; Pál, I.; Visy, J.; Kardos, J. Polyamidoamine dendrimer impairs mitochondrial oxidation in brain tissue. J. Nanobiotechnol. 2013, 11, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Studzian, M.; Petersen, J.F.; Ficker, M.; Christensen, J.B.; Klajnert-Maculewicz, B. PAMAM dendrimer with 4-carbomethoxypyrrolidone-In vitro assessment of neurotoxicity. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Barraza, L.F.; Jiménez, V.A.; Alderete, J.B. Association of Methotrexate with Native and PEGylated PAMAM-G4 Dendrimers: Effect of the PEGylation Degree on the Drug-Loading Capacity and Preferential Binding Sites. J. Phys. Chem. B 2017, 121, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, F.J.; Parodi, J.; Peoples, R.W.; Opazo, C.; Aguayo, L.G. Synaptotoxicity of Alzheimer beta amyloid can be explained by its membrane perforating property. PLoS ONE 2010, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Espinoza, M.P.; Gallegos, S.; Opazo, C.; Aguayo, L.G. Alzheimer’s Aβ interacts with cellular prion protein inducing neuronal membrane damage and synaptotoxicity. Neurobiol. Aging 2015, 36, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Kinns, H.; Mitchell, N.; Astier, Y.; Madathil, R.; Howorka, S. Nanoscale Protein Pores Modified with PAMAM Dendrimers. J. Am. Chem. Soc. 2007, 129, 9640–9649. [Google Scholar] [CrossRef] [PubMed]
- Gaffield, M.A.; Betz, W.J. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nat. Protoc. 2006, 1, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, L.G.; Pancetti, F.C. Ethanol modulation of the gamma-aminobutyric acidA- and glycine-activated Cl- current in cultured mouse neurons. J. Pharmacol. Exp. Ther. 1994, 270, 61–69. [Google Scholar] [PubMed]
- Benchaala, I.; Mishra, M.K.; Wykes, S.M.; Hali, M.; Kannan, R.M.; Whittum-Hudson, J.A. Folate-functionalized dendrimers for targeting Chlamydia-infected tissues in a mouse model of reactive arthritis. Int. J. Pharm. 2014, 466, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Kono, K.; Maruyama, K.; Takagishi, T. Synthesis of polyamidoamine dendrimers having poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjug. Chem. 2000, 11, 910–917. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, F.; Vásquez, P.; Cayumán, F.R.; Díaz, C.; Fuentealba, J.; Aguayo, L.G.; Yévenes, G.E.; Alderete, J.; Guzmán, L. Prevention of Synaptic Alterations and Neurotoxic Effects of PAMAM Dendrimers by Surface Functionalization. Nanomaterials 2018, 8, 7. https://doi.org/10.3390/nano8010007
Vidal F, Vásquez P, Cayumán FR, Díaz C, Fuentealba J, Aguayo LG, Yévenes GE, Alderete J, Guzmán L. Prevention of Synaptic Alterations and Neurotoxic Effects of PAMAM Dendrimers by Surface Functionalization. Nanomaterials. 2018; 8(1):7. https://doi.org/10.3390/nano8010007
Chicago/Turabian StyleVidal, Felipe, Pilar Vásquez, Francisca R. Cayumán, Carola Díaz, Jorge Fuentealba, Luis G. Aguayo, Gonzalo E. Yévenes, Joel Alderete, and Leonardo Guzmán. 2018. "Prevention of Synaptic Alterations and Neurotoxic Effects of PAMAM Dendrimers by Surface Functionalization" Nanomaterials 8, no. 1: 7. https://doi.org/10.3390/nano8010007
APA StyleVidal, F., Vásquez, P., Cayumán, F. R., Díaz, C., Fuentealba, J., Aguayo, L. G., Yévenes, G. E., Alderete, J., & Guzmán, L. (2018). Prevention of Synaptic Alterations and Neurotoxic Effects of PAMAM Dendrimers by Surface Functionalization. Nanomaterials, 8(1), 7. https://doi.org/10.3390/nano8010007