Covalent Organic Frameworks: From Materials Design to Biomedical Application
Abstract
1. Introduction
2. Developments of COFs
2.1. Dynamic Likages
2.1.1. B–O Linkages
2.1.2. C–N Linkages
2.1.3. C–C Linkages
2.1.4. Other Separate and Hetero Linkages
2.2. Synthetic Method
2.2.1. Solvothermal Synthesis
2.2.2. Ionothermal Synthesis
2.2.3. Microwave Synthesis
2.2.4. Mechanochemical Synthesis
2.2.5. Room-Temperature Synthesis
2.2.6. Interface Synthesis
2.2.7. Other Synthetic Methods
2.3. Pore Design
2.4. Morphological Control
2.4.1. 2D COF Films
2.4.2. Other Regular Morphologies
2.5. Functional Modification
2.5.1. Functionalized Skeleton
2.5.2. Incorporated Substituents
2.5.3. Doped Inorganic Ions or Particles
2.6. Properties for Biomedical Applications
3. COFs for Biomedical Applications
3.1. Drug Delivery
3.2. Photothermal and Photodynamic Therapy
3.3. Biosensing and Bioimaging
3.4. Other Biomedical Applications
4. Conclusions and Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, S.-J.; Choi, S.-J.; Jang, J.-S.; Cho, H.-J.; Kim, I.-D. Innovative nanosensor for disease diagnosis. Acc. Chem. Res. 2017, 50, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for in vivo imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Liu, J.; Wang, W.; Zhang, Y.; Zhao, F.; Kong, D.; Liu, J.; Dong, A. Zwitterionic nanoparticles constructed from bioreducible RAFT-ROP double head agent for shell shedding triggered intracellular drug delivery. Acta Biomater. 2016, 40, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, W.; Zhou, J.; Zhao, F.; Zhang, Y.; Liu, J.; Liu, J.; Dong, A.; Kong, D.; Zhang, J. Amphiphilic polyelectrolyte/prodrug nanoparticles constructed by synergetic electrostatic and hydrophobic interactions with cooperative pH-sensitivity for controlled doxorubicin delivery. ACS Appl. Mater. Interfaces 2015, 7, 6340–6350. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.K.; Vermerris, W. Recent advances in nanomaterials for gene delivery—A review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, Y.; Wang, C.; Cheng, Q.; Han, S.; Wang, X.; Zhang, J.; Deng, L.; Zhao, D.; Du, L.; et al. pH-sensitive nanomicelles for high-efficiency siRNA delivery in vitro and in vivo: An insight into the design of polycations with robust cytosolic release. Nano Lett. 2016, 16, 6916–6923. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, L.; Zhou, J.; Meng, L.; Cheng, Q.; Wang, C.; Wang, X.; Zhao, D.; Huang, Y.; Zheng, S.; et al. Elaboration on the distribution of hydrophobic segments in the chains of amphiphilic cationic polymers for small interfering rna delivery. ACS Appl. Mater. Interfaces 2017, 9, 32463–32474. [Google Scholar] [CrossRef] [PubMed]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA delivery strategies: A comprehensive review of recent developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, J.; Huang, P.; Zhang, C.; Wang, W.; Li, C.; Kong, D. Self-assembled PEG-b-PDPA-b-PGEM copolymer nanoparticles as protein antigen delivery vehicles to dendritic cells: Preparation, characterization and cellular uptake. Regen. Biomater. 2017, 4, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, H.; Zhang, H.; Yang, P.; Zhang, C.; Huang, P.; Kong, D.; Wang, W. Engineering biodegradable guanidyl-decorated PEG-PCL nanoparticles as robust exogenous activators of DCs and antigen cross-presentation. Nanoscale 2017, 9, 13413–13418. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.; Van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Cherukula, K.; Manickavasagam Lekshmi, K.; Uthaman, S.; Cho, K.; Cho, C.S.; Park, I.K. Multifunctional inorganic nanoparticles: Recent progress in thermal therapy and imaging. Nanomaterials 2016, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Pattani, V.P.; Tunnell, J.W. Nanoparticle-mediated photothermal therapy: A comparative study of heating for different particle types. Lasers Surg. Med. 2012, 44, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Amezcua, R.; Shirolkar, A.; Fraze, C.; Stout, D.A. Nanomaterials for cardiac myocyte tissue engineering. Nanomaterials 2016, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomaterials for tissue engineering in dentistry. Nanomaterials 2016, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Song, K.; Zhao, X.; Li, Y.; Wang, F.; Zhang, J.; Dong, A.; Qin, Z. Tumor microenvironment activated membrane fusogenic liposome with speedy antibody and doxorubicin delivery for synergistic treatment of metastatic tumors. ACS Appl. Mater. Interfaces 2017, 9, 9315–9326. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Calandra, P.; Barreca, D.; Magazu, S.; Kiselev, M.A. Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials 2016, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Campani, V.; Salzano, G.; Lusa, S.; De Rosa, G. Lipid nanovectors to deliver RNA oligonucleotides in cancer. Nanomaterials 2016, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Truong-Dinh Tran, T.; Zhang, J.; Kong, L. Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Amirmahani, N.; Mahmoodi, N.O.; Mohammadi Galangash, M.; Ghavidast, A. Advances in nanomicelles for sustained drug delivery. J. Ind. Eng. Chem. 2017, 55, 21–34. [Google Scholar] [CrossRef]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Lyer, A.K. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov. Today 2015, 20, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Sikwal, D.R.; Kalhapure, R.S.; Govender, T. An emerging class of amphiphilic dendrimers for pharmaceutical and biomedical applications: Janus amphiphilic dendrimers. Eur. J. Pharm. Sci. 2017, 97, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.; Thanh, N.; Jones, S.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.G.; Bachmatiuk, A.; Büchner, B.; Cuniberti, G.; Rümmeli, M.H. Carbon nanostructures as multi-functional drug delivery platforms. J. Mater. Chem. B 2013, 1, 401–428. [Google Scholar] [CrossRef]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Carmona, M.; Colilla, M.; Vallet-Regi, M. Smart mesoporous nanomaterials for antitumor therapy. Nanomaterials 2015, 5, 1906–1937. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, J.; Chen, F.; Zhu, M.; Zhang, L. An anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles. Biomaterials 2010, 31, 3335–3346. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-X.; Yang, Y.-W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef] [PubMed]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-organic framework nanoparticles in photodynamic therapy: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- He, C.; Liu, D.; Lin, W. Nanomedicine applications of hybrid nanomaterials built from metal-ligand coordination bonds: Nanoscale metal-organic frameworks and nanoscale coordination polymers. Chem. Rev. 2015, 115, 11079–11108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Urban, M.W. Stimuli-responsive polymeric nanoparticles. Macromol. Rapid Commun. 2017, 38, 1700030. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, J.; Kong, X.; Hyeon, T.; Ling, D. Dynamic nanoparticle assemblies for biomedical applications. Adv. Mater. 2017, 29, 1605897. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, M.; Livney, Y.D.; Assaraf, Y.G. Targeted nanomedicine for cancer therapeutics: Towards precision medicine overcoming drug resistance. Drug Resist. Updates 2017, 31, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Bauer, L.; Hoimes, C.; Ghaghada, K.B.; Karathanasis, E. Targeted nanotechnology for cancer imaging. Adv. Drug Deliv. Rev. 2014, 76, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef] [PubMed]
- Huxford, R.C.; Della Rocca, J.; Lin, W. Metal-organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Yaghi, O.M. Reticular chemistry-construction, properties and precision reactions of frameworks. J. Am. Chem. Soc. 2016, 138, 15507–15509. [Google Scholar] [CrossRef] [PubMed]
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortés, J.L.; Côté, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed synthesis of 3D covalent organic frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Kamai, R.; Hashimoto, K.; Nakanishi, S. Platinum-modified covalent triazine frameworks hybridized with carbon nanoparticles as methanol-tolerant oxygen reduction electrocatalysts. Nat. Commun. 2014, 5, 5040. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.G.; Su, C.Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.B. Crystallization of covalent organic frameworks for gas storage applications. Molecules 2017, 22, 1149. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zou, R.; Zhao, Y. Covalent organic frameworks for CO2 capture. Adv. Mater. 2016, 28, 2855–2873. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. Recent advances in the synthesis of covalent organic frameworks for CO2 capture. J. CO2 Util. 2017, 17, 137–161. [Google Scholar] [CrossRef]
- Qian, H.L.; Yang, C.X.; Yan, X.P. Bottom-up synthesis of chiral covalent organic frameworks and their bound capillaries for chiral separation. Nat. Commun. 2016, 7, 12104. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.S.; Haase, F.; Stegbauer, L.; Savasci, G.; Podjaski, F.; Ochsenfeld, C.; Lotsch, B.V. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation. Nat. Commun. 2015, 6, 8508. [Google Scholar] [CrossRef] [PubMed]
- Ascherl, L.; Sick, T.; Margraf, J.T.; Lapidus, S.H.; Calik, M.; Hettstedt, C.; Karaghiosoff, K.; Döblinger, M.; Clark, T.; Chapman, K.W.; et al. Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 2016, 8, 310–316. [Google Scholar] [CrossRef]
- Xu, H.; Tao, S.; Jiang, D. Proton conduction in crystalline and porous covalent organic frameworks. Nat. Mater. 2016, 15, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Mahmood, J.; Baek, J.-B. Two-dimensional covalent organic frameworks for optoelectronics and energy storage. ChemNanoMat 2017, 3, 373–391. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, J.; Gu, S.; Kaspar, R.B.; Zhuang, Z.; Zheng, J.; Guo, H.; Qiu, S.; Yan, Y. 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 2015, 137, 8352–8355. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Sasmal, H.S.; Kundu, T.; Kandambeth, S.; Illath, K.; Diaz Diaz, D.; Banerjee, R. Targeted drug delivery in covalent organic nanosheets (CONs) via sequential postsynthetic modification. J. Am. Chem. Soc. 2017, 139, 4513–4520. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Díaz, U.; Corma, A. Ordered covalent organic frameworks, COFs and PAFs. From preparation to application. Coord. Chem. Rev. 2016, 311, 85–124. [Google Scholar] [CrossRef]
- Bisbey, R.P.; Dichtel, W.R. Covalent organic frameworks as a platform for multidimensional polymerization. ACS Cent. Sci. 2017, 6, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Beuerle, F.; Gole, B. Covalent Organic Frameworks and Cage Compounds: Design and Applications of Polymeric and Discrete Organic Scaffolds. Angew. Chem. Int. Ed. 2017. [Google Scholar] [CrossRef]
- DeBlase, C.R.; Dichtel, W.R. Moving beyond boron: The emergence of new linkage chemistries in covalent organic frameworks. Macromolecules 2016, 49, 5297–5305. [Google Scholar] [CrossRef]
- Sakaushi, K.; Antonietti, M. Carbon- and nitrogen-based organic frameworks. ACC. Chem. Res. 2015, 48, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Puthiaraj, P.; Lee, Y.-R.; Zhang, S.; Ahn, W.-S. Triazine-based covalent organic polymers: Design, synthesis and applications in heterogeneous catalysis. J. Mater. Chem. A 2016, 4, 16288–16311. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheno, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, H.; Meng, X.; Wang, C. Two-dimensional porphyrin- and phthalocyanine-based covalent organic frameworks. Chin. Chem. Lett. 2016, 27, 1376–1382. [Google Scholar] [CrossRef]
- Nishiyabu, R.; Kubo, Y.; James, T.D.; Fossey, J.S. Boronic acid building blocks: Tools for self assembly. Chem. Commun. 2011, 47, 1124–1150. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Hu, Y.; Zhang, W. Tessellated multiporous two-dimensional covalent organic frameworks. Nat. Rev. Chem. 2017, 1, 0056. [Google Scholar] [CrossRef]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of covalent organic frameworks. ACC. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef] [PubMed]
- Alahakoon, S.B.; Thompson, C.M.; Occhialini, G.; Smaldone, R.A. Design principles for covalent organic frameworks in energy storage applications. ChemSusChem 2017, 10, 2116–2129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Riduan, S.N.; Wang, J. Redox active metal- and covalent organic frameworks for energy storage: Balancing porosity and electrical conductivity. Chemistry (Easton) 2017, 23, 16419–16431. [Google Scholar]
- Germain, J.; Frechet, J.M.; Svec, F. Nanoporous polymers for hydrogen storage. Small 2009, 5, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Mendoza-Cortes, J.L.; Goddard, W.A., 3rd. Recent advances on simulation and theory of hydrogen storage in metal-organic frameworks and covalent organic frameworks. Chem. Soc. Rev. 2009, 38, 1460–1476. [Google Scholar] [CrossRef] [PubMed]
- Tylianakis, E.; Klontzas, E.; Froudakis, G.E. Multi-scale theoretical investigation of hydrogen storage in covalent organic frameworks. Nanoscale 2011, 3, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepulveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal-organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Zhang, D.; Zhao, Z.; Xia, Z. Covalent organic framework electrocatalysts for clean energy conversion. Adv. Mater. 2017, 1703646. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, S.; Feng, X.; Wang, B. Recent advances of covalent organic frameworks in electronic and optical applications. Chin. Chem. Lett. 2016, 27, 1383–1394. [Google Scholar] [CrossRef]
- Xue, R.; Guo, H.; Wang, T.; Gong, L.; Wang, Y.; Ai, J.; Huang, D.; Chen, H.; Yang, W. Fluorescence properties and analytical applications of covalent organic frameworks. Anal. Methods 2017, 9, 3737–3750. [Google Scholar] [CrossRef]
- Yang, T.; Cui, Y.; Chen, H.; Li, W. Controllable preparation of two dimensional metal-or covalent organic frameworks for chemical sensing and biosensing. Acta Chim. Sin. 2017, 75, 339–350. [Google Scholar] [CrossRef]
- Wu, M.-X.; Yang, Y.-W. Applications of covalent organic frameworks (COFs): From gas storage and separation to drug delivery. Chin. Chem. Lett. 2017, 28, 1135–1143. [Google Scholar] [CrossRef]
- Zhang, P.; Dai, S. Mechanochemical synthesis of porous organic materials. J. Mater. Chem. A 2017, 5, 16118–16127. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, C.; Denman, R.J.; Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 2013, 42, 6634–6654. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R.; Doonan, C.J.; Levangie, J.D.; Côté, A.P.; Yaghi, O.M. Reticular synthesis of covalent organic borosilicate frameworks. J. Am. Chem. Soc. 2008, 130, 11872–11873. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yang, H.; Whiteley, J.M.; Wan, S.; Jin, Y.; Lee, S.H.; Zhang, W. Ionic covalent organic frameworks with spiroborate linkage. Angew. Chem. Int. Ed. Engl. 2016, 55, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klock, C.; O’Keeffe, M.; Yaghi, O.M. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 2009, 131, 4570–4571. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Romo, F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhuang, Z.; Gu, S.; Kaspar, R.B.; Zheng, J.; Wang, J.; Qiu, S.; Yan, Y. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 2014, 5, 4503. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Lyle, S.J.; Osborn Popp, T.M.; Diercks, C.S.; Reimer, J.A.; Yaghi, O.M. Chemical conversion of linkages in covalent organic frameworks. J. Am. Chem. Soc. 2016, 138, 15519–15522. [Google Scholar] [CrossRef] [PubMed]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Skorjanc, T.; Sharma, S.K.; Gandara, F.; Lusi, M.; Shankar Rao, D.S.; Vimala, S.; Krishna Prasad, S.; Raya, J.; Han, D.S.; et al. Viologen-based conjugated covalent organic networks via Zincke reaction. J. Am. Chem. Soc. 2017, 139, 9558–9565. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Jin, S.; Gao, J.; Xu, Y.; Nagai, A.; Jiang, D. An azine-linked covalent organic framework. J. Am. Chem. Soc. 2013, 135, 17310–17313. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, Y.; Jin, S.; Chen, L.; Kaji, T.; Honsho, Y.; Addicoat, M.A.; Kim, J.; Saeki, A.; Ihee, H.; et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized pi clouds. Nat. Commun. 2013, 4, 2736. [Google Scholar] [CrossRef] [PubMed]
- Nagai, A.; Chen, X.; Feng, X.; Ding, X.; Guo, Z.; Jiang, D. A squaraine-linked mesoporous covalent organic framework. Angew. Chem. Int. Ed. Engl. 2013, 52, 3770–3774. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xing, F.; Bai, Y.-L.; Hu, M.; Zhao, Y.; Li, M.-X.; Zhu, S. High sensitivity viologen for a facile and versatile sensor of base and solvent polarity in solution and solid state in air atmosphere. ACS Appl. Mater. Interfaces 2015, 7, 14493–14500. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. Engl. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiu, L.G.; Yuan, Y.P.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Microwave-assisted synthesis of highly fluorescent nanoparticles of a melamine-based porous covalent organic framework for trace-level detection of nitroaromatic explosives. J. Hazard. Mater. 2012, 221, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Gutzler, R.; Walch, H.; Eder, G.; Kloft, S.; Heckl, W.M.; Lackinger, M. Surface mediated synthesis of 2D covalent organic frameworks: 1,3,5-tris(4-bromophenyl)benzene on graphite(001), Cu(111) and Ag(110). Chem. Commun. 2009, 7, 4456–4458. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Asada, M.; Xu, Q.; Dalapati, S.; Addicoat, M.A.; Brady, M.A.; Xu, H.; Nakamura, T.; Heine, T.; Chen, Q.; et al. Two-dimensional sp2 carbon–conjugated covalent organic frameworks. Science 2017, 357, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.T.; Reich, T.E.; El-Kaderi, H.M. Targeted synthesis of a porous borazine-linked covalent organic framework. Chem. Commun. 2012, 48, 8823–8825. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, D.; Maris, T.; Wuest, J.D. Constructing monocrystalline covalent organic networks by polymerization. Nat. Chem. 2013, 5, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chavez, A.D.; Li, H.; Li, H.; Dichtel, W.R.; Bredas, J.L. Nucleation and growth of covalent organic frameworks from solution: The example of COF-5. J. Am. Chem. Soc. 2017, 139, 16310–16318. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Hwang, N.; Chavez, A.D.; Novotney, J.L.; Dichtel, W.R. Growth rates and water stability of 2D boronate ester covalent organic frameworks. Chem. Commun. 2015, 51, 7532–7535. [Google Scholar] [CrossRef] [PubMed]
- Lanni, L.M.; Tilford, R.W.; Bharathy, M.; Lavigne, J.J. Enhanced hydrolytic stability of self-assembling alkylated two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 2011, 133, 13975–13983. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zheng, C.; Wang, L.; Hu, Y.; Guo, H.; Song, Q.; Zhang, H.; Zhang, Z.; Zhang, Y. Covalent self-assembled nanoparticles with pH-dependent enhanced tumor retention and drug release for improving tumor therapeutic efficiency. J. Mater. Chem. B 2017, 5, 2133–2144. [Google Scholar] [CrossRef]
- Kandambeth, S.; Shinde, D.B.; Panda, M.K.; Lukose, B.; Heine, T.; Banerjee, R. Enhancement of chemical stability and crystallinity in porphyrin-containing covalent organic frameworks by intramolecular hydrogen bonds. Angew. Chem. Int. Ed. Engl. 2013, 52, 13052–13056. [Google Scholar] [CrossRef] [PubMed]
- Kandambeth, S.; Venkatesh, V.; Shinde, D.B.; Kumari, S.; Halder, A.; Verma, S.; Banerjee, R. Self-templated chemically stable hollow spherical covalent organic framework. Nat. Commun. 2015, 6, 6786. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Addicoat, M.; Jin, E.; Zhai, L.; Xu, H.; Huang, N.; Guo, Z.; Liu, L.; Irle, S.; Jiang, D. Locking covalent organic frameworks with hydrogen bonds: General and remarkable effects on crystalline structure, physical properties and photochemical activity. J. Am. Chem. Soc. 2015, 137, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Feng, X.L.; Cui, X.H.; Ma, Y.X.; Ding, S.Y.; Wang, W. Salen-based covalent organic framework. J. Am. Chem. Soc. 2017, 139, 6042–6045. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, J.; Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Kandambeth, S.; Biswal, B.P.; Lukose, B.; Kunjir, S.M.; Chaudhary, M.; Babarao, R.; Heine, T.; Banerjee, R. Chemically stable multilayered covalent organic nanosheets from covalent organic frameworks via mechanical delamination. J. Am. Chem. Soc. 2013, 135, 17853–17861. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Kundu, T.; Kandambeth, S.; Babarao, R.; Marathe, Y.; Kunjir, S.M.; Banerjee, R. Phosphoric acid loaded azo (–N=N–) based covalent organic framework for proton conduction. J. Am. Chem. Soc. 2014, 136, 6570–6573. [Google Scholar] [CrossRef] [PubMed]
- Pachfule, P.; Kandambeth, S.; Diaz Diaz, D.; Banerjee, R. Highly stable covalent organic framework-Au nanoparticles hybrids for enhanced activity for nitrophenol reduction. Chem. Commun. 2014, 50, 3169–3172. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.P.; Chandra, S.; Kandambeth, S.; Lukose, B.; Heine, T.; Banerjee, R. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. [Google Scholar] [CrossRef] [PubMed]
- Karak, S.; Kandambeth, S.; Biswal, B.P.; Sasmal, H.S.; Kumar, S.; Pachfule, P.; Banerjee, R. Constructing ultraporous covalent organic frameworks in seconds via an organic terracotta process. J. Am. Chem. Soc. 2017, 139, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.; Fang, Y.; De Feyter, S.; Perepichka, D.F. Conjugated covalent organic frameworks via Michael addition-elimination. J. Am. Chem. Soc. 2017, 139, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Bojdys, M.J.; Jeromenok, J.; Thomas, A.; Antonietti, M. Rational extension of the family of layered, covalent, triazine-based frameworks with regular porosity. Adv. Mater. 2010, 22, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Palkovits, R.; Antonietti, M.; Kuhn, P.; Thomas, A.; Schuth, F. Solid catalysts for the selective low-temperature oxidation of methane to methanol. Angew. Chem. Int. Ed. Engl. 2009, 48, 6909–6912. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Bojdys, M.J.; Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Porous, fluorescent, covalent triazine-based frameworks via room-temperature and microwave-assisted synthesis. Adv. Mater. 2012, 24, 2357–2361. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, L.; Wang, X.; Guo, L.; Cheng, G.; Zhang, C.; Jin, S.; Tan, B.; Cooper, A. Covalent triazine frameworks via a low temperature polycondensation approach. Angew. Chem. Int. Ed. Engl. 2017, 56, 14149–14153. [Google Scholar] [CrossRef] [PubMed]
- Troschke, E.; Grätz, S.; Lübken, T.; Borchardt, L. Mechanochemical Friedel–Crafts alkylation—A sustainable pathway towards porous organic polymers. Angew. Chem. 2017, 129, 6963–6967. [Google Scholar] [CrossRef]
- Rengaraj, A.; Puthiaraj, P.; Haldorai, Y.; Heo, N.S.; Hwang, S.K.; Han, Y.K.; Kwon, S.; Ahn, W.S.; Huh, Y.S. Porous covalent triazine polymer as a potential nanocargo for cancer therapy and imaging. ACS Appl. Mater. Interfaces 2016, 8, 8947–8955. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, J.; Liu, Y.; Lyu, Y. Porphyrin-based covalent triazine frameworks: Porosity, adsorption performance and drug delivery. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2594–2600. [Google Scholar] [CrossRef]
- Kuhn, P.; Forget, A.; Su, D.; Thomas, A.; Antonietti, M. From microporous regular frameworks to mesoporous materials with ultrahigh surface area: Dynamic reorganization of porous polymer networks. J. Am. Chem. Soc. 2008, 130, 13333–13337. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Xiao, J.; Liu, L.; Qiu, L.; Jiang, X. Facile microwave-assisted production of Fe3O4 decorated porous melamine-based covalent organic framework for highly selective removal of Hg2+. J. Porous Mater. 2016, 23, 791–800. [Google Scholar] [CrossRef]
- Xue, R.; Guo, H.; Wang, T.; Wang, X.; Ai, J.; Yue, L.; Wei, Y.; Yang, W. Synthesis and characterization of a new covalent organic framework linked by NH linkage. Mater. Lett. 2017, 209, 171–174. [Google Scholar] [CrossRef]
- Grill, L.; Dyer, M.; Lafferentz, L.; Persson, M.; Peters, M.V.; Hecht, S. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2007, 2, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Blunt, M.O.; Russell, J.C.; Champness, N.R.; Beton, P.H. Templating molecular adsorption using a covalent organic framework. Chem. Commun. 2010, 46, 7157–7159. [Google Scholar] [CrossRef] [PubMed]
- Larrea, C.R.; Baddeley, C.J. Fabrication of a high-quality, porous, surface-confined covalent organic framework on a reactive metal surface. ChemPhysChem 2016, 17, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Faury, T.; Clair, S.; Abel, M.; Dumur, F.; Gigmes, D.; Porte, L. Sequential linking to control growth of a surface covalent organic framework. J. Phys. Chem. C 2012, 116, 4819–4823. [Google Scholar] [CrossRef]
- Shi, K.J.; Yuan, D.W.; Wang, C.X.; Shu, C.H.; Li, D.Y.; Shi, Z.L.; Wu, X.Y.; Liu, P.N. Ullmann reaction of aryl chlorides on various surfaces and the application in stepwise growth of 2D covalent organic frameworks. Org. Lett. 2016, 18, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Marele, A.C.; Mas-Balleste, R.; Terracciano, L.; Rodriguez-Fernandez, J.; Berlanga, I.; Alexandre, S.S.; Otero, R.; Gallego, J.M.; Zamora, F.; Gomez-Rodriguez, J.M. Formation of a surface covalent organic framework based on polyester condensation. Chem. Commun. 2012, 48, 6779–6781. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Addicoat, M.; Jin, E.; Xu, H.; Hayashi, T.; Xu, F.; Huang, N.; Irle, S.; Jiang, D. Designed synthesis of double-stage two-dimensional covalent organic frameworks. Sci. Rep. 2015, 5, 14650. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zou, R.; Luo, Z.; Zhang, H.; Yao, X.; Ma, X.; Zou, R.; Zhao, Y. Covalent organic frameworks formed with two types of covalent bonds based on orthogonal reactions. J. Am. Chem. Soc. 2015, 137, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.L.; Clowes, R.; Ritchie, L.K.; Cooper, A.I. Rapid microwave synthesis and purification of porous covalent organic frameworks. Chem. Mater. 2009, 21, 204–206. [Google Scholar] [CrossRef]
- Dogru, M.; Sonnauer, A.; Gavryushin, A.; Knochel, P.; Bein, T. A covalent organic framework with 4 nm open pores. Chem. Commun. 2011, 47, 1707–1709. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Cui, X.H.; Feng, J.; Lu, G.; Wang, W. Facile synthesis of –C=N– linked covalent organic frameworks under ambient conditions. Chem. Commun. 2017, 53, 11956–11959. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Dasari, R.R.; Ji, W.; Feriante, C.H.; Parker, T.C.; Marder, S.R.; Dichtel, W.R. Rapid, low temperature formation of imine-linked covalent organic frameworks catalyzed by metal triflates. J. Am. Chem. Soc. 2017, 139, 4999–5002. [Google Scholar] [CrossRef] [PubMed]
- Colson, J.W.; Woll, A.R.; Mukherjee, A.; Levendorf, M.P.; Spitler, E.L.; Shields, V.B.; Spencer, M.G.; Park, J.; Dichtel, W.R. Oriented 2D covalent organic framework thin films on single-layer graphene. Science 2011, 332, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Spitler, E.L.; Colson, J.W.; Uribe-Romo, F.J.; Woll, A.R.; Giovino, M.R.; Saldivar, A.; Dichtel, W.R. Lattice expansion of highly oriented 2D phthalocyanine covalent organic framework films. Angew. Chem. Int. Ed. 2012, 51, 2623–2627. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, C.; Lee, M.; Song, I.; Kim, J.; Lee, M.; Jung, J.; Kim, Y.; Lim, H.; Choi, H.C. Rapid photochemical synthesis of sea-urchin-shaped hierarchical porous COF-5 and its lithography-free patterned growth. Adv. Funct. Mater. 2017, 27, 1700925. [Google Scholar] [CrossRef]
- Ding, X.; Guo, J.; Feng, X.; Honsho, Y.; Guo, J.; Seki, S.; Maitarad, P.; Saeki, A.; Nagase, S.; Jiang, D. Synthesis of metallophthalocyanine covalent organic frameworks that exhibit high carrier mobility and photoconductivity. Angew. Chem. Int. Ed. Engl. 2011, 50, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, L.; Dong, Y.; Jiang, D. Porphyrin-based two-dimensional covalent organic frameworks: Synchronized synthetic control of macroscopic structures and pore parameters. Chem. Commun. 2011, 47, 1979–1981. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Overholts, A.C.; Hwang, N.; Dichtel, W.R. Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks. Chem. Commun. 2016, 52, 3690–3693. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, W.; Wang, J.; Wu, Q.; Wang, H.; Pan, L.; Liu, X. Green, scalable and morphology controlled synthesis of nanofibrous covalent organic frameworks and their nanohybrids through a vapor-assisted solid-state approach. J. Mater. Chem. A 2014, 2, 8201–8204. [Google Scholar] [CrossRef]
- Thote, J.; Barike Aiyappa, H.; Rahul Kumar, R.; Kandambeth, S.; Biswal, B.P.; Balaji Shinde, D.; Chaki Roy, N.; Banerjee, R. Constructing covalent organic frameworks in water via dynamic covalent bonding. IUCrJ 2016, 3, 402–407. [Google Scholar] [CrossRef] [PubMed]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, L.K.; Trewin, A.; Reguera-Galan, A.; Hasell, T.; Cooper, A.I. Synthesis of COF-5 using microwave irradiation and conventional solvothermal routes. Microporous Mesoporous Mater. 2010, 132, 132–136. [Google Scholar] [CrossRef]
- Hao, D.; Zhang, J.; Lu, H.; Leng, W.; Ge, R.; Dai, X.; Gao, Y. Fabrication of a COF-5 membrane on a functionalized alpha-Al2O3 ceramic support using a microwave irradiation method. Chem. Commun. 2014, 50, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chai, S.; Hu, N.; Yang, Z.; Wei, L.; Wang, L. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 2015, 51, 12178–12181. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Dichtel, W.R. Synthesis of 2D imine-linked covalent organic frameworks through formal transimination reactions. J. Am. Chem. Soc. 2017, 139, 12911–12914. [Google Scholar] [CrossRef] [PubMed]
- Friscic, T. Supramolecular concepts and new techniques in mechanochemistry: Cocrystals, cages, rotaxanes, open metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 3493–3510. [Google Scholar] [CrossRef] [PubMed]
- Friscic, T.; James, S.L.; Boldyreva, E.V.; Bolm, C.; Jones, W.; Mack, J.; Steed, J.W.; Suslick, K.S. Highlights from Faraday discussion 170: Challenges and opportunities of modern mechanochemistry, Montreal, Canada, 2014. Chem. Commun. 2015, 51, 6248–6256. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friscic, T.; Grepioni, F.; Harris, K.D.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Balaji Shinde, D.; Kandambeth, S.; Biswal, B.P.; Banerjee, R. Mechanosynthesis of imine, beta-ketoenamine and hydrogen-bonded imine-linked covalent organic frameworks using liquid-assisted grinding. Chem. Commun. 2014, 50, 12615–12618. [Google Scholar] [CrossRef] [PubMed]
- De la Pena Ruigomez, A.; Rodriguez-San-Miguel, D.; Stylianou, K.C.; Cavallini, M.; Gentili, D.; Liscio, F.; Milita, S.; Roscioni, O.M.; Ruiz-Gonzalez, M.L.; Carbonell, C.; et al. Direct on-surface patterning of a crystalline laminar covalent organic framework synthesized at room temperature. Chemistry (Easton) 2015, 21, 10666–10670. [Google Scholar] [CrossRef] [PubMed]
- Montoro, C.; Rodriguez-San-Miguel, D.; Polo, E.; Escudero-Cid, R.; Ruiz-Gonzalez, M.L.; Navarro, J.A.R.; Ocon, P.; Zamora, F. Ionic conductivity and potential application for fuel cell of a modified imine-based covalent organic framework. J. Am. Chem. Soc. 2017, 139, 10079–10086. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-San-Miguel, D.; Abrishamkar, A.; Navarro, J.A.; Rodriguez-Trujillo, R.; Amabilino, D.B.; Mas-Balleste, R.; Zamora, F.; Puigmarti-Luis, J. Crystalline fibres of a covalent organic framework through bottom-up microfluidic synthesis. Chem. Commun. 2016, 52, 9212–9215. [Google Scholar] [CrossRef] [PubMed]
- DeBlase, C.R.; Hernandez-Burgos, K.; Silberstein, K.E.; Rodríguez-Calero, G.G.; Bisbey, R.P.; Abruna, H.D.; Dichtel, W.R. Rapid and efficient redox processes within 2D covalent organic framework thin films. ACS Nano 2015, 9, 3178–3183. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.Z.; Wang, D.; Wan, L.J. Construction and repair of highly ordered 2D covalent networks by chemical equilibrium regulation. Chem. Commun. 2012, 48, 2943–2945. [Google Scholar] [CrossRef] [PubMed]
- Spitler, E.L.; Koo, B.T.; Novotney, J.L.; Colson, J.W.; Uribe-Romo, F.J.; Gutierrez, G.D.; Clancy, P.; Dichtel, W.R. A 2D covalent organic framework with 4.7-nm pores and insight into its interlayer stacking. J. Am. Chem. Soc. 2011, 133, 19416–19421. [Google Scholar] [CrossRef] [PubMed]
- Colson, J.W.; Mann, J.A.; Deblase, C.R.; Dichtel, W.R. Patterned growth of oriented 2D covalent organic framework thin films on single-layer graphene. J. Polym. Sci. Pol. Chem. 2015, 53, 378–384. [Google Scholar] [CrossRef]
- Sun, B.; Zhu, C.-H.; Liu, Y.; Wang, C.; Wan, L.-J.; Wang, D. Oriented covalent organic framework film on graphene for robust ambipolar vertical organic field-effect transistor. Chem. Mater. 2017, 29, 4367–4374. [Google Scholar] [CrossRef]
- Zha, Z.; Xu, L.; Wang, Z.; Li, X.; Pan, Q.; Hu, P.; Lei, S. 3D graphene functionalized by covalent organic framework thin film as capacitive electrode in alkaline media. ACS Appl. Mater. Interfaces 2015, 7, 17837–17843. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.-L.; Zhang, Y.-B.; Pun, A.B.; He, B.; Yang, J.; Toma, F.M.; Sharp, I.D.; Yaghi, O.M.; Fan, J.; Zheng, S.-R.; et al. Tunable electrical conductivity in oriented thin films of tetrathiafulvalene-based covalent organic framework. Chem. Sci. 2014, 5, 4693–4700. [Google Scholar] [CrossRef]
- Wang, P.; Kang, M.; Sun, S.; Liu, Q.; Zhang, Z.; Fang, S. Imine-linked covalent organic framework on surface for biosensor. Chin. J. Chem. 2014, 32, 838–843. [Google Scholar] [CrossRef]
- Bisbey, R.P.; DeBlase, C.R.; Smith, B.J.; Dichtel, W.R. Two-dimensional covalent organic framework thin films grown in flow. J. Am. Chem. Soc. 2016, 138, 11433–11436. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, X.; Yu, Y.; Tian, W.Q.; Ma, J.; Lei, S. Surface-confined crystalline two-dimensional covalent organic frameworks via on-surface schiff-base coupling. ACS Nano 2013, 7, 8066–8073. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Pal, M.; Rout, K.C.; Kunjattu, H.S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 2017, 139, 13083–13091. [Google Scholar] [CrossRef] [PubMed]
- Khayum, M.A.; Kandambeth, S.; Mitra, S.; Nair, S.B.; Das, A.; Nagane, S.S.; Mukherjee, R.; Banerjee, R. Chemically delaminated free-standing ultrathin covalent organic nanosheets. Angew. Chem. Int. Ed. Engl. 2016, 55, 15604–15608. [Google Scholar] [CrossRef] [PubMed]
- Zwaneveld, N.A.; Pawlak, R.; Abel, M.; Catalin, D.; Gigmes, D.; Bertin, D.; Porte, L. Organized formation of 2D extended covalent organic frameworks at surfaces. J. Am. Chem. Soc. 2008, 130, 6678–6679. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.J.; Zhang, X.; Shu, C.H.; Li, D.Y.; Wu, X.Y.; Liu, P.N. Ullmann coupling reaction of aryl chlorides on Au(111) using dosed Cu as a catalyst and the programmed growth of 2D covalent organic frameworks. Chem. Commun. 2016, 52, 8726–8729. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, X.; Tian, W.Q.; Gao, T.; Zhang, Y.F.; Lei, S.; Liu, Z.F. Surface-confined single-layer covalent organic framework on single-layer graphene grown on copper foil. Angew. Chem. Int. Ed. Engl. 2014, 53, 9564–9568. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.Y.; Liu, X.H.; Sun, B.; Wang, D. The on-surface synthesis of imine-based covalent organic frameworks with non-aromatic linkage. Chem. Commun. 2015, 51, 14318–14321. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.D.; Rotter, J.M.; Hu, Y.; Dogru, M.; Werner, V.; Auras, F.; Markiewicz, J.T.; Knochel, P.; Bein, T. Room temperature synthesis of covalent-organic framework films through vapor-assisted conversion. J. Am. Chem. Soc. 2015, 137, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, Z.B.; Wang, D. Construction of boronate ester based single-layered covalent organic frameworks. Chem. Commun. 2016, 52, 13771–13774. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Guan, C.Z.; Ding, S.Y.; Wang, W.; Yan, H.J.; Wang, D.; Wan, L.J. On-surface synthesis of single-layered two-dimensional covalent organic frameworks via solid-vapor interface reactions. J. Am. Chem. Soc. 2013, 135, 10470–10474. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, S.; Rastgoo-Lahrood, A.; Macknapp, K.; Ritter, V.; Sotier, S.; Heckl, W.M.; Lackinger, M. Solvent-free on-surface synthesis of boroxine COF monolayers. Chem. Commun. 2017, 53, 5147–5150. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.Y.; Mo, Y.P.; Li, S.Y.; Dong, W.L.; Chen, T.; Wang, D. Simultaneous construction of two linkages for the on-surface synthesis of imine-boroxine hybrid covalent organic frameworks. Chem. Sci. 2017, 8, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Feldblyum, J.I.; McCreery, C.H.; Andrews, S.C.; Kurosawa, T.; Santos, E.J.; Duong, V.; Fang, L.; Ayzner, A.L.; Bao, Z. Few-layer, large-area, 2D covalent organic framework semiconductor thin films. Chem. Commun. 2015, 51, 13894–13897. [Google Scholar] [CrossRef] [PubMed]
- Sahabudeen, H.; Qi, H.; Glatz, B.A.; Tranca, D.; Dong, R.; Hou, Y.; Zhang, T.; Kuttner, C.; Lehnert, T.; Seifert, G.; et al. Wafer-sized multifunctional polyimine-based two-dimensional conjugated polymers with high mechanical stiffness. Nat. Commun. 2016, 7, 13461. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zan, W.; Li, K.; Yang, Y.; Bu, F.; Xu, Y. Solution synthesis of semiconducting two-dimensional polymer via trimerization of carbonitrile. J. Am. Chem. Soc. 2017, 139, 11666–11669. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Shao, F.; Szczerbinski, J.; McCaffrey, R.; Zenobi, R.; Jin, Y.; Schluter, A.D.; Zhang, W. Synthesis of a two-dimensional covalent organic monolayer through dynamic imine chemistry at the air/water interface. Angew. Chem. Int. Ed. Engl. 2016, 55, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Namuangruk, S.; Kong, W.; Kungwan, N.; Guo, J.; Wang, C. Manipulation of amorphous-to-crystalline transformation: Towards the construction of covalent organic framework hybrid microspheres with nir photothermal conversion ability. Angew. Chem. Int. Ed. Engl. 2016, 55, 13979–13984. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Gao, C.; Zheng, Q.; Lei, Z.; Geng, H.; Lin, Z.; Yang, H.; Cai, Z. Room-temperature synthesis of core-shell structured magnetic covalent organic frameworks for efficient enrichment of peptides and simultaneous exclusion of proteins. Chem. Commun. 2017, 53, 3649–3652. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, L.; Pei, Q.; He, S.; Liu, S.; Xie, Z. Metal–organic framework@porous organic polymer nanocomposite for photodynamic therapy. Chem. Mater. 2017, 29, 2374–2381. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, M.; Chen, B.; Zhang, Z.; Huang, Y.; Dai, F.; Lai, Z.; Cui, X.; Tan, C.; Zhang, H. Hybridization of MOFs and COFs: A new strategy for construction of MOF@COF core-shell hybrid materials. Adv. Mater. 2017, 1705454. [Google Scholar] [CrossRef] [PubMed]
- Tilford, R.W.; Gemmill, W.R.; zur Loye, H.-C.; Lavigne, J.J. Facile synthesis of a highly crystalline, covalently linked porous boronate network. Chem. Mater. 2006, 18, 5296–5301. [Google Scholar] [CrossRef]
- Kandambeth, S.; Biswal, B.P.; Chaudhari, H.D.; Rout, K.C.; Kunjattu, H.S.; Mitra, S.; Karak, S.; Das, A.; Mukherjee, R.; Kharul, U.K.; et al. Selective molecular sieving in self-standing porous covalent-organic-framework membranes. Adv. Mater. 2017, 29, 1603945. [Google Scholar] [CrossRef] [PubMed]
- Spitler, E.L.; Dichtel, W.R. Lewis acid-catalysed formation of two-dimensional phthalocyanine covalent organic frameworks. Nat. Chem. 2010, 2, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Spitler, E.L.; Giovino, M.R.; White, S.L.; Dichtel, W.R. A mechanistic study of Lewis acid-catalyzed covalent organic framework formation. Chem. Sci. 2011, 2, 1588–1593. [Google Scholar] [CrossRef]
- Dogru, M.; Sonnauer, A.; Zimdars, S.; Döblinger, M.; Knochel, P.; Bein, T. Facile synthesis of a mesoporous benzothiadiazole-COF based on a transesterification process. CrystEngComm 2013, 15, 1500–1502. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, L.; Gandara, F.; Ma, Y.; Liu, Z.; Zhu, C.; Lyu, H.; Trickett, C.A.; Kapustin, E.A.; Terasaki, O.; et al. A synthetic route for crystals of woven structures, uniform nanocrystals and thin films of imine covalent organic frameworks. J. Am. Chem. Soc. 2017, 139, 13166–13172. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Ding, S.Y.; Xue, H.D.; Cao, W.; Wang, W. Synthesis of –C=N– linked covalent organic frameworks via the direct condensation of acetals and amines. Chem. Commun. 2016, 52, 7217–7220. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Y.; Chung, T.-S.; Ng, N.P. Morphology, drug distribution and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials 2001, 22, 231–241. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.; Coupry, D.E.; Addicoat, M.A.; Okushita, K.; Nishimura, K.; Heine, T.; Jiang, D. Multiple-component covalent organic frameworks. Nat. Commun. 2016, 7, 12325. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.P.; El-Kaderi, H.M.; Furukawa, H.; Hunt, J.R.; Yaghi, O.M. Reticular synthesis of microporous and mesoporous 2D covalent organic frameworks. J. Am. Chem. Soc. 2007, 129, 12914–12915. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Addicoat, M.; Irle, S.; Nagai, A.; Jiang, D. Control of crystallinity and porosity of covalent organic frameworks by managing interlayer interactions based on self-complementary pi-electronic force. J. Am. Chem. Soc. 2013, 135, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Addicoat, M.; Jin, S.; Sakurai, T.; Gao, J.; Xu, H.; Irle, S.; Seki, S.; Jiang, D. Rational design of crystalline supermicroporous covalent organic frameworks with triangular topologies. Nat. Commun. 2015, 6, 7786. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-B.; Lyu, H.; Tian, J.; Wang, H.; Zhang, D.-W.; Liu, Y.; Li, Z.-T. A polycationic covalent organic framework: A robust adsorbent for anionic dye pollutants. Polym. Chem. 2016, 7, 3392–3397. [Google Scholar] [CrossRef]
- Feng, X.; Dong, Y.; Jiang, D. Star-shaped two-dimensional covalent organic frameworks. CrystEngComm 2013, 15, 1508–1511. [Google Scholar] [CrossRef]
- Jin, S.; Furukawa, K.; Addicoat, M.; Chen, L.; Takahashi, S.; Irle, S.; Nakamura, T.; Jiang, D. Large pore donor-acceptor covalent organic frameworks. Chem. Sci. 2013, 4, 4505–4511. [Google Scholar] [CrossRef]
- Tilford, R.W.; Mugavero, S.J.; Pellechia, P.J.; Lavigne, J.J. Tailoring microporosity in covalent organic frameworks. Adv. Mater. 2008, 20, 2741–2746. [Google Scholar] [CrossRef] [PubMed]
- Nagai, A.; Guo, Z.; Feng, X.; Jin, S.; Chen, X.; Ding, X.; Jiang, D. Pore surface engineering in covalent organic frameworks. Nat. Commun. 2011, 2, 536. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.Y.; Xu, S.Q.; Wen, Q.; Pang, Z.F.; Zhao, X. One-step construction of two different kinds of pores in a 2D covalent organic framework. J. Am. Chem. Soc. 2014, 136, 15885–15888. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, S.Q.; Qian, C.; Pang, Z.F.; Jiang, G.F.; Zhao, X. Two-dimensional dual-pore covalent organic frameworks obtained from the combination of two D2h symmetrical building blocks. Chem. Commun. 2016, 52, 11704–11707. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.F.; Zhou, T.Y.; Liang, R.R.; Qi, Q.Y.; Zhao, X. Regulating the topology of 2D covalent organic frameworks by the rational introduction of substituents. Chem. Sci. 2017, 8, 3866–3870. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, S.-Q.; Liang, R.-R.; Qian, C.; Jiang, G.-F.; Zhao, X. Construction of two heteropore covalent organic frameworks with Kagome lattices. CrystEngComm 2017, 19, 4877–4881. [Google Scholar] [CrossRef]
- Pang, Z.F.; Xu, S.Q.; Zhou, T.Y.; Liang, R.R.; Zhan, T.G.; Zhao, X. Construction of covalent organic frameworks bearing three different kinds of pores through the heterostructural mixed linker strategy. J. Am. Chem. Soc. 2016, 138, 4710–4713. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wen, Q.; Zhan, T.G.; Qi, Q.Y.; Xu, J.Q.; Zhao, X. A case study on the influence of substitutes on interlayer stacking of 2D covalent organic frameworks. Chemistry (Easton) 2017, 23, 5668–5672. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Qi, Q.Y.; Jiang, G.F.; Cui, F.Z.; Tian, Y.; Zhao, X. Toward covalent organic frameworks bearing three different kinds of pores: The strategy for construction and COF-to-COF transformation via heterogeneous linker exchange. J. Am. Chem. Soc. 2017, 139, 6736–6743. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.J.; Xu, S.Q.; Zhan, T.G.; Qi, Q.Y.; Wu, Z.Q.; Zhao, X. Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem. Commun. 2017, 53, 7266–7269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wan, S.; Jin, Y.; Zhang, W. Desymmetrized vertex design for the synthesis of covalent organic frameworks with periodically heterogeneous pore structures. J. Am. Chem. Soc. 2015, 137, 13772–13775. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Jin, E.; Addicoat, M.; Heine, T.; Jiang, D. Highly emissive covalent organic frameworks. J. Am. Chem. Soc. 2016, 138, 5797–5800. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, L.A.; Crowe, J.W.; Shannon, M.D.; Jaroniec, C.P.; McGrier, P.L. 2D covalent organic frameworks with alternating triangular and hexagonal pores. Chem. Mater. 2015, 27, 6169–6172. [Google Scholar] [CrossRef]
- Mo, Y.P.; Liu, X.H.; Wang, D. Concentration-directed polymorphic surface covalent organic frameworks: Rhombus, parallelogram and kagome. ACS Nano 2017, 11, 11694–11700. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.T.; Dichtel, W.R.; Clancy, P. A classification scheme for the stacking of two-dimensional boronate ester-linked covalent organic frameworks. J. Mater. Chem. 2012, 22, 17460–17469. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.; Holland, B.; Byrne, M.; Gun’Ko, Y.K. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, I.; Ruiz-Gonzalez, M.L.; Gonzalez-Calbet, J.M.; Fierro, J.L.; Mas-Balleste, R.; Zamora, F. Delamination of layered covalent organic frameworks. Small 2011, 7, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Bunck, D.N.; Dichtel, W.R. Bulk synthesis of exfoliated two-dimensional polymers using hydrazone-linked covalent organic frameworks. J. Am. Chem. Soc. 2013, 135, 14952–14955. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Biswal, B.P.; Kandambeth, S.; Venkatesh, V.; Kaur, G.; Addicoat, M.; Heine, T.; Verma, S.; Banerjee, R. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem. Sci. 2015, 6, 3931–3939. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Hu, Z.; Tee, L.; Guo, Z.; Zhao, D. Mixed matrix membranes (MMMs) comprising exfoliated 2D covalent organic frameworks (COFs) for efficient CO2 separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Li, G.; Zhang, K.; Tsuru, T. Two-dimensional covalent organic framework (COF) membranes fabricated via the assembly of exfoliated COF nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 8433–8436. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Kandambeth, S.; Biswal, B.P.; Khayum, M.A.; Choudhury, C.K.; Mehta, M.; Kaur, G.; Banerjee, S.; Prabhune, A.; Verma, S.; et al. Self-exfoliated guanidinium-based ionic covalent organic nanosheets (iCONs). J. Am. Chem. Soc. 2016, 138, 2823–2828. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A belt-shaped, blue luminescent and semiconducting covalent organic framework. Angew. Chem. Int. Ed. Engl. 2008, 47, 8826–8830. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Jiang, Y.; Li, X.; Li, X.; Wang, J.; Wu, Q.; Liu, X. Solvothermal synthesis of microporous, crystalline covalent organic framework nanofibers and their colorimetric nanohybrid structures. ACS Appl. Mater. Interfaces 2013, 5, 8845–8849. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chen, Z.; Deng, W.; Yan, G.; Liu, X. Morphology controlled synthesis of octahedral covalent imine frameworks through acid modulated aldehyde-amine polycondensation. Macromol. Res. 2016, 24, 366–370. [Google Scholar] [CrossRef]
- Halder, A.; Kandambeth, S.; Biswal, B.P.; Kaur, G.; Roy, N.C.; Addicoat, M.; Salunke, J.K.; Banerjee, S.; Vanka, K.; Heine, T.; et al. Decoding the morphological diversity in two dimensional crystalline porous polymers by core planarity modulation. Angew. Chem. Int. Ed. Engl. 2016, 128, 7937–7941. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Zhao, Y.; Sun, X.; Gándara, F.; Furukawa, H.; Liu, Z.; Zhu, H.; Zhu, C.; Suenaga, K.; et al. Weaving of organic threads into a crystalline covalent organic framework. Science 2016, 351, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Parent, L.R.; Overholts, A.C.; Beaucage, P.A.; Bisbey, R.P.; Chavez, A.D.; Hwang, N.; Park, C.; Evans, A.M.; Gianneschi, N.C.; et al. Colloidal covalent organic frameworks. ACS Cent. Sci. 2017, 3, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.D.; Smith, B.J.; Smith, M.K.; Beaucage, P.A.; Northrop, B.H.; Dichtel, W.R. Discrete, hexagonal boronate ester-linked macrocycles related to two-dimensional covalent organic frameworks. Chem. Mater. 2016, 28, 4884–4888. [Google Scholar] [CrossRef]
- Yang, C.X.; Liu, C.; Cao, Y.M.; Yan, X.P. Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem. Commun. 2015, 51, 12254–12257. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tang, Y.; Zhai, L.; Chen, Q.; Jiang, D. Pyrolysis of covalent organic frameworks: A general strategy for template converting conventional skeletons into conducting microporous carbons for high-performance energy storage. Chem. Commun. 2017, 53, 11690–11693. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Occhialini, G.; McCandless, G.T.; Alahakoon, S.B.; Cameron, V.; Nielsen, S.O.; Smaldone, R.A. Computational and experimental studies on the effects of monomer planarity on covalent organic framework formation. J. Am. Chem. Soc. 2017, 139, 10506–10613. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Gándara, F.; Asano, A.; Furukawa, H.; Saeki, A.; Dey, S.K.; Liao, L.; Ambrogio, M.W.; Botros, Y.Y.; Duan, X.; et al. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 2011, 23, 4094–4097. [Google Scholar] [CrossRef]
- Jin, S.; Ding, X.; Feng, X.; Supur, M.; Furukawa, K.; Takahashi, S.; Addicoat, M.; El-Khouly, M.E.; Nakamura, T.; Irle, S.; et al. Charge dynamics in a donor-acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions. Angew. Chem. Int. Ed. Engl. 2013, 52, 2017–2021. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, X.; Gao, J.; Lin, J.; Addicoat, M.; Irle, S.; Jiang, D. Catalytic covalent organic frameworks via pore surface engineering. Chem. Commun. 2014, 50, 1292–1294. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Krishna, R.; Jiang, D. Tailor-made pore surface engineering in covalent organic frameworks: Systematic functionalization for performance screening. J. Am. Chem. Soc. 2015, 137, 7079–7082. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Lan, J.; Wang, W.; Smit, B. Lithium-doped 3D covalent organic frameworks: High-capacity hydrogen storage materials. Angew. Chem. Int. Ed. Engl. 2009, 48, 4730–4733. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ding, H.; Li, B.; Ai, X.; Wang, C. Covalent-organic frameworks: Potential host materials for sulfur impregnation in lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 8854–8858. [Google Scholar] [CrossRef]
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A photoconductive covalent organic framework: Self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chem. Int. Ed. Engl. 2009, 48, 5439–5442. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Ding, H.; Yuan, D.; Wang, B.; Wang, C. A pyrene-based, fluorescent three-dimensional covalent organic framework. J. Am. Chem. Soc. 2016, 138, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Osuka, A. Conjugated porphyrin arrays: Synthesis, properties and applications for functional materials. Chem. Soc. Rev. 2015, 44, 943–969. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, L.; Honsho, Y.; Saeki, A.; Seki, S.; Irle, S.; Dong, Y.; Nagai, A.; Jiang, D. High-rate charge-carrier transport in porphyrin covalent organic frameworks: Switching from hole to electron to ambipolar conduction. Angew. Chem. Int. Ed. 2012, 51, 2618–2622. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Ding, H.; Chen, R.; Peng, Z.; Wang, B.; Wang, C. 3D porphyrin-based covalent organic frameworks. J. Am. Chem. Soc. 2017, 139, 8705–8709. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.M. Surface chemistry of porphyrins and phthalocyanines. Surf. Sci. Rep. 2015, 70, 259–379. [Google Scholar] [CrossRef]
- Ding, X.; Feng, X.; Saeki, A.; Seki, S.; Nagai, A.; Jiang, D. Conducting metallophthalocyanine 2D covalent organic frameworks: The role of central metals in controlling pi-electronic functions. Chem. Commun. 2012, 48, 8952–8954. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ding, X.; Chen, L.; Wu, Y.; Liu, L.; Addicoat, M.; Irle, S.; Dong, Y.; Jiang, D. Two-dimensional artificial light-harvesting antennae with predesigned high-order structure and robust photosensitising activity. Sci. Rep. 2016, 6, 32944. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hijikata, Y.; Page, A.; Jiang, D.; Irle, S. Theoretical analysis of structural diversity of covalent organic framework: Stacking isomer structures thermodynamics and kinetics. Chem. Phys. Lett. 2016, 664, 101–107. [Google Scholar] [CrossRef]
- Jin, S.; Supur, M.; Addicoat, M.; Furukawa, K.; Chen, L.; Nakamura, T.; Fukuzumi, S.; Irle, S.; Jiang, D. Creation of superheterojunction polymers via direct polycondensation: Segregated and bicontinuous donor-acceptor π-columnar arrays in covalent organic frameworks for long-lived charge separation. J. Am. Chem. Soc. 2015, 137, 7817–7827. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Jin, S.; Zhong, H.; Wu, D.; Yang, X.; Chen, X.; Wei, H.; Fu, R.; Jiang, D. Electrochemically active, crystalline, mesoporous covalent organic frameworks on carbon nanotubes for synergistic lithium-ion battery energy storage. Sci. Rep. 2015, 5, 8225. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, L.; Honsho, Y.; Saengsawang, O.; Liu, L.; Wang, L.; Saeki, A.; Irle, S.; Seki, S.; Dong, Y.; et al. An ambipolar conducting covalent organic framework with self-sorted and periodic electron donor-acceptor ordering. Adv. Mater. 2012, 24, 3026–3031. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Rong, Q.; Niu, H.; Cai, Y. Construction of a superior visible-light-driven photocatalyst based on a C3N4 active centre-photoelectron shift platform-electron withdrawing unit triadic structure covalent organic framework. Chem. Commun. 2017, 53, 9636–9639. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Zeng, Q.; Lei, S. A photoresponsive surface covalent organic framework: Surface-confined synthesis, isomerization and controlled guest capture and release. Chemistry (Easton) 2016, 22, 6768–6773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Li, N.; Liu, J.; Zhang, W.; Zhang, Z.; Zhou, N.; Zhu, X. A novel azobenzene covalent organic framework. CrystEngComm 2014, 16, 6547–6551. [Google Scholar] [CrossRef]
- Ge, R.; Hao, D.; Shi, Q.; Dong, B.; Leng, W.; Wang, C.; Gao, Y. Target synthesis of an azo (N=N) based covalent organic framework with high CO2-over-N2 selectivity and benign gas storage capability. J. Chem. Eng. Data 2016, 61, 1904–1909. [Google Scholar] [CrossRef]
- Huang, N.; Ding, X.; Kim, J.; Ihee, H.; Jiang, D. A photoresponsive smart covalent organic framework. Angew. Chem. Int. Ed. Engl. 2015, 54, 8704–8707. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Sakurai, T.; Kowalczyk, T.; Dalapati, S.; Xu, F.; Wei, H.; Chen, X.; Gao, J.; Seki, S.; Irle, S.; et al. Two-dimensional tetrathiafulvalene covalent organic frameworks: Towards latticed conductive organic salts. Chemistry (Easton) 2014, 20, 14608–14613. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, Y.; Hu, H.; Sun, Y.; Wang, J.; Wang, C.; Wang, C.; Zhang, G.; Wang, B.; Xu, W.; et al. A tetrathiafulvalene-based electroactive covalent organic framework. Chemistry (Easton) 2014, 20, 14614–14618. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Roy Chowdhury, D.; Addicoat, M.; Heine, T.; Paul, A.; Banerjee, R. Molecular level control of the capacitance of two-dimensional covalent organic frameworks: Role of hydrogen bonding in energy storage materials. Chem. Mater. 2017, 29, 2074–2080. [Google Scholar] [CrossRef]
- Feng, S.; Xu, H.; Zhang, C.; Chen, Y.; Zeng, J.; Jiang, D.; Jiang, J.X. Bicarbazole-based redox-active covalent organic frameworks for ultrahigh-performance energy storage. Chem. Commun. 2017, 53, 11334–11337. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Shao, P.; Han, Y.; Gao, X.; Ma, L.; Yuan, S.; Ma, X.; Zhou, J.; Feng, X.; et al. Exfoliation of covalent organic frameworks into few-layer redox-active nanosheets as cathode materials for lithium-ion batteries. J. Am. Chem. Soc. 2017, 139, 4258–4261. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Bessinger, D.; Reuter, S.; Calik, M.; Ascherl, L.; Hanusch, F.C.; Auras, F.; Bein, T. Oligothiophene-bridged conjugated covalent organic frameworks. J. Am. Chem. Soc. 2017, 139, 8194–8199. [Google Scholar] [CrossRef] [PubMed]
- Khattak, A.M.; Ghazi, Z.A.; Liang, B.; Khan, N.A.; Iqbal, A.; Li, L.; Tang, Z. A redox-active 2D covalent organic framework with pyridine moieties capable of faradaic energy storage. J. Mater. Chem. A 2016, 4, 16312–16317. [Google Scholar] [CrossRef]
- Deng, W.; Li, Y.; Zheng, S.; Liu, X.; Li, P.; Sun, L.; Yang, R.; Wang, S.; Wu, Z.; Bao, X. Conductive microporous covalent triazine-based framework for high-performance electrochemical capacitive energy storage. Angew. Chem. Int. Ed. Engl. 2017, 139, 8194–8199. [Google Scholar]
- Zhang, Y.; Duan, J.; Ma, D.; Li, P.; Li, S.; Li, H.; Zhou, J.; Ma, X.; Feng, X.; Wang, B. Three-dimensional anionic cyclodextrin-covalent organic frameworks. Angew. Chem. Int. Ed. 2017. [Google Scholar] [CrossRef]
- Doonan, C.J.; Tranchemontagne, D.J.; Glover, T.G.; Hunt, J.R.; Yaghi, O.M. Exceptional ammonia uptake by a covalent organic framework. Nat. Chem. 2010, 2, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, P.; Addicoat, M.A.; Heine, T.; Jiang, D. Ionic covalent organic frameworks: Design of a charged interface aligned on 1D channel walls and its unusual electrostatic functions. Angew. Chem. Int. Ed. Engl. 2017, 56, 4982–4986. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Huang, N.; Xu, H.; Chen, Q.; Jiang, D. A backbone design principle for covalent organic frameworks: The impact of weakly interacting units on CO2 adsorption. Chem. Commun. 2017, 53, 4242–4245. [Google Scholar] [CrossRef] [PubMed]
- Ning, G.H.; Chen, Z.; Gao, Q.; Tang, W.; Chen, Z.; Liu, C.; Tian, B.; Li, X.; Loh, K.P. Salicylideneanilines-based covalent organic frameworks as chemoselective molecular sieves. J. Am. Chem. Soc. 2017, 139, 8897–8904. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xu, H.; Chen, X.; Wu, D.; Wu, Y.; Liu, H.; Gu, C.; Fu, R.; Jiang, D. Radical covalent organic frameworks: A general strategy to immobilize open-accessible polyradicals for high-performance capacitive energy storage. Angew. Chem. Int. Ed. Engl. 2015, 54, 6814–6818. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Furukawa, K.; Gao, J.; Nagai, A.; Nakamura, T.; Dong, Y.; Jiang, D. Photoelectric covalent organic frameworks: Converting open lattices into ordered donor-acceptor heterojunctions. J. Am. Chem. Soc. 2014, 136, 9806–9809. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, D.W. The advent and development of organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, X.; Wu, X.; Liu, Y.; Cui, Y. Multivariate chiral covalent organic frameworks with controlled crystallinity and stability for asymmetric catalysis. J. Am. Chem. Soc. 2017, 139, 8277–8285. [Google Scholar] [CrossRef] [PubMed]
- Bunck, D.N.; Dichtel, W.R. Internal functionalization of three-dimensional covalent organic frameworks. Angew. Chem. Int. Ed. 2012, 51, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.S.; Stassin, T.; Naudin, G.; Wuttke, S.; Ameloot, R.; De Vos, D.; Medina, D.D.; Bein, T. Sequential pore wall modification in a covalent organic framework for application in lactic acid adsorption. Chem. Mater. 2016, 28, 626–631. [Google Scholar] [CrossRef]
- Bunck, D.N.; Dichtel, W.R. Postsynthetic functionalization of 3D covalent organic frameworks. Chem. Commun. 2013, 49, 2457–2459. [Google Scholar] [CrossRef] [PubMed]
- Calik, M.; Sick, T.; Dogru, M.; Doblinger, M.; Datz, S.; Budde, H.; Hartschuh, A.; Auras, F.; Bein, T. From highly crystalline to outer surface-functionalized covalent organic frameworks—A modulation approach. J. Am. Chem. Soc. 2016, 138, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Aguila, B.; Perman, J.; Earl, L.D.; Abney, C.W.; Cheng, Y.; Wei, H.; Nguyen, N.; Wojtas, L.; Ma, S. Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal. J. Am. Chem. Soc. 2017, 139, 2786–2793. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Dong, M.; Wang, Y.W.; Chen, Y.T.; Wang, H.Z.; Su, C.Y.; Wang, W. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(II). J. Am. Chem. Soc. 2016, 138, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Zhai, L.; Xu, H.; Jiang, D. Stable covalent organic frameworks for exceptional mercury removal from aqueous solutions. J. Am. Chem. Soc. 2017, 139, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Merí-Bofí, L.; Royuela, S.; Zamora, F.; Ruiz-González, M.L.; Segura, J.L.; Muñoz-Olivas, R.; Mancheño, M.J. Thiol grafted imine-based covalent organic frameworks for water remediation through selective removal of Hg(II). J. Mater. Chem. A 2017, 5, 17973–17981. [Google Scholar] [CrossRef]
- Alahakoon, S.B.; McCandless, G.T.; Karunathilake, A.A.; Thompson, C.M.; Smaldone, R.A. Enhanced structural organization in covalent organic frameworks through fluorination. Chemistry (Easton) 2017, 23, 4255–4259. [Google Scholar]
- Alahakoon, S.B.; Occhialini, G.; McCandless, G.T.; Karunathilake, A.A.K.; Nielsen, S.O.; Smaldone, R.A. Experimental and theoretical insight into the effect of fluorine substituents on the properties of azine linked covalent organic frameworks. CrystEngComm 2017, 19, 4882–4885. [Google Scholar] [CrossRef]
- Mendoza-Cortes, J.L.; Han, S.S.; Goddard, W.A., 3rd. High H2 uptake in Li-, Na-, K-metalated covalent organic frameworks and metal organic frameworks at 298 K. J. Phys. Chem. A 2012, 116, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, N.; Gao, J.; Xu, H.; Xu, F.; Jiang, D. Towards covalent organic frameworks with predesignable and aligned open docking sites. Chem. Commun. 2014, 50, 6161–6163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, P.; Wang, Y.; Zhang, J.; Gao, Y.; Zhang, P. Bottom-up approach to engineer a molybdenum-doped covalent-organic framework catalyst for selective oxidation reaction. RSC Adv. 2014, 4, 51544–51547. [Google Scholar] [CrossRef]
- Aiyappa, H.B.; Thote, J.; Shinde, D.B.; Banerjee, R.; Kurungot, S. Cobalt-modified covalent organic framework as a robust water oxidation electrocatalyst. Chem. Mater. 2016, 28, 4375–4379. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kamiya, K.; Hashimoto, K.; Nakanishi, S. Ru atom-modified covalent triazine framework as a robust electrocatalyst for selective alcohol oxidation in aqueous electrolytes. Chem. Commun. 2017, 53, 10437–10440. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xia, Q.; Huang, J.; Liu, Y.; Tan, C.; Cui, Y. Chiral covalent organic frameworks with high chemical stability for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 2017, 139, 8693–8697. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, M.; Sasmal, H.S.; Basu, A.; Midya, S.P.; Kandambeth, S.; Pachfule, P.; Balaraman, E.; Banerjee, R. Predesigned metal-anchored building block for in situ generation of Pd nanoparticles in porous covalent organic framework: Application in heterogeneous tandem catalysis. ACS Appl. Mater. Interfaces 2017, 9, 13785–13792. [Google Scholar] [CrossRef] [PubMed]
- Thote, J.; Aiyappa, H.B.; Deshpande, A.; Diaz Diaz, D.; Kurungot, S.; Banerjee, R. A covalent organic framework-cadmium sulfide hybrid as a prototype photocatalyst for visible-light-driven hydrogen production. Chemistry (Easton) 2014, 20, 15961–15965. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Haase, F.; Savasci, G.; Gottschling, K.; Ochsenfeld, C.; Lotsch, B.V. Single-site photocatalytic H2 evolution from covalent organic frameworks with molecular cobaloxime co-catalysts. J. Am. Chem. Soc. 2017, 139, 16228–16234. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, T.; Liu, L.; Chen, W.; Zhang, M.; Wu, G.; Chen, P. Covalent triazine framework supported non-noble metal nanoparticles with superior activity for catalytic hydrolysis of ammonia borane: From mechanistic study to catalyst design. Chem. Sci. 2017, 8, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-C.; Kan, J.-L.; Chen, G.-J.; Chen, C.-X.; Dong, Y.-B. Pd NPs-loaded homochiral covalent organic framework for heterogeneous asymmetric catalysis. Chem. Mater. 2017, 29, 6518–6524. [Google Scholar] [CrossRef]
- Baldwin, L.A.; Crowe, J.W.; Pyles, D.A.; McGrier, P.L. Metalation of a mesoporous three-dimensional covalent organic framework. J. Am. Chem. Soc. 2016, 138, 15134–15137. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dong, B.; Zhang, H.; Ge, R.; Gao, Y.; Zhang, H. Sulfur impregnated in a mesoporous covalent organic framework for high performance lithium–sulfur batteries. RSC Adv. 2015, 5, 86137–86143. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; Zhu, L.; Wang, H.; Naeem, A.; Khattak, A.M.; Liang, B.; Khan, N.A.; Wei, Z.; Li, L.; Tang, Z. Efficient polysulfide chemisorption in covalent organic frameworks for high-performance lithium-sulfur batteries. Adv. Energy Mater. 2016, 6, 1601250. [Google Scholar] [CrossRef]
- Mulzer, C.R.; Shen, L.; Bisbey, R.P.; McKone, J.R.; Zhang, N.; Abruna, H.D.; Dichtel, W.R. Superior charge storage and power density of a conducting polymer-modified covalent organic framework. ACS Cent. Sci. 2016, 2, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Dalapati, S.; Jiang, D. Charge up in wired covalent organic frameworks. ACS Cent. Sci. 2016, 2, 586–587. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Kundu, T.; Dey, K.; Addicoat, M.; Heine, T.; Banerjee, R. Interplaying intrinsic and extrinsic proton conductivities in covalent organic frameworks. Chem. Mater. 2016, 28, 1489–1494. [Google Scholar] [CrossRef]
- Shinde, D.B.; Aiyappa, H.B.; Bhadra, M.; Biswal, B.P.; Wadge, P.; Kandambeth, S.; Garai, B.; Kundu, T.; Kurungot, S.; Banerjee, R. A mechanochemically synthesized covalent organic framework as a proton-conducting solid electrolyte. J. Mater. Chem. A 2016, 4, 2682–2690. [Google Scholar] [CrossRef]
- Ma, H.; Liu, B.; Li, B.; Zhang, L.; Li, Y.-G.; Tan, H.-Q.; Zang, H.-Y.; Zhu, G. Cationic covalent organic frameworks: A simple platform of anionic exchange for porosity tuning and proton conduction. J. Am. Chem. Soc. 2016, 138, 5897–5903. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Jiang, D. Covalent organic frameworks with spatially confined guest molecules in nanochannels and their impacts on crystalline structures. Chem. Commun. 2016, 52, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Su, Q.; Guo, R.; Zhang, J.; Dong, A.; Lin, C.; Zhang, J. A multitasking hydrogel based on double dynamic network with quadruple-stimuli sensitiveness, autonomic self-healing property and biomimetic adhesion ability. Macromol. Chem. Phys. 2017, 218, 1700166. [Google Scholar] [CrossRef]
- Guo, R.; Su, Q.; Zhang, J.; Dong, A.; Lin, C.; Zhang, J. Facile access to multisensitive and self-healing hydrogels with reversible and dynamic boronic ester and disulfide linkages. Biomacromolecules 2017, 18, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, D.; Yao, D.; Guo, R.; Wang, W.; Dong, A.; Kong, D.; Zhang, J. An injectable particle-hydrogel hybrid system for glucose-regulatory insulin delivery. Acta Biomater. 2017, 64, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.B.; Choe, Y.H.; McGuire, J.; Conover, C.D. Effective drug delivery by PEGylated drug conjugates. Adv. Drug Deliv. Rev. 2003, 55, 217–250. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, Y.; Chu, J.; Zhang, X.; Deng, H. Multivariate metal-organic frameworks for dialing-in the binding and programming the release of drug molecules. J. Am. Chem. Soc. 2017, 139, 14209–14216. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Phua, S.Z.; Lim, W.Q.; Jana, A.; Luo, Z.; Tham, H.P.; Zhao, L.; Gao, Q.; Zhao, Y. Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem. Commun. 2016, 52, 4128–4131. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.S.; Vishwakarma, M.; Moudrakovski, I.; Haase, F.; Savasci, G.; Ochsenfeld, C.; Spatz, J.P.; Lotsch, B.V. Exploiting noncovalent interactions in an imine-based covalent organic framework for quercetin delivery. Adv. Mater. 2016, 28, 8749–8754. [Google Scholar] [CrossRef] [PubMed]
- Wybranowski, T.; Kruszewski, S. Optical spectroscopy study of the interaction between quercetin and human serum albumin. Acta Phys. Pol. A 2014, 125, A-57–A-60. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Park, K. Facing the truth about nanotechnology in drug delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, P.; Mishra, S.; Manna, K.; Mallick, A.; Das Saha, K.; Bhaumik, A. Covalent organic framework material bearing phloroglucinol building units as a potent anticancer agent. ACS Appl. Mater. Interfaces 2017, 9, 31411–31423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, M.; Zu, Y.; Fu, Y.; Gu, C.; Wang, W.; Yao, L.; Efferth, T. Dryofragin, a phloroglucinol derivative, induces apoptosis in human breast cancer MCF-7 cells through ROS-mediated mitochondrial pathway. Chem. Biol. Interact. 2012, 199, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wan, J.; Guo, J.; Wang, C. Self-sacrificial template-induced modulation of conjugated microporous polymer microcapsules and shape-dependent enhanced photothermal efficiency for ablation of cancer cells. Chem. Commun. 2015, 51, 17394–17397. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. Int. Ed. Engl. 2013, 52, 13958–13964. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, C.X.; Yan, X.P. A versatile covalent organic framework-based platform for sensing biomolecules. Chem. Commun. 2017, 53, 11469–11471. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Huang, Y.; Zhu, Y.; Chen, B.; Wang, L.; Lai, Z.; Zhang, Z.; Zhao, M.; Tan, C.; Yang, N.; et al. Ultrathin two-dimensional covalent organic framework nanosheets: Preparation and application in highly sensitive and selective DNA detection. J. Am. Chem. Soc. 2017, 139, 8698–8704. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xue, R.; Chen, H.; Shi, P.; Lei, X.; Wei, Y.; Guo, H.; Yang, W. Preparation of two new polyimide bond linked porous covalent organic frameworks and their fluorescence sensing application for sensitive and selective determination of Fe3+. New J. Chem. 2017, 41, 14272–14278. [Google Scholar] [CrossRef]
- Lei, C.; Shin, Y.; Liu, J.; Ackerman, E.J. Entrapping enzyme in a functionalized nanoporous support. J. Am. Chem. Soc. 2002, 124, 11242–11243. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wheatley, P.S.; Zhao, X.; Fletcher, A.J.; Fox, S.; Rossi, A.G.; Megson, I.L.; Bordiga, S.; Regli, L.; Thomas, K.M. High-capacity hydrogen and nitric oxide adsorption and storage in a metal–organic framework. J. Am. Chem. Soc. 2007, 129, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
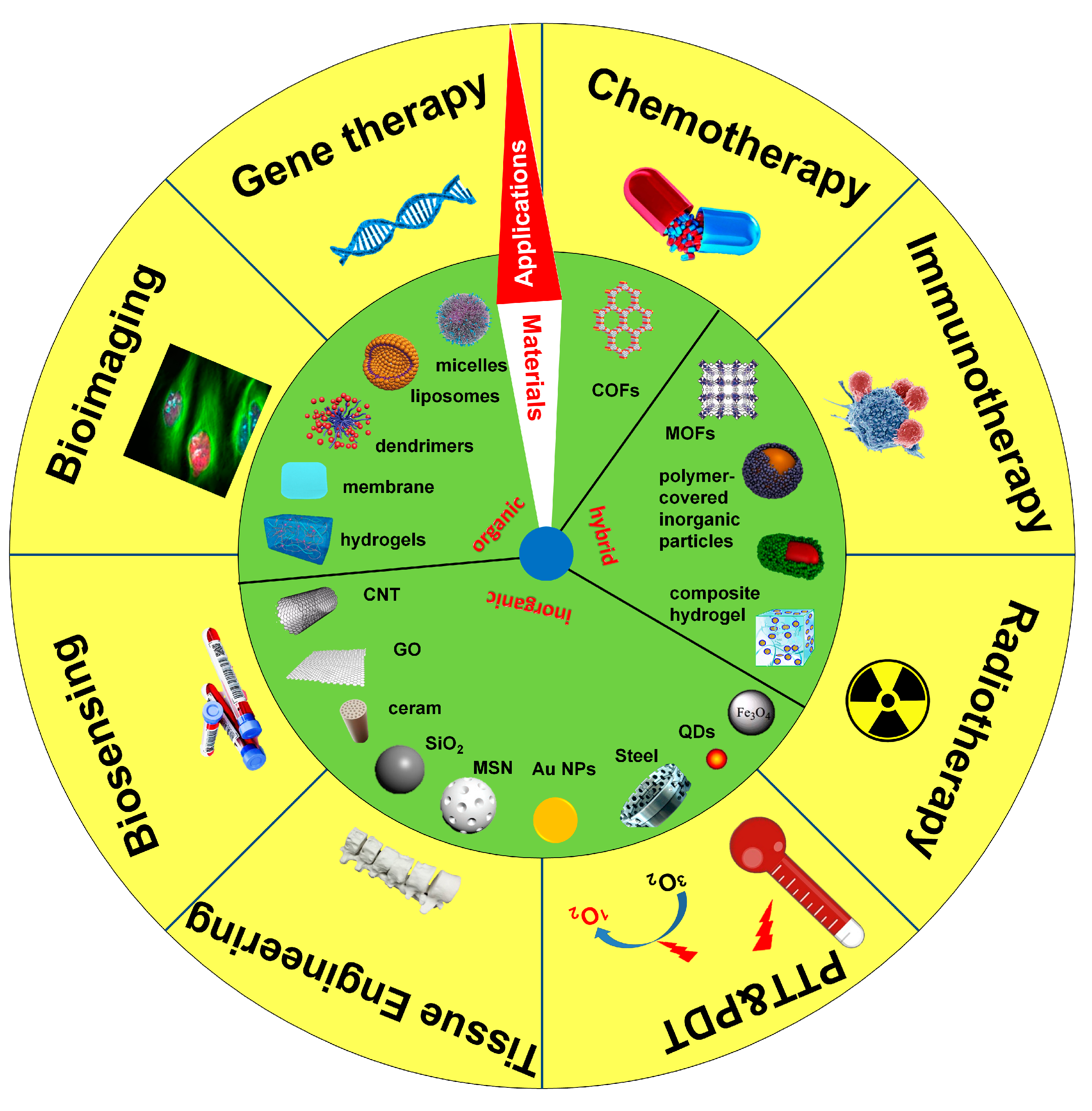
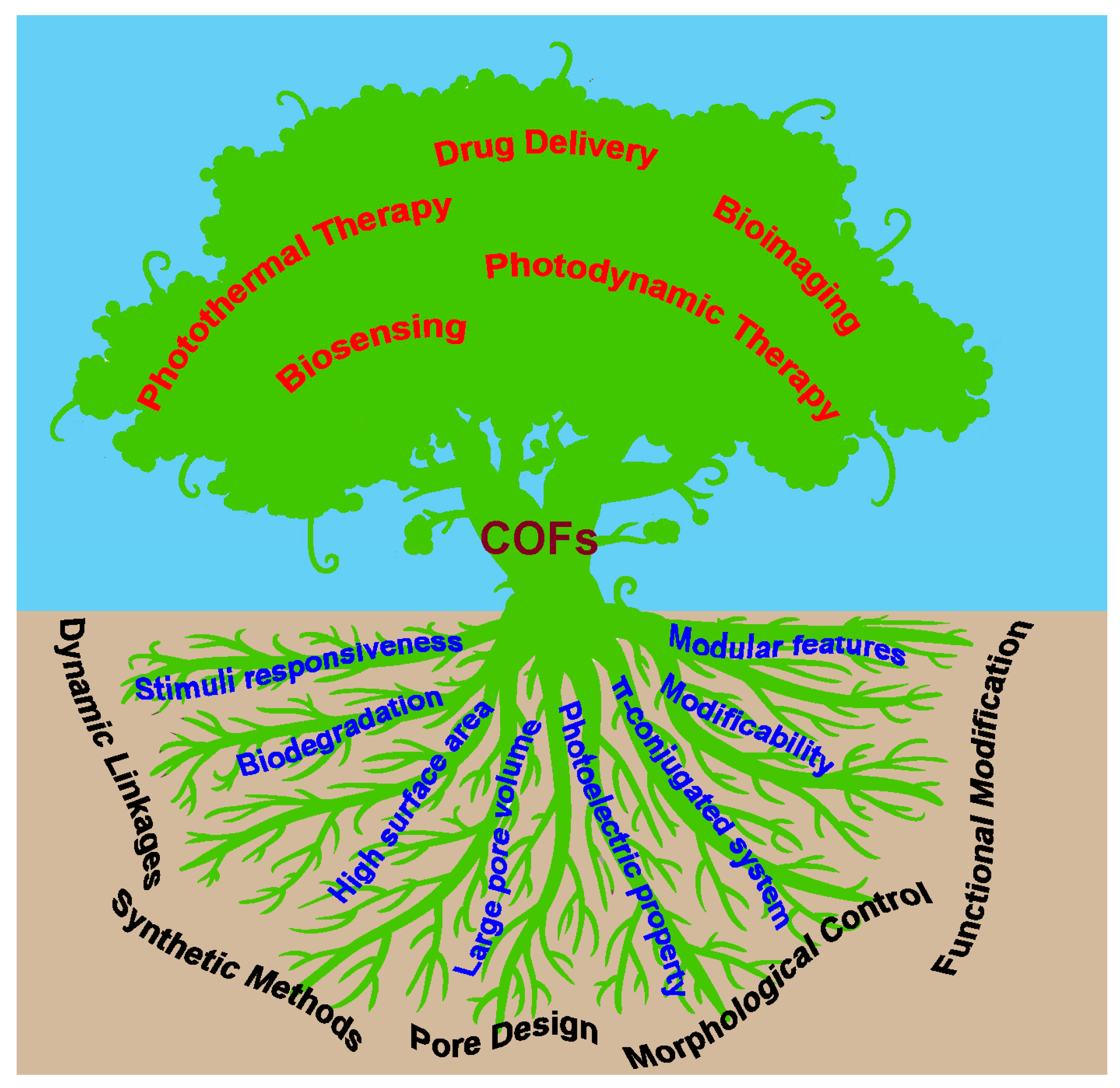
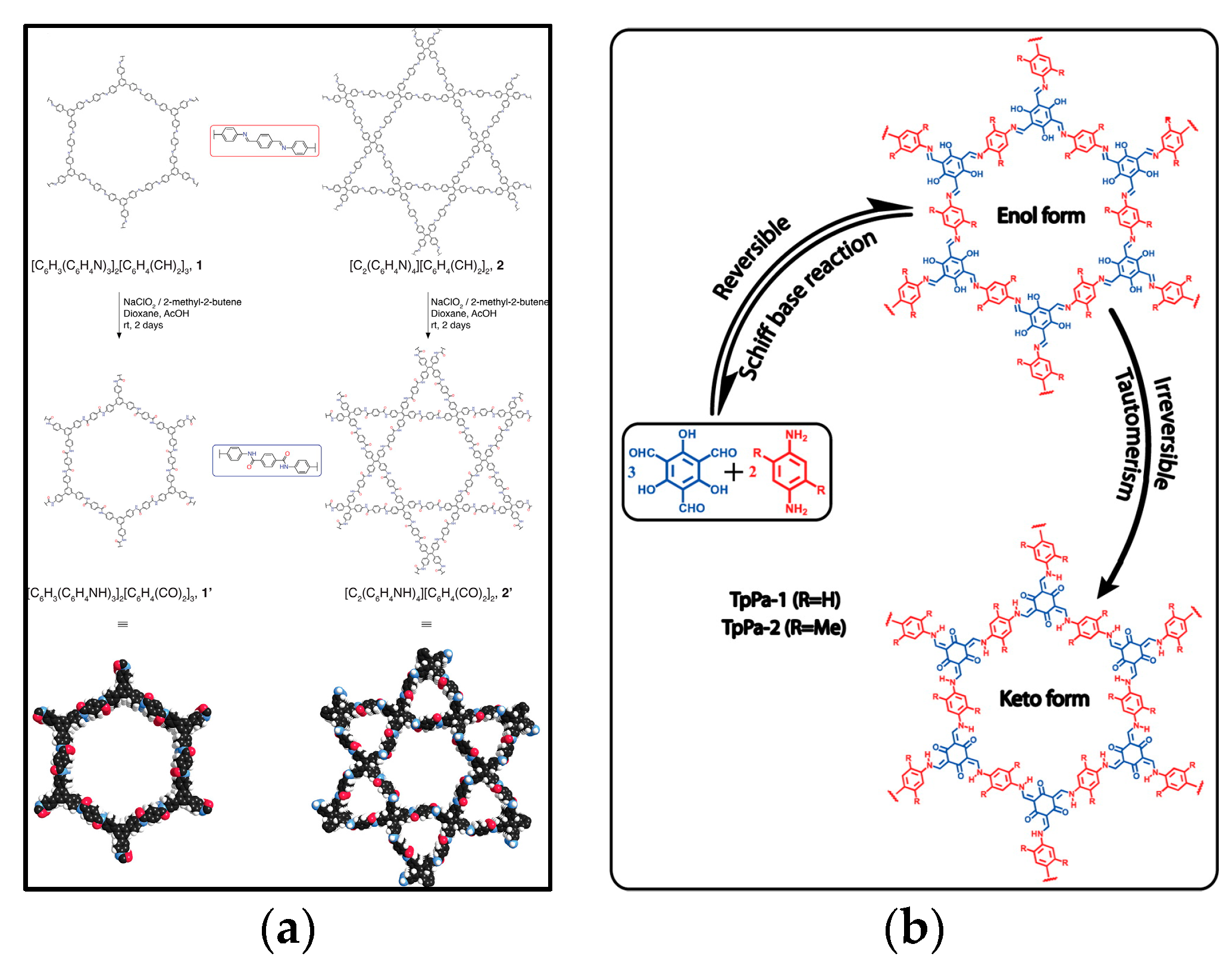

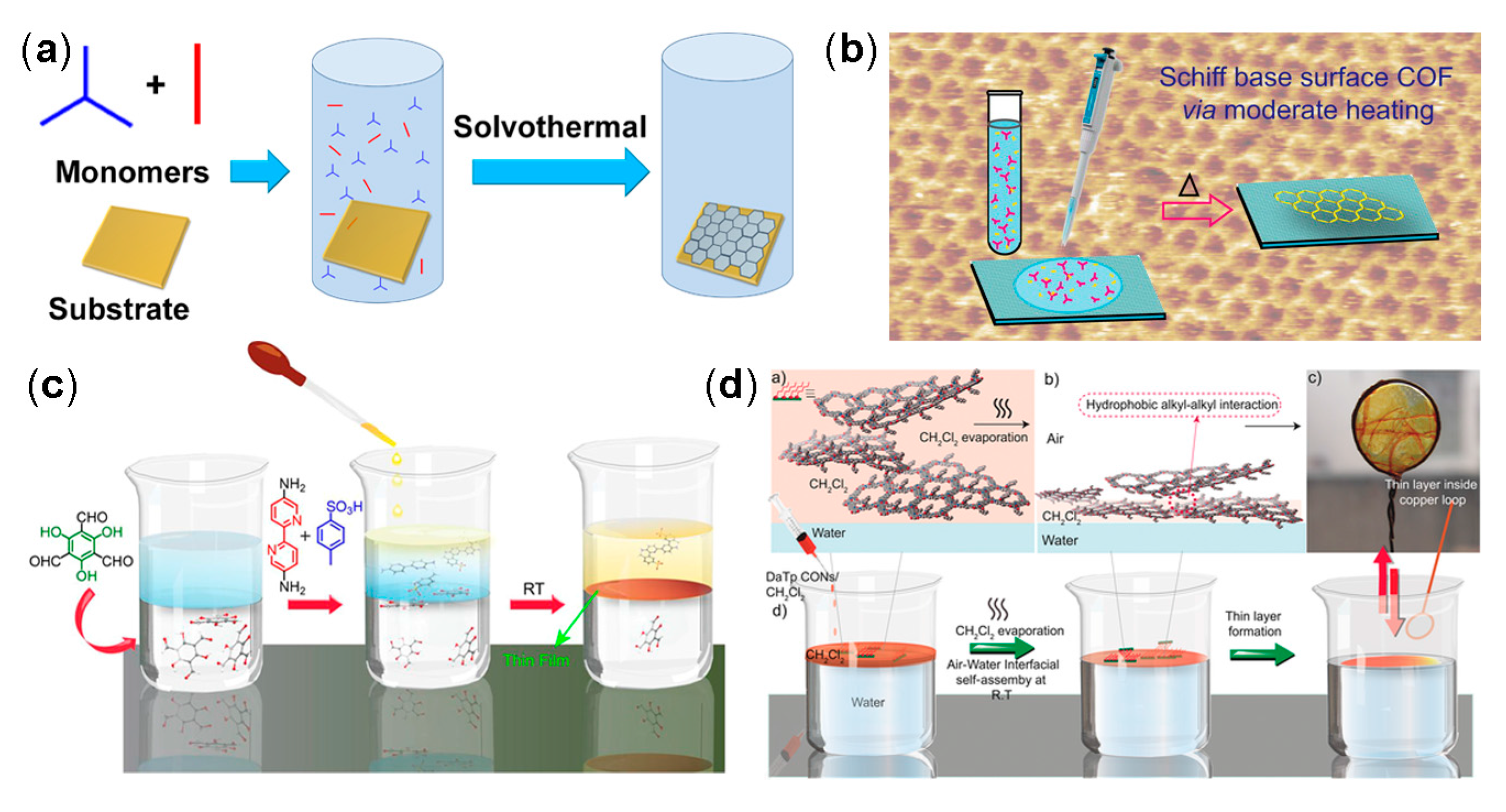
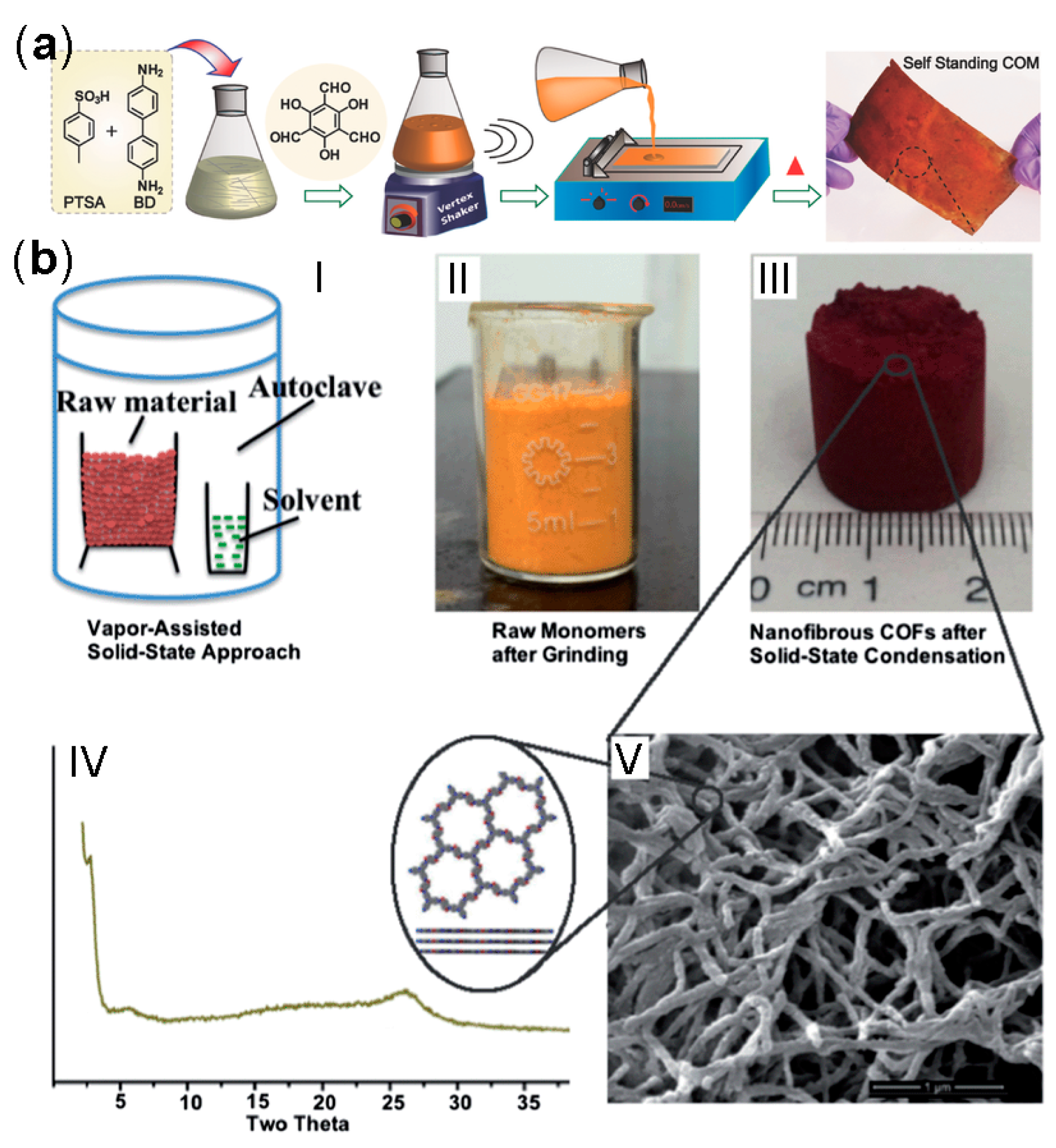

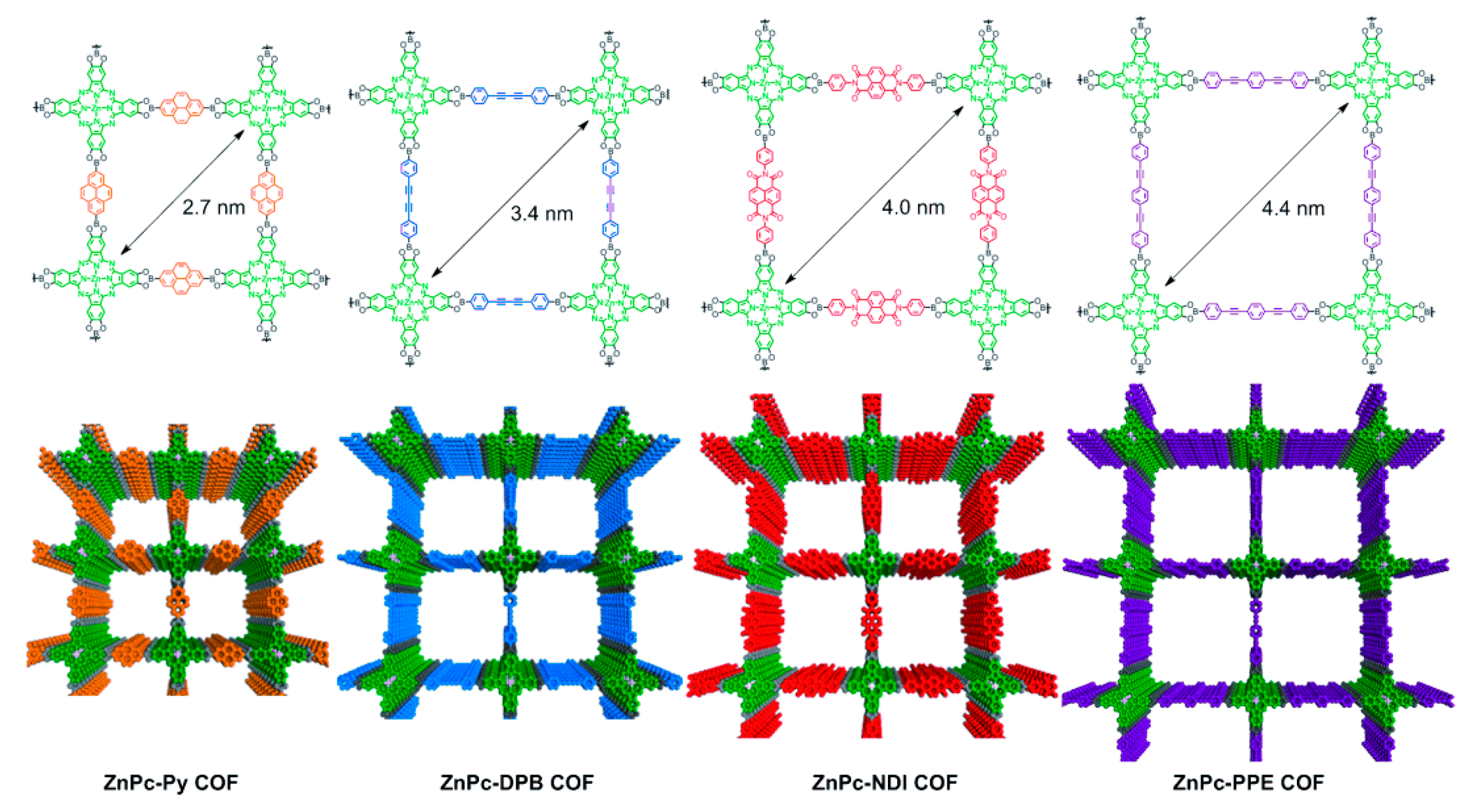
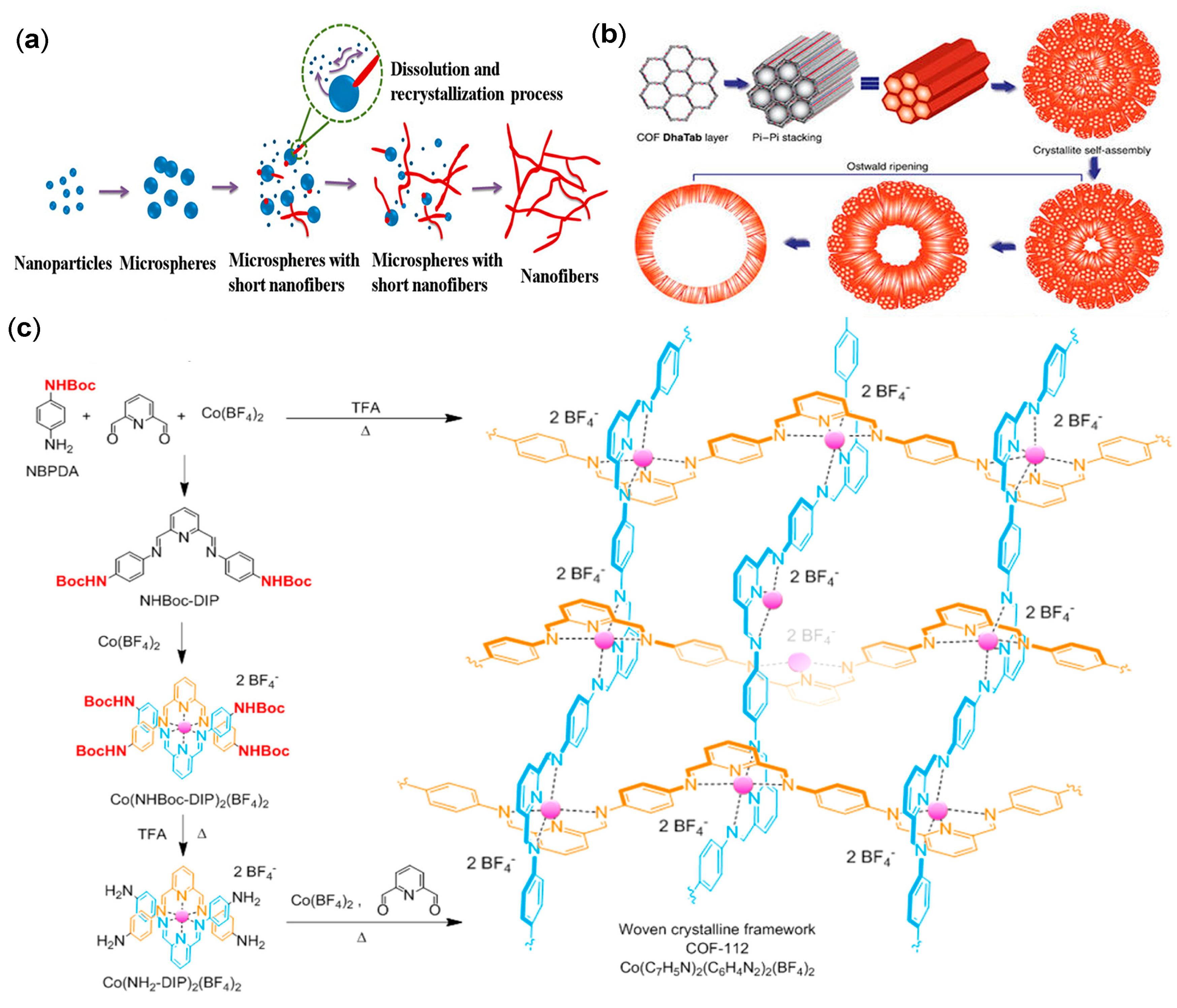
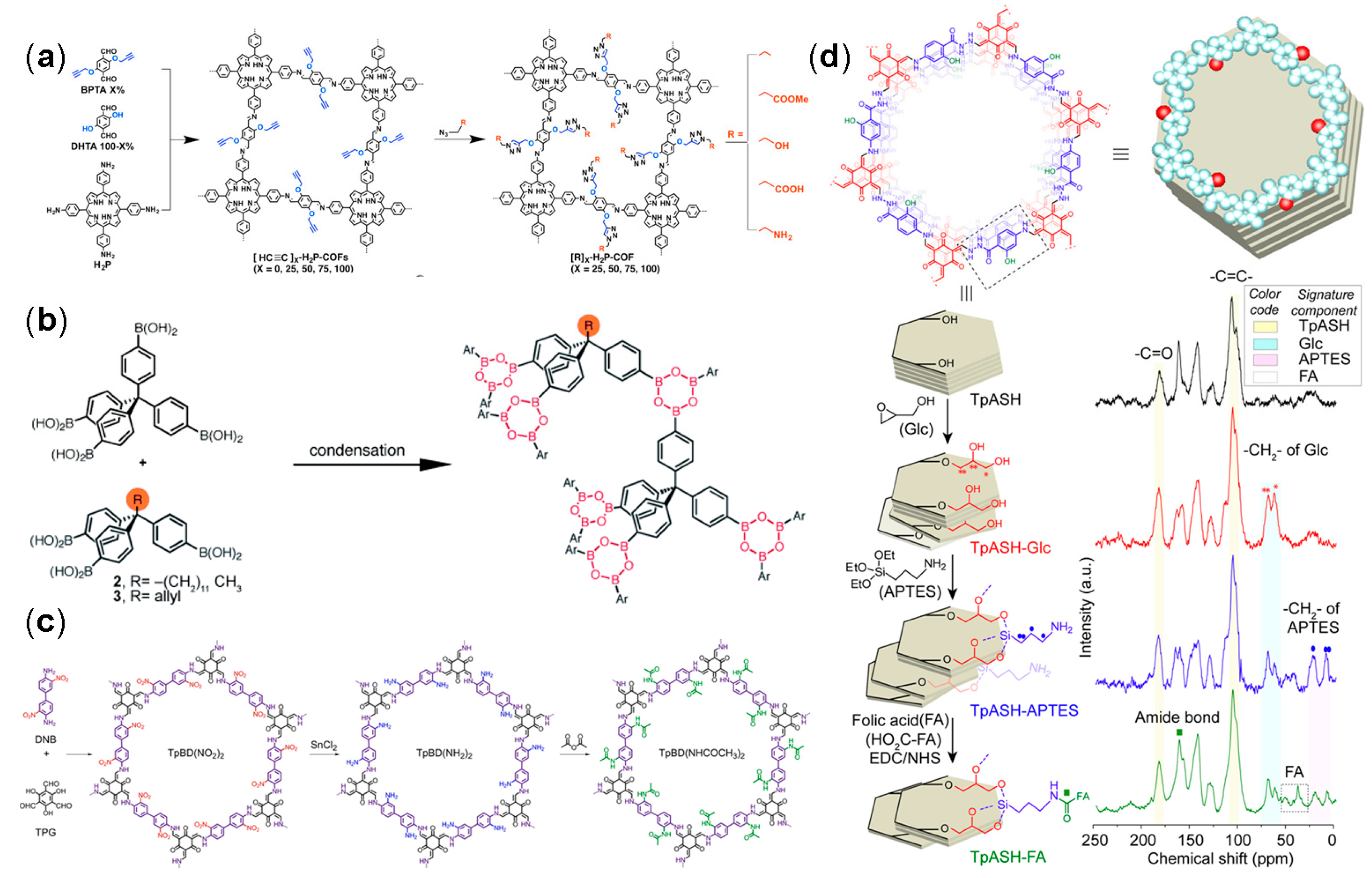
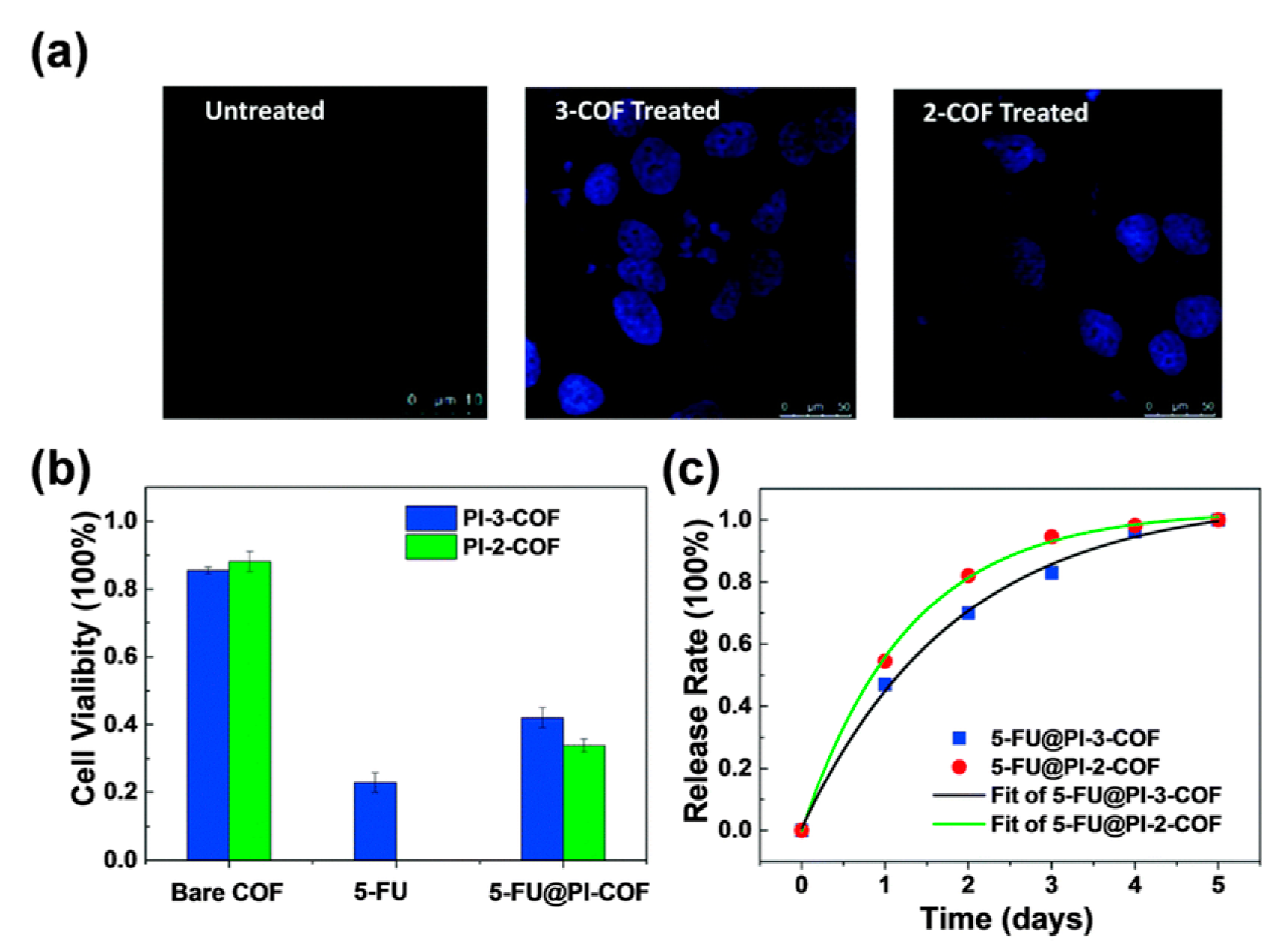
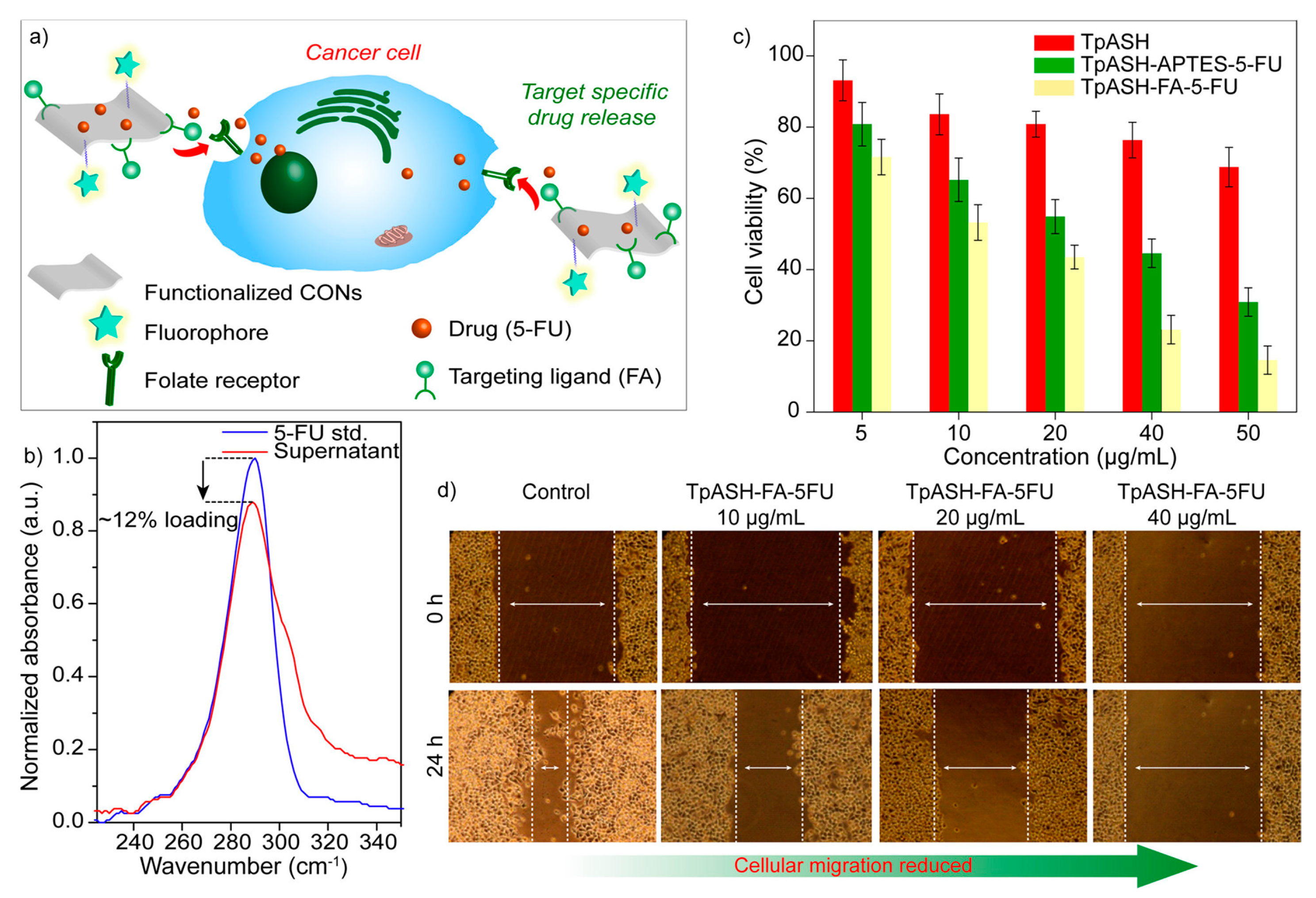
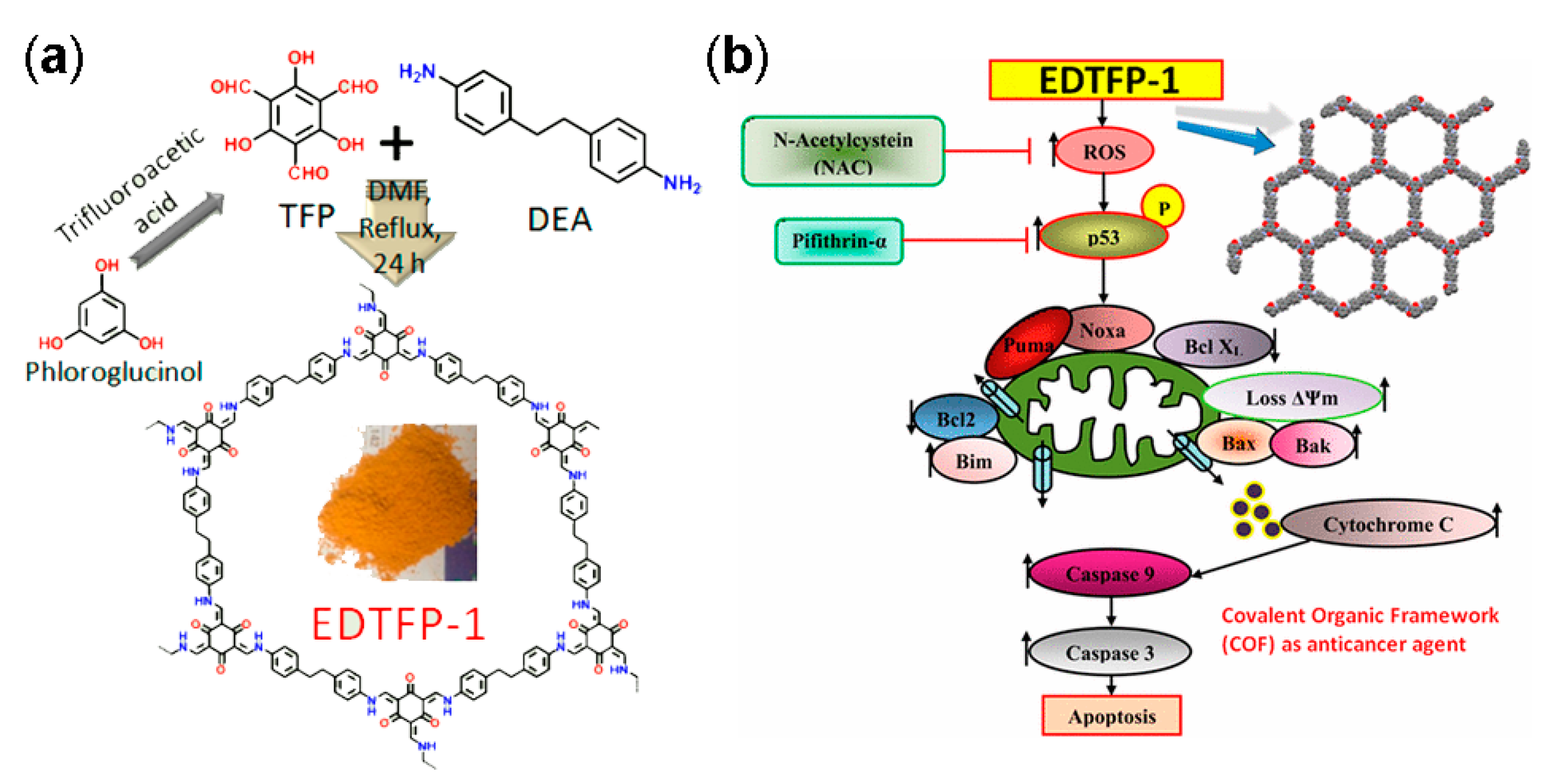
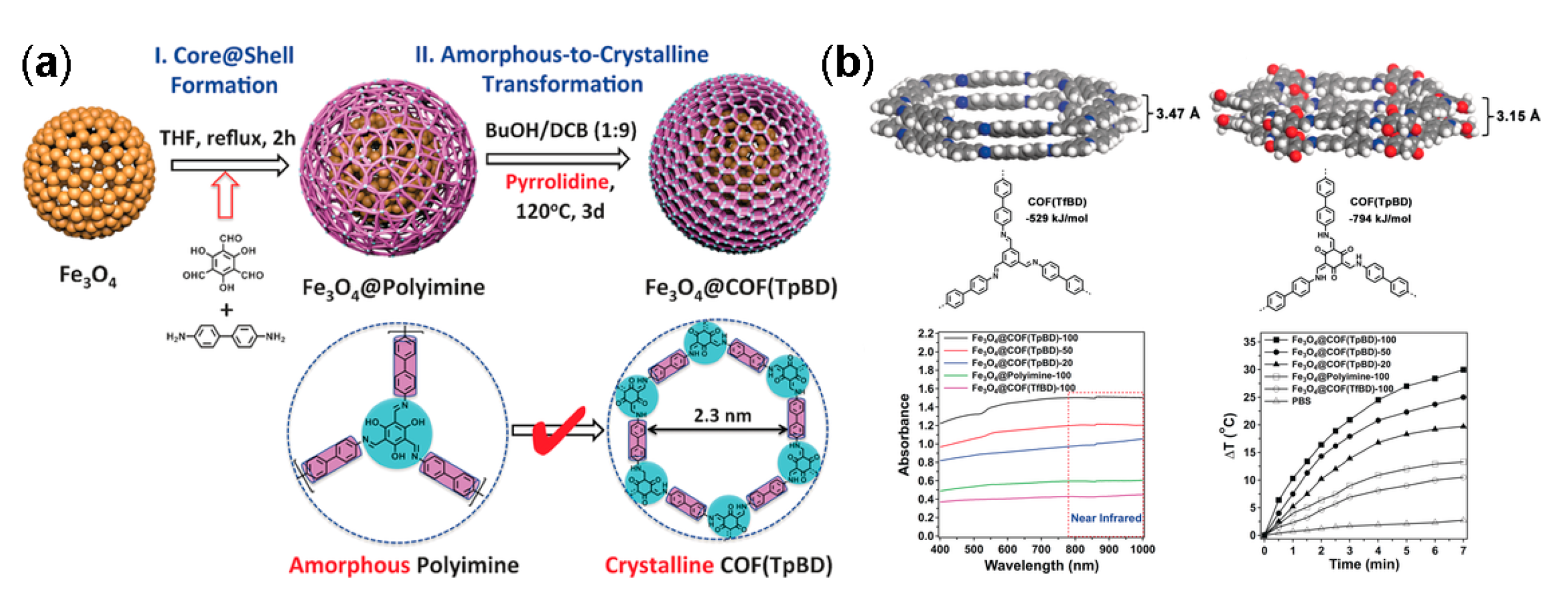
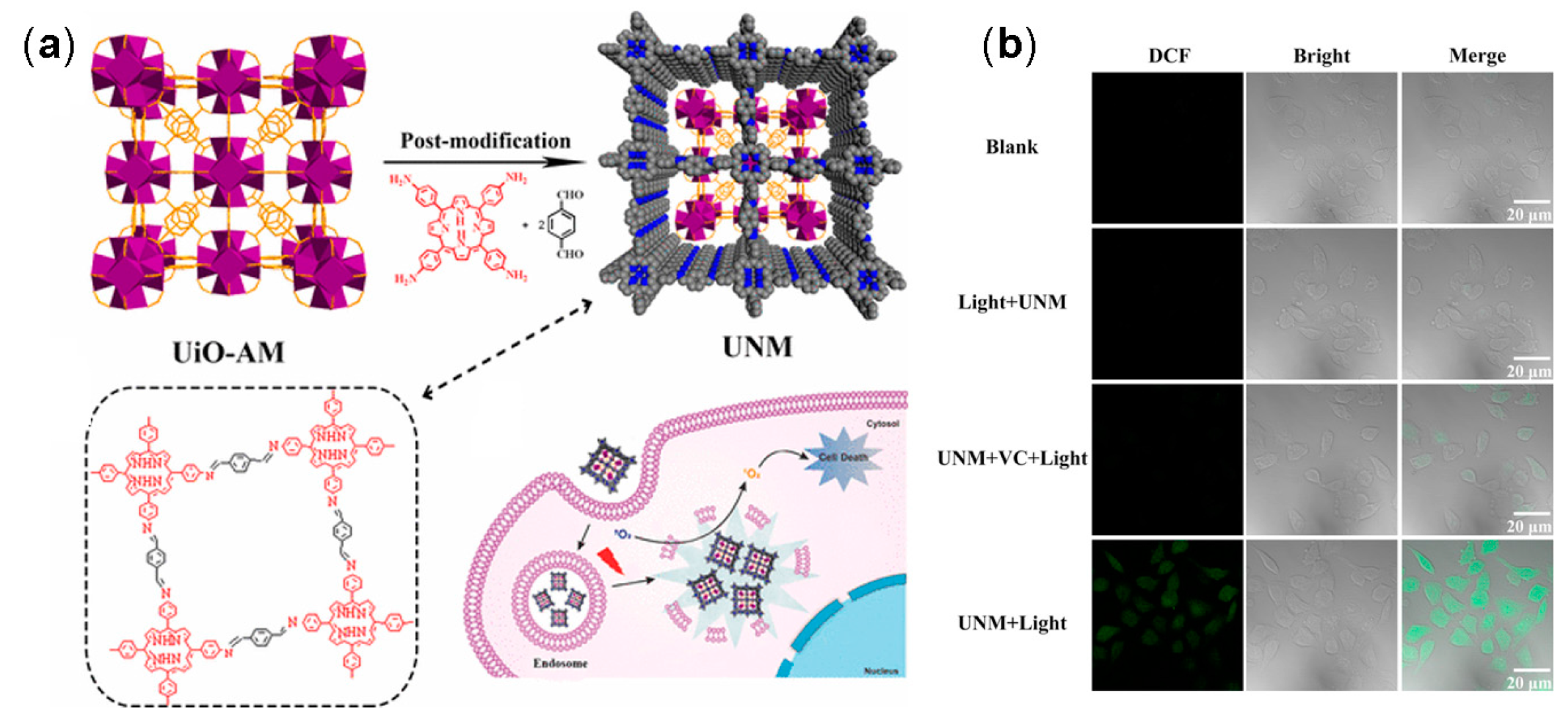
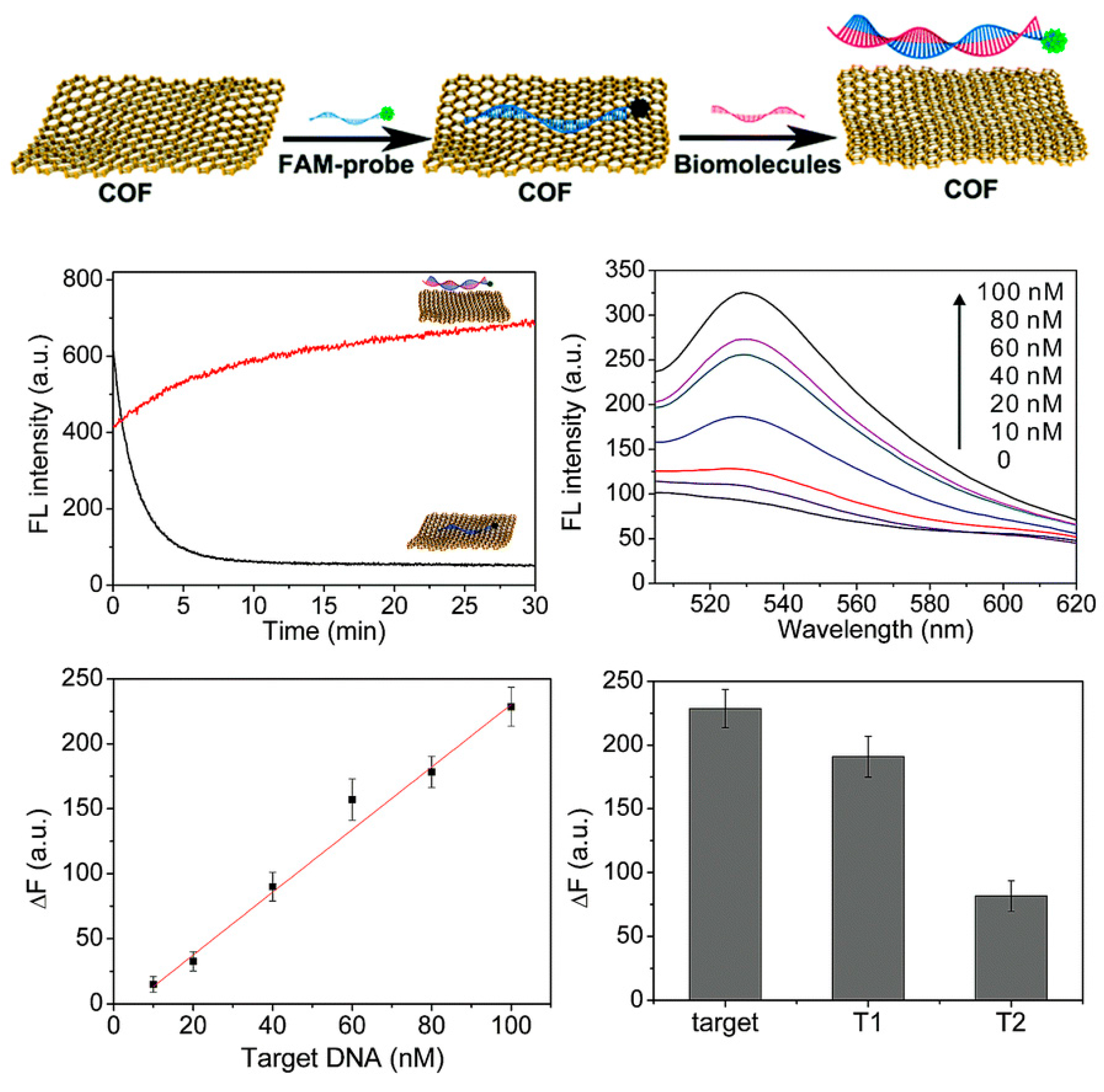
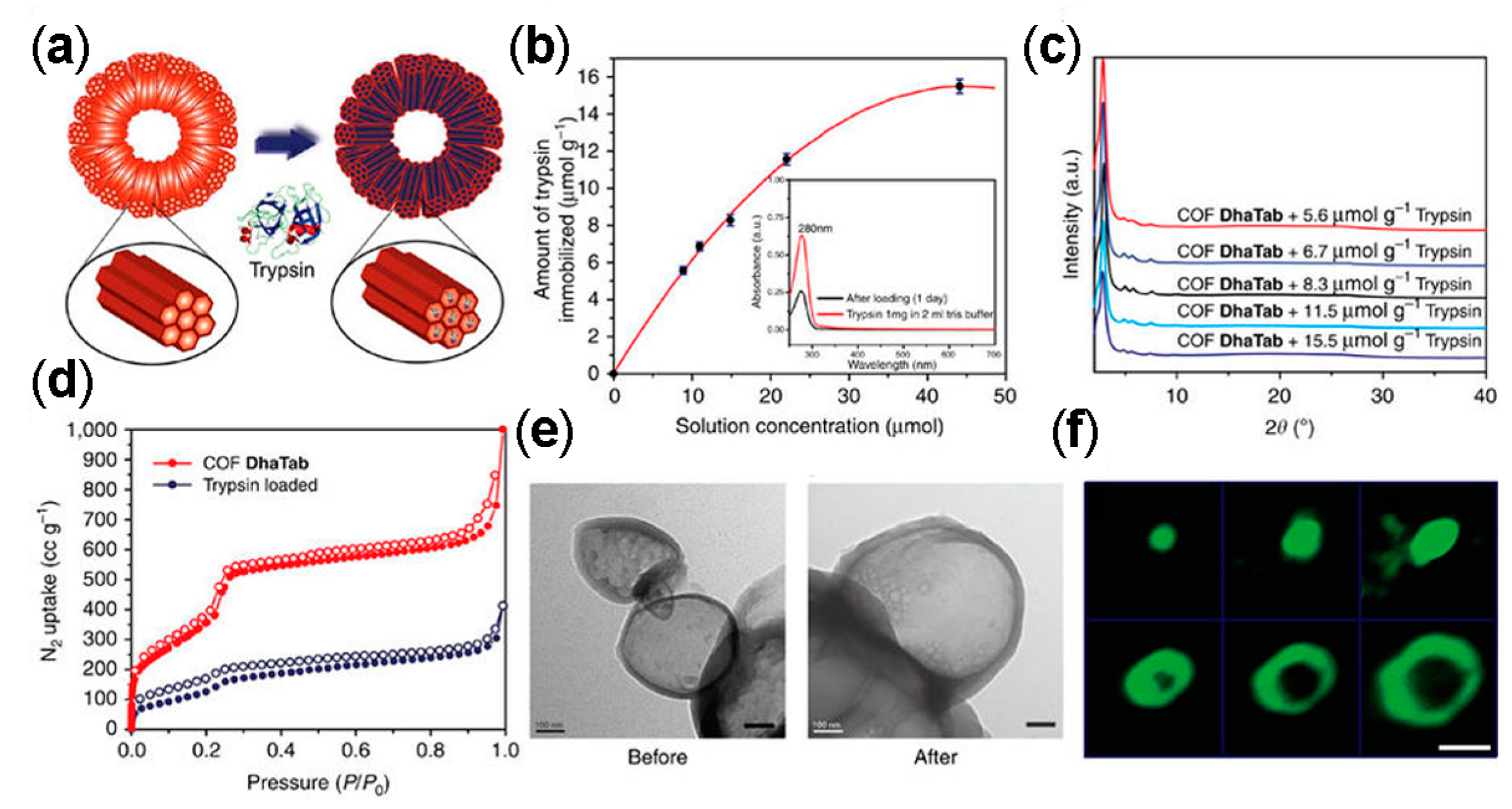
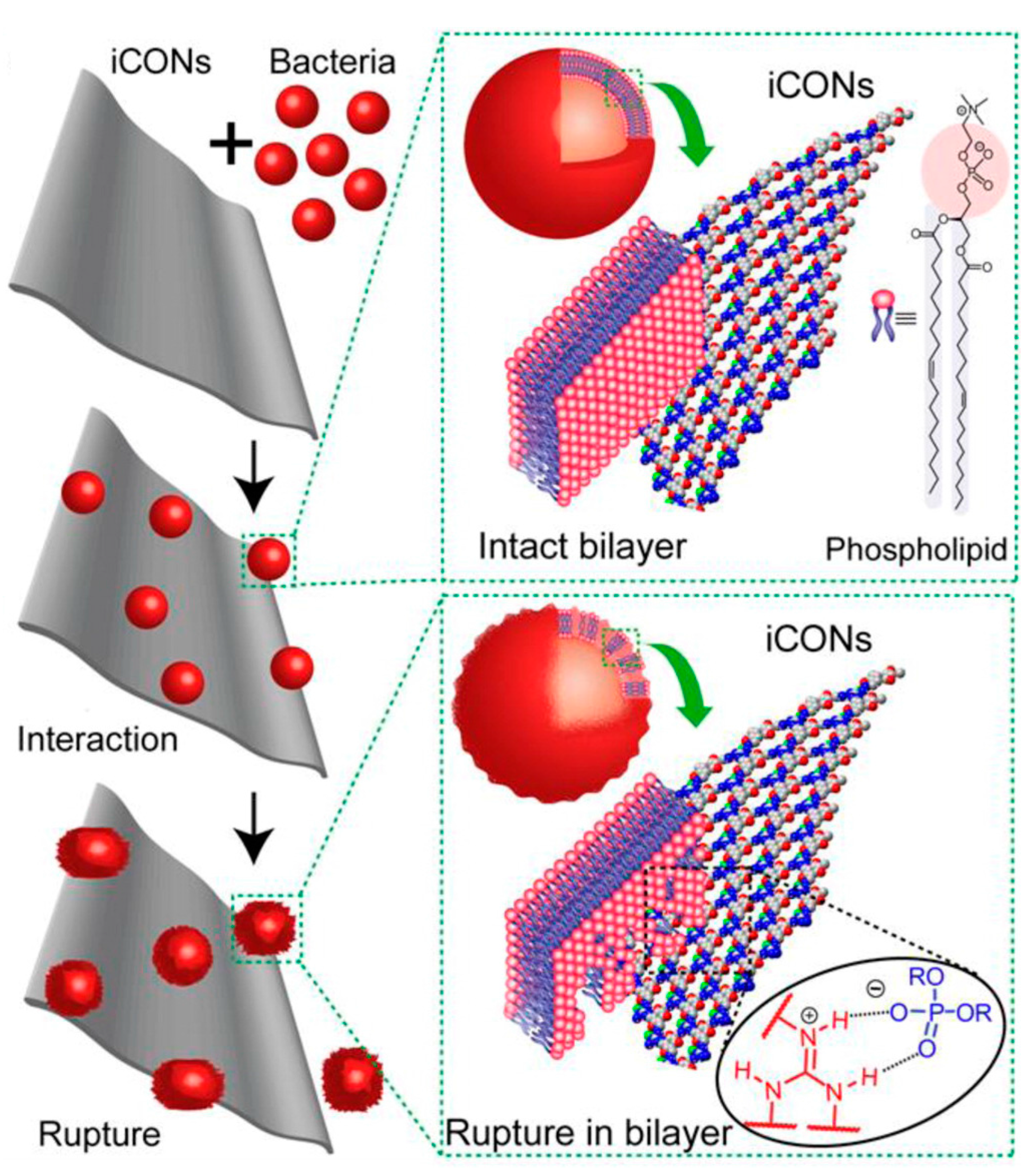
| Categories | Main Contents | |
|---|---|---|
| Comprehensive Reviews | Structure designs, synthetic methods, characterizations and applications [65,66,67,68,69] | |
| Structures | Linkages | Different linkages [55,56,57]; New linkages beyond boron chemistry [70]; C–N based COFs [71]; Triazine-based COFs [72]; Schiff-based COFs [73]; imine-based COFs [74] |
| Building Blocks | Porphyrin- and phthalocyanine-based COFs [75]; Boronic acid-based COFs [76] | |
| Pore Designs | Multiparous 2D COF [77] | |
| Architectures | Structure design [48,78,79] | |
| Applications | Absorption & Storage | Energy storage [62,80,81]; Gas storage [55]; Hydrogen storage [82,83,84]; CO2 capture [56,57] |
| Catalysis | Heterogeneous catalysis [72]; single-site catalysts [85]; Electrocatalysts [86] | |
| Analysis & Detection | Electronic and optical applications [62,87]; Analytical applications [88]; Chemical sensing [89] | |
| Comprehensiveness | Various applications [78,90] | |
| Synthetic Method | Various synthetic methods [73]; Mechanochemical synthesis [91] | |
| Bonds | Linkages | Characters | Reference |
|---|---|---|---|
| B–O | Boronate ester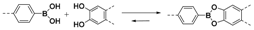 | Crystallinity: Excellent; Thermostability: 600 °C; Chemical stability: Sensitive to water, acid, base, alcohols and atmospheric moisture. | [51] |
Boronxine | Crystallinity: Excellent; Thermostability: 500 °C; Chemical stability: Sensitive to water, acid, base, alcohols and atmospheric moisture. | [51] | |
Borosilicate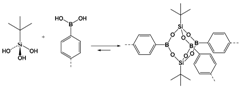 | Crystallinity: Excellent; Thermostability: 450 °C; Chemical stability: 1 h in air. | [93] | |
Spiroborate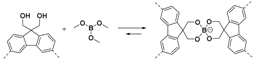 | Crystallinity: Excellent; Thermostability: Loss 7–12% at 400 °C; Chemical stability: 2 d in water and 1 M LiOH; sensitive to acid. | [94] | |
| C–N | Imine | Crystallinity: Good; Thermostability: 500 °C; Chemical stability: Better than boron-based COFs. | [95] |
Hydrazone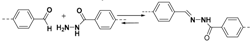 | Crystallinity: Good; Thermostability: 280 °C; Chemical stability: Better than imine-linked COFs. | [96] | |
Imide 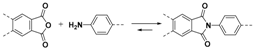 | Crystallinity: Good; Thermostability: 530 °C; Chemical stability: Better than boron-based COFs. | [97] | |
Amide  | Crystallinity: Good; Thermostability: 400 °C; Chemical stability: 1 d in 12 M HCl and 1 M NaOH. | [98] | |
| C–N | β-ketoenamine 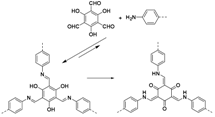 | Crystallinity: Good; Thermostability: 350 °C; Chemical stability: More than 7 d in boing water, 9 M HCl and 9 M NaOH. | [99] |
β-ketoenamine  | Crystallinity: Moderate; Thermostability: 300 °C; Chemical stability: 7 d in hot water (50 °C) and 9 M HCl; partial hydrolysis in 9 M NaOH. | [100] | |
Azine 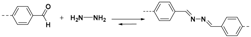 | Crystallinity: Moderate; Thermostability: 250 °C; Chemical stability: 1 d in water, 1 M HCl, 1 M NaOH and organic solvents. | [101] | |
Phenazine 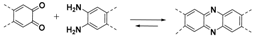 | Crystallinity: Moderate; Thermostability: More than 1000 °C; Chemical stability: 1 d in water, 1 M HCl, 1 M NaOH and organic solvents. | [102] | |
Squaraine 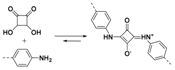 | Crystallinity: Moderate; Thermostability: 300 °C; Chemical stability: 1 d in water, 1 M HCl, 1 M NaOH and organic solvents. | [103] | |
Viologen 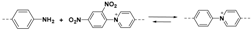 | Crystallinity: Moderate; Thermostability: 400 °C; Chemical stability: 3 d in boiling water and 6 M HCl; sensitive to 1 M NaOH. | [104] | |
Triazine  | Crystallinity: Poor; Thermostability: 400 °C; Chemical stability: High stability (no precise description). | [105] | |
Melamine | Crystallinity: Poor; Thermostability: 400 °C; Chemical stability: Stable in water and common organic solvents. | [106] | |
| C–C | C–C  | Crystallinity: No reported; Thermostability: 250 °C; Chemical stability: No reported. | [107] |
C=C 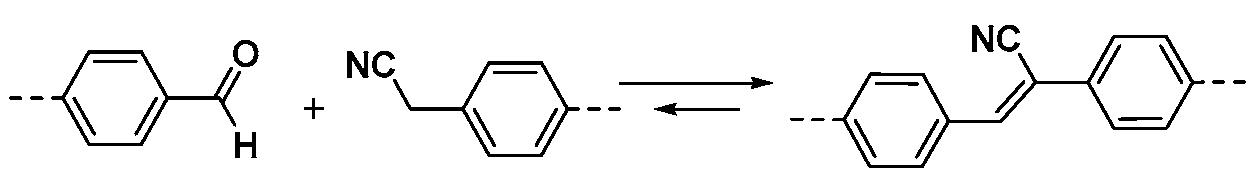 | Crystallinity: Moderate; Thermostability: 350 °C; Chemical stability: High stability (no precise description). | [108] | |
| B–N | Borazine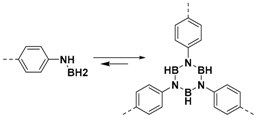 | Crystallinity: Good; Thermostability: 420 °C; Chemical stability: No reported. | [109] |
| N–N | Azodioxide 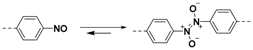 | Crystallinity: Monocrystalline; Thermostability: Less than 130 °C; Chemical stability: No reported. | [110] |
| Year | COFs | Linkages | Pore Sizes (nm) | Morphologies | Model Drugs | Characters | Reference |
|---|---|---|---|---|---|---|---|
| 2015 | PI-COF-4 PI-COF-5 | Imide | 1.3 1.0 | Rectangular; Length: Hundreds of nanometers | IBU, caffeine and captopril | DLE a: 24 wt %; DRR: 95% for 6 days. DLE a: 20 wt %; DRR: 95% for 6 days. | [63] |
| 2015 | COF-DhaTab | Imine | 3.7 | Submicron hollow spheres | DOX | DLE b: 0.35 mg/g; DRR: 42% after 7 days at pH 5 | [116] |
| 2016 | PI-2-COF PI-3-COF | Imine | 1.4 1.1 | Spherical nanoparticles; 50 nm | 5-FU, IBU and captopril | DLE a: 30 wt %; DRR: 85% for 5 days; Good biocompatibility. | [323] |
| 2016 | COFABBA | Boroxine | 2.1 | Single-layer | CuPc | Photoresponsive release; No DLE nor DRR has been measured. | [266] |
| 2016 | TTI-COF | Imine | 2.4 | Elongated rods | Quercetin | DLE b: fail to measure; DRR: fail to measure; Good biocompatibility. | [324] |
| 2016 | NCTP | Triazine | 1.21 | Spherical nanoparticles; 50–70 nm | DOX | DLE a: 20 wt %; DRR: 60% for 2 days at pH 7.4 & 80% for 2 days at pH 4.8; Good biocompatibility. | [131] |
| 2017 | EDTFP-1 | β-ketoenamine | 1.5 | Nanofiber; Diameter: 22–30 nm | TFP | TFP works as model drug as well as building block; Kill cancer cells by COFs themselves. | [328] |
| 2017 | TpASH-FA | β-ketoenamine | 1.3 | Nanosheets | 5-FU | DLE b: 12 wt %; DRR: 50% for 75 h at pH 7.4 & 75% for 75 h at pH 5; Good biocompatibility. | [64] |
| 2017 | PCTF PCTF-Mn | Triazine | 0.8~2.7 0.7~4.2 | Irregular nanoparticles; Plate-shaped | IBU | DLE a: 19 wt %; DRR: 90% for 48 h. DLE a: 23 wt %; DRR: 95% for 48 h. | [132] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Liu, H.; Mathe, S.D.R.; Dong, A.; Zhang, J. Covalent Organic Frameworks: From Materials Design to Biomedical Application. Nanomaterials 2018, 8, 15. https://doi.org/10.3390/nano8010015
Zhao F, Liu H, Mathe SDR, Dong A, Zhang J. Covalent Organic Frameworks: From Materials Design to Biomedical Application. Nanomaterials. 2018; 8(1):15. https://doi.org/10.3390/nano8010015
Chicago/Turabian StyleZhao, Fuli, Huiming Liu, Salva D. R. Mathe, Anjie Dong, and Jianhua Zhang. 2018. "Covalent Organic Frameworks: From Materials Design to Biomedical Application" Nanomaterials 8, no. 1: 15. https://doi.org/10.3390/nano8010015
APA StyleZhao, F., Liu, H., Mathe, S. D. R., Dong, A., & Zhang, J. (2018). Covalent Organic Frameworks: From Materials Design to Biomedical Application. Nanomaterials, 8(1), 15. https://doi.org/10.3390/nano8010015






