Versatility of Pyridoxal Phosphate as a Coating of Iron Oxide Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
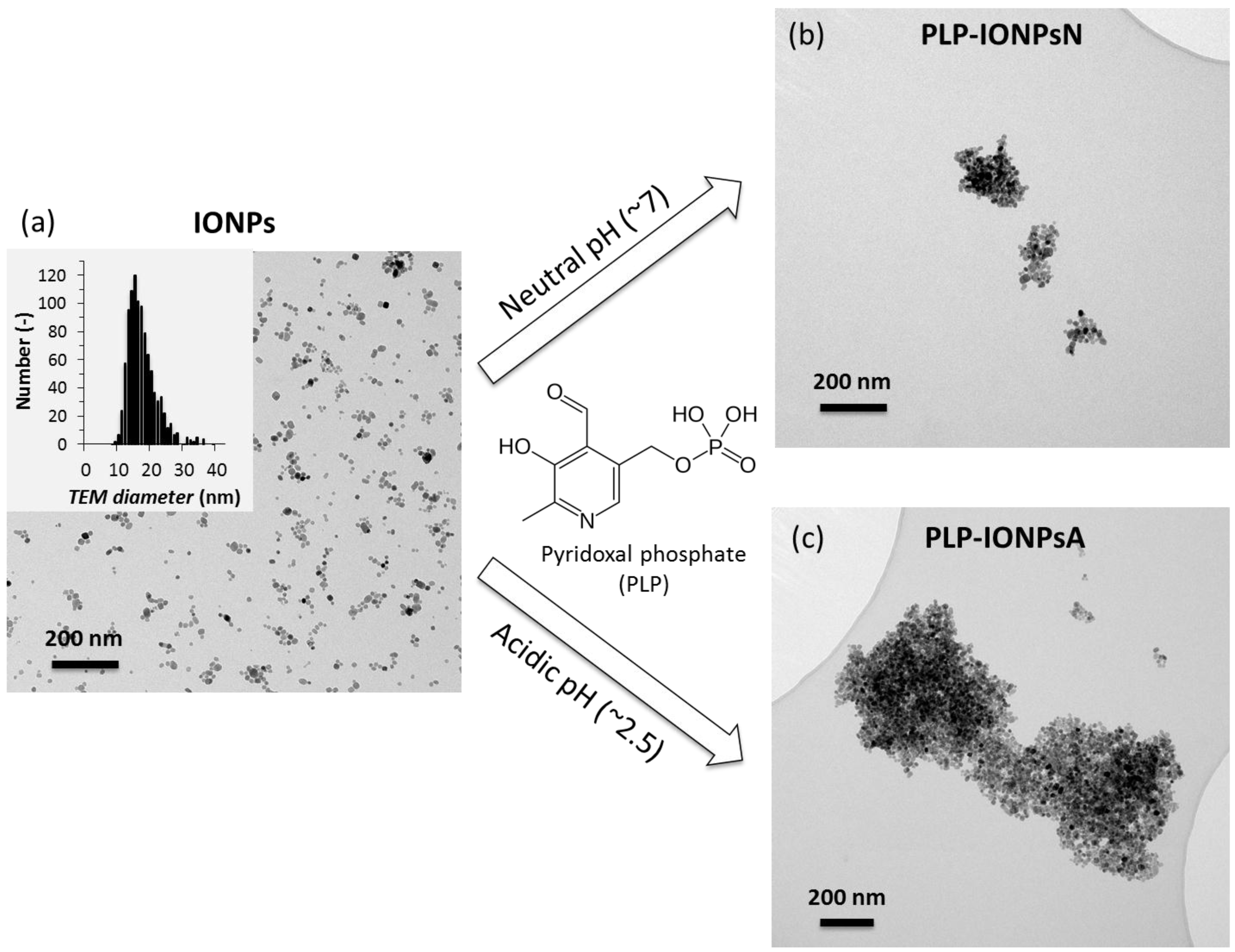
2.1. Binding of PLP to IONPs
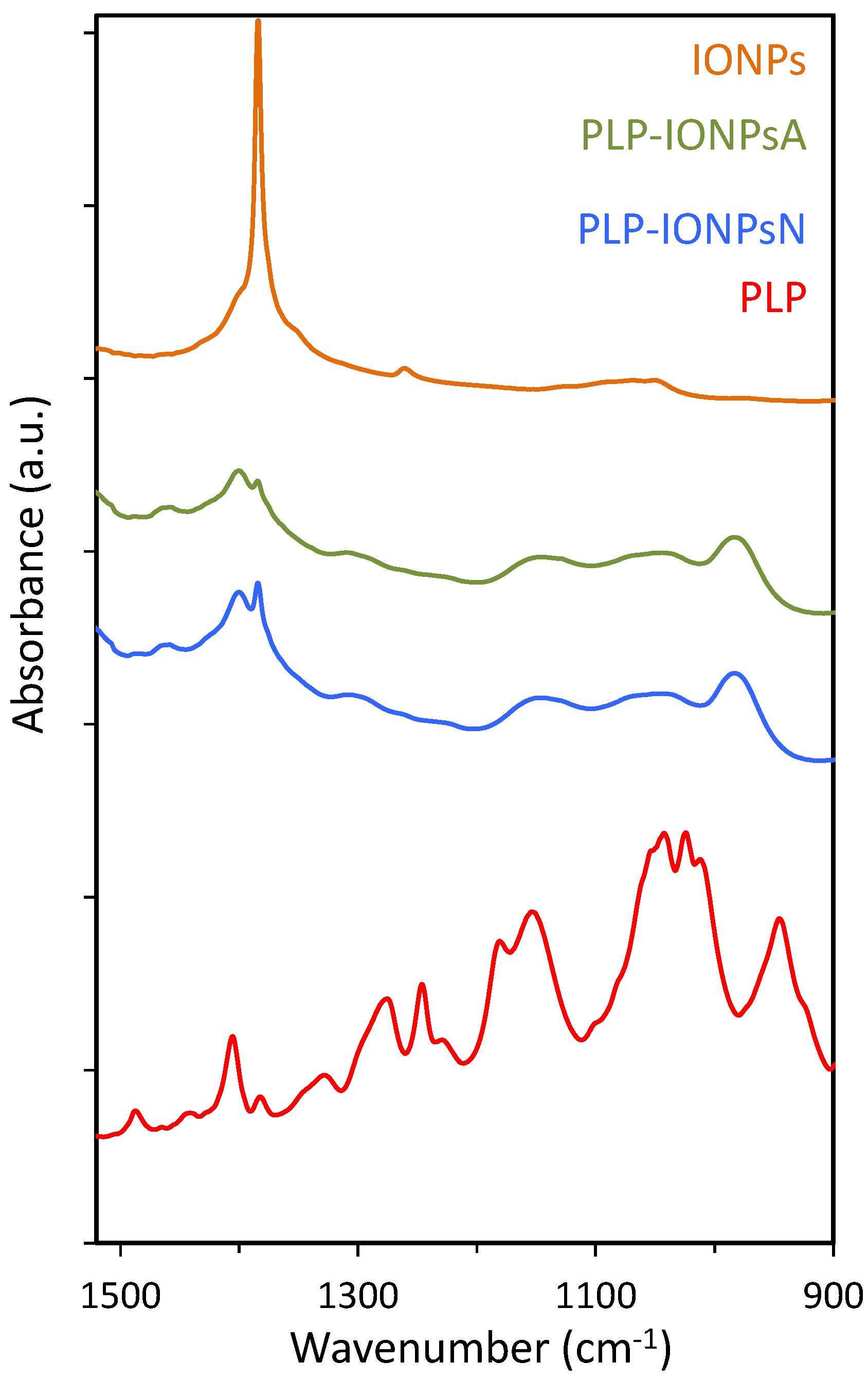
2.2. Surface Characterization
2.3. Stability of PLP-Coated IONPs
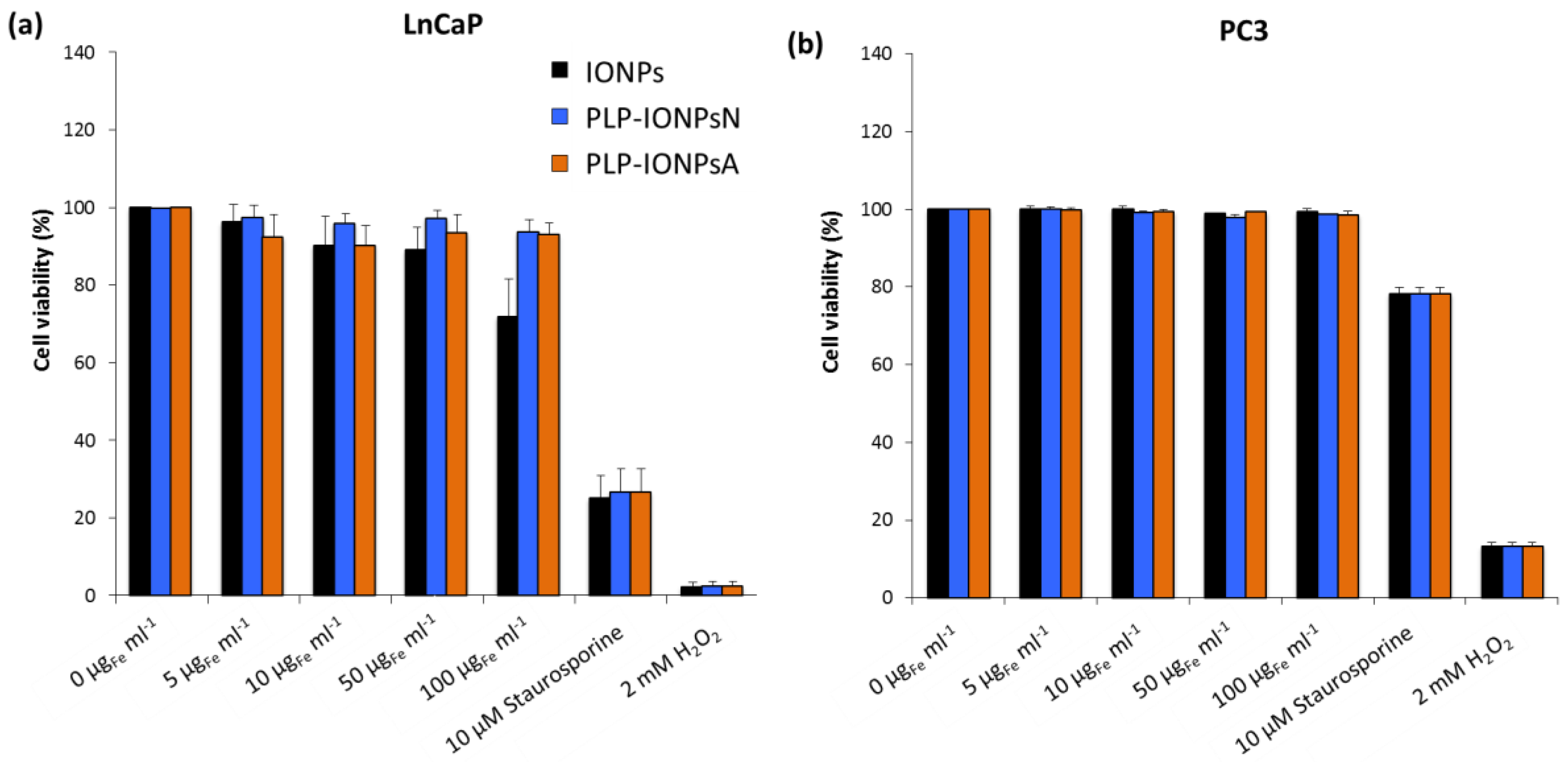
2.4. Toxicity Evaluation
2.5. Interaction of IONPs with Cells
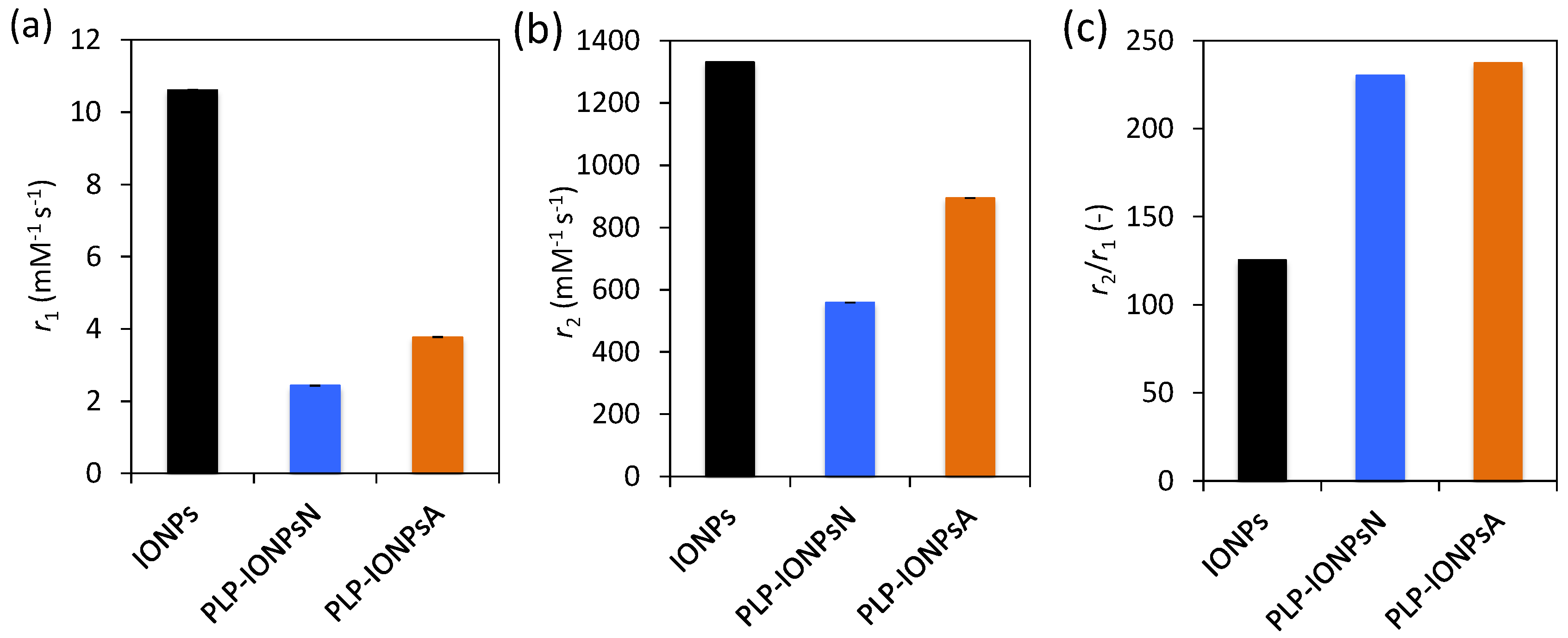
2.6. Potential as an MRI Contrast Agent
3. Materials and Methods
3.1. Synthesis of IONPs
3.2. Coating of IONPs with PLP
3.3. Characterization of PLP-Coated IONPs
3.4. Binding Strength of PLP Chemical Groups on IONPs
3.5. Cellular Uptake Study by TEM
3.6. In Vitro Toxicity Study by the MTS Assay
3.7. Cellular Uptake and Annexin V/PI In Vitro Toxicity Study by Flow Cytometry
3.8. MRI
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Clift, M.J.D.; Rothen-Rutishauser, B.; Brown, D.M.; Duffin, R.; Donaldson, K.; Proudfoot, L.; Guy, K.; Stone, V. The impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell line. Toxicol. Appl. Pharmacol. 2008, 232, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Chouly, C.; Pouliquen, D.; Lucet, I.; Jeune, J.J.; Jallet, P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. J. Microencapsul. 1996, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 2001, 53, 283. [Google Scholar] [PubMed]
- Lunov, O.; Syrovets, T.; Röcker, C.; Tron, K.; Ulrich Nienhaus, G.; Rasche, V.; Mailänder, V.; Landfester, K.; Simmet, T. Lysosomal degradation of the carboxydextran shell of coated superparamagnetic iron oxide nanoparticles and the fate of professional phagocytes. Biomaterials 2010, 31, 9015–9022. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, C.; Kumar, C.S.; Hansel, W.; Soboyejo, W.; Zhou, J.; Hormes, J. LHRH-conjugated Magnetic Iron Oxide Nanoparticles for Detection of Breast Cancer Metastases. Breast Cancer Res. Treat. 2006, 99, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Artemov, D. Molecular magnetic resonance imaging with targeted contrast agents. J. Cell. Biochem. 2003, 90, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, S.R.; Zhou, R.; Kung, H.F.; Chen, I.-W. Iron oxide nanoparticles as magnetic resonance contrast agent for tumor imaging via folate receptor-targeted delivery1. Acad. Radiol. 2004, 11, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Percudani, R.; Peracchi, A. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep. 2003, 4, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F. The Vitamins; The Vitamins Series; Elsevier Science: Amsterdam, The Netherland, 2007; ISBN 9780080561301. [Google Scholar]
- Richard, J.P.; Amyes, T.L.; Crugeiras, J.; Rios, A. Pyridoxal 5’-Phosphate: Electrophilic Catalyst Extraordinaire. Curr. Opin. Chem. Biol. 2009, 13, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Waldoefner, N.; Decken, K.; Scholz, R. Nanoparticle-Active Ingredient Conjugates. U.S. Patent 20080268061, 30 October 2008. Available online: https://www.google.ms/patents/US20080268061 (accessed on 28 July 2017).
- Bothra, S.; Upadhyay, Y.; Kumar, R.; Sahoo, S.K. Applications of vitamin B6 cofactor pyridoxal 5′-phosphate and pyridoxal 5′-phosphate crowned gold nanoparticles for optical sensing of metal ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 174, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, X.-M.; Chen, X.; Zhu, G.-T.; Wang, R.-Q.; Feng, Y.-Q. Pyridoxal 5′-phosphate mediated preparation of immobilized metal affinity material for highly selective and sensitive enrichment of phosphopeptides. J. Chromatogr. A 2017, 1499, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bonvin, D.; Hofmann, H.; Mionic Ebersold, M. Optimisation of aqueous synthesis of iron oxide nanoparticles for biomedical applications. J. Nanoparticle Res. 2016, 18, 376. [Google Scholar] [CrossRef]
- Bonvin, D.; Arakcheeva, A.; Millan, A.; Pinol, R.; Hofmann, H.; Mionic Ebersold, M. Controlling structural and magnetic properties of IONPs by aqueous synthesis for improved hyperthermia. RSC Adv. 2017, 7, 13159–13170. [Google Scholar] [CrossRef]
- Ogawa, H.; Fujioka, M. The reaction of pyridoxal 5′-phosphate with an essential lysine residue of saccharopine dehydrogenase (L-lysine-forming). J. Biol. Chem. 1980, 255, 7420–7425. [Google Scholar] [PubMed]
- Williams, V.R.; Neilands, J.B. Apparent ionization constants, spectral properties and metal chelation of the cotransaminases and related compounds. Arch. Biochem. Biophys. 1954, 53, 56–70. [Google Scholar] [CrossRef]
- Tudisco, C.; Oliveri, V.; Cantarella, M.; Vecchio, G.; Condorelli, G.G. Cyclodextrin Anchoring on Magnetic Fe3O4 Nanoparticles Modified with Phosphonic Linkers. Eur. J. Inorg. Chem. 2012, 2012, 5323–5331. [Google Scholar] [CrossRef]
- Textor, M.; Ruiz, L.; Hofer, R.; Rossi, A.; Feldman, K.; Hähner, G.; Spencer, N.D. Structural Chemistry of Self-Assembled Monolayers of Octadecylphosphoric Acid on Tantalum Oxide Surfaces. Langmuir 2000, 16, 3257–3271. [Google Scholar] [CrossRef]
- Zhang, B.; Kong, T.; Xu, W.; Su, R.; Gao, Y.; Cheng, G. Surface Functionalization of Zinc Oxide by Carboxyalkylphosphonic Acid Self-Assembled Monolayers. Langmuir 2010, 26, 4514–4522. [Google Scholar] [CrossRef] [PubMed]
- Bonvin, D.; Duncan, T.L.A.; Diego, C.; Aschauer, U.; Moniatte, M.; Hofmann, H.; Mionić Ebersold, M. Protein corona: Impact of lymph vs blood in a complex in vitro environment. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Peralvarez-Marin, A.; Minelli, C.; Faraudo, J.; Roig, A.; Laromaine, A. Albumin-coated SPIONs: An experimental and theoretical evaluation of protein conformation, binding affinity and competition with serum proteins. Nanoscale 2016, 8, 14393–14405. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.Z.; Ma, X.; Chen, T.; Zhang, L.; Ren, W.; Xiang, L.; Wu, A. Silica-coated super-paramagnetic iron oxide nanoparticles (SPIONPs): A new type contrast agent of T1 magnetic resonance imaging (MRI). J. Mater. Chem. B 2015, 3, 5172–5181. [Google Scholar] [CrossRef]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)-and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Aberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, C.C.; Payne, C.K. Nanoparticle Surface Charge Mediates the Cellular Receptors Used by Protein–Nanoparticle Complexes. J. Phys. Chem. B 2012, 116, 8901–8907. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, A.; Palchetti, S.; Pozzi, D.; Hormozi-Nezhad, M.R.; Baldelli Bombelli, F.; Caracciolo, G.; Mahmoudi, M. Exploring Cellular Interactions of Liposomes Using Protein Corona Fingerprints and Physicochemical Properties. ACS Nano 2016, 10, 3723–3737. [Google Scholar] [CrossRef] [PubMed]
- Palchetti, S.; Digiacomo, L.; Pozzi, D.; Peruzzi, G.; Micarelli, E.; Mahmoudi, M.; Caracciolo, G. Nanoparticles-cell association predicted by protein corona fingerprints. Nanoscale 2016, 8, 12755–12763. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhang, S.; Yang, Q.; Zhang, T.; Wei, X.-Q.; Jiang, L.; Zhang, C.-L.; Chen, Q.-M.; Zhang, Z.-R.; Lin, Y.-F. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials 2013, 34, 8521–8530. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Choi, E.-J.; Webster, T.J.; Kim, S.-H.; Khang, D. Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int. J. Nanomed. 2014, 10, 97–113. [Google Scholar] [CrossRef]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M.; et al. Regulation of Macrophage Recognition through the Interplay of Nanoparticle Surface Functionality and Protein Corona. ACS Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef] [PubMed]
- Petri-Fink, A.; Steitz, B.; Finka, A.; Salaklang, J.; Hofmann, H. Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (SPIONs): Colloidal stability, cytotoxicity, and cellular uptake studies. Interact. Polym. Pharm. Biomed. Appl. 2008, 68, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Bonvin, D.; Hofmann, H.; Ebersold, M.M. Assessment of nanoparticles’ safety: Corrected absorbance-based toxicity test. Analyst 2017, 142, 2338–2342. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.P.; Janko, C.; Poettler, M.; Tripal, P.; Zaloga, J.; Cicha, I.; Dürr, S.; Nowak, J.; Odenbach, S.; Slabu, I.; et al. Flow cytometry for intracellular SPION quantification: Specificity and sensitivity in comparison with spectroscopic methods. Int. J. Nanomed. 2015, 10, 4185–4201. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singh, G.; Arora, V.; Mewar, S.; Sharma, U.; Jagannathan, N.; Sapra, S.; Dinda, A.K.; Kharbanda, S.; Singh, H. Cellular interaction of folic acid conjugated superparamagnetic iron oxide nanoparticles and its use as contrast agent for targeted magnetic imaging of tumor cells. Int. J. Nanomed. 2012, 7, 3503–3516. [Google Scholar] [CrossRef] [Green Version]
- Tse, B.W.-C.; Cowin, G.J.; Soekmadji, C.; Jovanovic, L.; Vasireddy, R.S.; Ling, M.-T.; Khatri, A.; Liu, T.; Thierry, B.; Russell, P.J. PSMA-targeting iron oxide magnetic nanoparticles enhance MRI of preclinical prostate cancer. Nanomedicine 2014, 10, 375–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Wang, X.; Klein, E.; Heston, W.D.W. Novel Role of Prostate-Specific Membrane Antigen in Suppressing Prostate Cancer Invasiveness. Cancer Res. 2005, 65, 727. [Google Scholar] [PubMed]
- Hrkach, J.; Von Hoff, D.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef] [PubMed]
- Maresca, K.P.; Hillier, S.M.; Femia, F.J.; Keith, D.; Barone, C.; Joyal, J.L.; Zimmerman, C.N.; Kozikowski, A.P.; Barrett, J.A.; Eckelman, W.C.; et al. A Series of Halogenated Heterodimeric Inhibitors of Prostate Specific Membrane Antigen (PSMA) as Radiolabeled Probes for Targeting Prostate Cancer. J. Med. Chem. 2009, 52, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Fonda, M.L.; Trauss, C.; Guempel, U.M. The binding of pyridoxal 5′-phosphate to human serum albumin. Arch. Biochem. Biophys. 1991, 288, 79–86. [Google Scholar] [CrossRef]
- Whittaker, J.W. Intracellular Trafficking of the Pyridoxal Cofactor. Implications for Health and Metabolic Disease. Arch. Biochem. Biophys. 2016, 592, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M.; Ortiz, A.; Ma, T.Y. A carrier-mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco-2 cells: Regulation by a PKA-mediated pathway. Am. J. Physiol. Cell Physiol. 2003, 285, C1219. [Google Scholar] [CrossRef] [PubMed]
- Said, Z.M.; Subramanian, V.S.; Vaziri, N.D.; Said, H.M. Pyridoxine uptake by colonocytes: A specific and regulated carrier-mediated process. Am. J. Physiol. Cell Physiol. 2008, 294, C1192. [Google Scholar] [CrossRef] [PubMed]
- Schenker, S.; Johnson, R.F.; Mahuren, J.D.; Henderson, G.I.; Coburn, S.P. Human placental vitamin B6 (pyridoxal) transport: Normal characteristics and effects of ethanol. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992, 262, R966. [Google Scholar]
- Suzue, R.; Tachibana, M. The Uptake of Pyridoxal Phosphate by Human Red Blood Cells. J. Vitaminol. (Kyoto) 1970, 16, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Chiappi, M.; Lazaro-Carrillo, A.; Rodríguez, M.J.; Chichón, F.J.; Crosbie-Staunton, K.; Prina-Mello, A.; Volkov, Y.; Villanueva, A.; Carrascosa, J.L. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnol. 2015, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Luther, E.M.; Petters, C.; Bulcke, F.; Kaltz, A.; Thiel, K.; Bickmeyer, U.; Dringen, R. Endocytotic uptake of iron oxide nanoparticles by cultured brain microglial cells. Acta Biomater. 2013, 9, 8454–8465. [Google Scholar] [CrossRef] [PubMed]
- Kurzhals, S.; Gal, N.; Zirbs, R.; Reimhult, E. Controlled aggregation and cell uptake of thermoresponsive polyoxazoline-grafted superparamagnetic iron oxide nanoparticles. Nanoscale 2017, 9, 2793–2805. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity Assessment of Silica Coated Iron Oxide Nanoparticles and Biocompatibility Improvement by Surface Engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Colombo, M.; Prosperi, D.; Gregori, M.; Panariti, A.; Rivolta, I.; Masserini, M.; Cazzaniga, E. Iron oxide nanoparticles surface coating and cell uptake affect biocompatibility and inflammatory responses of endothelial cells and macrophages. J. Nanoparticle Res. 2015, 17, 351. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Wang, Y.-X.J.; Leung, K.C.-F.; Lee, S.-F.; Zhao, F.; Wang, D.-W.; Lai, J.M.; Wan, C.; Cheng, C.H.; Ahuja, A.T. Enhanced cellular uptake of aminosilane-coated superparamagnetic iron oxide nanoparticles in mammalian cell lines. Int. J. Nanomed. 2012, 7, 953–964. [Google Scholar] [CrossRef]
- Geinguenaud, F.; Banissi, C.; Carpentier, A.F.; Motte, L. Iron Oxide Nanoparticles Coated with a Phosphorothioate Oligonucleotide and a Cationic Peptide: Exploring Four Different Ways of Surface Functionalization. Nanomaterials 2015, 5, 1588–1609. [Google Scholar] [CrossRef] [PubMed]
- LaConte, L.E. W.; Nitin, N.; Zurkiya, O.; Caruntu, D.; O’Connor, C.J.; Hu, X.; Bao, G. Coating thickness of magnetic iron oxide nanoparticles affects R2 relaxivity. J. Magn. Reson. Imaging 2007, 26, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Hou, S.; Zheng, Z.; Zhou, J.; Bao, G. Coating Optimization of Superparamagnetic Iron Oxide Nanoparticles for High T2 Relaxivity. Nano Lett. 2010, 10, 4607–4613. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali, M.; Rostamizadeh, K.; Sadighian, S.; Khoeini, F.; Naghibi, M.; Hamidi, M. The impact of polymer coatings on magnetite nanoparticles performance as MRI contrast agents: A comparative study. DARU J. Pharm. Sci. 2015, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, E.D.; Marjanska, M.; Pierre, V.C. A responsive particulate MRI contrast agent for copper(I): A cautionary tale. Dalton Trans. 2012, 41, 8039–8046. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Key, J.; Stigliano, C.; Ananta, J.S.; Zhong, M.; Decuzzi, P. Engineered magnetic hybrid nanoparticles with enhanced relaxivity for tumor imaging. Biomaterials 2013, 34, 7725–7732. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Awasthi, R.; Gajbhiye, N.S.; Agarwal, V.; Singh, A.; Yadav, A.; Gupta, R.K. Innovative synthesis of citrate-coated superparamagnetic Fe3O4 nanoparticles and its preliminary applications. J. Colloid Interface Sci. 2011, 359, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.H.; Bauer, J.; Saborovski, O.; Fu, Y.; Corot, C.; Wendland, M.F.; Daldrup-Link, H.E. T1 and T2 relaxivity of intracellular and extracellular USPIO at 1.5T and 3T clinical MR scanning. Eur. Radiol. 2006, 16, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Aschauer, U.; Selloni, A. Adsorption of biomedical coating molecules, amino acids, and short peptides on magnetite (110). J. Chem. Phys. 2015, 143, 044705. [Google Scholar] [CrossRef] [PubMed]


| dh in Water (nm) | dh in RPMI (nm) | dh in DMEM (nm) | ξ in Water (mV) | ξ in RPMI (mV) | ξ in DMEM (mV) | |
|---|---|---|---|---|---|---|
| IONPs | 23 ± 8 | 114 ± 39 | 1352 ± 562 | 5.0 ± 2.0 | −7.6 ± 0.6 | −9.2 ± 0.7 |
| PLP-IONPsN | 1065 ± 296 | 1893 ± 440 | 1657 ± 552 | −16.7 ± 0.6 | −7.6 ± 0.7 | −7.5 ± 0.6 |
| PLP-IONPsA | 1592 ± 537 | 1752 ± 379 | 1685 ± 1074 | −3.9 ± 0.8 | −7.9 ± 0.8 | −8.0 ± 0.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonvin, D.; Aschauer, U.J.; Bastiaansen, J.A.M.; Stuber, M.; Hofmann, H.; Mionić Ebersold, M. Versatility of Pyridoxal Phosphate as a Coating of Iron Oxide Nanoparticles. Nanomaterials 2017, 7, 202. https://doi.org/10.3390/nano7080202
Bonvin D, Aschauer UJ, Bastiaansen JAM, Stuber M, Hofmann H, Mionić Ebersold M. Versatility of Pyridoxal Phosphate as a Coating of Iron Oxide Nanoparticles. Nanomaterials. 2017; 7(8):202. https://doi.org/10.3390/nano7080202
Chicago/Turabian StyleBonvin, Debora, Ulrich J. Aschauer, Jessica A. M. Bastiaansen, Matthias Stuber, Heinrich Hofmann, and Marijana Mionić Ebersold. 2017. "Versatility of Pyridoxal Phosphate as a Coating of Iron Oxide Nanoparticles" Nanomaterials 7, no. 8: 202. https://doi.org/10.3390/nano7080202
APA StyleBonvin, D., Aschauer, U. J., Bastiaansen, J. A. M., Stuber, M., Hofmann, H., & Mionić Ebersold, M. (2017). Versatility of Pyridoxal Phosphate as a Coating of Iron Oxide Nanoparticles. Nanomaterials, 7(8), 202. https://doi.org/10.3390/nano7080202






