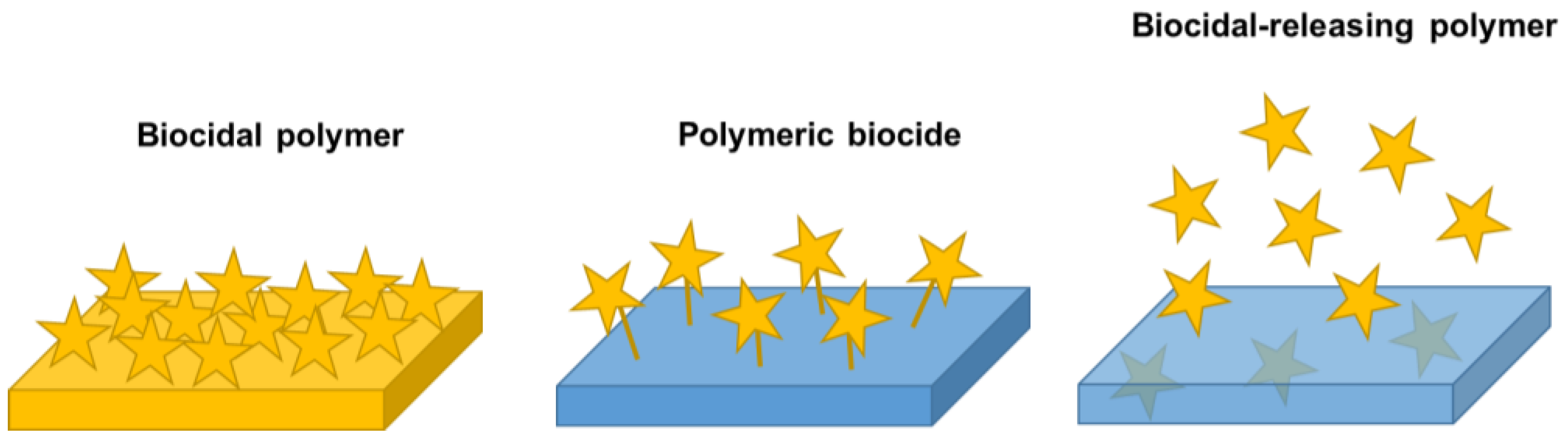
Antimicrobial Polymers in the Nano-World
Abstract
:1. Introduction
2. Areas of Application
2.1. Health Care and Biomedical Devices
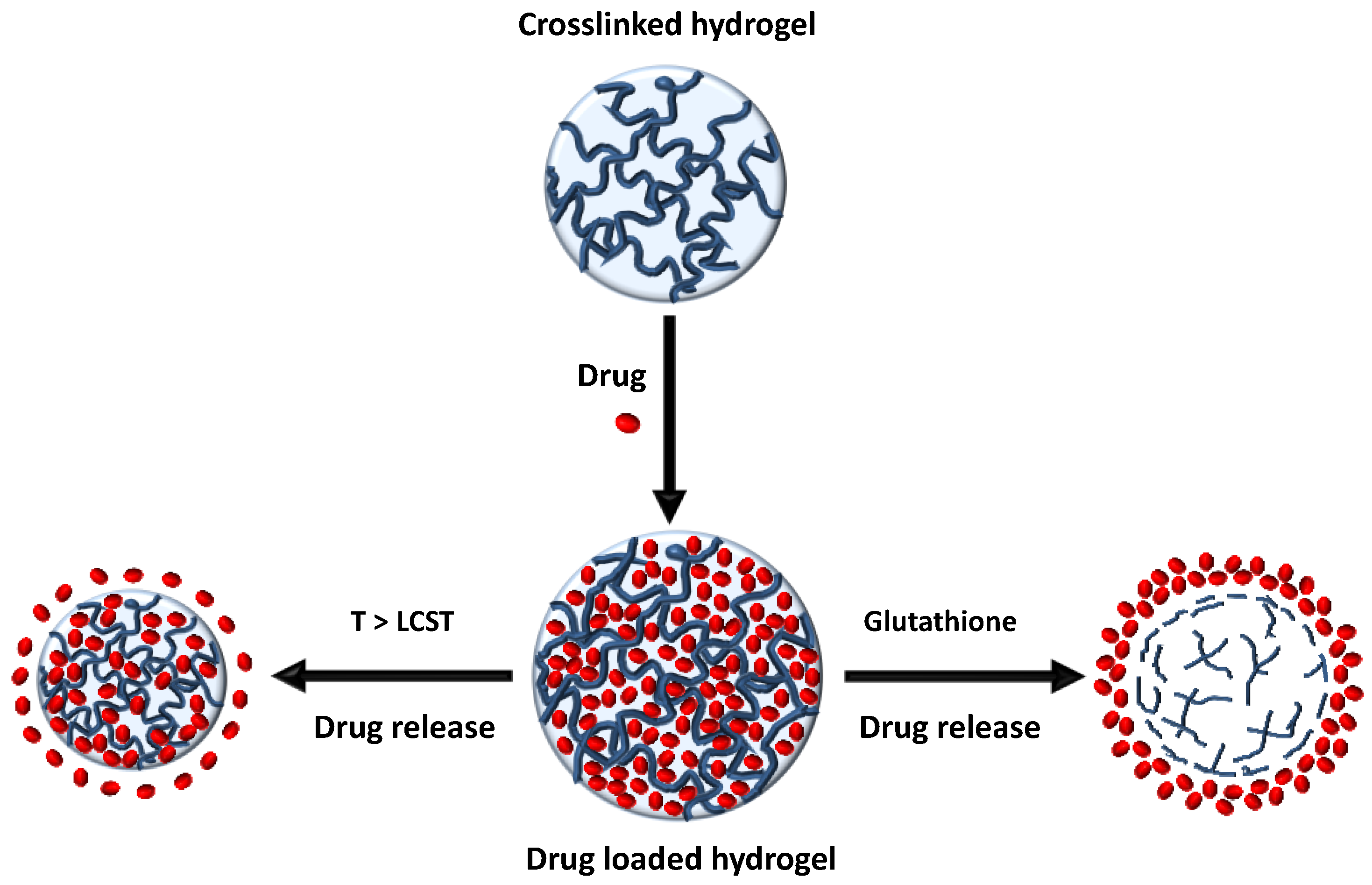
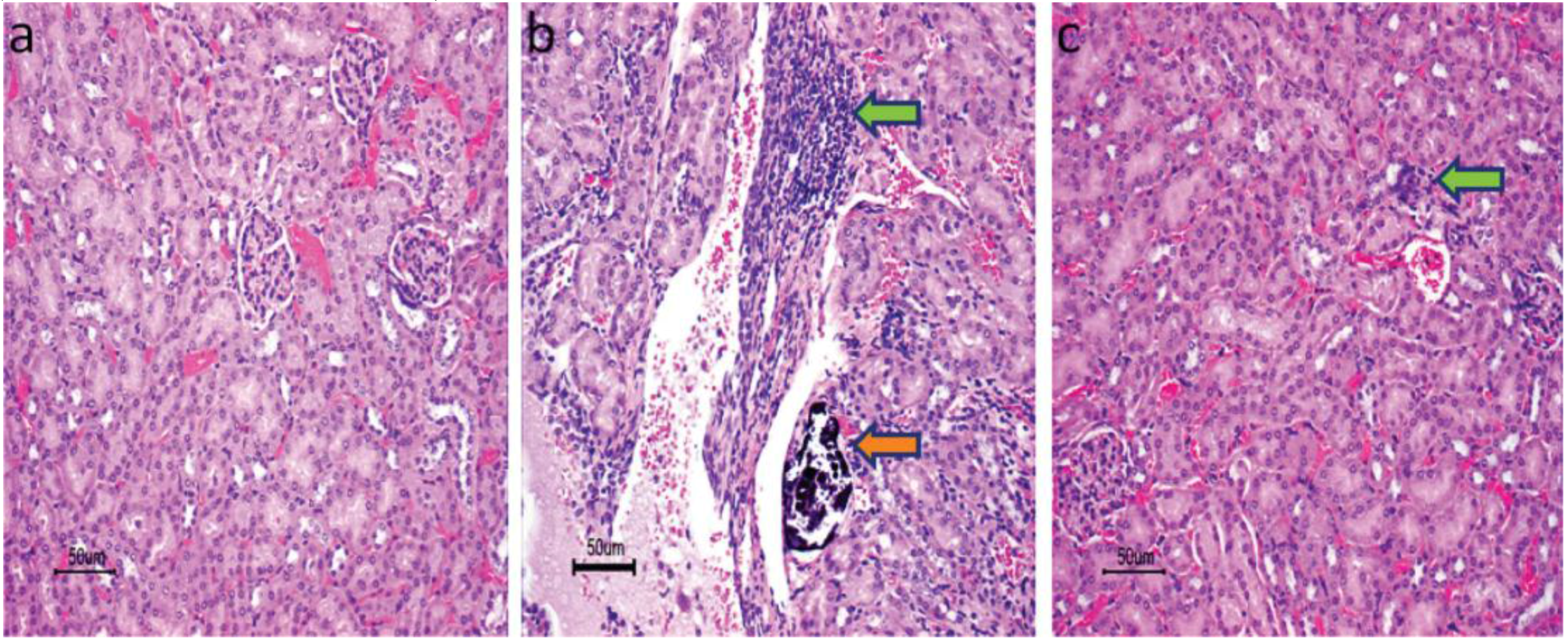
2.1.1. Drug Delivery
2.1.2. Wound Healing or Dressing
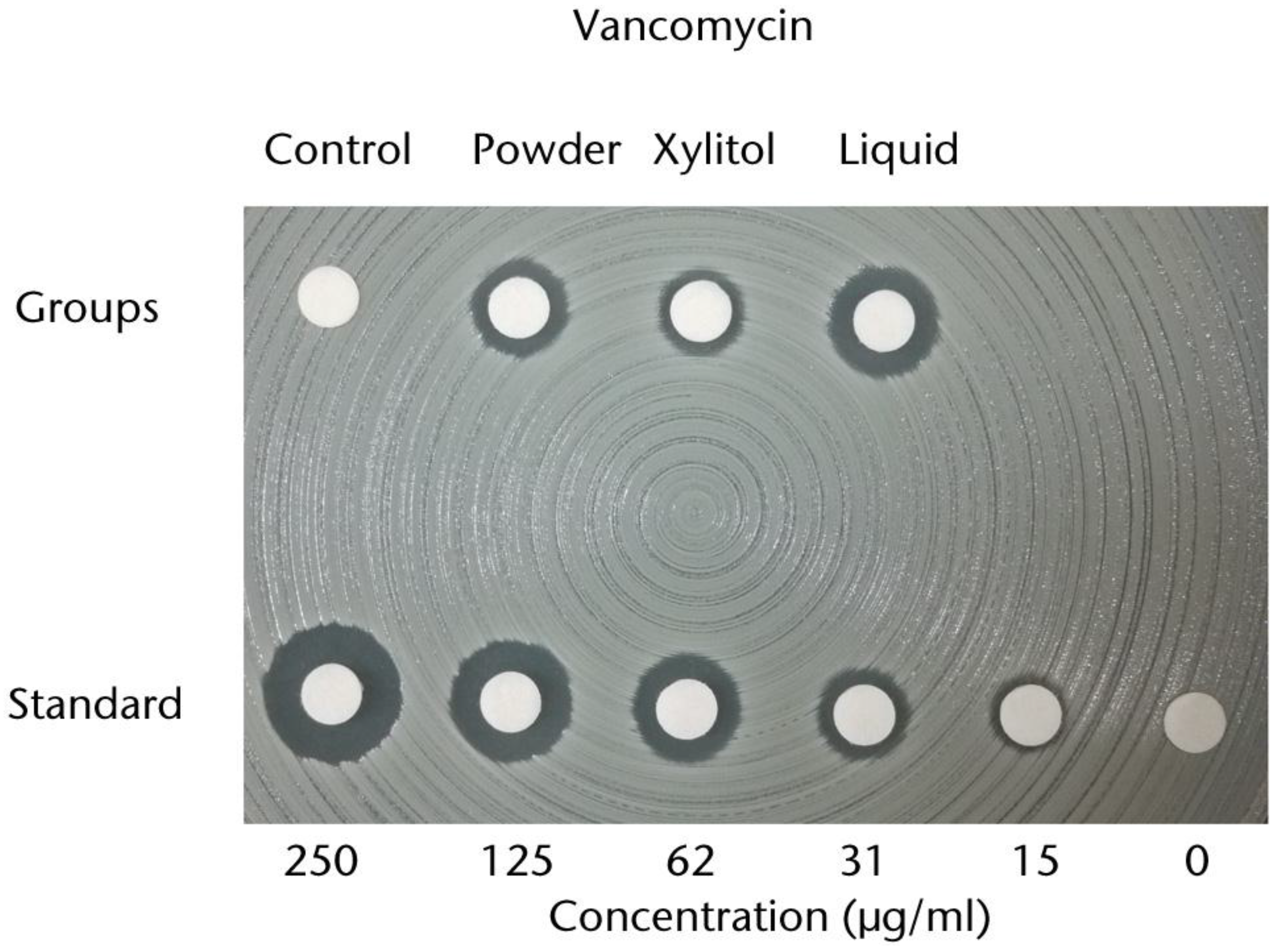
2.1.3. Sutures and Prosthesis
2.1.4. Dental Applications
2.2. Food
2.2.1. Food-Packaging
2.2.2. Edible Films
2.2.3. Food Additives
2.3. Environmental Science: Agriculture and Water Purification
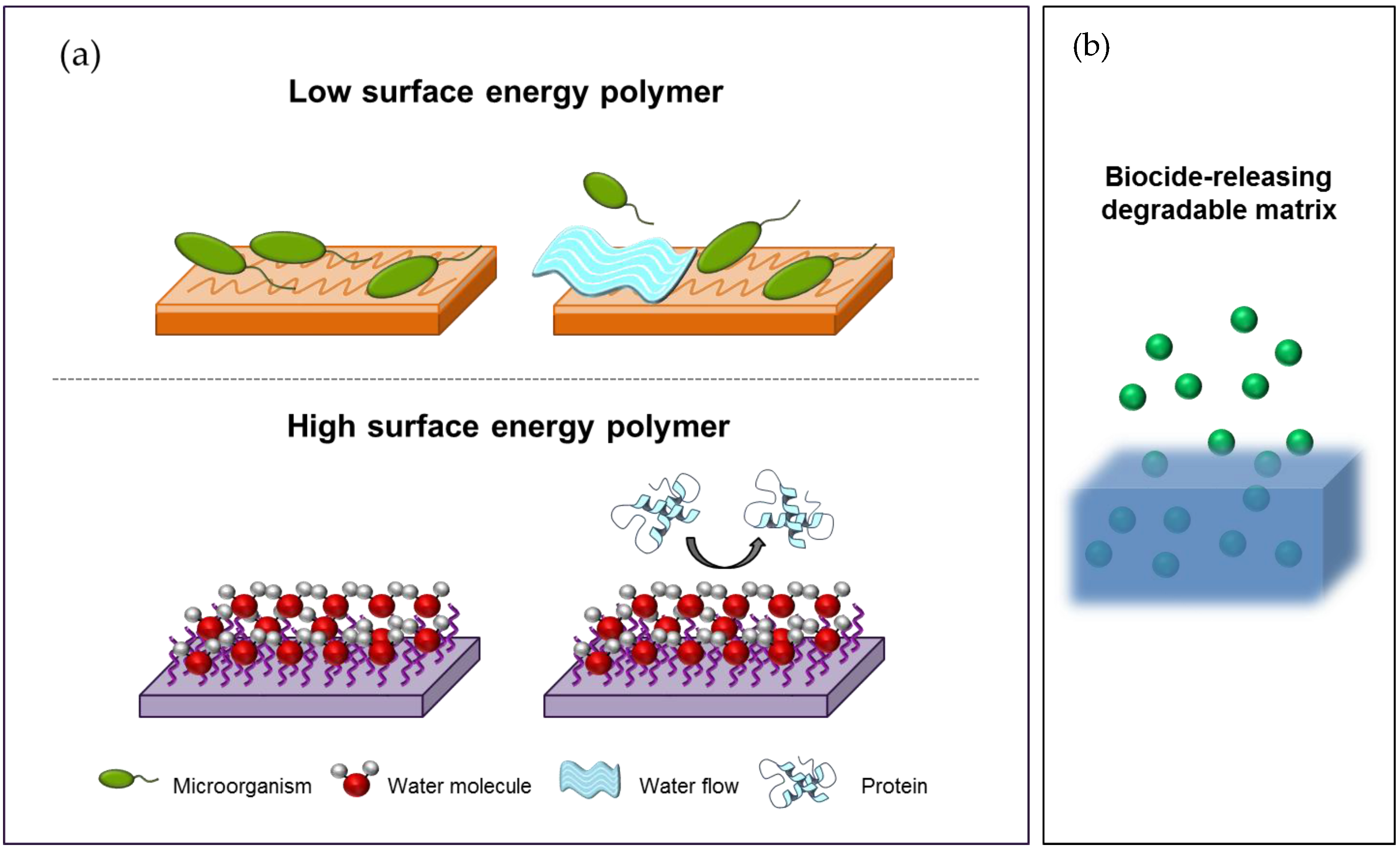
2.3.1. Antibiofouling Surfaces
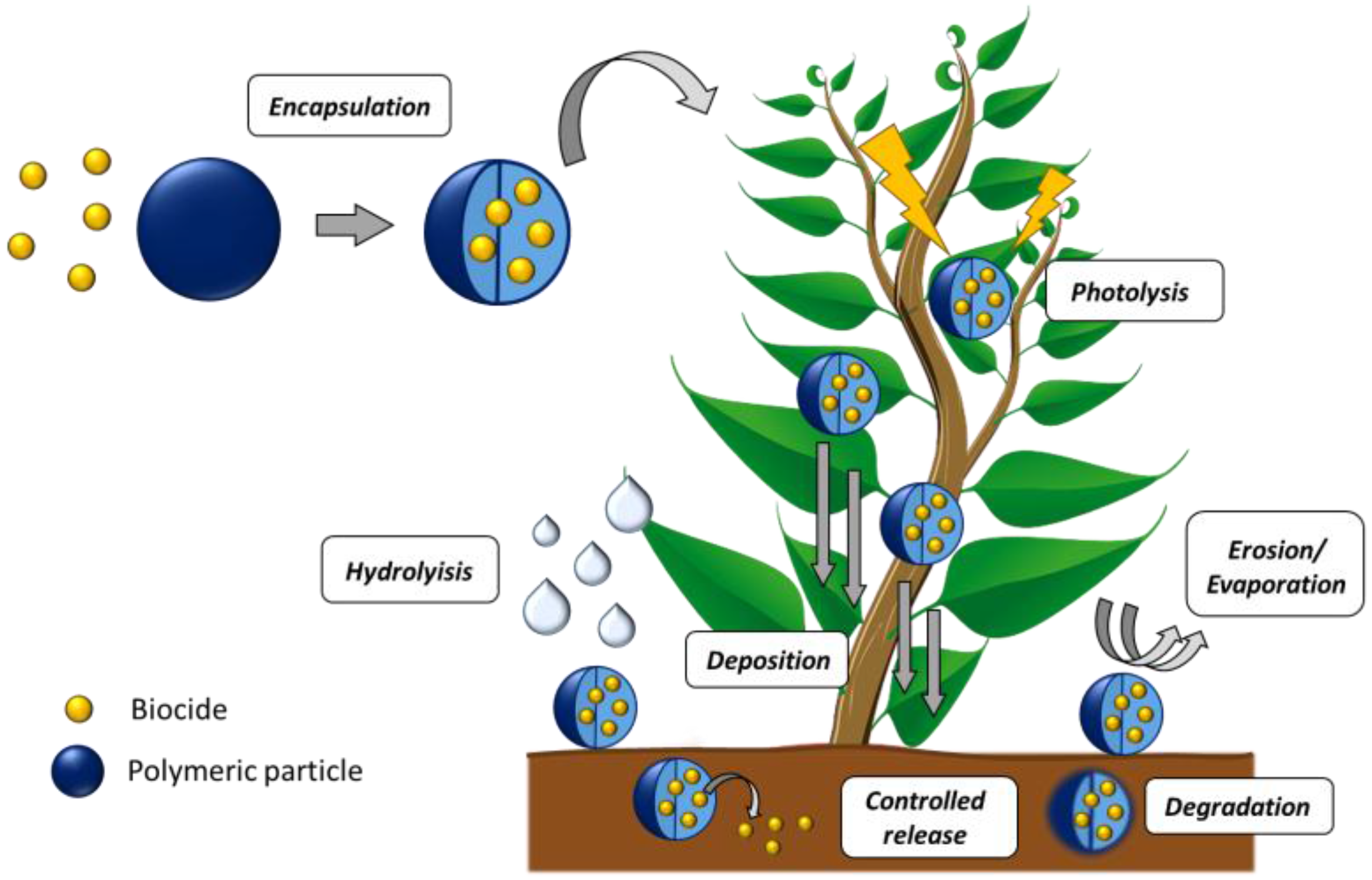
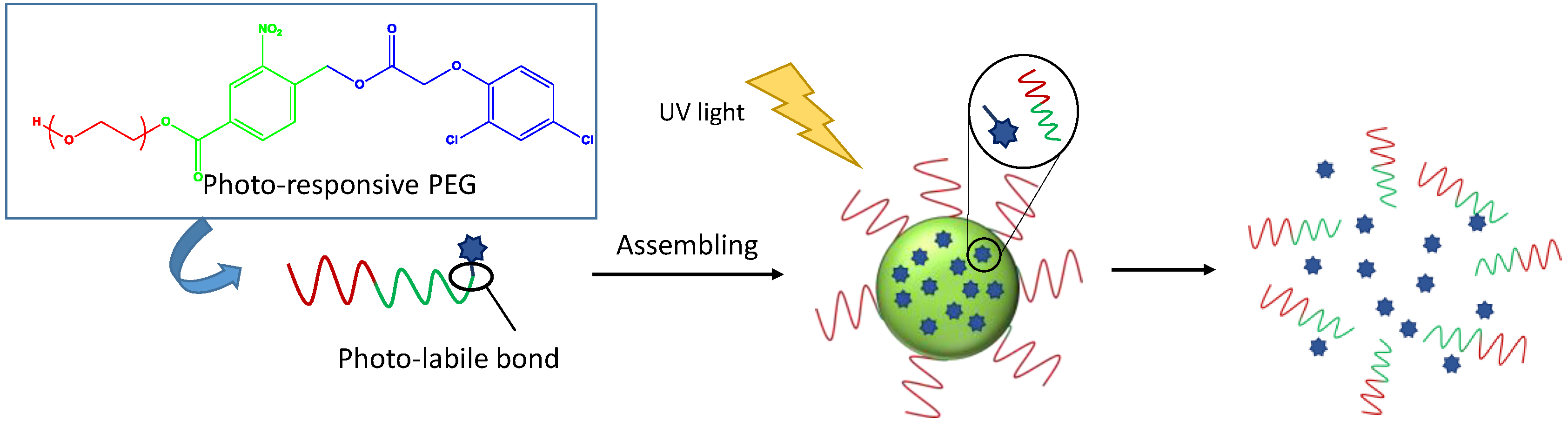
2.3.2. Agriculture
2.3.3. Water Purification
2.3.4. Air Purification
2.4. Fabrics
3. Conclusions and Future Development
Acknowledgments
Conflicts of Interest
Abbreviations
| ACP | amorphous calcium phosphate |
| Alg | alginate |
| ALM | alveolar like macrophages |
| AM | ampicillin |
| Arg | arginine |
| AZ | azithromycin |
| Bla | β-lactamase |
| BSA | bovine serum albumin |
| CaP | calcium phosphate |
| CD | cyclodextrin |
| CFU | Colony-forming unit |
| Ch | chitosan |
| CHX | chlorhexidine |
| CL | clarithromycin |
| CP | ciprofloxacin |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane chloride salt |
| DS | dextran sulfate |
| EVA | ethylene/vinyl acetate copolymer |
| EVOH | ethylene-vinyl alcohol copolymer |
| FDA | Food and Drug Administration |
| F-POSS | fluorinated-decyl polyhedral oligomeric silsesquioxane |
| Gg | gellan gum |
| GRAS | Generally Recognized as Safe |
| GT | gentamicin |
| HA | hydroxyapatite |
| HEPA | high efficiency particle arresting |
| Hy | hyaluronic acid |
| IFN-γ | interferon-gamma |
| IL-12 | interleukin-12 |
| LCST | lower critical solution temperature |
| LDPE | low-density polyethylene |
| LV | levofloxacin |
| MBC | minimum bactericidal concentration |
| MIC | minimum inhibitory concentration |
| MRSA | methicillin-resistant S. aureus |
| MSN | mesoporous silica nanoparticles |
| MSSA | methicillin susceptible S. aureus |
| MX | moxifloxacin |
| PNIPAM | poly(N-isopropyl acrylamide) |
| NPs | nanoparticles |
| NR | norfloxacin |
| OFX | ofloxacin |
| PAA | polyacid acrylic |
| PAANa | poly(sodium acrylate) |
| PALA | polyallylamine |
| PAN | polyacrylonitrile |
| pBA | poly(n-butyl acrylate) |
| PBCA | poly(butyl cyanoacrylate) |
| PCL | polycaprolactone |
| PDAEMA | poly(2-aminoethyl methacrylate) |
| PDMS | poly(dimethylsiloxane) |
| PDO | polydioxanone |
| PE | polyethylene |
| PEG | poly(ethylene glycol) |
| PEI | polyethyleneimine |
| PEO | poly(ethylene oxide) |
| PET | poly(ethylene terephthalate) |
| PGa | penicillin G amidase |
| PGA | poly(glycolic acid) |
| PGCL | poly(glycolide-co-caprolactone) |
| PLA | poly(lactide) |
| PLGA | poly(lactic-co-glycolic acid) |
| PMMA | poly(methyl methacrylate) |
| p-(NβGlcEAM) | poly(N-2-(β-d-glucosyloxy)ethyl acrylamide) |
| p(NβGalEAM) | poly(N-2-(β-d-galactosyloxy)ethyl acrylamide) |
| Por | Porphyrins |
| PPO | poly(propylene oxide) |
| PP | polypropylene |
| PS | polystyrene |
| PU | polyurethane |
| PVA | poly(vinyl alcohol) |
| PVP | poly(N-vinylpyrrolidone) |
| RIF | Rifampicin |
| ROS | reactive oxygen species |
| TDI | toluene-2,4-diisocyanate |
| TNF-α | tumour necrosis factor-α |
| TOB | tobramycin |
| TPP | tripolyphosphate |
| VC | vancomycin |
References
- Muñoz-Bonilla, A.; Cerrada, M.; Fernández-García, M. Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications; RSC: London, UK, 2014. [Google Scholar]
- Palermo, E.F.; Kuroda, K. Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biotechnol. 2010, 87, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Engler, A.C.; Wiradharma, N.; Ong, Z.Y.; Coady, D.J.; Hedrick, J.L.; Yang, Y.-Y. Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today 2012, 7, 201–222. [Google Scholar] [CrossRef]
- Fik, C.P.; Krumm, C.; Muennig, C.; Baur, T.I.; Salz, U.; Bock, T.; Tiller, J.C. Impact of functional satellite groups on the antimicrobial activity and hemocompatibility of telechelic poly(2-methyloxazoline)s. Biomacromolecules 2012, 13, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Krumm, C.; Harmuth, S.; Hijazi, M.; Neugebauer, B.; Kampmann, A.-L.; Geltenpoth, H.; Sickmann, A.; Tiller, J.C. Antimicrobial poly(2-methyloxazoline)s with bioswitchable activity through satellite group modification. Angew. Chem. Int. Ed. 2014, 53, 3830–3834. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur. Polym. J. 2015, 65, 46–62. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.; Hosseini-Nasr, M.; Rad-Malekshahi, M.; Samadi, N.; Atyabi, F.; Dinarvand, R. Preparation and antibacterial activity evaluation of rifampicin-loaded poly lactide-co-glycolide nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Reynolds, J.L.; Law, W.-C.; Maponga, C.C.; Prasad, P.N.; Morse, G.D. Multimodal nanoparticles that provide immunomodulation and intracellular drug delivery for infectious diseases. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Kisich, K.O.; Gelperina, S.; Higgins, M.P.; Wilson, S.; Shipulo, E.; Oganesyan, E.; Heifets, L. Encapsulation of moxifloxacin within poly(butyl cyanoacrylate) nanoparticles enhances efficacy against intracellular mycobacterium tuberculosis. Int. J. Pharm. 2007, 345, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Mahor, A.; Prajapati, S.K.; Verma, A.; Gupta, R.; Iyer, A.K.; Kesharwani, P. Moxifloxacin loaded gelatin nanoparticles for ocular delivery: Formulation and in vitro, in vivo evaluation. J. Colloid Interface Sci. 2016, 483, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retin. Eye Res. 2010, 29, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Kaskoos, R.A. Investigation of moxifloxacin loaded chitosan-dextran nanoparticles for topical instillation into eye: in vitro and ex vivo evaluation. Int. J. Pharm. Investig. 2014, 4, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Duxfield, L.; Sultana, R.; Wang, R.; Englebretsen, V.; Deo, S.; Swift, S.; Rupenthal, I.; Al-Kassas, R. Development of gatifloxacin-loaded cationic polymeric nanoparticles for ocular drug delivery. Pharm. Dev. Technol. 2016, 21, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Sparfloxacin-loaded plga nanoparticles for sustained ocular drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. Antibacterial efficacy of inhalable levofloxacin-loaded polymeric nanoparticles against E. coli biofilm cells: The effect of antibiotic release profile. Pharm. Res. 2010, 27, 1597–1609. [Google Scholar] [PubMed]
- Cheow, W.S.; Hadinoto, K. Enhancing encapsulation efficiency of highly water-soluble antibiotic in poly(lactic-co-glycolic acid) nanoparticles: Modifications of standard nanoparticle preparation methods. Colloids Surf. Physicochem. Eng. Aspects 2010, 370, 79–86. [Google Scholar] [CrossRef]
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. The roles of lipid in anti-biofilm efficacy of lipid–polymer hybrid nanoparticles encapsulating antibiotics. Colloids Surf. Physicochem. Eng. Aspects 2011, 389, 158–165. [Google Scholar] [CrossRef]
- Cheow, W.S.; Hadinoto, K. Green preparation of antibiotic nanoparticle complex as potential anti-biofilm therapeutics via self-assembly amphiphile–polyelectrolyte complexation with dextran sulfate. Colloids Surf. B Biointerfaces 2012, 92, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kho, K.; Cheow, W.S.; Lie, R.H.; Hadinoto, K. Aqueous re-dispersibility of spray-dried antibiotic-loaded polycaprolactone nanoparticle aggregates for inhaled anti-biofilm therapy. Powder Technol. 2010, 203, 432–439. [Google Scholar] [CrossRef]
- Kumar, G.; Sharma, S.; Shafiq, N.; Khuller, G.K.; Malhotra, S. Optimization, in vitro–in vivo evaluation, and short-term tolerability of novel levofloxacin-loaded plga nanoparticle formulation. J. Pharm. Sci. 2012, 101, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J. Drug Target. 2011, 19, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.A.; Sabry, S.A.; Abdallah, M.H.; El-damasy, D.A. Formulation and in vitro characterization of poly(DL-lactide-co-glycolide)/eudragit RLPO or RS30D nanoparticles as an oral carrier of levofloxacin hemihydrate. Pharm. Dev. Technol. 2016, 21, 655–663. [Google Scholar] [PubMed]
- Montanari, E.; D’Arrigo, G.; di Meo, C.; Virga, A.; Coviello, T.; Passariello, C.; Matricardi, P. Chasing bacteria within the cells using levofloxacin-loaded hyaluronic acid nanohydrogels. Eur. J. Pharm. Biopharm. 2014, 87, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Lorimer, C.P.; McCoy, C.P.; Gorman, S.P. Characterization of the physicochemical, antimicrobial, and drug release properties of thermoresponsive hydrogel copolymers designed for medical device applications. J. Biomed. Mater. Res. Part B 2008, 85B, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Guan, X.; Zheng, J.; Guo, H.; Wu, K.; Liang, L.; Lu, M. Biodegradable, thermoresponsive pnipam-based hydrogel scaffolds for the sustained release of levofloxacin. RSC Adv. 2016, 6, 32967–32978. [Google Scholar] [CrossRef]
- Su, Y.-R.; Yu, S.-H.; Chao, A.-C.; Wu, J.-Y.; Lin, Y.-F.; Lu, K.-Y.; Mi, F.-L. Preparation and properties of ph-responsive, self-assembled colloidal nanoparticles from guanidine-containing polypeptide and chitosan for antibiotic delivery. Colloids Surf. Physicochem. Eng. Aspects 2016, 494, 9–20. [Google Scholar] [CrossRef]
- Xiao, P.; Dudal, Y.; Corvini, P.F.X.; Shahgaldian, P. Polymeric cyclodextrin-based nanoparticles: Synthesis, characterization and sorption properties of three selected pharmaceutically active ingredients. Polym. Chem. 2011, 2, 120–125. [Google Scholar] [CrossRef]
- Hernandez-Montelongo, J.; Naveas, N.; Degoutin, S.; Tabary, N.; Chai, F.; Spampinato, V.; Ceccone, G.; Rossi, F.; Torres-Costa, V.; Manso-Silvan, M.; et al. Porous silicon-cyclodextrin based polymer composites for drug delivery applications. Carbohydr. Polym. 2014, 110, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Shady, S.F.; Gaines, P.; Garhwal, R.; Leahy, C.; Ellis, E.; Crawford, K.; Schmidt, D.F.; McCarthy, S.P. Synthesis and characterization of pullulan-polycaprolactone core-shell nanospheres encapsulated with ciprofloxacin. J. Biomed. Nanotechnol. 2013, 9, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Ghari, T.; Mortazavi, S.A.; Khoshayand, M.R.; Kobarfard, F.; Gilani, K. Preparation, optimization, and in vitro evaluation of azithromycin encapsulated nanoparticles by using response surface methodology. J. Drug Deliv. Sci. Technol. 2014, 24, 352–360. [Google Scholar] [CrossRef]
- Mohammadi, G.; Nokhodchi, A.; Barzegar-Jalali, M.; Lotfipour, F.; Adibkia, K.; Ehyaei, N.; Valizadeh, H. Physicochemical and anti-bacterial performance characterization of clarithromycin nanoparticles as colloidal drug delivery system. Colloids Surf. B 2011, 88, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Payab, S.; Jafari-Aghdam, N.; Barzegar-Jalali, M.; Mohammadi, G.; Lotfipour, F.; Gholikhani, T.; Adibkia, K. Preparation and physicochemical characterization of the azithromycin-eudragit RS100 nanobeads and nanofibers using electrospinning method. J. Drug Deliv. Sci. Technol. 2014, 24, 585–590. [Google Scholar] [CrossRef]
- Toti, U.S.; Guru, B.R.; Hali, M.; McPharlin, C.M.; Wykes, S.M.; Panyam, J.; Whittum-Hudson, J.A. Targeted delivery of antibiotics to intracellular chlamydial infections using PLGA nanoparticles. Biomaterials 2011, 32, 6606–6613. [Google Scholar] [CrossRef] [PubMed]
- Sheybani, S.; Hosseinifar, T.; Abdouss, M.; Mazinani, S. Mesoporous molecularly imprinted polymer nanoparticles as a sustained release system of azithromycin. RSC Adv. 2015, 5, 98880–98891. [Google Scholar] [CrossRef]
- Xiao, P.; Dudal, Y.; Corvini, P.F.X.; Spahr, P.; Shahgaldian, P. Synthesis and characterization of fluoroquinolone-imprinted polymeric nanoparticles. React. Funct. Polym. 2012, 72, 287–293. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, X.; Liu, M.; He, N. Chitosan/polylactic acid/tripolyphosphate nanocapsules for encapsulation of water-insoluble drugs: in vitro drug release and cytotoxicity. Sci. Adv. Mater. 2013, 5, 2053–2057. [Google Scholar] [CrossRef]
- Turos, E.; Reddy, G.S.K.; Greenhalgh, K.; Ramaraju, P.; Abeylath, S.C.; Jang, S.; Dickey, S.; Lim, D.V. Penicillin-bound polyacrylate nanoparticles: Restoring the activity of β-lactam antibiotics against mrsa. Bioorg. Med. Chem. Lett. 2007, 17, 3468–3472. [Google Scholar] [CrossRef] [PubMed]
- Turos, E.; Shim, J.-Y.; Wang, Y.; Greenhalgh, K.; Reddy, G.S.K.; Dickey, S.; Lim, D.V. Antibiotic-conjugated polyacrylate nanoparticles: New opportunities for development of anti-mrsa agents. Bioorg. Med. Chem. Lett. 2007, 17, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Abeylath, S.C.; Turos, E.; Dickey, S.; Lim, D.V. Glyconanobiotics: Novel carbohydrated nanoparticle antibiotics for MRSA and Bacillus anthracis. Biorg. Med. Chem. 2008, 16, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, N.D.; Ouimet, M.A.; Uhrich, K.E. Antibiotic-containing polymers for localized, sustained drug delivery. Adv. Drug Del. Rev. 2014, 78, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.M.; Abdulkarim, A.; Sharples, G.J.; Cameron, N.R. Glycosylated nanoparticles as efficient antimicrobial delivery agents. Biomacromolecules 2016, 17, 2672–2679. [Google Scholar] [CrossRef] [PubMed]
- Crisante, F.; Francolini, I.; Bellusci, M.; Martinelli, A.; D’Ilario, L.; Piozzi, A. Antibiotic delivery polyurethanes containing albumin and polyallylamine nanoparticles. Eur. J. Pharm. Sci. 2009, 36, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; la Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Pothayee, N.; Seleem, M.N.; Tyler, R.D.; Brenseke, B.; Sriranganathan, N.; Riffle, J.S.; Kasimanickam, R. Antibacterial efficacy of core-shell nanostructures encapsulating gentamicin against an in vivo intracellular Salmonella model. Int. J. Nanomed. 2009, 4, 289–297. [Google Scholar] [CrossRef]
- Umeyor, C.; Attama, A.; Uronnachi, E.; Kenechukwu, F.; Nwakile, C.; Nzekwe, I.; Okoye, E.; Esimone, C. Formulation design and in vitro physicochemical characterization of surface modified self-nanoemulsifying formulations (SNEFS) of gentamicin. Int. J. Pharm. 2016, 497, 161–198. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Desai, N.; Dixit, R.; Nagarsenker, M. Self-nanoemulsifying drug delivery systems: Formulation insights, applications and advances. Nanomedicine 2010, 5, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Suresh, P.K.; Desmukh, R. Design of eudragit RL100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Saúde, A.C.M.; Ombredane, A.S.; Silva, O.N.; Barbosa, J.A.R.G.; Moreno, S.E.; Guerra Araujo, A.C.; Falcão, R.; Silva, L.P.; Dias, S.C.; Franco, O.L. Clavanin bacterial sepsis control using a novel methacrylate nanocarrier. Int. J. Nanomed. 2014, 9, 5055–5069. [Google Scholar]
- Li, Y.; Liu, G.; Wang, X.; Hu, J.; Liu, S. Enzyme-responsive polymeric vesicles for bacterial-strain-selective delivery of antimicrobial agents. Angew. Chem. Int. Ed. 2016, 55, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Meli, A.; Sutera, A.; Gallo, G.; Chillura Martino, D.; Lo Meo, P.; Noto, R. Photosynthesized silver-polyaminocyclodextrin nanocomposites as promising antibacterial agents with improved activity. RSC Adv. 2016, 6, 40090–40099. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Effect of different carboxylic acids in cyclodextrin functionalization of cellulose nanocrystals for prolonged release of carvacrol. Mater. Sci. Eng. C 2016, 69, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. in vivo evaluation of chitosan–PVP–titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. Chitosan-PVP-nano silver oxide wound dressing: in vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.M.G.; El-Aassar, M.R.; Al-Deyab, S.S. Antimicrobial activity of carboxymethyl chitosan/polyethylene oxide nanofibers embedded silver nanoparticles. Carbohydr. Polym. 2013, 92, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Rashkov, I. Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) prepared by electrospinning. Eur. Polym. J. 2007, 43, 1112–1122. [Google Scholar] [CrossRef]
- Mat Amin, K.A.; Gilmore, K.J.; Matic, J.; Poon, S.; Walker, M.J.; Wilson, M.R.; Panhuis, M. Polyelectrolyte complex materials consisting of antibacterial and cell-supporting layers. Macromol. Biosci. 2012, 12, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, R.; Shaabani, A. Electrospun biocompatible core/shell polymer-free core structure nanofibers with superior antimicrobial potency against multi drug resistance organisms. Polymer 2016, 101, 151–157. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun non-woven nanofibrous hybrid mats based on chitosan and PLA for wound-dressing applications. Macromol. Biosci. 2009, 9, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-X.; Mo, X.-M.; Zhang, K.-H.; Fan, L.-P.; Yin, A.-L.; He, C.-L.; Wang, H.-S. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int. J. Mol. Sci. 2010, 11, 3529. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Luo, Y.; Wang, Q. Development of silver-zein composites as a promising antimicrobial agent. Biomacromolecules 2010, 11, 2366–2375. [Google Scholar] [CrossRef] [PubMed]
- Rujitanaroj, P.-O.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly(vinyl alcohol)/cellulose nanowhiskers nanocomposite hydrogels for potential wound dressings. Mater. Sci. Eng. C 2014, 34, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H.; Park, J.L.; Sul, I.H.; Youk, J.H.; Kang, T.J. Preparation of antimicrobial poly(vinyl alcohol) nanofibers containing silver nanoparticles. J. Polym. Sci. Part B 2006, 44, 2468–2474. [Google Scholar] [CrossRef]
- Jalvandi, J.; White, M.; Truong, Y.B.; Gao, Y.; Padhye, R.; Kyratzis, I.L. Release and antimicrobial activity of levofloxacin from composite mats of poly(ɛ-caprolactone) and mesoporous silica nanoparticles fabricated by core–shell electrospinning. J. Mater. Sci. 2015, 50, 7967–7974. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Rashkov, I. Electrospinning of poly(vinyl pyrrolidone)-iodine complex and poly(ethylene oxide)/poly(vinyl pyrrolidone)-iodine complex—a prospective route to antimicrobial wound dressing materials. Eur. Polym. J. 2007, 43, 1609–1623. [Google Scholar] [CrossRef]
- Lalani, R.; Liu, L. Electrospun zwitterionic poly(sulfobetaine methacrylate) for nonadherent, superabsorbent, and antimicrobial wound dressing applications. Biomacromolecules 2012, 13, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Mohan, Y.M.; Vimala, K.; Mohana Raju, K. Synthesis and characterization of hydrogel-silver nanoparticle-curcumin composites for wound dressing and antibacterial application. J. Appl. Polym. Sci. 2011, 121, 784–796. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Barakat, N.A.M.; Tirupathi Pichiah, P.B.; Gnanasekaran, G.; Nirmala, R.; Cha, Y.-S.; Jung, C.-H.; El-Newehy, M.; Kim, H.Y. Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin hcl. Carbohydr. Polym. 2012, 90, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, A.R.; Gnanasekaran, G.; Sathishkumar, Y.; Lee, Y.S.; Kim, C.S. Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing. Carbohydr. Polym. 2014, 102, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, Y.; Liu, X.; Chen, C.; Guiltinan, M.J.; Allcock, H.R. Biodegradable polyphosphazenes containing antibiotics: Synthesis, characterization, and hydrolytic release behavior. Polym. Chem. 2013, 4, 1826–1835. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Tummalapalli, M.; Anjum, S.; Kumari, S.; Gupta, B. Antimicrobial surgical sutures: Recent developments and strategies. Polymer Rev. 2016, 56, 607–630. [Google Scholar] [CrossRef]
- Hoshino, S.; Yoshida, Y.; Tanimura, S.; Yamauchi, Y.; Noritomi, T.; Yamashita, Y. A study of the efficacy of antibacterial sutures for surgical site infection: A retrospective controlled trial. Int. Surg. 2013, 98, 129–132. [Google Scholar] [CrossRef] [PubMed]
- García-Vargas, M.; González-Chomón, C.; Magariños, B.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Acrylic polymer-grafted polypropylene sutures for covalent immobilization or reversible adsorption of vancomycin. Int. J. Pharm. 2014, 461, 286–295. [Google Scholar] [CrossRef] [PubMed]
- He, C.-L.; Huang, Z.-M.; Han, X.-J. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J. Biomed. Mater. Res. A 2009, 89A, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zheng-Ming, H.; Xiang-Yang, L. Development of braided drug-loaded nanofiber sutures. Nanotechnology 2010, 21, 315104. [Google Scholar]
- Saravanan, S.; Nethala, S.; Pattnaik, S.; Tripathi, A.; Moorthi, A.; Selvamurugan, N. Preparation, characterization and antimicrobial activity of a bio-composite scaffold containing chitosan/nano-hydroxyapatite/nano-silver for bone tissue engineering. Int. J. Biol. Macromol. 2011, 49, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Botta, L.; Sanfilippo, M.; Gallo, G.; Palazzolo, G.; Puglia, A.M. Combining in the melt physical and biological properties of poly(caprolactone) and chlorhexidine to obtain antimicrobial surgical monofilaments. Appl. Microbiol. Biotechnol. 2013, 97, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Hortigüela, M.; Yuste, L.; Rojo, F.; Aranaz, I. Green synthesis of hierarchically structured silver-polymer nanocomposites with antibacterial activity. Nanomaterials 2016, 6, 137. [Google Scholar] [CrossRef]
- Padmakumar, S.; Joseph, J.; Neppalli, M.H.; Mathew, S.E.; Nair, S.V.; Shankarappa, S.A.; Menon, D. Electrospun polymeric core–sheath yarns as drug eluting surgical sutures. ACS Appl. Mater. Interfaces 2016, 8, 6925–6934. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumar, K.N.; Dabkowski, J.M.; Corrigan, M.; Scott, R.W.; Nüsslein, K.; Tew, G.N. New bactericidal surgical suture coating. Langmuir 2012, 28, 12134–12139. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.M.; Jiang, Z.; Safdar, W.; Xie, Z.; Ren, X. N-halamine-modified polyglycolide (PGA) multifilament as a potential bactericidal surgical suture: in vitro study. J. Appl. Polym. Sci. 2015, 132, 42483. [Google Scholar] [CrossRef]
- Ho, C.H.; Odermatt, E.K.; Berndt, I.; Tiller, J.C. Long-term active antimicrobial coatings for surgical sutures based on silver nanoparticles and hyperbranched polylysine. J. Biomater. Sci. Polym. Ed. 2013, 24, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.; García-Fernández, L.; Fernández-Blázquez, J.P.; Barbeck, M.; Ghanaati, S.; Unger, R.; Kirkpatrick, J.; Arzt, E.; Funk, L.; Turón, P.; et al. Nanostructured medical sutures with antibacterial properties. Biomaterials 2015, 52, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Ter Boo, G.-J.A.; Grijpma, D.W.; Moriarty, T.F.; Richards, R.G.; Eglin, D. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials 2015, 52, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jiang, B.; Dietz, M.J.; Smith, E.S.; Clovis, N.B.; Rao, K.M.K. Evaluation of local MCP-1 and IL-12 nanocoatings for infection prevention in open fractures. J. Orthop. Res. 2010, 28, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bistolfi, A.; Massazza, G.; Verné, E.; Massé, A.; Deledda, D.; Ferraris, S.; Miola, M.; Galetto, F.; Crova, M. Antibiotic-loaded cement in orthopedic surgery: A review. ISRN Orthop. 2011, 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Tai, C.L.; Hsu, H.Y.; Hsieh, P.H.; Lee, M.S.; Ueng, S.W.N. Liquid antibiotics in bone cement. Bone Joint Res. 2014, 3, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Slane, J.; Vivanco, J.; Rose, W.; Ploeg, H.-L.; Squire, M. Mechanical, material, and antimicrobial properties of acrylic bone cement impregnated with silver nanoparticles. Mater. Sci. Eng. C 2015, 48, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Prokopovich, P.; Leech, R.; Carmalt, C.J.; Parkin, I.P.; Perni, S. A novel bone cement impregnated with silver–tiopronin nanoparticles: Its antimicrobial, cytotoxic, and mechanical properties. Int. J. Nanomed. 2013, 8, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Prokopovich, P.; Köbrick, M.; Brousseau, E.; Perni, S. Potent antimicrobial activity of bone cement encapsulating silver nanoparticles capped with oleic acid. J. Biomed. Mater. Res. B 2015, 103, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Beyth, S.; Polak, D.; Milgrom, C.; Weiss, E.I.; Matanis, S.; Beyth, N. Antibacterial activity of bone cement containing quaternary ammonium polyethyleneimine nanoparticles. J. Antimicrob. Chemother. 2013, 69, 854–855. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Abid, C.K.V.Z.; Jain, S.; Jackeray, R.; Chattopadhyay, S.; Singh, H. Formulation and characterization of antimicrobial quaternary ammonium dendrimer in poly(methyl methcarylate) bone cement. J. Biomed. Mater. Res. B 2015. [Google Scholar] [CrossRef] [PubMed]
- Sahithi, K.; Swetha, M.; Prabaharan, M.; Moorthi, A.; Saranya, N.; Ramasamy, K.; Srinivasan, N.; Partridge, N.C.; Selvamurugan, N. Synthesis and characterization of nanoscalehydroxyapatite-copper for antimicrobial activity towards bone tissue engineering applications. J. Biomed. Nanotechnol. 2010, 6, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Saravanan, S.; Pattnaik, S.; Moorthi, A.; Partridge, N.C.; Selvamurugan, N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper-zinc for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Fernández, A.; Lopez-Esteban, S.; Malpartida, F.; Moya, J.S.; Torrecillas, R. Ceramic/metal biocidal nanocomposites for bone-related applications. J. Mater. Sci. Mater. Med. 2012, 23, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Hamlekhan, A.; Moztarzadeh, F.; Mozafari, M.; Azami, M.; Nezafati, N. Preparation of laminated poly(ε-caprolactone)-gelatin-hydroxyapatite nanocomposite scaffold bioengineered via compound techniques for bone substitution. Biomatter 2011, 1, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Bastari, K.; Arshath, M.; NG, Z.H.M.; Chia, J.H.; Yow, Z.X.D.; Sana, B.; Tan, M.F.C.; Lim, S.; Loo, S.C.J. A controlled release of antibiotics from calcium phosphate-coated poly(lactic-co-glycolic acid) particles and their in vitro efficacy against Staphylococcus aureus biofilm. J. Mater. Sci. Mater. Med. 2014, 25, 747–757. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhou, P.; Wang, L.; Xiong, X.; Zhang, Y.; Deng, Y.; Wei, S. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J. R. Soc. Interface 2014, 11, 20140169. [Google Scholar] [CrossRef] [PubMed]
- Grischke, J.; Eberhard, J.; Stiesch, M. Antimicrobial dental implant functionalization strategies—A systematic review. Dent. Mater. J. 2016, 35, 545–558. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V.J. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules 2011, 16, 9755–9763. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Liao, S.; Wen, Z.T.; Fan, Y. Synthesis and characterization of antibacterial dental monomers and composites. J. Biomed. Mater. Res. B 2012, 100B, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, S.; Zhou, X.; Wang, H.; Xu, H.; Cheng, L. The use of quaternary ammonium to combat dental caries. Materials 2015, 8, 3532–3549. [Google Scholar] [CrossRef] [PubMed]
- Fik, C.P.; Konieczny, S.; Pashley, D.H.; Waschinski, C.J.; Ladisch, R.S.; Salz, U.; Bock, T.; Tiller, J.C. Telechelic poly(2-oxazoline)s with a biocidal and a polymerizable terminal as collagenase inhibiting additive for long-term active antimicrobial dental materials. Macromol. Biosci. 2014, 14, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Zaltsman, N.; Kesler-Shvero, D.; Weiss, E.I.; Beyth, N. Synthesis variants of quaternary ammonium polyethyleneimine nanoparticles and their antibacterial efficacy in dental materials. J. Appl. Biomater. Funct. Mater. 2016, 14, e205–e211. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.-Q.; Niu, L.-N.; Kemp, L.K.; Yiu, C.K.Y.; Ryou, H.; Qi, Y.-P.; Blizzard, J.D.; Nikonov, S.; Brackett, M.G.; Messer, R.L.W.; et al. Quaternary ammonium silane-functionalized, methacrylate resin composition with antimicrobial activities and self-repair potential. Acta Biomater. 2012, 8, 3270–3282. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ghaemy, M.; Ghadiri, A.A.; Mohseni, M. Photocurable, antimicrobial quaternary ammonium–modified nanosilica. J. Dent. Res. 2015, 94, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H.K. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent. Mater. 2013, 29, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Ma, S.; Chen, J.; Xu, H.H.K. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dent. Mater. 2014, 30, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.T.; Xu, X. Antibacterial dental composites with chlorhexidine and mesoporous silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Leung, K.C.-F.; Wong, C.-H.; Lee, S.-F.; Li, X.; Leung, P.C.; Lau, C.B.S.; Wat, E.; Jin, L. Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS ONE 2014, 9, e103234. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Xu, H.H.K.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial and physical properties of calcium–phosphate and calcium–fluoride nanocomposites with chlorhexidine. Dent. Mater. 2012, 28, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Xu, H.H.K.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X.; Weir, M.D.; Fouad, A.F.; Zhao, L.; Xu, H.H.K. Effect of bioactive dental adhesive on periodontal and endodontic pathogens. J. Mater. Sci. Mater. Med. 2016, 27, 168. [Google Scholar] [CrossRef] [PubMed]
- Aydin Sevinç, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. B 2010, 94B, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Hojati, S.T.; Alaghemand, H.; Hamze, F.; Babaki, F.A.; Rajab-Nia, R.; Rezvani, M.B.; Kaviani, M.; Atai, M. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent. Mater. 2013, 29, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and lactobacillus. Restor Dent. Endod 2014, 39, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Lu, Y.; Broadnax, A.D.; Hunter, R.A.; Carpenter, A.W.; Schoenfisch, M.H. Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 9322–9329. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Reighard, K.P.; Saavedra, J.E.; Schoenfisch, M.H. O2-protected diazeniumdiolate-modified silica nanoparticles for extended nitric oxide release from dental composites. Biomater. Sci. 2013, 1, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Progress in polymer science food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-romero, M.; Cummins, E. Nanotechnologies in the food industry-recent developments, risks and regulation. Trends Food Sci. Technol. 2012, 24, 30–46. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Li, X.; Zhou, W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci. Technol. 2015, 45, 187–199. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar]
- Van Long, N.N.; Joly, C.; Dantigny, P. Active packaging with antifungal activities. Int. J. Food Microbiol. 2016, 220, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.-Y.; Tin, L.; Tan, A.-C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.D.; Agroindustry, E.T.; Mesquita, R.D.S. Antimicrobial nanostructures in food packaging. Trends Food Sci. Technol. 2013, 30, 56–69. [Google Scholar] [CrossRef]
- Joerger, R.D. Antimicrobial films for food applications: A quantitative analysis of their effectiveness and science. Packag. Technol. Sci. 2007, 20, 231–273. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R. Metallic-based micro and nanocomposites in food contact materials and active. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; Jesus, M.B.D.; Seabra, A.B.; Fc!varo, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, N.V.; Mosavian, M.T.H.; Mortazavi, S.A. Effect of polyethylene packaging modified with silver particles on the microbial, sensory and appearance of dried barberry. Packag. Technol. Sci. 2013, 26, 39–49. [Google Scholar] [CrossRef]
- Martinez-Abad, A.; Lagaron, J.M.; Ocio, M.J. Development and characterization of silver-based antimicrobial ethylene-vinyl alcohol copolymer (EVOH) films for food-packaging applications. J. Agric. Food Chem. 2012, 60, 5350–5359. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; Abdel-Aziz, M.S. Preparation of polystyrene nanocomposites based on silver nanoparticles using marine bacterium for packaging. Polym. Plast. Technol. Eng. 2013, 52, 607–613. [Google Scholar] [CrossRef]
- Yoksan, R.; Chirachanchai, S. Silver nanoparticle-loaded chitosan–starch based films: Fabrication and evaluation of tensile, barrier and antimicrobial properties. Mater. Sci. Eng. C 2010, 30, 891–897. [Google Scholar] [CrossRef]
- Fernandez, A.; Soriano, E.; Hernández-Muñoz, P.; Gavara, R. Migration of antimicrobial silver from composites of polylactide with silver zeolites. J. Food Sci. 2010, 75, E186–E193. [Google Scholar] [CrossRef] [PubMed]
- Incoronato, A.L.; Buonocore, G.G.; Conte, A.; Lavorgna, M.; Nobile, M.A.D. Active systems based on silver-montmorillonite nanoparticles embedded into bio-based polymer matrices for packaging applications. J. Food Prot. 2010, 73, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Chawengkijwanich, C.; Hayata, Y. Development of TiO2 powder-coated food packaging film and its ability to inactivate Escherichia coli in vitro and in actual tests. Int. J. Food Microbiol. 2008, 123, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Cerrada, M.L.; Serrano, C.; Sanchez-Chaves, M.; Fernandez-García, M.; Fernandez-Martin, F.; de Andres, A.; Jimenez-Rioboo, R.J.; Kubacka, A.; Ferrer, M.; Fernandez-García, M. Self-sterilized EVOH-TiO2 nanocomposites: Interface effects on biocidal properties. Adv. Funct. Mater. 2008, 18, 1949–1960. [Google Scholar] [CrossRef]
- Kubacka, A.; Cerrada, M.L.; Serrano, C.; Fernández-García, M.; Ferrer, M.; Fernández-García, M. Light-driven novel properties of TiO2-modified polypropylene-based nanocomposite films. J. Nanosci. Nanotechnol. 2008, 8, 3241–3246. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Ferrer, M.; Cerrada, M.L.; Serrano, C.; Sánchez-Chaves, M.; Fernández-García, M.; de Andres, A.; Rioboo, R.J.J.; Fernández-Martín, F.; Fernández-García, M. Boosting TiO2-anatase antimicrobial activity: Polymer-oxide thin films. Appl. Catal. B 2009, 89, 441–447. [Google Scholar] [CrossRef]
- Kubacka, A.; Ferrer, M.; Fernández-García, M.; Serrano, C.; Cerrada, M.L.; Fernández-García, M. Tailoring polymer-TiO2 film properties by presence of metal (Ag, Cu, Zn) species: Optimization of antimicrobial properties. Appl. Catal. B 2011, 104, 346–352. [Google Scholar] [CrossRef]
- Kubacka, A.; Cerrada, M.L.; Serrano, C.; Fernández-García, M.; Ferrer, M.; Fernández-García, M. Plasmonic nanoparticle/polymer nanocomposites with enhanced photocatalytic antimicrobial properties. J. Phys. Chem. C 2009, 113, 9182–9190. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Cerrada, M.L.; Fernández-García, M.; Kubacka, A.; Ferrer, M.; Fernández-García, M. Biodegradable polycaprolactone-titania nanocomposites: Preparation, characterization and antimicrobial properties. Int. J. Mol. Sci. 2013, 14, 9249–9266. [Google Scholar] [CrossRef] [PubMed]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-zad, S. Evaluation of nanocomposite packaging containing ag and ZnO on shelf life of fresh orange juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Li, X.H.; Li, W.L.; Xing, Y.G.; Jiang, Y.H.; Ding, Y.L.; Zhang, P.P. Effects of nano-zno power-coated pvc film on the physiological properties and microbiological changes of fresh-cut “fuji” apple. Adv. Mater. Res. 2011, 152–153, 450–453. [Google Scholar] [CrossRef]
- Jin, T.; Gurtler, J.B. Inactivation of salmonella in liquid egg albumen by antimicrobial bottle coatings infused with allyl isothiocyanate, nisin and zinc oxide nanoparticles. J. Appl. Microbiol. 2011, 110, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-I.; Rhim, J.-W. Antimicrobial activity of organically modified nano-clays. J. Nanosci. Nanotechnol. 2008, 8, 5818–5824. [Google Scholar] [CrossRef] [PubMed]
- Sothornvit, R.; Rhim, J.-W.; Hong, S.-I. Effect of nano-clay type on the physical and antimicrobial properties of whey protein isolate/clay composite films. J. Food Eng. 2009, 91, 468–473. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT—Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Mascheroni, E.; Chalier, P.; Gontard, N.; Gastaldi, E. Designing of a wheat gluten/montmorillonite based system as carvacrol carrier: Rheological and structural properties. Food Hydrocoll. 2010, 24, 406–413. [Google Scholar] [CrossRef]
- Khan, I.; Oh, D.-H. Integration of nisin into nanoparticles for application in foods. Innov. Food Sci. Emerg. Technol. 2016, 34, 376–384. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.-M.; René, N.; Jamshidian, M.; Akhtar, M.J.; Arab-Tehrany, E.; Jacquot, M.; Desobry, S. Microstructure and physico-chemical evaluation of nano-emulsion-based antimicrobial peptides embedded in bioactive packaging films. Food Hydrocoll. 2012, 29, 407–419. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of edible films and coatings with antimicrobial activity. Food Bioprocess. Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Lorevice, M.V.; Mattoso, L.H.C.; Zucolotto, V. Highly stable, edible cellulose films incorporating chitosan nanoparticles. J. Food Sci. 2011, 76, 25–29. [Google Scholar]
- Judith, P.; Espitia, P.C.R.; Du, W.-X.; Avena-bustillos, R.D.J.S.; Fátima, N.D.; Soares, F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar]
- Olle, C.P.; Gerschenson, L.C.A.N.; Jagus, R.J. Natamycin and nisin supported on starch edible fi lms for controlling mixed culture growth on model systems and port salut cheese. Food Control 2014, 44, 146–151. [Google Scholar] [CrossRef]
- Otoni, C.G.; Pontes, S.F.O.; Medeiros, E.A.A. Edible films from methylcellulose and nanoemulsions of clove bud (syzygium aromaticum) and oregano (origanum vulgare) essential oils as shelf life extenders for sliced bread. J. Agric. Food Chem. 2014, 62, 5214–5219. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-fani, A.; Salvia-trujillo, L.; Rojasgra, C.; Martin-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Tavassoli-kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Orsuwan, A.; Shankar, S.; Wang, L.-F.; Sothornvit, R.; Rhim, J.-W. Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocoll. 2016, 60, 476–485. [Google Scholar] [CrossRef]
- Morsy, M.K.; Khalaf, H.H.; Sharoba, A.M.; El-tanahi, H.H.; Cutter, C.N. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J. Food Sci. 2014, 79, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Abugoch, L.; Caro, N.; Medina, E.-A.; Díaz-dosque, M.; Luis, L.; Tapia, C. Novel active packaging based on films of chitosan and chitosan/quinoa protein printed with chitosan-tripolyphosphate-thymol nanoparticles via thermal ink-jet printing. Food Hydrocoll. 2016, 52, 520–532. [Google Scholar]
- Cerqueira, M.A.; Costa, M.J.; Fuciños, C.; Pastrana, L.M.; Vicente, A.A. Development of active and nanotechnology-based smart edible packaging systems: Physical-chemical characterization. Food Bioprocess. Technol. 2014, 7, 1472–1482. [Google Scholar] [CrossRef]
- Zohri, M.; Shafiee, M.; Ismaeil, A. A comparative study between the antibacterial effect of nisin and nisin-loaded chitosan/alginate nanoparticles on the growth of staphylococcus aureus in raw and pasteurized milk samples. Probiotics Antimicrob. Proteins 2010, 2, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Zohri, M.; Shafiee Alavidjeh, M.; Mirdamadi, S.S.; Behmadi, H.; Hossaini Nasr, S.M.; Eshghi Gonbaki, S.; Shafiee Ardestani, M.; Jabbari Arabzadeh, A. Nisin-loaded chitosan/alginate nanoparticles: A hopeful hybrid biopreservative. J. Food Saf. 2013, 33, 40–49. [Google Scholar]
- Bernela, M.; Kaur, P.; Chopra, M.; Thakur, R. Synthesis, characterization of nisin loaded alginate-chitosan-pluronic composite nanoparticles and evaluation against microbes. LWT—Food Sci. Technol. 2014, 59, 1093–1099. [Google Scholar] [CrossRef]
- Donsí, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT—Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf. B 2014, 114, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Y.; Huang, Q. Synthesis and characterization of novel antimicrobial emulsifiers from ε-polylysine. J. Agric. Food Chem. 2010, 58, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Hui, F.; Debiemme-Chouvy, C. Antimicrobial n-halamine polymers and coatings: A review of their synthesis, characterization, and applications. Biomacromolecules 2013, 14, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.J.; Goddard, J.M. Self-healing antimicrobial polymer coating with efficacy in the presence of organic matter. Appl. Surf. Sci. 2016, 378, 479–488. [Google Scholar] [CrossRef]
- Farah, S.; Aviv, O.; Daif, M.; Kunduru, K.R.; Laout, N.; Ratner, S.; Beyth, N.; Domb, A.J. N-bromo-hydantoin grafted polystyrene beads: Synthesis and nano-micro beads characteristics for achieving controlled release of active oxidative bromine and extended microbial inactivation efficiency. J. Polym. Sci. Part A 2016, 54, 596–610. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, S.; Jing, Z. Preparation of Cu (II)/PEI–QPEI/SiO2 nanopowder as antibacterial material. J. Sol-Gel Sci. Technol. 2014, 72, 351–358. [Google Scholar] [CrossRef]
- Chang, L.; Wang, J.; Tong, C.; Zhao, L.; Liu, X. Comparison of antimicrobial activities of polyacrylonitrile fibers modified with quaternary phosphonium salts having different alkyl chain lengths. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Cuthbert, T.J.; Harrison, T.D.; Ragogna, P.J.; Gillies, E.R. Synthesis, properties, and antibacterial activity of polyphosphonium semi-interpenetrating networks. J. Mater. Chem. B 2016, 4, 4872–4883. [Google Scholar] [CrossRef]
- Alvarez-Paino, M.; Munoz-Bonilla, A.; Lopez-Fabal, F.; Gomez-Garces, J.L.; Heuts, J.P.A.; Fernandez-Garcia, M. Functional surfaces obtained from emulsion polymerization using antimicrobial glycosylated block copolymers as surfactants. Polym. Chem. 2015, 6, 6171–6181. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, C.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial silica particles synthesized via ring-opening grafting of cationic amphiphilic cyclic carbonates: Effects of hydrophobicity and structure. Polym. Chem. 2016, 7, 2192–2201. [Google Scholar] [CrossRef]
- Aviv, O.; Amir, N.; Laout, N.; Ratner, S.; Basu, A.; Domb, A.J. Poly(hexamethylene guanidine)-poly(ethylene glycol) solid blend for water microbial deactivation. Polym. Degrad. Stab. 2016, 129, 239–245. [Google Scholar] [CrossRef]
- Rogalsky, S.; Bardeau, J.-F.; Wu, H.; Lyoshina, L.; Bulko, O.; Tarasyuk, O.; Makhno, S.; Cherniavska, T.; Kyselov, Y.; Koo, J.H. Structural, thermal and antibacterial properties of polyamide 11/polymeric biocide polyhexamethylene guanidine dodecylbenzenesulfonate composites. J. Mater. Sci. 2016, 51, 7716–7730. [Google Scholar] [CrossRef]
- Villanueva, M.E.; González, J.A.; Rodríguez-Castellón, E.; Teves, S.; Copello, G.J. Antimicrobial surface functionalization of pvc by a guanidine based antimicrobial polymer. Mater. Sci. Eng. C 2016, 67, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nam, J.A.; Lee, S.; In, I.; Park, S.Y. Antimicrobial activity of water resistant surface coating from catechol conjugated polyquaternary amine on versatile substrates. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.; In, I.; Park, S.Y. Synthesis and antibacterial activity of surface-coated catechol-conjugated polymer with silver nanoparticles on versatile substrate. Surf. Interface Anal. 2016, 48, 995–1001. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Palmucci, J.; Pettinari, C.; Pettinari, R.; Condello, F.; Ferraro, S.; Marangoni, M.; Crispini, A.; Scuri, S.; Grappasonni, I.; et al. Novel composite plastics containing silver(I) acylpyrazolonato additives display potent antimicrobial activity by contact. Chem. Eur. J. 2015, 21, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Hubauer-Brenner, M.; Hinterdorfer, P. Influence of surface morphology on the antimicrobial effect of transition metal oxides in polymer surface. J. Nanosci. Nanotechnol. 2015, 15, 7853–7859. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Fonseca, A.; Mendonça, P.; Branco, R.; Serra, A.; Morais, P.; Coelho, J. Recent developments in antimicrobial polymers: A review. Materials 2016, 9, 599. [Google Scholar] [CrossRef]
- Sun, G.; Hong, K.H. Photo-induced antimicrobial and decontaminating agents: Recent progresses in polymer and textile applications. Text. Res. J. 2013, 83, 532–542. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Z.; Tay, S.L.; Gao, W. Photocatalytic TiO2 nanoparticles enhanced polymer antimicrobial coating. Appl. Surf. Sci. 2014, 290, 274–279. [Google Scholar] [CrossRef]
- Hynek, J.; Rathousky, J.; Demel, J.; Lang, K. Design of porphyrin-based conjugated microporous polymers with enhanced singlet oxygen productivity. RSC Adv. 2016, 6, 44279–44287. [Google Scholar] [CrossRef]
- Naik, A.J.T.; Ismail, S.; Kay, C.; Wilson, M.; Parkin, I.P. Antimicrobial activity of polyurethane embedded with methylene blue, toluidene blue and gold nanoparticles against Staphylococcus aureus; illuminated with white light. Mater. Chem. Phys. 2011, 129, 446–450. [Google Scholar] [CrossRef]
- Masilela, N.; Kleyi, P.; Tshentu, Z.; Priniotakis, G.; Westbroek, P.; Nyokong, T. Photodynamic inactivation of Staphylococcus aureus using low symmetrically substituted phthalocyanines supported on a polystyrene polymer fiber. Dyes Pigm. 2013, 96, 500–508. [Google Scholar] [CrossRef]
- Noimark, S.; Bovis, M.; MacRobert, A.J.; Correia, A.; Allan, E.; Wilson, M.; Parkin, I.P. Photobactericidal polymers; the incorporation of crystal violet and nanogold into medical grade silicone. RSC Adv. 2013, 3, 18383–18394. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Wang, S. Flexible antibacterial film deposited with polythiophene–porphyrin composite. Adv. Healthc. Mater. 2013, 2, 1582–1585. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Niu, R.; Qi, J.; Yuan, H.; Fan, Y.; An, H.; Yan, W.; Li, H.; Zhan, Y.; Xing, C. Conjugated polythiophene for rapid, simple, and high-throughput screening of antimicrobial photosensitizers. ACS Appl. Mater. Interfaces 2015, 7, 14569–14572. [Google Scholar] [CrossRef] [PubMed]
- Elashnikov, R.; Lyutakov, O.; Ulbrich, P.; Svorcik, V. Light-activated polymethylmethacrylate nanofibers with antibacterial activity. Mater. Sci. Eng. C 2016, 64, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.; Scholle, F.; Zhu, J.; Lu, Y.; Zhang, X.; Situ, X.; Ghiladi, R. Photosensitizer-embedded polyacrylonitrile nanofibers as antimicrobial non-woven textile. Nanomaterials 2016, 6, 77. [Google Scholar] [CrossRef]
- Ozkan, E.; Allan, E.; Parkin, I.P. The antibacterial properties of light-activated polydimethylsiloxane containing crystal violet. RSC Adv. 2014, 4, 51711–51715. [Google Scholar] [CrossRef]
- Ozkan, E.; Ozkan, F.T.; Allan, E.; Parkin, I.P. The use of zinc oxide nanoparticles to enhance the antibacterial properties of light-activated polydimethylsiloxane containing crystal violet. RSC Adv. 2015, 5, 8806–8813. [Google Scholar] [CrossRef]
- Ismail, S.; Perni, S.; Pratten, J.; Parkin, I.; Wilson, M. Efficacy of a novel light-activated antimicrobial coating for disinfecting hospital surfaces. Infect. Control. Hosp. Epidemiol. 2011, 32, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Pappas, H.C.; Hill, E.H.; Huang, Y.; Whitten, D.G.; Schanze, K.S. Conjugated polyelectrolytes with imidazolium solubilizing groups. Properties and application to photodynamic inactivation of bacteria. ACS Appl. Mater. Interfaces 2015, 7, 28027–28034. [Google Scholar] [CrossRef] [PubMed]
- Corbitt, T.S.; Sommer, J.R.; Chemburu, S.; Ogawa, K.; Ista, L.K.; Lopez, G.P.; Whitten, D.G.; Schanze, K.S. Conjugated polyelectrolyte capsules: Light-activated antimicrobial micro “roach motels”. ACS Appl. Mater. Interfaces 2009, 1, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.H.; Whitten, D.G.; Evans, D.G. Computational study of bacterial membrane disruption by cationic biocides: Structural basis for water pore formation. J. Phys. Chem. B 2014, 118, 9722–9732. [Google Scholar] [CrossRef] [PubMed]
- Tew, G.N.; Scott, R.W.; Klein, M.L.; DeGrado, W.F. De novo design of antimicrobial polymers, foldamers, and small molecules: From discovery to practical applications. Acc. Chem. Res. 2010, 43, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jang, J. Antimicrobial polymer nanostructures: Synthetic route, mechanism of action and perspective. Adv. Colloid Interface Sci. 2014, 203, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Narayan, R.; Aminabhavi, T.M.; Raju, K.V.S.N. Nitrogen rich hyperbranched polyol via A3 + B3 polycondensation: Thermal, mechanical, anti-corrosive and antimicrobial properties of poly(urethane-urea). J. Polym. Res. 2014, 21, 547. [Google Scholar] [CrossRef]
- Yagci, M.B.; Bolca, S.; Heuts, J.P.A.; Ming, W.; de With, G. Self-stratifying antimicrobial polyurethane coatings. Prog. Org. Coat. 2011, 72, 305–314. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Heine, E.; Keul, H.; Moeller, M. Azetidinium functionalized polytetrahydrofurans: Antimicrobial properties in solution and application to prepare non leaching antimicrobial surfaces. Polymers 2014, 6, 1618–1630. [Google Scholar] [CrossRef]
- Wynne, J.H.; Fulmer, P.A.; McCluskey, D.M.; Mackey, N.M.; Buchanan, J.P. Synthesis and development of a multifunctional self-decontaminating polyurethane coating. ACS Appl. Mater. Interfaces 2011, 3, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Dorner, F.; Boschert, D.; Schneider, A.; Hartleb, W.; Al-Ahmad, A.; Lienkamp, K. Toward self-regenerating antimicrobial polymer surfaces. ACS Macro Lett. 2015, 4, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Luan, S.; Shi, H.; Xu, X.; Zhang, J.; Yuan, S.; Yang, Y.; Yin, J. Hierarchical polymer brushes with dominant antibacterial mechanisms switching from bactericidal to bacteria repellent. Biomacromolecules 2016, 17, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Gittens, J.E.; Smith, T.J.; Suleiman, R.; Akid, R. Current and emerging environmentally-friendly systems for fouling control in the marine environment. Biotechnol. Adv. 2013, 31, 1738–1753. [Google Scholar] [CrossRef] [PubMed]
- Bekiari, V.; Nikolaou, K.; Koromilas, N.; Lainioti, G.; Avramidis, P.; Hotos, G.; Kallitsis, J.K.; Bokias, G. Release of polymeric biocides from synthetic matrices for marine biofouling applications. Agric. Agric. Sci. Procedia 2015, 4, 445–450. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Li, J.; Kleintschek, T.; Rieder, A.; Cheng, Y.; Baumbach, T.; Obst, U.; Schwartz, T.; Levkin, P.A. Hydrophobic liquid-infused porous polymer surfaces for antibacterial applications. ACS Appl. Mater. Interfaces 2013, 5, 6704–6711. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Cai, T.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Barnacle cement as surface anchor for “clicking” of antifouling and antimicrobial polymer brushes on stainless steel. Biomacromolecules 2013, 14, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ma, C.; Zhang, G. Biodegradable polymers for marine antibiofouling: Poly(ε-caprolactone)/poly(butylene succinate) blend as controlled release system of organic antifoulant. Polymer 2016, 90, 215–221. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Iatridi, Z.; Moschakou, M.; Damigos, P.; Bokias, G.; Kallitsis, J.K. Development of Cu2+- and/or phosphonium-based polymeric biocidal materials and their potential application in antifouling paints. Prog. Org. Coat. 2012, 75, 190–199. [Google Scholar] [CrossRef]
- Anyaogu, K.C.; Fedorov, A.V.; Neckers, D.C. Synthesis, characterization, and antifouling potential of functionalized copper nanoparticles. Langmuir 2008, 24, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, A.; Zhang, J.; Kann, N.; Moth-Poulsen, K.; Nydén, M. Copper-coordinating polymers for marine anti-fouling coatings: A physicochemical and electrochemical study of ternary system of copper, pmma and poly(TBTA). Prog. Org. Coat. 2016, 97, 216–221. [Google Scholar] [CrossRef]
- Hajji, S.; Chaker, A.; Jridi, M.; Maalej, H.; Jellouli, K.; Boufi, S.; Nasri, M. Structural analysis, and antioxidant and antibacterial properties of chitosan-poly(vinyl alcohol) biodegradable films. Environ. Sci. Pollut. Res. Int. 2016, 23, 15310–15320. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Malinconico, M.; Petti, L.; Romano, G. Physical behavior of biodegradable alginate–poly(vinyl alcohol) blend films. J. Polym. Sci. Part B 2005, 43, 1205–1213. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, H.; Qian, Y.; Lü, L.; Wang, B.; Qiu, X. Hollow lignin azo colloids encapsulated avermectin with high anti-photolysis and controlled release performance. Ind. Crops Prod. 2016, 87, 191–197. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Li, B.-X.; Zhang, X.-P.; Zhang, Z.-Q.; Wang, W.-C.; Liu, F. Phoxim microcapsules prepared with polyurea and urea–formaldehyde resins differ in photostability and insecticidal activity. J. Agric. Food Chem. 2016, 64, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Boyandin, A.N.; Zhila, N.O.; Kiselev, E.G.; Volova, T.G. Constructing slow-release formulations of metribuzin based on degradable poly(3-hydroxybutyrate). J. Agric. Food Chem. 2016, 64, 5625–5632. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Zhila, N.; Vinogradova, O.; Shumilova, A.; Prudnikova, S.; Shishatskaya, E. Characterization of biodegradable poly-3-hydroxybutyrate films and pellets loaded with the fungicide tebuconazole. Environ. Sci. Pollut. Res. Int. 2016, 23, 5243–5254. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Gao, Z.; Zhang, S.; Li, Y.; Hu, S.; Ren, X. Preparation and anti-UV property of modified cellulose membranes for biopesticides controlled release. Ind. Crops Prod. 2016, 89, 176–181. [Google Scholar] [CrossRef]
- Tan, S.; Cui, J.; Fu, Q.; Nam, E.; Ladewig, K.; Ren, J.M.; Wong, E.H.H.; Caruso, F.; Blencowe, A.; Qiao, G.G. Photocontrolled cargo release from dual cross-linked polymer particles. ACS Appl. Mater. Interfaces 2016, 8, 6219–6228. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Shi, L.; Zhang, L.; Zeng, T.; Yin, Y.; Yi, Y. Synthesis of photoresponsive polymeric propesticide micelles based on peg for the controlled release of a herbicide. Polym. Chem. 2016, 7, 899–904. [Google Scholar] [CrossRef]
- Turalija, M.; Bischof, S.; Budimir, A.; Gaan, S. Antimicrobial pla films from environment friendly additives. Compos. Part B 2016, 102, 94–99. [Google Scholar] [CrossRef]
- Golberg, K.; Emuna, N.; Vinod, T.P.; van Moppes, D.; Marks, R.S.; Arad, S.M.; Kushmaro, A. Novel anti-adhesive biomaterial patches: Preventing biofilm with metal complex films (MCF) derived from a microalgal polysaccharide. Adv. Mater. Interf. 2016, 3, 1500486. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Mansour Lakouraj, M.; Mohseni, M. Biodegradable polypyrrole/dextrin conductive nanocomposite: Synthesis, characterization, antioxidant and antibacterial activity. Synth. Met. 2014, 187, 9–16. [Google Scholar] [CrossRef]
- Shaik, M.R.; Alam, M.; Alandis, N.M. Development of castor oil based poly(urethane-esteramide)/TiO2 nanocomposites as anticorrosive and antimicrobial coatings. J. Nanomater. 2015, 2015, 10. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tirgir, F.; Sabzalian, M.R. Synthesis, characterization and in vitro antimicrobial and biodegradability study of pseudo-poly(amino acid)s derived from N,N′-(pyromellitoyl)-bis-L-tyrosine dimethyl ester as a chiral bioactive diphenolic monomer. Amino Acids 2011, 40, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Varona, S.; Kareth, S.; Martín, Á.; Cocero, M.J. Formulation of lavandin essential oil with biopolymers by PGSS for application as biocide in ecological agriculture. J. Supercrit. Fluids 2010, 54, 369–377. [Google Scholar] [CrossRef]
- Abid, C.K.V.Z.; Chattopadhyay, S.; Mazumdar, N.; Singh, H. Synthesis and characterization of quaternary ammonium PEGDA dendritic copolymer networks for water disinfection. J. Appl. Polym. Sci. 2010, 116, 1640–1649. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Naushad, M.; Ahamad, T.; Alothman, Z.A.; Aldalbahi, A. Synthesis, characterization of curcumin based ecofriendly antimicrobial bio-adsorbent for the removal of phenol from aqueous medium. Chem. Eng. J. 2014, 254, 181–189. [Google Scholar] [CrossRef]
- Alamri, A.; El-Newehy, M.H.; Al-Deyab, S.S. Biocidal polymers: Synthesis and antimicrobial properties of benzaldehyde derivatives immobilized onto amine-terminated polyacrylonitrile. Chem. Cent. J. 2012, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bonenfant, D.; Bourgeois, F.R.; Mimeault, M.; Monette, F.; Niquette, P.; Hausler, R. Synthesis and structure-activity study of quaternary ammonium functionalized β-cyclodextrin-carboxymethyl cellulose polymers. Water Sci. Technol. 2011, 63, 2827–2832. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Z.; Sun, Y. N-halamine-based antimicrobial additives for polymers: Preparation, characterization and antimicrobial activity. Ind. Eng. Chem. Res. 2006, 45, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.J.; McLandsborough, L.A.; Peleg, M.; Goddard, J.M. Antimicrobial N-halamine modified polyethylene: Characterization, biocidal efficacy, regeneration, and stability. J. Food Sci. 2014, 79, E887–E897. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Demir, B.; Broughton, R.M.; Ren, X.; Huang, T.S.; Worley, S.D. Antimicrobial silica and sand particles functionalized with an N-halamine acrylamidesiloxane copolymer. J. Appl. Polym. Sci. 2016, 133, 43413. [Google Scholar] [CrossRef]
- Aviv, O.; Farah, S.; Amir, N.; Laout, N.; Ratner, S.; Domb, A.J. N-bromo-dimethylhydantoin polystyrene resin for water microbial decontamination. Biomacromolecules 2015, 16, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.G.; Almeida, C.A.; Fernandez-Baldo, M.A.; Felici, E.; Raba, J.; Sanz, M.I. Development of nitrocellulose membrane filters impregnated with different biosynthesized silver nanoparticles applied to water purification. Talanta 2016, 146, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Livi, K.J.T.; Chen, K.L. Polysulfone membranes modified with bioinspired polydopamine and silver nanoparticles formed in situ to mitigate biofouling. Environ. Sci. Technol. 2015, 2, 59–65. [Google Scholar] [CrossRef]
- Liu, X.; Qi, S.; Li, Y.; Yang, L.; Cao, B.; Tang, C.Y. Synthesis and characterization of novel antibacterial silver nanocomposite nanofiltration and forward osmosis membranes based on layer-by-layer assembly. Water Res. 2013, 47, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Jewrajka, S.K.; Haldar, S. Amphiphilic poly(acrylonitrile-co-acrylic acid)/silver nanocomposite additives for the preparation of antibiofouling membranes with improved properties. Polym. Compos. 2011, 32, 1851–1861. [Google Scholar] [CrossRef]
- Blin, T.; Purohit, V.; Leprince, J.; Jouenne, T.; Glinel, K. Bactericidal microparticles decorated by an antimicrobial peptide for the easy disinfection of sensitive aqueous solutions. Biomacromolecules 2011, 12, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Lubasova, D.; Netravali, A.; Parker, J.; Ingel, B. Bacterial filtration efficiency of green soy protein based nanofiber air filter. J. Nanosci. Nanotechnol. 2014, 14, 4891–4898. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, T.S.; Tsai, C.H.; Huang, H.L.; Ho, K.S.; Lin, I.; Wang, Y.F. Fabrication of polyaniline coated plasma modified polypropylene filter for antibioaerosol application. Aerosol Air Qual. Res. 2016, 16, 1911–1921. [Google Scholar] [CrossRef]
- Taylor, M.; McCollister, B.; Park, D. Highly bactericidal polyurethane effective against both normal and drug-resistant bacteria: Potential use as an air filter coating. Appl. Biochem. Biotechnol. 2016, 178, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Akdag, A.; Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. N-halamine-coated cotton for antimicrobial and detoxification applications. Carbohydr. Polym. 2009, 78, 220–226. [Google Scholar] [CrossRef]
- Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Antimicrobial coatings for polyester and polyester/cotton blends. Prog. Org. Coat. 2013, 76, 1082–1087. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Du, J.; Ren, X.; Worley, S.D.; Huang, T.S. Improved UV stability of antibacterial coatings with n-halamine/TiO2. Cellulose 2013, 20, 2151–2161. [Google Scholar] [CrossRef]
- Karimi, L.; Yazdanshenas, M.E.; Khajavi, R.; Rashidi, A.; Mirjalili, M. Using graphene/TiO2 nanocomposite as a new route for preparation of electroconductive, self-cleaning, antibacterial and antifungal cotton fabric without toxicity. Cellulose 2014, 21, 3813–3827. [Google Scholar] [CrossRef]
- Pant, H.R.; Pandeya, D.R.; Nam, K.T.; Baek, W.; Hong, S.T.; Kim, H.Y. Photocatalytic and antibacterial properties of a TiO2/nylon-6 electrospun nanocomposite mat containing silver nanoparticles. J. Hazard. Mater. 2011, 189, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, X.L.; Chen, Y.Y.; Lin, H. Application of silver nanoparticles to cotton fabric as an antibacterial textile finish. Fibers Polym. 2009, 10, 496–501. [Google Scholar] [CrossRef]
- Shahid ul, I.; Butola, B.S.; Mohammad, F. Silver nanomaterials as future colorants and potential antimicrobial agents for natural and synthetic textile materials. RSC Adv. 2016, 6, 44232–44247. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Chwastowski, J.; Kucharski, A.; Banach, M. Functionalization of textiles with silver and zinc oxide nanoparticles. Appl. Surf. Sci. 2016, 385, 543–553. [Google Scholar] [CrossRef]
- Wu, M.; Ma, B.; Pan, T.; Chen, S.; Sun, J. Silver-nanoparticle-colored cotton fabrics with tunable colors and durable antibacterial and self-healing superhydrophobic properties. Adv. Funct. Mater. 2016, 26, 569–576. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, X.; Chen, Y.; Guo, M.; Zhang, Y.; Guo, X.; Gu, H. Antibiotic-loaded, silver core-embedded mesoporous silica nanovehicles as a synergistic antibacterial agent for the treatment of drug-resistant infections. Biomaterials 2016, 101, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Akkapeddi, P.; Yadav, V.; Manjunath, G.B.; Uppu, D.S.S.M.; Konai, M.M.; Yarlagadda, V.; Sanyal, K.; Haldar, J. Broad spectrum antibacterial and antifungal polymeric paint materials: Synthesis, structure–activity relationship, and membrane-active mode of action. ACS Appl. Mater. Interfaces 2015, 7, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Paino, M.; Juan-Rodríguez, R.; Cuervo-Rodríguez, R.; Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial films obtained from latex particles functionalized with quaternized block copolymers. Colloids Surf. B 2016, 140, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.L.; An, Q.Z.; Li, X.; Qian, L.Y.; He, B.H.; Xiao, H.N. Synergistic effects of chitosan-guanidine complexes on enhancing antimicrobial activity and wet-strength of paper. Bioresour. Technol. 2010, 101, 5693–5700. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Guan, Y.; He, B.; Xiao, H. Synergy of wet strength and antimicrobial activity of cellulose paper induced by a novel polymer complex. Mater. Lett. 2008, 62, 3610–3612. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the antimicrobial activities of graphene materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]

| Nanoparticles | Polymer | Tested Food | Reference |
|---|---|---|---|
| Ag NPs | LDPE | Barberry | [154] |
| Ag NPs | EVOH | Chicken, pork, cheese, lettuce, apples, peels, eggshells | [155] |
| Ag NPs | PS | - | [156] |
| Ag NPs | Chitosan | - | [157] |
| Ag-Zeolite | PLA | - | [158] |
| Ag-Clay | Agar, zein, PCL | - | [159] |
| TiO2 | PP | Lettuce | [160] |
| TiO2 | EVOH, PP, PCL | - | [161,162,163,164,165,166] |
| ZnO | LDPE | Orange juice | [167] |
| ZnO | PVC | Apple | [168] |
| ZnO, nisin | PLA | Liquid egg | [169] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Paino, M.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial Polymers in the Nano-World. Nanomaterials 2017, 7, 48. https://doi.org/10.3390/nano7020048
Álvarez-Paino M, Muñoz-Bonilla A, Fernández-García M. Antimicrobial Polymers in the Nano-World. Nanomaterials. 2017; 7(2):48. https://doi.org/10.3390/nano7020048
Chicago/Turabian StyleÁlvarez-Paino, Marta, Alexandra Muñoz-Bonilla, and Marta Fernández-García. 2017. "Antimicrobial Polymers in the Nano-World" Nanomaterials 7, no. 2: 48. https://doi.org/10.3390/nano7020048
APA StyleÁlvarez-Paino, M., Muñoz-Bonilla, A., & Fernández-García, M. (2017). Antimicrobial Polymers in the Nano-World. Nanomaterials, 7(2), 48. https://doi.org/10.3390/nano7020048







