One-Step Reduction and Surface Modification of Graphene Oxide by 3-Hydroxy-2-Naphthoic Acid Hydrazide and Its Polypropylene Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
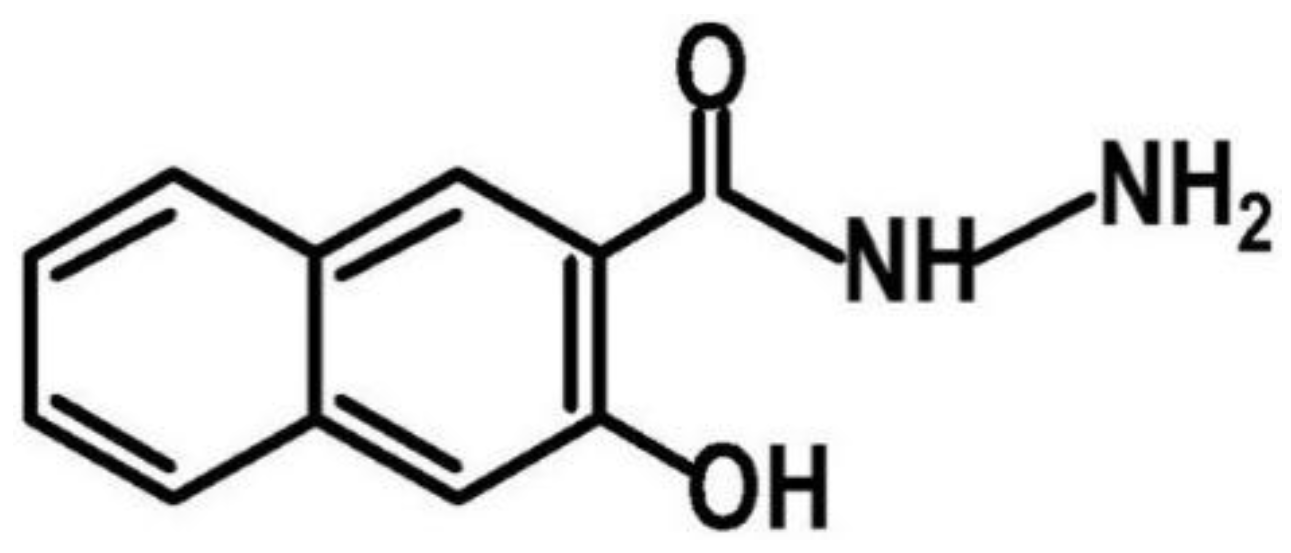
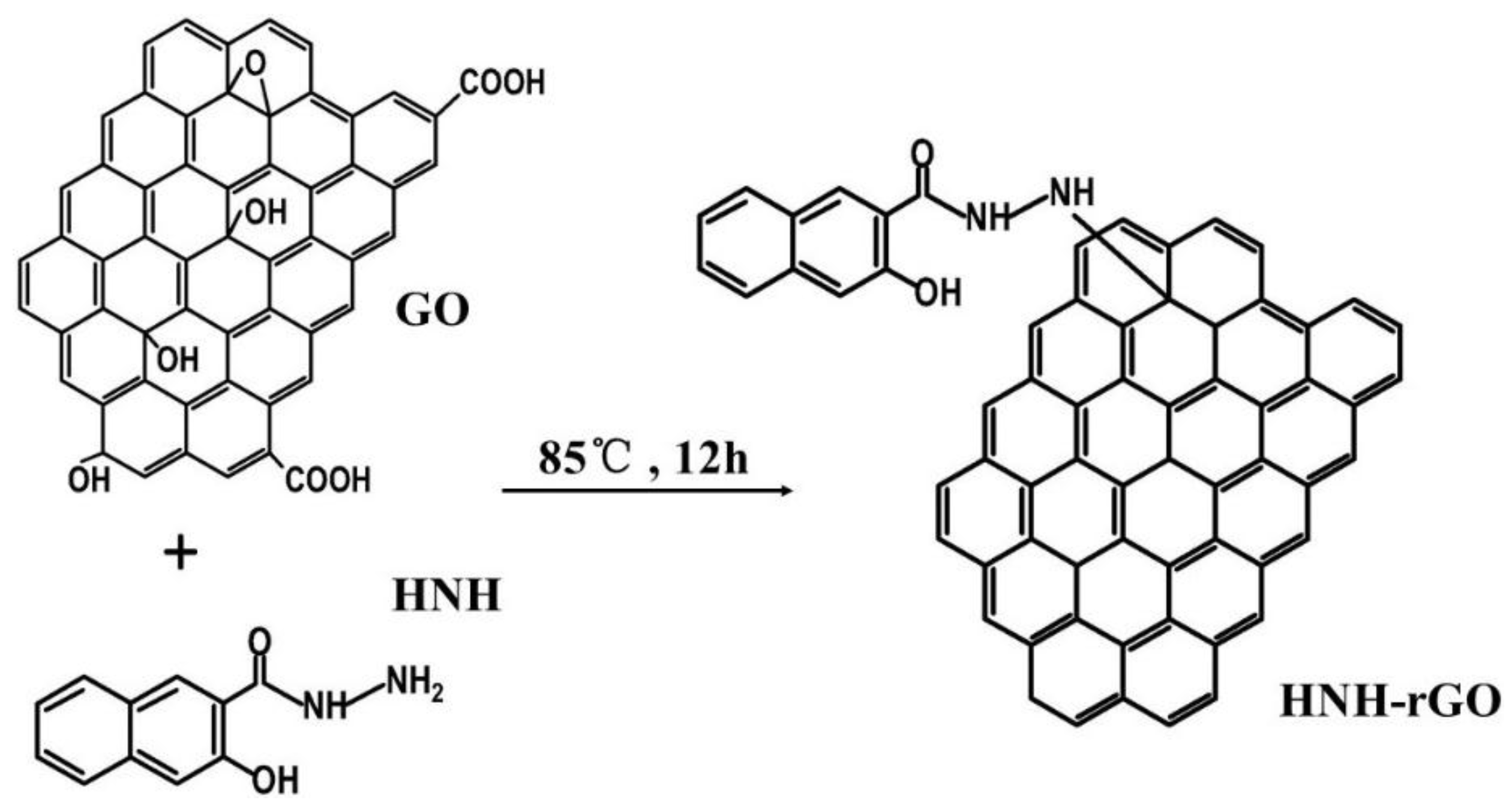
2.2. Synthesis of HNH Reduced Graphene from GO
2.3. Synthesis of HNH-rGO/PP and G/PP Composites
2.4. Characterization
3. Results and Discussions
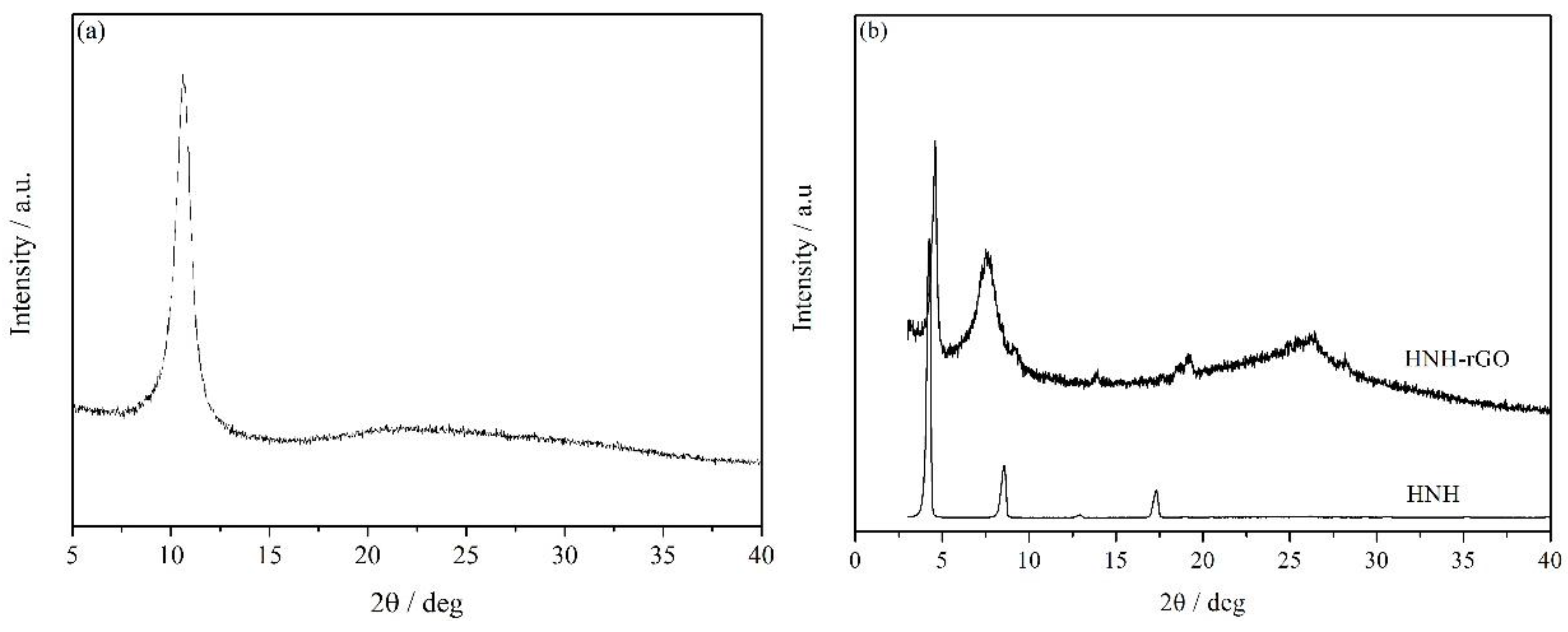
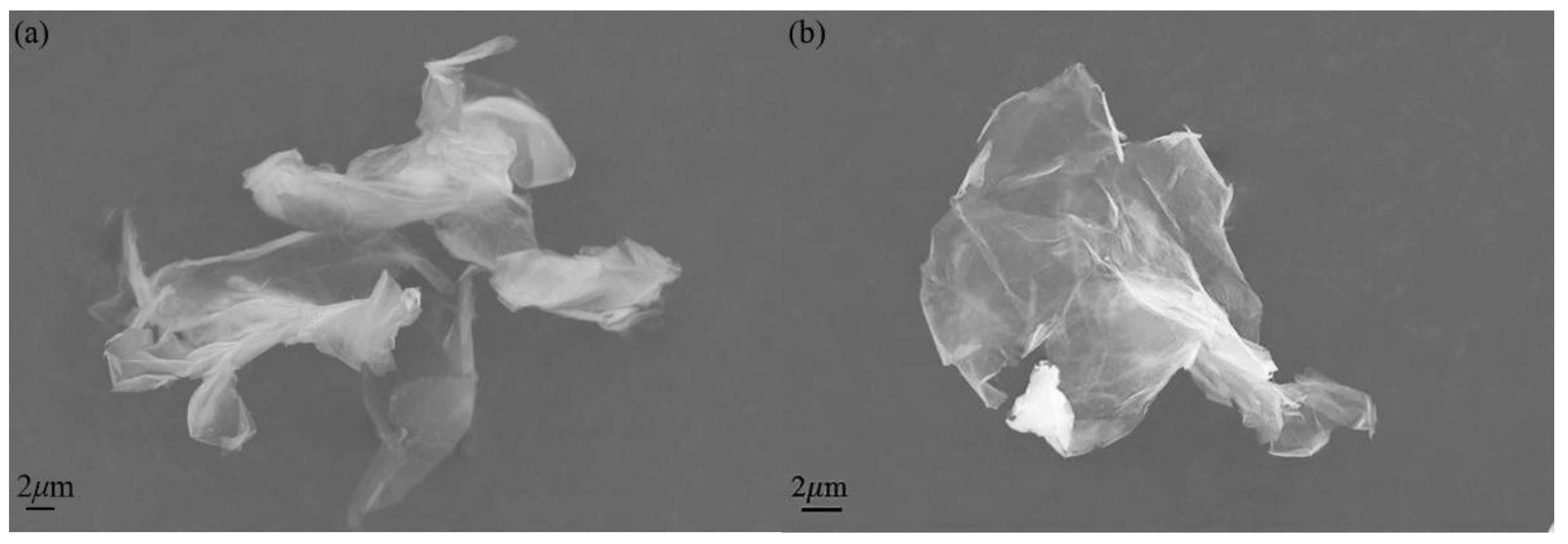
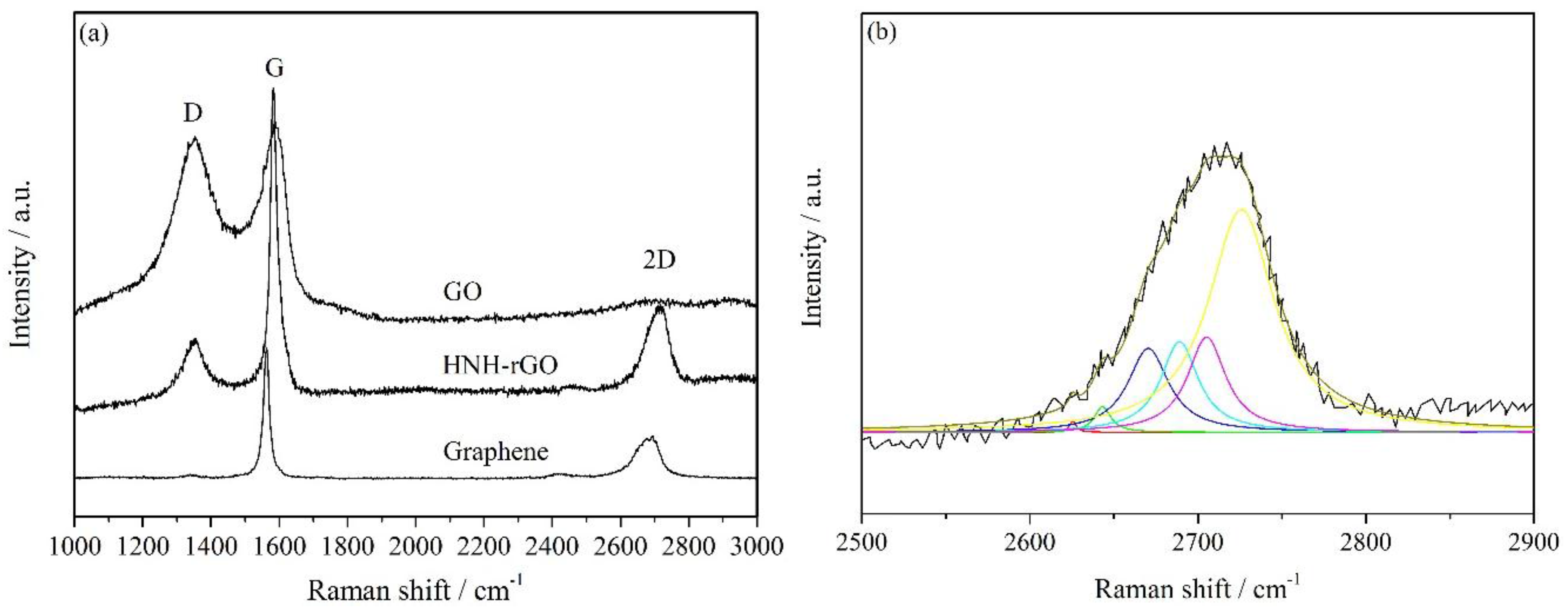
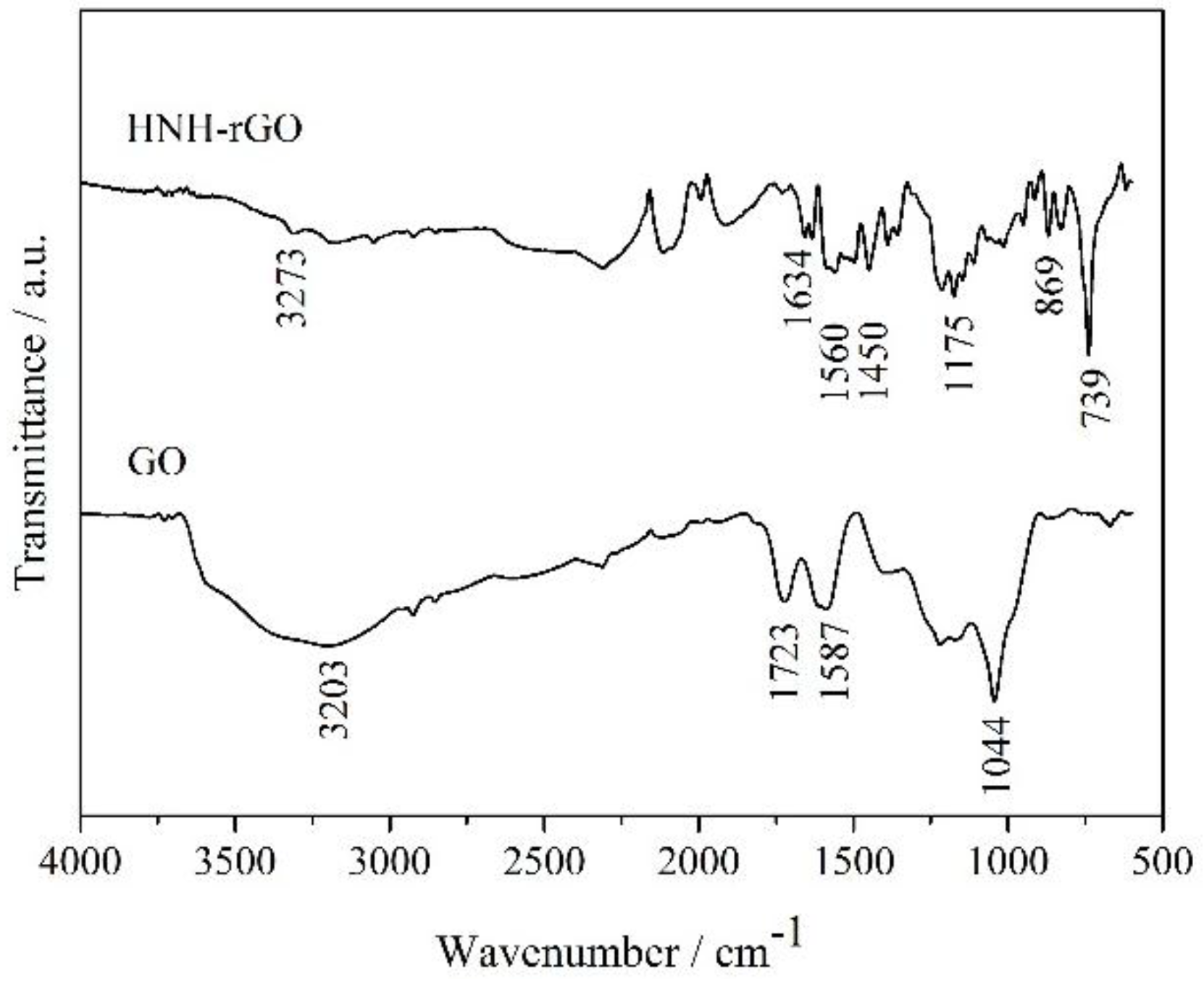
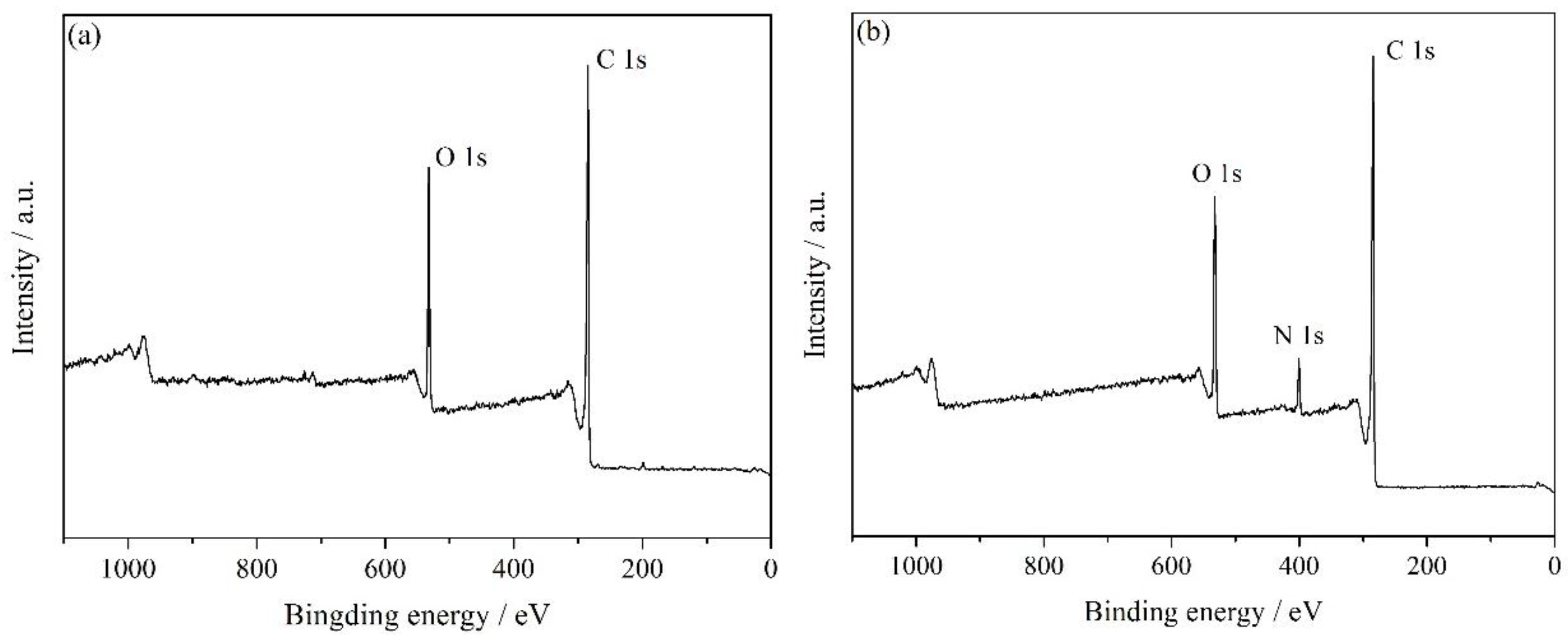
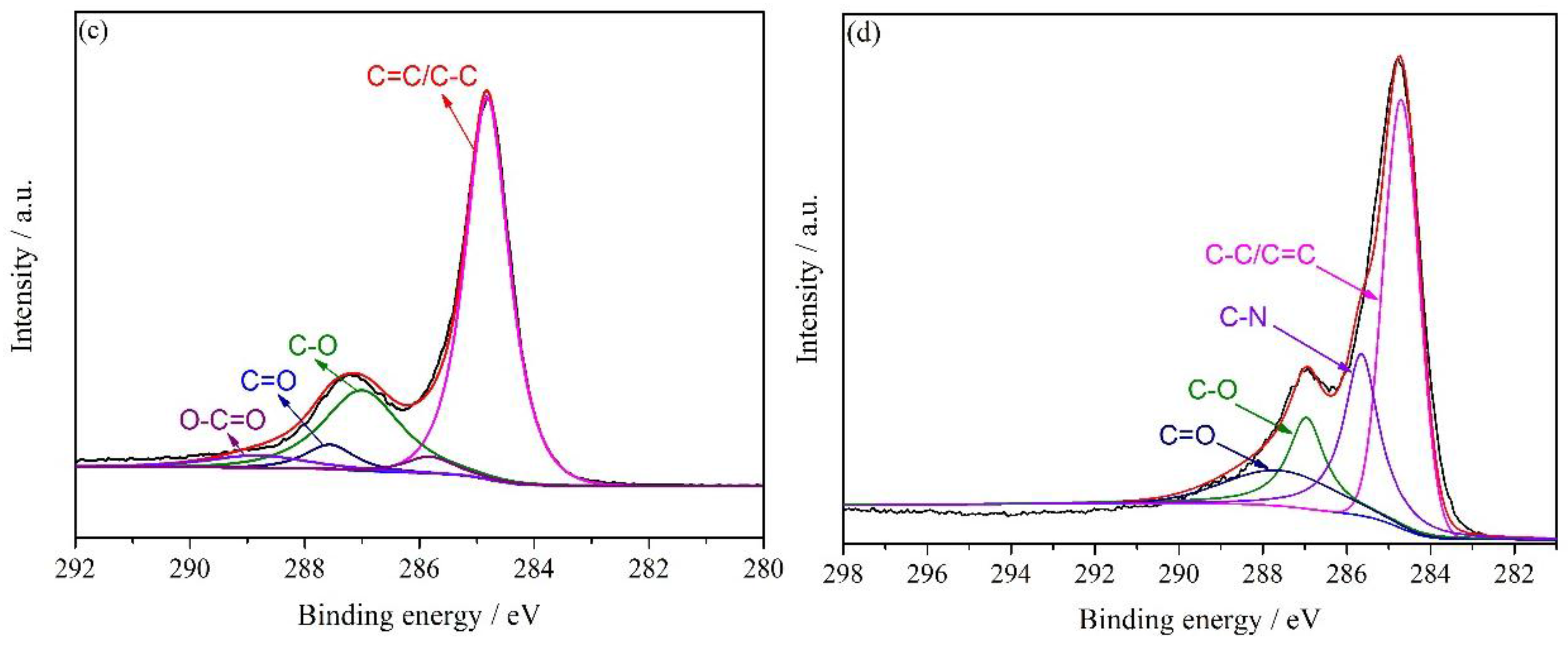
3.1. Characterization of HNH-rGO
3.2. Rheological Behavior of HNH-rGO/Polypropylene Composites
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; An, X.H.; Shah, R.; Rawat, D.; Dave, B.; Kar, S.; Talapatra, S. Effect of 1-Pyrene Carboxylic-Acid functionalization of graphene on its capacitive energy storage. J. Phys. Chem. C 2012, 116, 20688–20693. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.J.; Clarke, K.; Li, K.C. Covalent functionalization of graphene with polysaccharides. Ind. Eng. Chem. Res. 2012, 51, 310–317. [Google Scholar] [CrossRef]
- Narayan, R.; Kim, J.E.; Kim, J.Y.; Lee, K.E.; Kim, S.O. Liquid crystals: Graphene oxide liquid crystals: Discovery, evolution and applications. Adv. Mater. 2016, 28, 3044. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; EI-Ads, E.H.; Ahmed, Y.M.; Galal, A. Determination of some neurotransmitters at cyclodextrin/ionic liquid crystal/graphene composite electrode. Electrochim. Acta 2016, 199, 319–331. [Google Scholar] [CrossRef]
- Xu, X.T.; Liu, Y.; Wang, M.; Zhu, C.; Lu, T.; Zhao, R.; Pan, L.K. Hierarchical hybrids with microporous carbon spheres decorated three-dimensional graphene frameworks for capacitive applications in supercapacitor and deionization. Electrochim. Acta 2016, 193, 88–95. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Chua, C.K.; Pumera, M. Facile labelling of graphene oxide for superior capacitive energy storage and fluorescence applications. Phys. Chem. Chem. Phys. 2016, 18, 9673–9681. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhi, L.J.; Mullen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stret alhable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kim, M.; Cha, H.Y.; Spencer, M.G.; Lee, J.W. Metal free growth of graphene on quartz substrate using chemical vapor deposition (CVD). J. Nanosci. Nanotechnol. 2014, 14, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Rong, Y.; He, Z.; Fan, Z.; Warner, J.H. Uniformity of large-area bilayer graphene grown by chemical vapor deposition. Nanotechnology 2015, 26, 395601. [Google Scholar] [CrossRef] [PubMed]
- Ismach, A.; Druzgalski, C.; Penwell, S.; Schwartzberg, A.; Zheng, M.; Javey, A.; Bokor, J.; Zhang, Y.G. Direct chemical vapor deposition of graphene on dielectric surfaces. Nano Lett. 2010, 10, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Sun, H.J.; Peng, T.J. Synthesis and structural characterization of graphene by oxidation reduction. Chin. J. Inorg. Chem. 2010, 26, 2083–2090. [Google Scholar]
- Zhao, X.J.; Li, Y.; Wang, J.H.; Ouyang, Z.F.; Li, J.F.; Wei, G.; Su, Z.Q. Interactive oxidation-reduction reaction for the in situ synthesis of graphene-phenol formaldehyde composites with enhanced properties. ACS Appl. Mater. Interfaces 2014, 6, 4254–4263. [Google Scholar] [CrossRef] [PubMed]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Terrones, M. Materials science: Nanotubes unzipped. Nature 2009, 485, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Silva, R.; Morelos-Gomez, A.; Vega-Diaz, S.; Tristan-Lopez, F.; Elias, A.L.; Perea-Lopez, N.; Muramatsu, H.; Hayashi, T.; Fujisawa, K.; Kim, Y.A.; et al. Formation of nitrogen-doped graphene nanoribbons via chemical unzipping. ACS Nano 2013, 7, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; He, L.; Bo, X.J.; Yang, H.J.; Guo, L.P. Electrochemical preparation of free-standing few-layer graphene through oxidation-reduction cycling. Chem. Eng. J. 2011, 171, 340–344. [Google Scholar] [CrossRef]
- Wang, L.Q.; Wang, P.J.; Liu, Y.; Liu, C.J. Effects of phenylhydrazine-4-sulfonic acid on the reduction of GO and preparation of hydrophilic graphene with broad pH stability and antioxidant activity. RSC Adv. 2015, 5, 38696–38705. [Google Scholar] [CrossRef]
- Yu, C.; Li, J.; Liu, Q.B.; Feng, Z.H. Quasi-equilibrium growth of monolayer epitaxial graphene on SiC (0001). Acta Phys. Sin. 2014, 63. [Google Scholar] [CrossRef]
- De Heer, W.A.; Berger, C.; Wu, X.S.; First, P.N.; Conrad, E.H.; Li, X.B.; Li, T.B.; Sprinkle, M.; Hass, J.; Sadowski, M.L.; et al. Epitaxial graphene. Solid State Commun. 2007, 143, 92–100. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymer 2013, 54, 5087–5103. [Google Scholar] [CrossRef]
- Jang, J.; Pham, V.H.; Hur, S.H.; Chung, J.S. Dispersibility of reduced alkylamine-functionalized graphene oxides in organic solvents. J. Colloid Interface Sci. 2014, 424, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.J.; Kim, H.W.; Yoo, B.M.; Park, H.B. Highly soluble polyetheramine-functionalized graphene oxide and reduced graphene oxide both in aqueous and non-aqueous solvents. Carbon 2014, 75, 149–160. [Google Scholar] [CrossRef]
- Goods, J.B.; Sydlik, S.A.; Walish, J.J.; Swager, T.M. Phosphate functionalized graphene with tunable mechanical properties. Adv. Mater. 2014, 26, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chung, J.S.; Kong, B.; Kim, E.J.; Hur, S.H. Synthesis of grapheme-polyurethane nanocomposite using highly functionalized graphene oxide as pseudo-crosslinker. Mater. Lett. 2013, 106, 319–321. [Google Scholar] [CrossRef]
- Tang, G.; Jiang, Z.; Li, X.; Zhang, H.B.; Hong, S.; Yu, Z.Z. Electrically conductive rubbery epoxy/diamine-functionalized graphene nanocomposites with improved mechanical properties. Compos. Part B 2014, 67, 564–570. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Yin, J. Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. ACS Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.F.; Shi, M.; Ma, H.W.; Yan, B.; Li, N.; Hu, Y.Z.; Ye, M.X. Synthesis of hydrophilic and organophilic chemically modified graphene oxide sheets. J. Colloid Interface Sci. 2010, 352, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, K.G.; Lu, H.B.; Yang, Y.L.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Gracio, J. Surface modification of graphene nanosheets with gold nanoparticles: The role of oxygen moieties at graphene surface on gold nucleation and growth. J. Mater. Chem. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Layek, R.K.; Samanta, S.; Chatterjee, D.P.; Nandi, A.K. Physical and mechanical properties of poly(methyl methacrylate) -functionalized graphene/poly(vinylidine fluoride) nanocomposites Piezoelectric beta polymorph formation. Polymer 2010, 51, 5846–5856. [Google Scholar] [CrossRef]
- Kang, S.M.; Park, S.; Kim, D.; Park, S.Y.; Ruoff, R.S.; Lee, H. Simultaneous reduction and surface functionalization of graphene oxide by mussel-inspired chemistry. Adv. Funct. Mater. 2011, 21, 108–112. [Google Scholar] [CrossRef]
- Hu, H.W.; Allan, C.C.K.; Li, J.H.; Kong, Y.Y.; Wang, X.W.; Xin, J.H.; Hu, H. Multifunctional organically modified graphene with super-hydrophobicity. Nano Res. 2014, 7, 418–433. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Nie, S.Q.; Zhao, W.F.; Yang, H.; Sun, S.D.; Zhao, C.S. General and biomimetic approach to biopolymer-functionalized graphene oxide nanosheet through adhesive dopamine. Biomacromolecules 2012, 13, 4236–4246. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. The reduction of graphene oxide with hydrazine: Elucidating its reductive capability based on a reaction-model approach. Chem. Commun. 2016, 52, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.J.; Xu, X.N.; Qiu, Y.; Xiao, H.C.; Zhu, Y.F. Simultaneous reduction and functionalization of graphene oxide by 4-Hydrazinobenzenesulfonic acid for polymer nanocomposites. Nanomaterials 2016, 6, 29. [Google Scholar] [CrossRef]
- Li, B.; Zhou, L.; Wu, D.; Peng, H.L.; Yan, K.; Zhou, Y.; Liu, Z.F. Photochemical Chlorination of Graphene. ACS Nano 2011, 5, 5957–5961. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.-N.; Guan, X.-N.; Zhou, H.-H.; Zhu, Y.-F. One-Step Reduction and Surface Modification of Graphene Oxide by 3-Hydroxy-2-Naphthoic Acid Hydrazide and Its Polypropylene Nanocomposites. Nanomaterials 2017, 7, 25. https://doi.org/10.3390/nano7020025
Xu X-N, Guan X-N, Zhou H-H, Zhu Y-F. One-Step Reduction and Surface Modification of Graphene Oxide by 3-Hydroxy-2-Naphthoic Acid Hydrazide and Its Polypropylene Nanocomposites. Nanomaterials. 2017; 7(2):25. https://doi.org/10.3390/nano7020025
Chicago/Turabian StyleXu, Xiang-Nan, Xiao-Na Guan, Hui-Hua Zhou, and Yue-Feng Zhu. 2017. "One-Step Reduction and Surface Modification of Graphene Oxide by 3-Hydroxy-2-Naphthoic Acid Hydrazide and Its Polypropylene Nanocomposites" Nanomaterials 7, no. 2: 25. https://doi.org/10.3390/nano7020025
APA StyleXu, X.-N., Guan, X.-N., Zhou, H.-H., & Zhu, Y.-F. (2017). One-Step Reduction and Surface Modification of Graphene Oxide by 3-Hydroxy-2-Naphthoic Acid Hydrazide and Its Polypropylene Nanocomposites. Nanomaterials, 7(2), 25. https://doi.org/10.3390/nano7020025




