Visible Light-Responsive Platinum-Containing Titania Nanoparticle-Mediated Photocatalysis Induces Nucleotide Insertion, Deletion and Substitution Mutations
Abstract
:1. Introduction
2. Results
2.1. Involvement of Heat in VLRP-Induced Plasmid DNA Damage
2.2. VLRP Induces Plasmid DNA Damage
2.3. Application of VLRP-Induced DNA to Different Plasmids
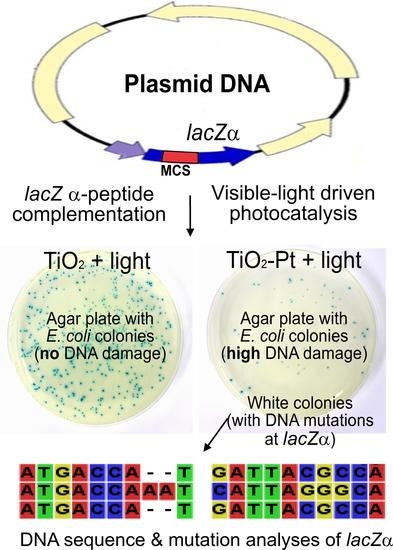
2.4. VLRP Induces Mutations in Plasmid DNA
2.5. VLRP-Induced DNA Mutations Involve Nucleotide Deletion and Substitution
3. Discussion
4. Materials and Methods
4.1. Photocatalyst Preparation
4.2. Bacterial Strains and Culture
4.3. Plasmids
4.4. Photocatalytic Reaction of Plasmid DNA
4.5. Blue-White Screen and Mutation Site Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TiO2 | titanium dioxide |
| TiO2-Pt | platinum-containing TiO2 |
| VLRP | visible light-driven photocatalyst |
| NPs | nanoparticles |
| UV | ultraviolet |
| ROS | reactive oxygen species |
| ·OH | hydroxyl radicals |
| O2− | superoxide anions |
| MCS | multiple cloning site |
References
- Arias, C.A.; Murray, B.E. Antibiotic-resistant bugs in the 21st century—A clinical super-challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.W.; Chang, H.H. Bactericidal effects and mechanisms of visible light-responsive titanium dioxide photocatalysts on pathogenic bacteria. Arch. Immunol. Ther. Exp. 2012, 60, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.M.; Chang, H.H.; Chang, K.C.; Liu, L.Y.; Tseng, C.C. A comparative study of the bactericidal effect of photocatalytic oxidation by TiO2 on antibiotic-resistant and antibiotic-sensitive bacteria. J. Chem. Technol. Biotechnol. 2010, 85, 1642–1653. [Google Scholar] [CrossRef]
- Tseng, C.C.; Tsai, Y.H.; Hu, A.; Liou, J.W.; Chang, K.C.; Chang, H.H. Altered susceptibility to the bactericidal effect of photocatalytic oxidation by TiO2 is related to colistin resistance development in acinetobacter baumannii. Appl. Microbiol. Biotechnol. 2016, 100, 8549–8561. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Sliney, D.H. Optical radiation safety of medical light sources. Phys. Med. Biol. 1997, 42, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Sun, D.S.; Lin, Y.C.; Cheng, C.L.; Hung, S.C.; Chen, P.K.; Yang, J.H.; Chang, H.H. Nanodiamonds protect skin from ultraviolet B-induced damage in mice. J. Nanobiotechnol. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.K.; Sun, D.S.; Chan, H.; Huang, P.T.; Wu, W.S.; Lin, C.H.; Tseng, Y.H.; Cheng, Y.H.; Tseng, C.C.; Chang, H.H. Visible light responsive core-shell structured In2O3@CaIn2O4 photocatalyst with superior bactericidal property and biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Sun, D.S.; Chu, W.C.; Tseng, Y.H.; Ho, H.C.; Wang, J.B.; Chung, P.H.; Chen, J.H.; Tsai, P.J.; Lin, N.T.; et al. The effects of the bacterial interaction with visible-light responsive titania photocatalyst on the bactericidal performance. J. Biomed. Sci. 2009, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.W.; Gu, M.H.; Chen, Y.K.; Chen, W.Y.; Chen, Y.C.; Tseng, Y.H.; Hung, Y.J.; Chang, H.H. Visible light responsive photocatalyst induces progressive and apical-terminus preferential damages on Escherichia coli surfaces. PLoS ONE 2011, 6, e19982. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Sun, D.S.; Wu, W.S.; Chan, H.; Syue, M.S.; Ho, H.C.; Chang, H.H. Antibacterial performance of nanoscaled visible-light responsive platinum-containing titania photocatalyst in vitro and in vivo. Biochim. Biophys. Acta 2013, 1830, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Chen, C.W.; Hsieh, C.C.; Hung, S.C.; Sun, D.S.; Chang, H.H. Antibacterial property of AG nanoparticle-impregnated N-doped titania films under visible light. Sci. Rep. 2015, 5, 11978. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Chu, W.C.; Sun, D.S.; Huang, H.S.; Chen, J.H.; Tsai, P.J.; Lin, N.T.; Yu, M.S.; Hsu, S.F.; Wang, S.L.; et al. Visible-light-induced bactericidal activity of a Nitrogen-Doped titanium photocatalyst against human pathogens. Appl. Environ. Microbiol. 2006, 72, 6111–6116. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Sun, D.S.; Chang, H.H. Bactericidal performance of visible-light responsive titania photocatalyst with silver nanostructures. PLoS ONE 2010, 5, e10394. [Google Scholar] [CrossRef] [PubMed]
- Kau, J.H.; Sun, D.S.; Huang, H.H.; Wong, M.S.; Lin, H.C.; Chang, H.H. Role of visible light-activated photocatalyst on the reduction of anthrax spore-induced mortality in mice. PLoS ONE 2009, 4, e4167. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Chen, Y.S.; Chan, H.; Tseng, Y.H.; Yang, S.R.; Tsai, H.Y.; Liu, H.Y.; Sun, D.S.; Chang, H.H. The use of nanoscale visible light-responsive photocatalyst TiO2-Pt for the elimination of soil-borne pathogens. PLoS ONE 2012, 7, e31212. [Google Scholar]
- Xie, J.; Pan, X.; Wang, M.; Yao, L.; Liang, X.; Ma, J.; Fei, Y.; Wang, P.N.; Mi, L. Targeting and photodynamic killing of cancer cell by Nitrogen-Doped titanium dioxide coupled with folic acid. Nanomaterials 2016, 6, 113. [Google Scholar] [CrossRef]
- Humayun, M.; Li, Z.; Sun, L.; Zhang, X.; Raziq, F.; Zada, A.; Qu, Y.; Jing, L. Coupling of nanocrystalline anatase TiO2 to porous nanosized LaFeO3 for efficient visible-light photocatalytic degradation of pollutants. Nanomaterials 2016, 6, 22. [Google Scholar] [CrossRef]
- Sun, D.S.; Kau, J.H.; Huang, H.H.; Tseng, Y.H.; Wu, W.S.; Chang, H.H. Antibacterial properties of visible-light-responsive carbon-containing titanium dioxide photocatalytic nanoparticles against anthrax. Nanomaterials 2016, 6, 237. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Lu, Z.X.; Zhang, Z.L.; Zhang, M.X.; Xie, H.Y.; Tian, Z.Q.; Chen, P.; Huang, H.; Pang, D.W. Core/shell quantum-dot-photosensitized nano-TiO2 films: Fabrication and application to the damage of cells and DNA. J. Phys. Chem. B 2005, 109, 22663–22666. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.C.; Zhang, Z.L.; Zhou, B.; Peng, J.; Xie, M.; Zhang, M.; Pang, D.W. Visible light-induced plasmid DNA damage catalyzed by a CdSe/ZnS-photosensitized nano-TiO2 film. Environ. Sci. Technol. 2008, 42, 5049–5054. [Google Scholar] [CrossRef] [PubMed]
- Markovitsi, D. UV-induced DNA damage: The role of electronic excited states. Photochem. Photobiol. 2016, 92, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sugasawa, K. Molecular mechanisms of DNA damage recognition for mammalian nucleotide excision repair. DNA Repair 2016, 44, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Malakhova, L.V.; Masalimov, Z.K.; Chernikov, A.V. Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nucleic Acids Res. 2002, 30, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Hu, S.T.; Huang, T.F.; Chen, S.H.; Lee, Y.H.; Lo, S.J. Rhodostomin, an RGD-containing peptide expressed from a synthetic gene in Escherichia coli, facilitates the attachment of human hepatoma cells. Biochem. Biophys. Res. Commun. 1993, 190, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Shyu, H.F.; Wang, Y.M.; Sun, D.S.; Shyu, R.H.; Tang, S.S.; Huang, Y.S. Facilitation of cell adhesion by immobilized dengue viral nonstructural protein 1 (NS1): Arginine-glycine-aspartic acid structural mimicry within the dengue viral ns1 antigen. J. Infect. Dis. 2002, 186, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Shih, K.N.; Lo, S.J. Receptor-mediated endocytosis as a selection force to enrich bacteria expressing rhodostomin on their surface. J. Biomed. Sci. 2000, 7, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Sheu, S.Y.; Lo, S.J. Expression of foreign antigens on the surface of Escherichia coli by fusion to the outer membrane protein trat. J. Biomed. Sci. 1999, 6, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chen, P.K.; Lin, G.L.; Wang, C.J.; Liao, C.H.; Hsiao, Y.C.; Dong, J.H.; Sun, D.S. Cell adhesion as a novel approach to determining the cellular binding motif on the severe acute respiratory syndrome coronavirus spike protein. J. Virol. Methods 2014, 201, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Chang, J.C.; Chang, H.H.; Tsai, W.J.; Lo, S.J. Positional importance of Pro53 adjacent to the Arg49-Gly50-Asp51 sequence of rhodostomin in binding to integrin αIIbβ3. Biochem. J. 2001, 357, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chang, C.P.; Chang, J.C.; Dung, S.Z.; Lo, S.J. Application of recombinant rhodostomin in studying cell adhesion. J. Biomed. Sci. 1997, 4, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Lin, C.H.; Lo, S.J. Recombinant rhodostomin substrates induce transformation and active calcium oscillation in human platelets. Exp. Cell Res. 1999, 250, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Lo, S.J. Full-spreading platelets induced by the recombinant rhodostomin are via binding to integrins and correlated with fak phosphorylation. Toxicon 1998, 36, 1087–1099. [Google Scholar] [CrossRef]
- Chang, H.H.; Tsai, W.J.; Lo, S.J. Glutathione S-transferase-rhodostomin fusion protein inhibits platelet aggregation and induces platelet shape change. Toxicon 1997, 35, 195–204. [Google Scholar] [CrossRef]
- Chang, H.; Lo, S.J. Modification with a phosphorylation tag of PKA in the trat-based display vector of Escherichia coli. J. Biotechnol. 2000, 78, 115–122. [Google Scholar] [CrossRef]
- Ahmad, J.; Dwivedi, S.; Alarifi, S.; Al-Khedhairy, A.A.; Musarrat, J. Use of β-galactosidase (lacZ) gene alpha-complementation as a novel approach for assessment of titanium oxide nanoparticles induced mutagenesis. Mutat. Res. 2012, 747, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Aruoma, O.I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991, 281, 9–19. [Google Scholar] [CrossRef]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Berquist, B.R.; Wilson, D.M., III. Pathways for repairing and tolerating the spectrum of oxidative DNA lesions. Cancer Lett. 2012, 327, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Greinert, R.; Volkmer, B.; Henning, S.; Breitbart, E.W.; Greulich, K.O.; Cardoso, M.C.; Rapp, A. UVA-induced DNA double-strand breaks result from the repair of clustered oxidative DNA damages. Nucleic Acids Res. 2012, 40, 10263–10273. [Google Scholar] [CrossRef] [PubMed]
- Jena, N.R. DNA damage by reactive species: Mechanisms, mutation and repair. J. Biosci. 2012, 37, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Hader, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.W.; Grossman, L. The role of Escherichia coli UvrB in nucleotide excision repair. J. Biol. Chem. 1990, 265, 7158–7165. [Google Scholar] [PubMed]
- Liu, B.; Xue, Q.; Tang, Y.; Cao, J.; Guengerich, F.P.; Zhang, H. Mechanisms of mutagenesis: DNA replication in the presence of DNA damage. Mutat. Res. Rev. Mutat. Res. 2016, 768, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiong, X.; Li, W. The breakage and damage of plasmid DNA photocatalized by TiO2/carbon nanotube composites. Surf. Interface Anal. 2012, 44, 84–88. [Google Scholar] [CrossRef]
- Van Gent, D.C.; Hoeijmakers, J.H.; Kanaar, R. Chromosomal stability and the DNA double-stranded break connection. Nat. Rev. Genet. 2001, 2, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Lieber, M.R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 2010, 79, 181–211. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Richa, K.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Besaratinia, A.; Lee, D.H.; Lee, C.S.; Pfeifer, G.P. The role of DNA polymerase I in UV mutational spectra. Mutat. Res. 2006, 599, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Kang, T.M.; Yuan, J.; Beppler, C.; Nguyen, C.; Mao, Z.; Nguyen, M.Q.; Yeh, P.; Miller, J.H. Synergistic interactions of vancomycin with different antibiotics against Escherichia coli: Trimethoprim and nitrofurantoin display strong synergies with vancomycin against wild-type E. Coli. Antimicrob. Agents Chemother. 2015, 59, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, Woodbury, NY, USA, 1989. [Google Scholar]
- Lien, T.S.; Sun, D.S.; Chang, C.M.; Wu, C.Y.; Dai, M.S.; Chan, H.; Wu, W.S.; Su, S.H.; Lin, Y.Y.; Chang, H.H. Dengue virus and antiplatelet autoantibodies synergistically induce haemorrhage through Nlrp3-inflammasome and FcγRIII. Thromb. Haemost. 2015, 113, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.S.; Chang, Y.C.; Lien, T.S.; King, C.C.; Shih, Y.L.; Huang, H.S.; Wang, T.Y.; Li, C.R.; Lee, C.C.; Hsu, P.N.; et al. Endothelial cell sensitization by death receptor fractions of an anti-dengue nonstructural protein 1 antibody induced plasma leakage, coagulopathy, and mortality in mice. J. Immunol. 2015, 195, 2743–2753. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Chang, A.C.; Sun, D.S.; Hsu, C.Y.; Chang, N.C. Restricted expression of LUZP in neural lineage cells: A study in embryonic stem cells. J. Biomed. Sci. 2001, 8, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.S.; Lee, P.C.; Kau, J.H.; Shih, Y.L.; Huang, H.H.; Li, C.R.; Lee, C.C.; Wu, Y.P.; Chen, K.C.; Chang, H.H. Acquired coagulant factor VIII deficiency induced by bacillus anthracis lethal toxin in mice. Virulence 2015, 6, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Jagessar, S.A.; Holtman, I.R.; Hofman, S.; Morandi, E.; Heijmans, N.; Laman, J.D.; Gran, B.; Faber, B.W.; van Kasteren, S.I.; Eggen, B.J.; et al. Lymphocryptovirus infection of nonhuman primate B cells converts destructive into productive processing of the pathogenic CD8 T cell epitope in myelin oligodendrocyte glycoprotein. J. Immunol. 2016, 197, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Luhung, I.; Wu, Y.; Ng, C.K.; Miller, D.; Cao, B.; Chang, V.W. Protocol improvements for low concentration DNA-based bioaerosol sampling and analysis. PLoS ONE 2015, 10, e0141158. [Google Scholar] [CrossRef] [PubMed]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Ou, D.S.; Lee, S.B.; Chang, L.H.; Lin, R.K.; Li, Y.S.; Upadhyay, A.K.; Cheng, X.; Wang, Y.C.; Hsu, H.S.; et al. HNaa10p contributes to tumorigenesis by facilitating DNMT1-mediated tumor suppressor gene silencing. J. Clin. Investig. 2010, 120, 2920–2930. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y.; Hsieh, C.H.; Chen, M.Y.; Tsai, J.P.; Liu, H.H.; Liu, S.J.; Chong, P.; Leng, C.H.; Chen, H.W. Recombinant lipidated dengue-4 envelope protein domain iii elicits protective immunity. Vaccine 2014, 32, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Translate DNA and RNA sequences to protein sequences. Available online: http://www.fr33.net/translator.php (accessed on 25 September 2016).






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.-S.; Tseng, Y.-H.; Wu, W.-S.; Wong, M.-S.; Chang, H.-H. Visible Light-Responsive Platinum-Containing Titania Nanoparticle-Mediated Photocatalysis Induces Nucleotide Insertion, Deletion and Substitution Mutations. Nanomaterials 2017, 7, 2. https://doi.org/10.3390/nano7010002
Sun D-S, Tseng Y-H, Wu W-S, Wong M-S, Chang H-H. Visible Light-Responsive Platinum-Containing Titania Nanoparticle-Mediated Photocatalysis Induces Nucleotide Insertion, Deletion and Substitution Mutations. Nanomaterials. 2017; 7(1):2. https://doi.org/10.3390/nano7010002
Chicago/Turabian StyleSun, Der-Shan, Yao-Hsuan Tseng, Wen-Shiang Wu, Ming-Show Wong, and Hsin-Hou Chang. 2017. "Visible Light-Responsive Platinum-Containing Titania Nanoparticle-Mediated Photocatalysis Induces Nucleotide Insertion, Deletion and Substitution Mutations" Nanomaterials 7, no. 1: 2. https://doi.org/10.3390/nano7010002
APA StyleSun, D.-S., Tseng, Y.-H., Wu, W.-S., Wong, M.-S., & Chang, H.-H. (2017). Visible Light-Responsive Platinum-Containing Titania Nanoparticle-Mediated Photocatalysis Induces Nucleotide Insertion, Deletion and Substitution Mutations. Nanomaterials, 7(1), 2. https://doi.org/10.3390/nano7010002