Preparation of g-C3N4/Graphene Composite for Detecting NO2 at Room Temperature
Abstract
:1. Introduction
2. Results and Discussion
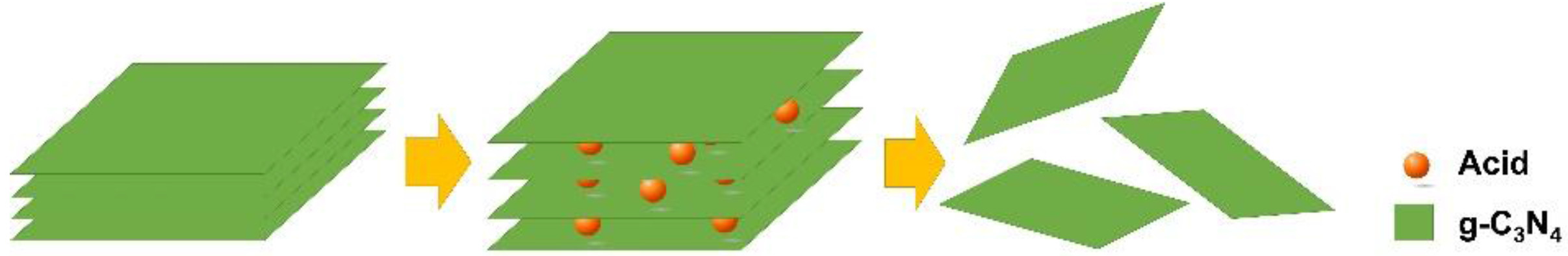
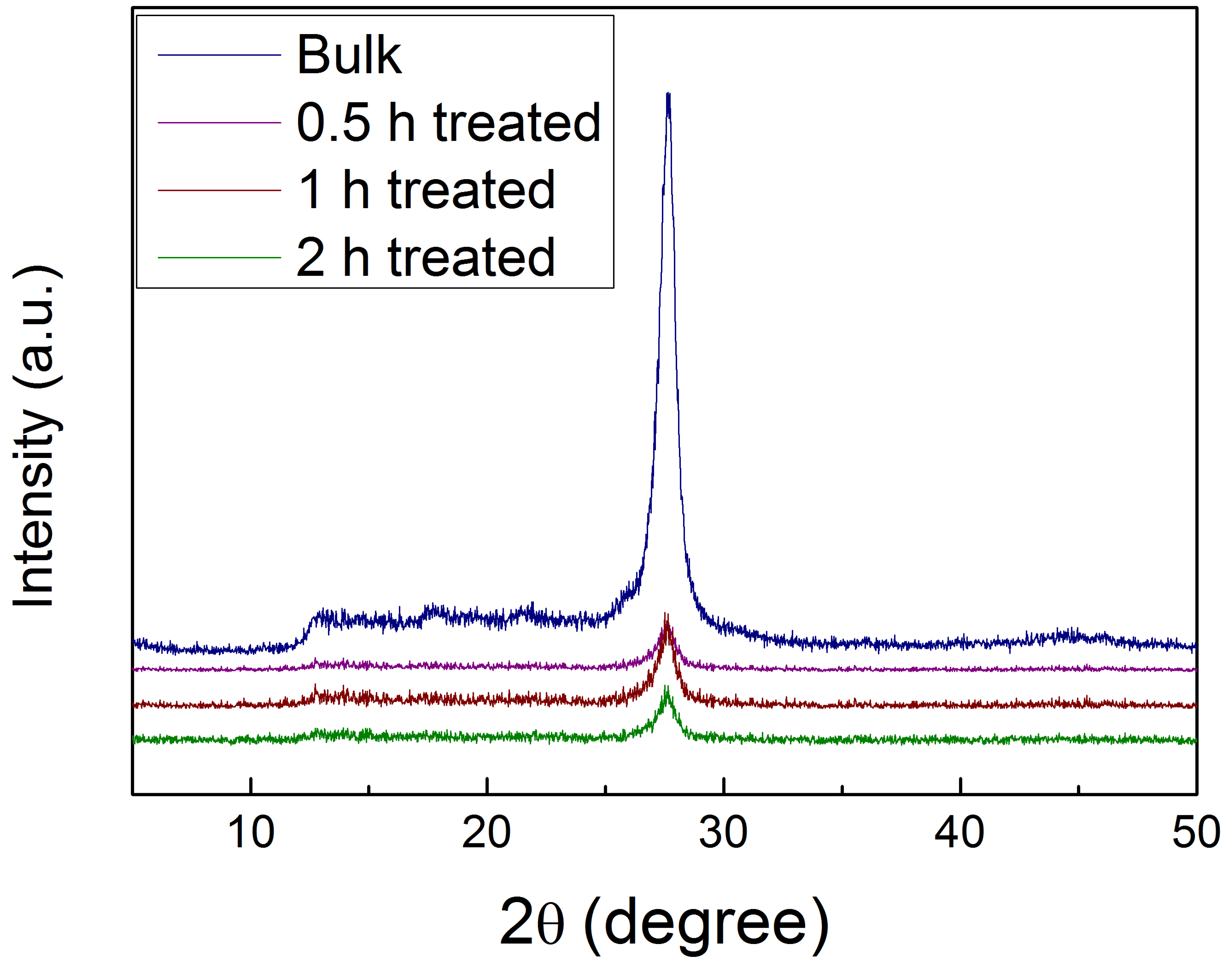
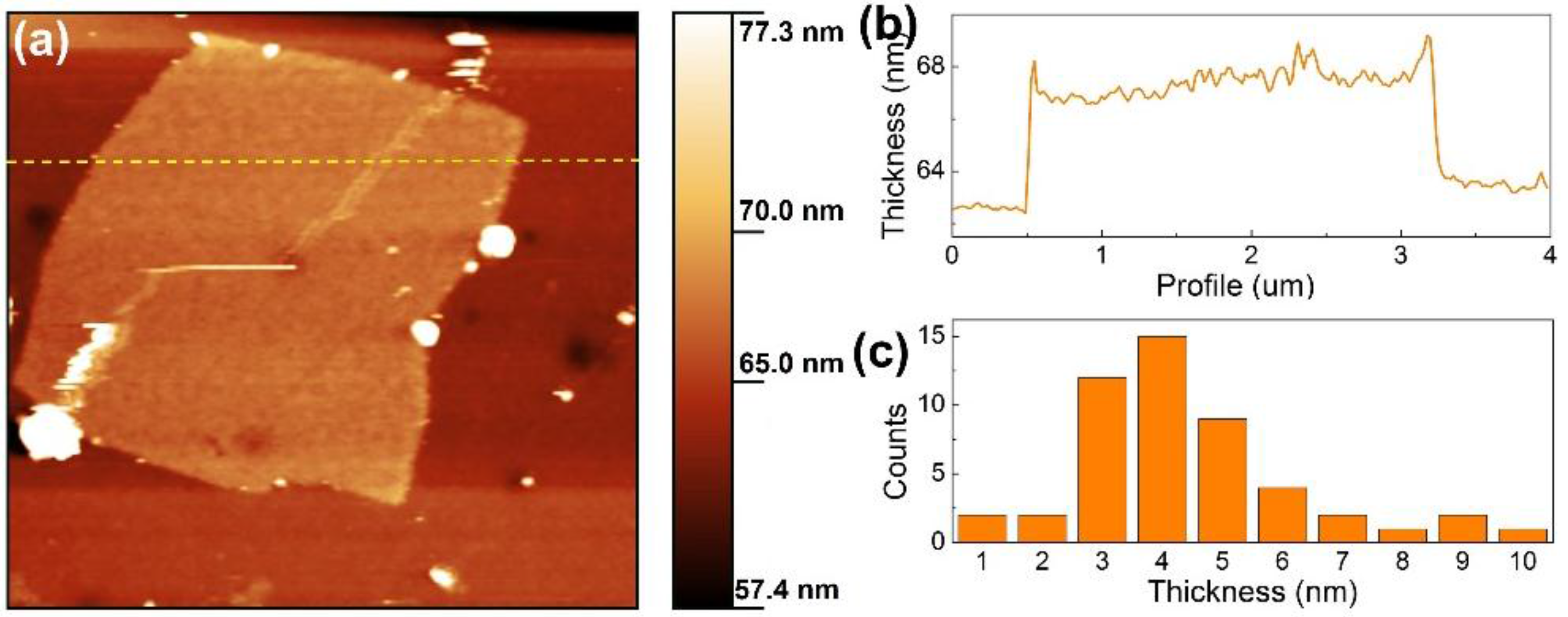
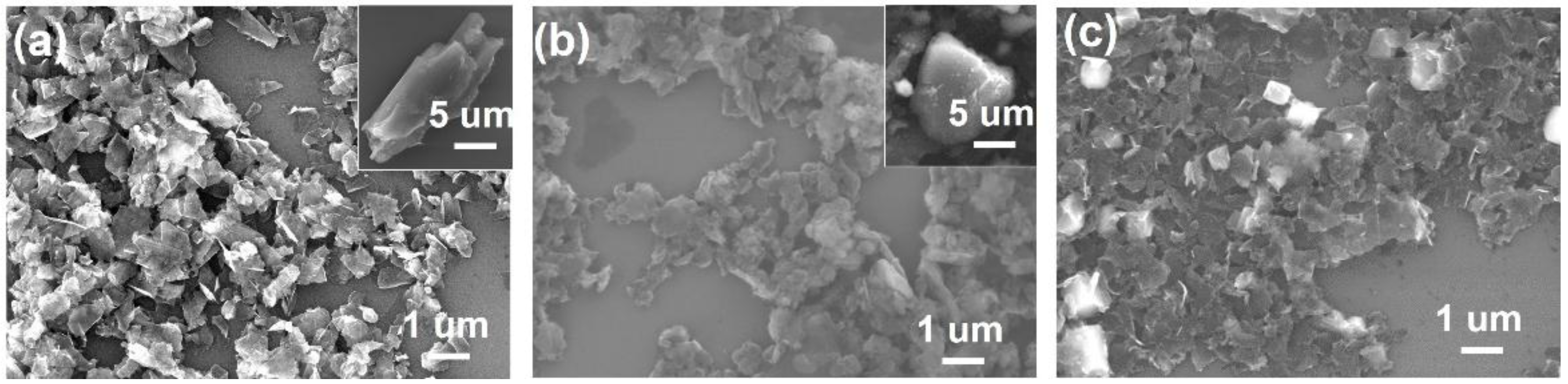
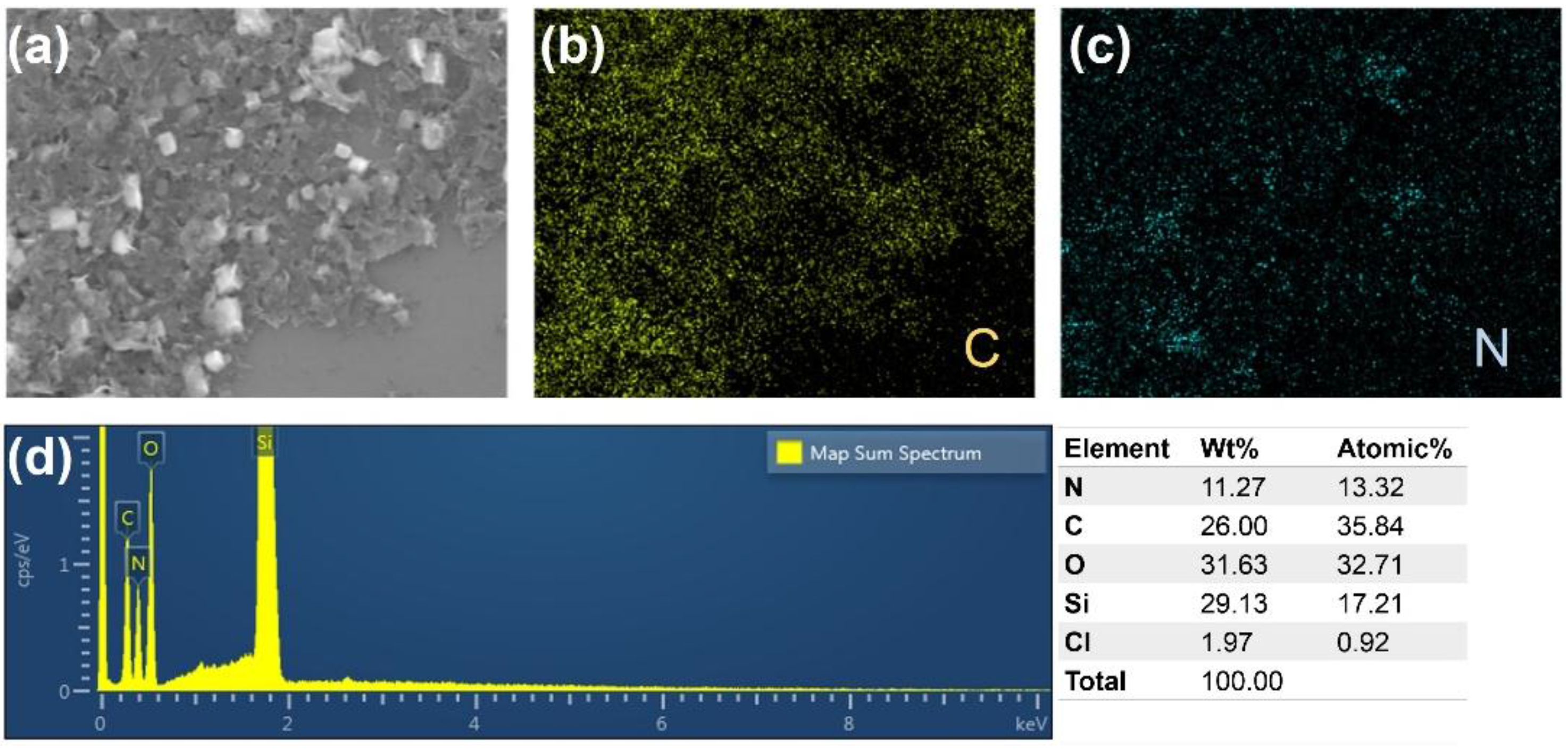
2.1. Acid Treatment Enhanced Exfoliation
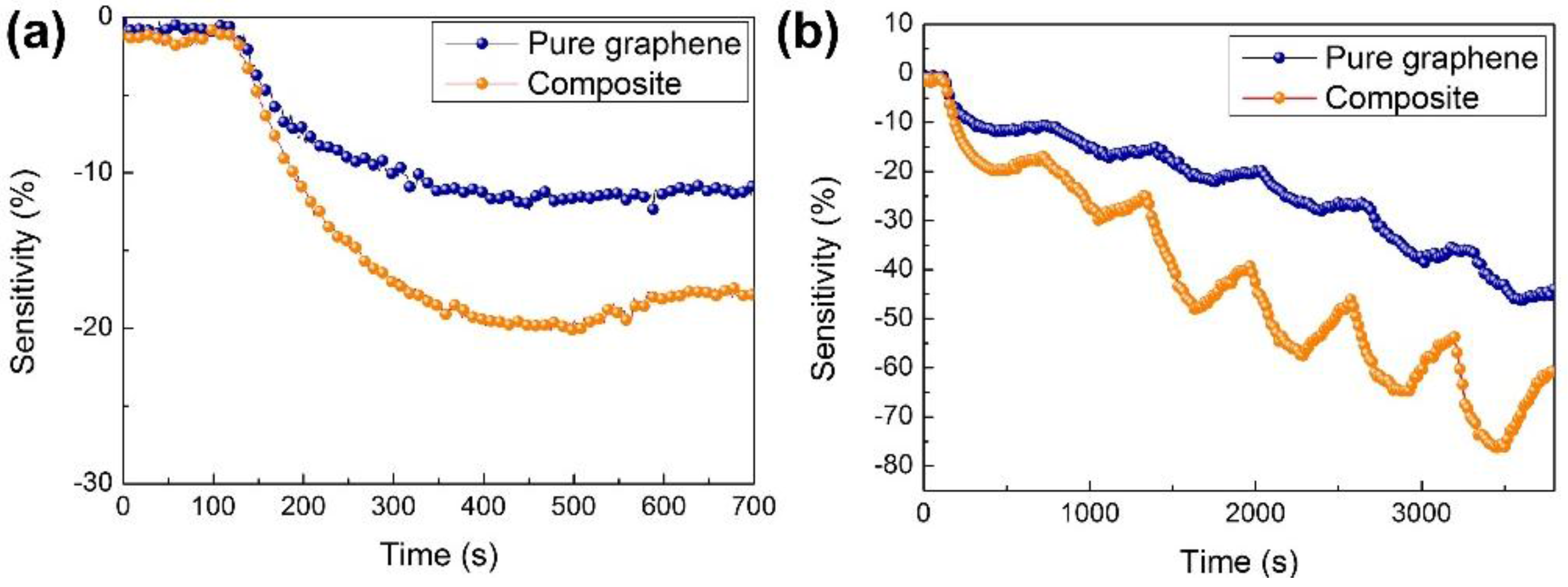
2.2. Gas Sensing Performance
3. Materials and Methods
3.1. Preparation of Bulk g-C3N4
3.2. Acid Treatment Enhanced Exfoliation of g-C3N4
3.3. Preparation of Graphene
3.4. Fabrication of g-C3N4/Graphene Composite Sensor
3.5. Measurement of g-C3N4/Graphene Composite Sensor
3.6. Material Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| g-C3N4 | Graphitic Carbon Nitride |
| HCl | Hydrochloric Acid |
| DI Water | Deionized Water |
| NO2 | Nitrogen Dioxide |
| 2D Material | Two Dimensional Material |
| H2SO4 | Sulphuric Acid |
| HNO3 | Nitric Acid |
| EDS | Energy-dispersive X-ray Spectroscopy |
| XRD | X-ray Diffraction |
| FE-SEM | Field Emission Scanning Electron Microscopy |
| AFM | Atomic Force Microscopy |
| UV-Vis spectroscopy | Ultraviolet-visible Spectroscopy |
| MFC | Mass Flow Controllers |
References
- Hjiri, M.; El Mir, L.; Leonardi, S.G.; Donato, N.; Neri, G. CO and NO2 Selective Monitoring by ZnO-Based Sensors. Nanomaterials 2013, 3, 357–369. [Google Scholar] [CrossRef]
- Arafat, M.M.; Dinan, B.; Akbar, S.A.; Haseeb, A.S.M.A. Gas Sensors Based on One Dimensional Nanostructured Metal-Oxides: A Review. Sensors 2012, 12, 7207–7258. [Google Scholar] [CrossRef] [PubMed]
- Tomchenko, A.A.; Harmer, G.P.; Marquis, B.T.; Allen, J.W. Semiconducting metal oxide sensor array for the selective detection of combustion gases. Sens. Actuators B 2003, 93, 126–134. [Google Scholar] [CrossRef]
- Wu, C.S. Hydrothermal Fabrication of WO3 Hierarchical Architectures: Structure, Growth and Response. Nanomaterials 2015, 5, 1250–1255. [Google Scholar] [CrossRef]
- Endres, H.E.; Gottler, W.; Hartinger, R.; Drost, S.; Hellmich, W.; Muller, G.; BoschvonBraunmuhl, C.; Krenkow, A.; Perego, C.; Sberveglieri, G. A thin-film SnO2 sensor system for simultaneous detection of CO and NO2 with neural signal evaluation. Sens. Actuators B 1996, 36, 353–357. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Capone, S.; Forleo, A.; Francioso, L.; Rella, R.; Siciliano, P.; Spadavecchia, J.; Presicce, D.S.; Taurino, A.M. Solid state gas sensors: State of the art and future activities. J. Optoelectron. Adv. Mater. 2003, 5, 1335–1348. [Google Scholar] [CrossRef]
- Varghese, S.S.; Varghese, S.H.; Swaminathan, S.; Singh, K.K.; Mittal, V. Two-Dimensional Materials for Sensing: Graphene and Beyond. Electronics 2015, 4, 651–687. [Google Scholar] [CrossRef]
- Zanjani, S.M.M.; Sadeghi, M.M.; Holt, M.; Chowdhury, S.F.; Tao, L.; Akinwande, D. Enhanced sensitivity of graphene ammonia gas sensors using molecular doping. Appl. Phys. Lett. 2016, 108, 033106. [Google Scholar] [CrossRef]
- Lv, R.; Li, Q.; Botello-Mendez, A.R.; Hayashi, T.; Wang, B.; Berkdemir, A.; Hao, Q.Z.; Elias, A.L.; Cruz-Silva, R.; Gutierrez, H.R.; et al. Nitrogen-doped graphene: Beyond single substitution and enhanced molecular sensing. Sci. Rep. 2012, 2, 586. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Liu, J.M.; Tao, L.M.; Wang, W.; Song, W.G. Nitrogen and silica co-doped graphene nanosheets for NO2 gas sensing. J. Mater. Chem. A 2013, 1, 6130–6133. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zou, X.M.; Xu, L.; Liao, L.; Liu, W.; Ho, J.; Xiao, X.H.; Jiang, C.Z.; Li, J.C. Hydrogen gas sensor based on metal oxide nanoparticles decorated graphene transistor. Nanoscale 2015, 7, 10078–10084. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.S.; Shen, F.P.; Tian, X.K.; Wang, D.H.; Zhang, T.; Chen, W. Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H2S gas sensing with ultrahigh sensitivity. Nanoscale 2013, 5, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Yoon, J.; Hahm, M.G.; Kim, D.H.; Kim, A.R.; Kahng, Y.H.; Park, S.W.; Lee, Y.J.; Park, S.G.; Kwon, J.D.; et al. Graphene-based gas sensor: Metal decoration effect and application to a flexible device. J. Mater. Chem. C 2014, 2, 5280–5285. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Li, J.; Aslan, H.; Li, Q.; Li, Y.; Chen, M.L.; Huang, Y.D.; Froning, J.P.; Otyepka, M.; Zboril, R.; et al. A high efficiency H2S gas sensor material: Paper like Fe2O3/graphene nanosheets and structural alignment dependency of device efficiency. J. Mater. Chem. A 2014, 2, 6714–6717. [Google Scholar] [CrossRef]
- Yang, W.; Gan, L.; Li, H.; Zhai, T. Two-dimensional layered nanomaterials for gas-sensing applications. Inorg. Chem. Front. 2016, 3, 433. [Google Scholar] [CrossRef]
- Li, W.W.; Geng, X.M.; Guo, Y.F.; Rong, J.Z.; Gong, Y.P.; Wu, L.Q.; Zhang, X.M.; Li, P.; Xu, J.B.; Cheng, G.S.; et al. Reduced Graphene Oxide Electrically Contacted Graphene Sensor for Highly Sensitive Nitric Oxide Detection. ACS Nano 2011, 5, 6955–6961. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.W.; Zeng, D.W.; Li, H.Y.; Xie, C.S. Room temperature formaldehyde sensors with enhanced performance, fast response and recovery based on zinc oxide quantum dots/graphene nanocomposites. Nanoscale 2012, 4, 5651–5658. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Graphitic Carbon Nitride Nanosheet-Carbon Nanotube Three-Dimensional Porous Composites as High-Performance Oxygen Evolution Electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 7281–7285. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Qiu, L.G.; Xiao, J.D.; Ye, S.; Jiang, X.; Yuan, Y.P. Inorganic-organic hybrid NiO-g-C3N4 photocatalyst for efficient methylene blue degradation using visible light. Rsc. Adv. 2014, 4, 22491–22496. [Google Scholar] [CrossRef]
- Sun, L.M.; Zhao, X.; Jia, C.J.; Zhou, Y.X.; Cheng, X.F.; Li, P.; Liu, L.; Fan, W.L. Enhanced visible-light photocatalytic activity of g-C3N4-ZnWO4 by fabricating a heterojunction: Investigation based on experimental and theoretical studies. J. Mater. Chem. 2012, 22, 23428–23438. [Google Scholar] [CrossRef]
- Tian, J.Q.; Ning, R.; Liu, Q.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X.P. Three-Dimensional Porous Supramolecular Architecture from Ultrathin g-C3N4 Nanosheets and Reduced Graphene Oxide: Solution Self-Assembly Construction and Application as a Highly Efficient Metal-Free Electrocatalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2014, 6, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Gu, W.; Zhang, Y.W.; Hu, Y.; Zhang, T.; Tao, X.M.; Chen, W. Novel C-rich carbon nitride for room temperature NO2 gas sensors. RSC Adv. 2014, 4, 18003–18006. [Google Scholar] [CrossRef]
- Jung, J.; Lee, S.P. Physical Properties of Nano-Structured Carbon Nitride Film for Integrated Humidity Sensors. J. Nanosci. Nanotechnol. 2013, 13, 7030–7032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Xie, X.; Wang, H.; Zhang, J.J.; Pan, B.C.; Xie, Y. Enhanced Photoresponsive Ultrathin Graphitic-Phase C3N4 Nanosheets for Bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, L.W.; Shi, R.; Zhu, Y.F. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Lin, L.S.; Cong, Z.X.; Li, J.; Ke, K.M.; Guo, S.S.; Yang, H.H.; Chen, G.N. Graphitic-phase C3N4 nanosheets as efficient photosensitizers and pH-responsive drug nanocarriers for cancer imaging and therapy. J. Mater. Chem. B 2014, 2, 1031–1037. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhang, Q.; Shi, Q.W.; Cai, Z.Y.; Yang, Z.J. Acid-treated g-C3N4 with improved photocatalytic performance in the reduction of aqueous Cr(VI) under visible-light. Sep. Purif. Technol. 2015, 142, 251–257. [Google Scholar] [CrossRef]
- Chang, C.; Fu, Y.; Hu, M.; Wang, C.; Shan, G. Photodegradation of bisphenol A by highly stable palladium-doped mesoporous graphite carbon nitride (Pd/mpg-C3N4) under simulated solar light irradiation. Appl. Catal. B 2013, 142–143, 553–560. [Google Scholar] [CrossRef]
- Cao, S.W.; Liu, X.F.; Yuan, Y.P.; Zhang, Z.Y. Solar-to-fuels conversion over In2O3/g-C3N4 hybrid photocatalysts. Appl. Catal. B 2014, 147, 940–946. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Romero, H.E.; Joshi, P.; Gupta, A.K.; Gutierrez, H.R.; Cole, M.W.; Tadigadapa, S.A.; Eklund, P.C. Adsorption of ammonia on graphene. Nanotechnology 2009, 20, 245501. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Hung, S.C.; Yang, M.D.; Yeh, C.W.; Wu, C.H.; Chi, G.C.; Ren, F.; Pearton, S.J. Oxygen sensors made by monolayer graphene under room temperature. Appl. Phys. Lett. 2011, 99, 243502. [Google Scholar] [CrossRef]
- Chen, G.; Paronyan, T.M.; Harutyunyan, A.R. Sub-ppt gas detection with pristine graphene. Appl. Phys. Lett. 2012, 101, 053119. [Google Scholar] [CrossRef]
- Zhong, Y.J.; Wang, Z.Q.; Feng, J.Y.; Yan, S.C.; Zhang, H.T.; Li, Z.S.; Zou, Z.G. Improvement in photocatalytic H2 evolution over g-C3N4 prepared from protonated melamine. Appl. Surf. Sci. 2014, 295, 253–259. [Google Scholar] [CrossRef]
- Martín-Ramos, P.; Martín-Gil, J.; Dante, R.C.; Vaquero, F.; Navarro, R.M.; Fierro, J.L.G. A simple approach to synthesize g-C3N4 with high visible light photoactivity for hydrogen production. Int. J. Hydrog. Energy 2015, 40, 7273–7281. [Google Scholar] [CrossRef]
- Dante, R.C.; Martín-Ramos, P.; Correa-Guimaraes, A.; Martín-Gil, J. Synthesis of graphitic carbon nitride by reaction of melamine and uric acid. Mater. Chem. Phys. 2011, 130, 1094–1102. [Google Scholar] [CrossRef]
- Zhang, S.L.; Zhang, Z.J.; Yang, W.C. High-yield exfoliation of graphene using ternary-solvent strategy for detecting volatile organic compounds. Appl. Surf. Sci. 2016, 360, 323–328. [Google Scholar] [CrossRef]


© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Hang, N.T.; Zhang, Z.; Yue, H.; Yang, W. Preparation of g-C3N4/Graphene Composite for Detecting NO2 at Room Temperature. Nanomaterials 2017, 7, 12. https://doi.org/10.3390/nano7010012
Zhang S, Hang NT, Zhang Z, Yue H, Yang W. Preparation of g-C3N4/Graphene Composite for Detecting NO2 at Room Temperature. Nanomaterials. 2017; 7(1):12. https://doi.org/10.3390/nano7010012
Chicago/Turabian StyleZhang, Shaolin, Nguyen Thuy Hang, Zhijun Zhang, Hongyan Yue, and Woochul Yang. 2017. "Preparation of g-C3N4/Graphene Composite for Detecting NO2 at Room Temperature" Nanomaterials 7, no. 1: 12. https://doi.org/10.3390/nano7010012
APA StyleZhang, S., Hang, N. T., Zhang, Z., Yue, H., & Yang, W. (2017). Preparation of g-C3N4/Graphene Composite for Detecting NO2 at Room Temperature. Nanomaterials, 7(1), 12. https://doi.org/10.3390/nano7010012





