Cholesterol-Modified Amino-Pullulan Nanoparticles as a Drug Carrier: Comparative Study of Cholesterol-Modified Carboxyethyl Pullulan and Pullulan Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
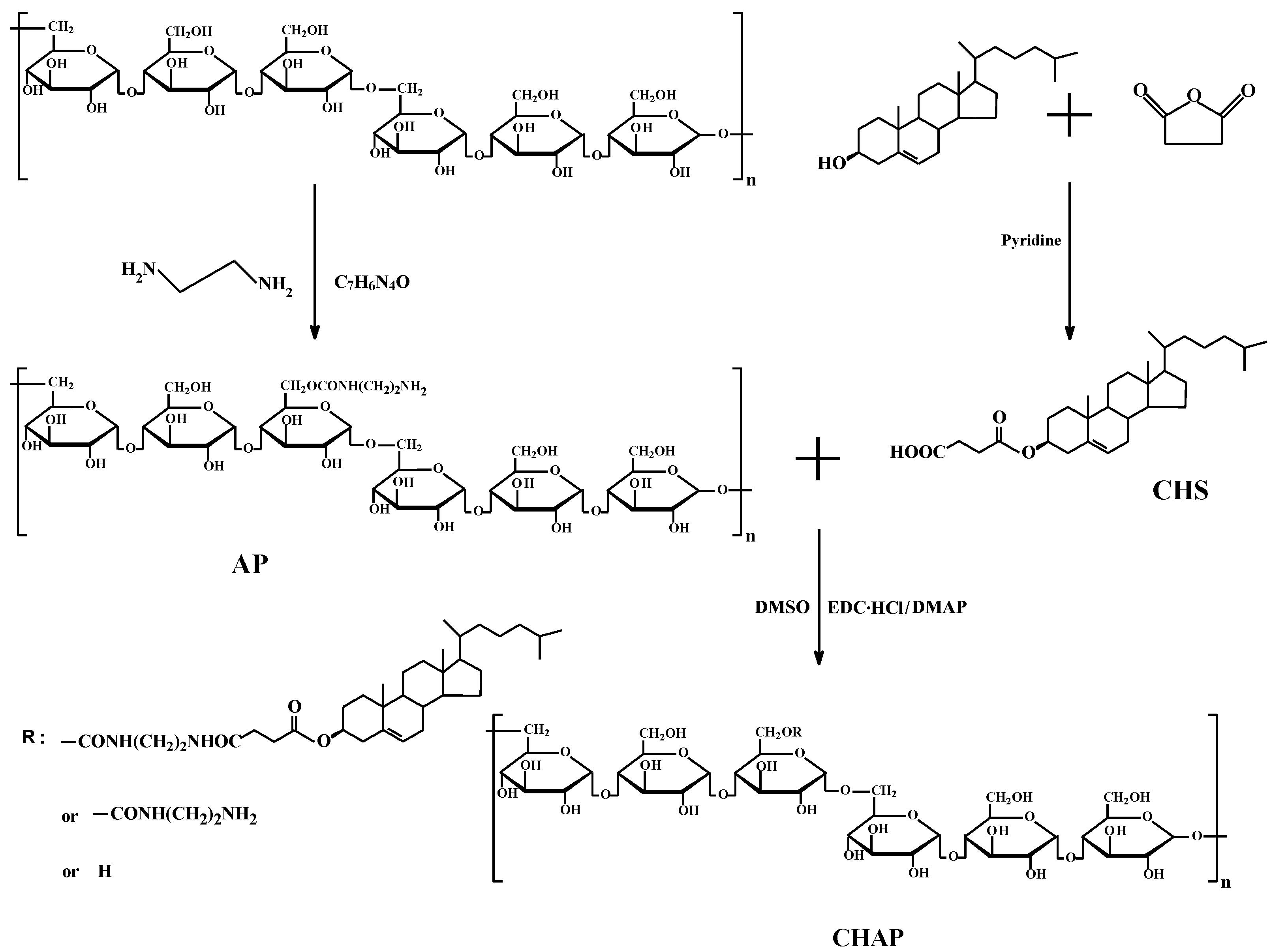
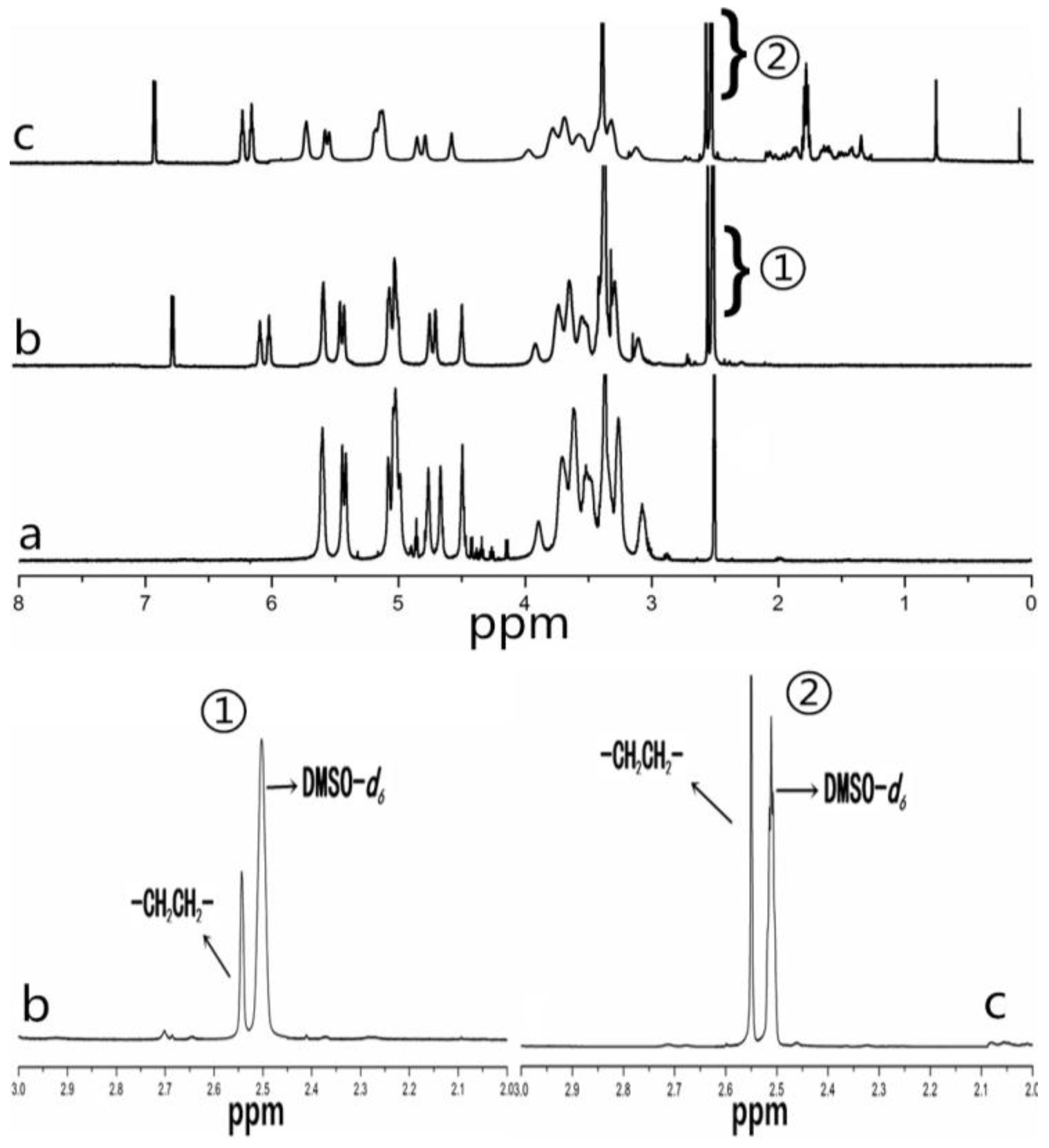
2.1. CHAP Conjugate
2.2. Property of CHAP NPs
2.3. Mitoxantrone (MTO) Loading of Pullulan NPs with Different Surface Charge
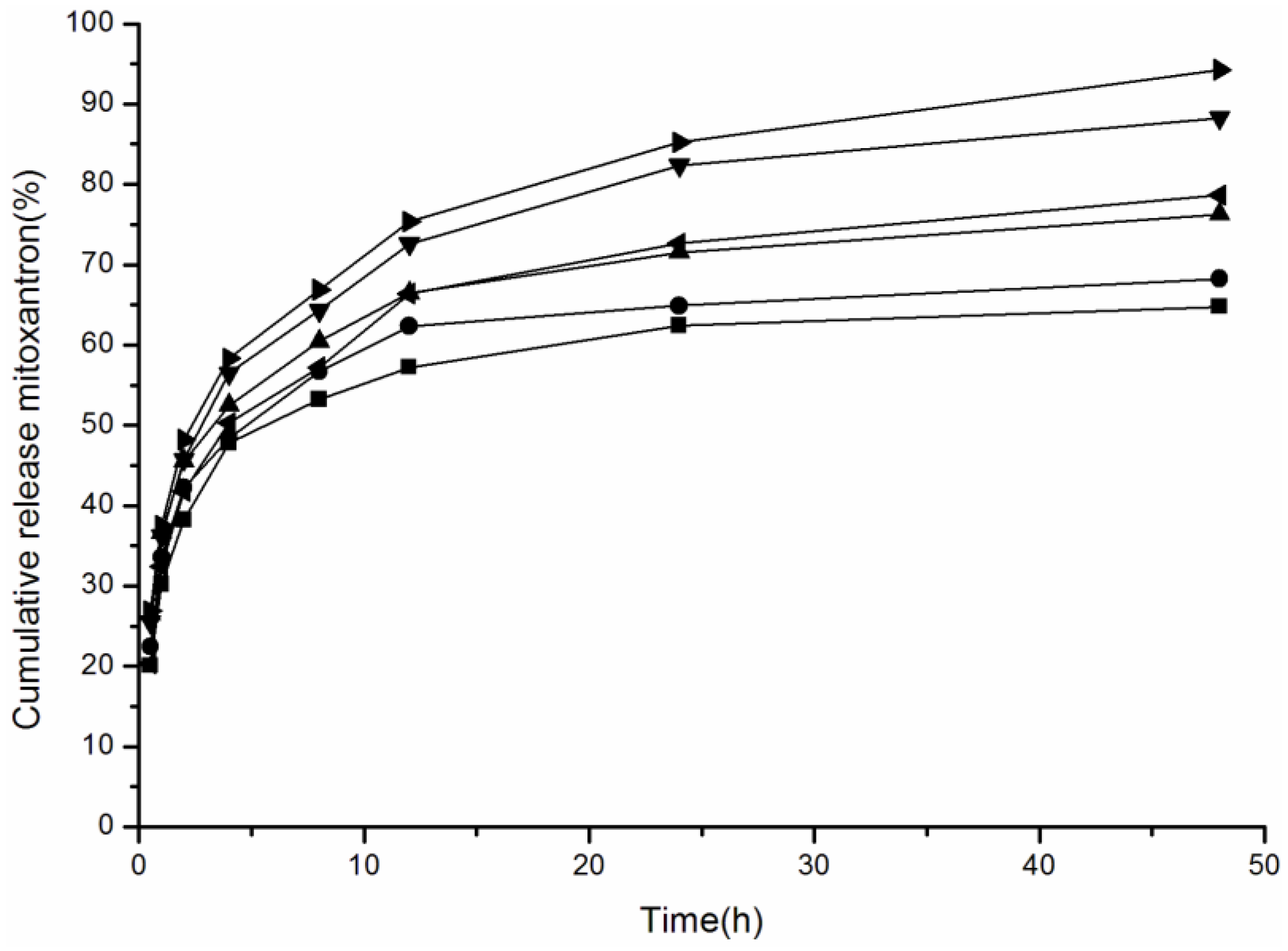
2.4. Effect of Media pH Value on Drug Release of Pullulan NPs with Different Surface Charge
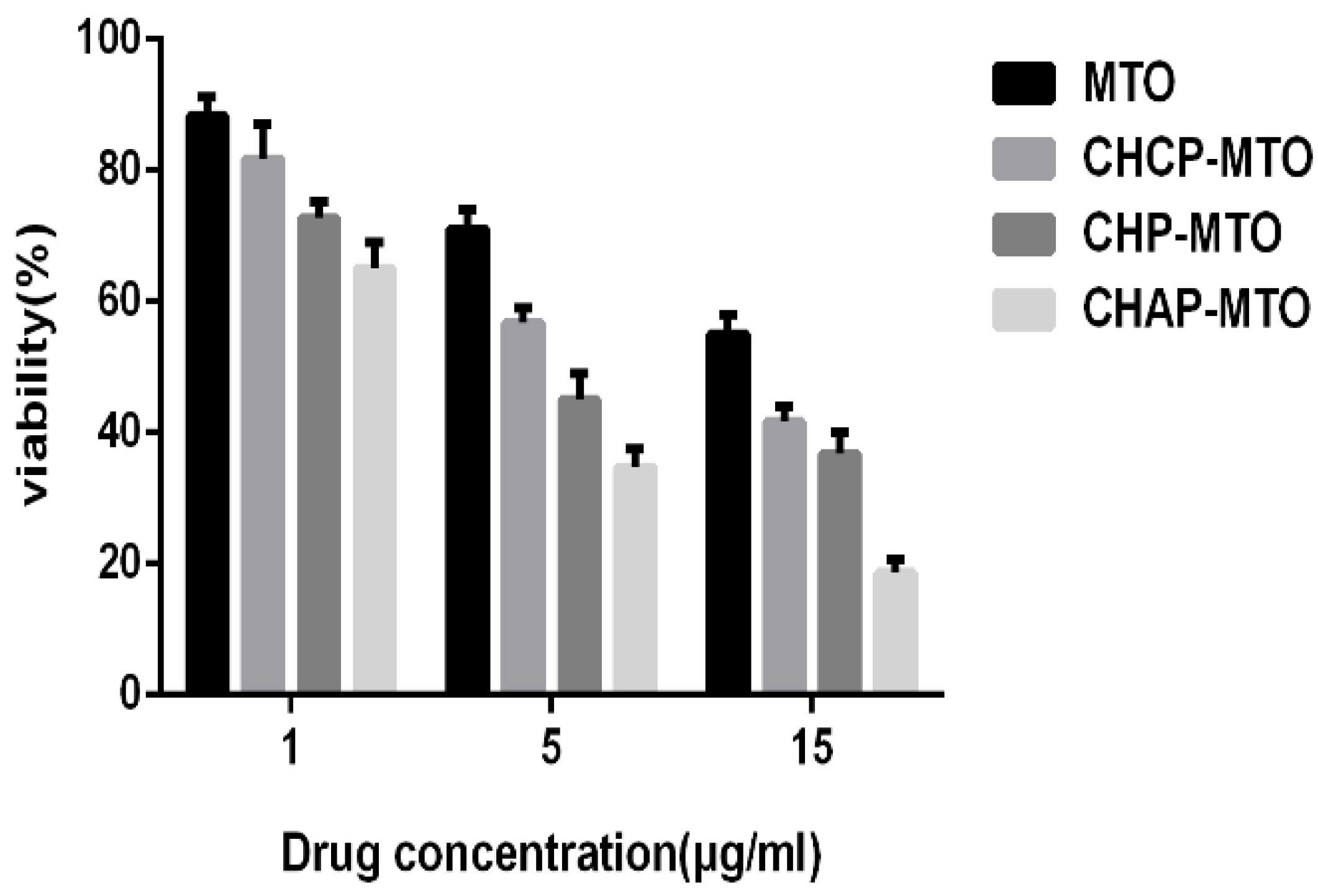
2.5. In Vitro Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of CHAP NPs
4.3. Fourier Transform Infrared (FTIR) Spectroscopy and NMR Spectroscopy
4.4. Preparation and Characterization of Pullulan NPs
4.5. Preparation and Characterization of MTO-Loaded NPs
- Drug loading efficiency (%) = NP drug-loading amount/initially added drug amount × 100%
- Loading content (%) = NP drug-loading amount/NP drug carrier amount × 100%
4.6. In Vitro Drug Release of Pullulan NPs with Different Properties
4.7. In Vitro Drug Release of Pullulan NPs in Release Media with Different pH
4.8. In Vitro Cytotoxicity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Lomis, A.; Westfall, S.; Farahdel, L.; Malhotra, M.; Shum-Tim, D.; Prakash, S. Human Serum Albumin Nanoparticles for Use in Cancer Drug Delivery: Process Optimization and in Vitro Characterization. Nanomaterials 2016, 6, 116. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced Materials and Processing for Drug Delivery: The Past and the Future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Pattni, B.S.; Abouzeid, A.H.; Torchilin, V.P. Nanopreparations to overcome multidrug resistance in cancer. Adv. Drug Deliv. Rev. 2013, 65, 1748–1762. [Google Scholar] [CrossRef] [PubMed]
- Rabanel, J.M.; Aoun, V.; Elkin, I.; Mokhtar, M.; Hildgen, P. Drug-loaded nanocarriers: Passive targeting and crossing of biological barriers. Curr. Med. Chem. 2012, 19, 3070–3102. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Oh, J.E.; Lee, K.H.; Park, T.G. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Pharm. Res. 1999, 16, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Patra, B.; Layek, B.; Mukherjee, A. Sustained release of acyclovir from nano-liposomes and nano-niosomes: An in vitro study. Int. J. Nanomed. 2007, 2, 213–225. [Google Scholar]
- Calatayud, M.P.; Sanz, B.; Raffa, V.; Riggio, C.; Ibarra, M.R.; Goya, G.F. The effect of surface charge of functionalized Fe3O4 nanoparticles on protein adsorption and cell uptake. Biomaterials 2014, 35, 6389–6399. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Dai, D.W.; Yuan, Y.; Qian, J.C.; Sha, S.; Shi, J.L.; Liu, C.S. Effect of size on the cellular endocytosis and controlled release of mesoporous silica nanoparticles for intracellular delivery. Biomed. Microdevices 2012, 14, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mccrate, J.M.; Lee, J.C.-M.; Li, H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011, 22, 105708–105720. [Google Scholar] [CrossRef] [PubMed]
- Baoum, A.; Dhillon, N.; Buch, S.; Berkland, C. Cationic Surface Modification of PLG Nanoparticles Offers Sustained Gene Delivery to Pulmonary Epithelial Cells. J. Pharm. Sci. 2010, 99, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Xie, J.W.; Wurm, P.A. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI Etchant. Nano Lett. 2009, 9, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, S.S. PLGA/TPGS Nanoparticles for Controlled Release of Paclitaxel: Effects of the Emulsifier and Drug Loading Ratio. Pharm. Res. 2003, 20, 1864–1872. [Google Scholar]
- Yang, W.Z.; Chen, H.L.; Gao, F.P.; Chen, M.M.; Li, X.M. Self-aggregated nanoparticles of cholesterol-modified pullulan conjugated as a novel carrier of mitoxantrone. Curr. Nanosci. 2010, 6, 298–306. [Google Scholar] [CrossRef]
- Tao, X.J.; Zhang, Q.F.; Ling, K.; Chen, Y.C.; Yang, W.Z.; Gao, F.F.; Shi, G.G. Effect of pullulan nanoparticle surface charges on HSA complexation and drug Release behavior of HSA-bound nanoparticles. PLoS ONE 2012, 7, 49304–49317. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, E.; Morimoto, N.; Kujawa, P.; Ozawa, Y.; Akiyoshi, K. Self-assembled nanogels of cholesteryl-modified polysaccharides: Effect of the polysaccharide structure on their association characteristics in the dilute and semidilute regimes. Biomacromolecules 2007, 8, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Wang, M.M.; Ma, L.L.; Li, H.Y.; Huang, L. Synthesis and characterization of biotin modified cholesteryl pullulan as a novel anticancer drug carrier. Carbohydr. Polym. 2014, 99, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, Y.P.; Jiao, Y.F.; Wang, C.R.; Zhou, Z.Y.; Zhang, Z.Y. Polypolysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar]
- Qinna, N.A.; Karwi, Q.G.; Al-Jbour, N.; Al-Remawi, M.A.; Alhussainy, T.M.; Al-So’ud, K.A.; Al-Omari, M.M.H.; Badwan, A.A. Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations. Mar. Drugs 2015, 13, 1710–1725. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.W.; Xue, M.D.; Chen, G.; Liu, I. Vitro Investigation of Self-assembled Nanoparticles Based on Hyaluronic Acid-Deoxycholic Acid Conjugates for Controlled Release Doxorubicin: Effect of Degree of Substitution of Deoxycholic Acid. Int. J. Mol. Sci. 2015, 16, 7195–7209. [Google Scholar]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 35, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.H.; Cao, C.; Sim, S.J.J. Application of citrate-stabilized gold coated ferric oxide composite nanoparticles for biological separations. J. Mag. Mag. Mater. 2008, 320, 2049–2055. [Google Scholar] [CrossRef]
- Du, L.V.; Cerf, D.L.; Picton, L.; Muller, G. Carboxymethylpullulan hydrogels with an ionic and/or amphiphilic behavior: Swelling properties and entrapment of cationic and/or hydrophobic molecules. Colloids Surf. A Physicochem. Eng. Asp. 2006, 274, 163–169. [Google Scholar]
- Ayame, H.; Morimoto, N.; Akiyoshi, K. Self-assembled cationic nanogels for intracellular protein delivery. Bioconjugate Chem. 2008, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Jiang, Q.; Li, R.S.; Liu, L.L.; Zhang, Q.Q.; Wang, Y.M.; Zhao, J. Self-assembled nanoparticles of cholesterol-modified O-carboxymethyl chitosan as a novel carrier for paclitaxel. Nanotechnology 2008, 19, 145101–145109. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.L.Z.; Wang, Y.; Cheng, H.Y.; Pervainz, S.; Yang, Y.Y. The co-delivery of paclitaxel and herceptin using cationic micellar nanoparticles. Biomaterials 2009, 30, 919–992. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Zuccheri, G.; Federica Belluti, F.; Provenzano, S.; Verardi, L.; Bigucci, F.; Cerchiara, T.; Luppi, B.; Calonghi, N. Chitosan nanoparticles for lipophilic anticancer drug delivery: Development, characterization and in vitro studies on HT29 cancer cells. Colloids Surf. B Biointerfaces 2016, 145, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Sallustio, S.; Galantini, L.; Gente, G.; Masci, G.; Mesa, C.L. Hydrophobically Modified Pullulans: Characterization and Physicochemical Properties. J. Phys. Chem. B 2004, 108, 18876–18883. [Google Scholar] [CrossRef]
- Shen, S.G.; Li, H.Y.; Yang, W.Z. The preliminary evaluation on cholesterol-modified pullulan as a drug nanocarrier. Drug Deliv. 2014, 21, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Kitano, S.; Kageyama, S.; Nagata, Y.; Miyahara, Y.; Hiasa, A.; Naota, H.; Okumura, S.; Imai, H.; Shiraishi, T.; Masuya, M.; et al. HER2-SpecificT-Cell Immune Responses in PatientsVaccinated with Truncated HER2 Protein Complexed with Nanogels of Cholesteryl Pullulan. Clin. Cancer Res. 2006, 12, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.J.; Shu, J.; De, H.W.; Kai, L.; Li, M.Y.; Ping, F.L.; Yong, C.X.; Xiao, P.Y. Effects of Particle Hydrophobicity, Surface Charge, Media pH Value and Complexation with Human Serum Albumin on Drug Release Behavior of Mitoxantrone-Loaded Pullulan Nanoparticles. Nanomaterials 2016, 6, 2–16. [Google Scholar] [CrossRef]
- Wang, Y.S.; Chen, H.L.; Liu, Y.Y.; Wu, J.; Zhou, P.; Wang, Y.; Li, R.S.; Yang, X.Y.; Zhang, N. pH-sensitive pullulan-based nanoparticle carrier of methotrexate and combretastatin A4 for the combination therapy against hepatocellular carcinoma. Biomaterials 2013, 34, 7181–7190. [Google Scholar] [CrossRef] [PubMed]
- Nitta, S.K.; Numata, K. Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [PubMed]
- Lindman, S.; Lynch, I.; Thulin, E.; Nilsson, H.; Dawson, H.A.; Linse, S. Systematic investigation of the thermodynamics of HSA adsorption to N-iso-propylacrylamide/N-tert-butylacrylamide copolymer nanoparticles: Effects of particle size and hydrophobicity. Nano Lett. 2007, 7, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Okadaa, T.; Sawadab, S.I.; Akiyoshib, K.; Matsuzakia, K. Inhibition of the formation of amyloid b-protein fibrils using biocompatible nanogels as artificial chaperones. FEBS Lett. 2006, 580, 6587–6595. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.J.; Zhang, Q.Q.; Yang, W.Z.; Zhang, Q.Q. The interaction between human serum albumin and cholesterol-modified pullulan nanoparticle. Curr. Nanosci. 2012, 8, 830–837. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, P.W.; Tran, T.T.D.; Zhang, J.; Kong, L.X. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials 2016, 6, 26–44. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Ascenzi, P. Preparation and characterization of pH- and temperature-sensitive pullulan microspheres for controlled release of drugs. Biomaterials 2008, 29, 2767–2775. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yang, J.; Ji, F.; Liu, Y.X.; Yao, F.L. Drug co-loading and pH-sensitive release core-shell nanoparticles via layer-by-layer assembly. J. Biomater. Sci. Polym. Ed. 2014, 25, 1573–1589. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Liu, Y.; Liu, Y.Y.; Wang, Y.; Wu, J.; Li, R.S.; Yang, J.R.; Zhang, N. pH-sensitive pullulan-based nanoparticles for intracellular drug delivery. Polym. Chem. 2014, 5, 423–432. [Google Scholar] [CrossRef]
- Na, K.; Lee, E.S.; Bae, Y.H. Self-organized nanogels responding to tumor extracellular pH: pH-dependent drug release and in vitro cytotoxicity against MCF-7 Cells. Bioconjugate Chem. 2007, 18, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.M.; Kang, Y.J.; Sohn, Y.J.; Kim, D.K. Dual targeting strategy of magnetic nanoparticle-loaded and RGD peptide-activated stimuli-sensitive polymeric micelles for delivery of paclitaxel. J. Nanopart. Res. 2015, 17, 248–257. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Li, X.M.; Liu, L.R.; Zhang, Q.Q. Cellular uptake mechanism and intracellular fate of hydrophobically modified pullulan nanoparticles. Int. J. Nanomed. 2013, 8, 1825–1834. [Google Scholar]
- Ptail, Y.; Sadhukha, T.; Ma, L.; Panyam, J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tarquidar overcomes tumor drug resistance. J. Control. Release 2009, 136, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bhirde, A.A.; Kapoor, A.; Liu, G.; Iglesias-Bartolome, R.; Jin, A.; Zhang, G.F.; Xing, R.J.; Lee, S.; Leapman, R.D.; Gutkind, S.J.; et al. Nuclear mapping of nano-drug delivery systems in dynamic cellular environments. ACS Nano 2012, 6, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef] [PubMed]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; Briesen, H.V.; Kreuter, J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurons. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A.; Danscher, G. Kupffer cells are central in the removal of nanoparticles from the Organism. Part. Fibre Toxicol. 2007, 4, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Hall, J.B.; Mcleland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Sundaram, S.K.; Teeguarden, J.G.; Riley, B.J.; Fifield, L.S.; Jacobs, M.J. Adsorbed Proteins Influence the Biological Activity and Molecular Targeting of Nanomaterials. Toxicol. Sci. 2007, 100, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Yuan, R.F.; Zheng, F.C.; Zhong, S.P.; Tao, X.J.; Zhang, Y.M.; Gao, F.F.; Yao, F.; Chen, J.X.; Chen, Y.C.; Shi, G.G. Self-Assembled Nanoparticles of Glycyrrhetic Acid-Modified Pullulan as a Novel Carrier of Curcumin. Molecules 2014, 19, 13305–13318. [Google Scholar] [CrossRef] [PubMed]






| Sample | D/C (w/w) a | EE (%) b | LC (%) c | ζ (mV) d |
|---|---|---|---|---|
| CHP | 1/5 | 75.2 ± 1.94 | 13.3 ± 0.22 | −1.21 ± 0.12 |
| CHCP | 1/5 | 72.4 ± 1.72 | 12.7 ± 0.20 | −19.9 ± 0.23 |
| CHNP | 1/5 | 70.8 ± 1.68 | 12.3 ± 0.20 | 7.22 ± 0.18 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.; Xie, Y.; Zhang, Q.; Qiu, X.; Yuan, L.; Wen, Y.; Li, M.; Yang, X.; Tao, T.; Xie, M.; et al. Cholesterol-Modified Amino-Pullulan Nanoparticles as a Drug Carrier: Comparative Study of Cholesterol-Modified Carboxyethyl Pullulan and Pullulan Nanoparticles. Nanomaterials 2016, 6, 165. https://doi.org/10.3390/nano6090165
Tao X, Xie Y, Zhang Q, Qiu X, Yuan L, Wen Y, Li M, Yang X, Tao T, Xie M, et al. Cholesterol-Modified Amino-Pullulan Nanoparticles as a Drug Carrier: Comparative Study of Cholesterol-Modified Carboxyethyl Pullulan and Pullulan Nanoparticles. Nanomaterials. 2016; 6(9):165. https://doi.org/10.3390/nano6090165
Chicago/Turabian StyleTao, Xiaojun, Yongchao Xie, Qiufang Zhang, Ximin Qiu, Liming Yuan, Yi Wen, Min Li, Xiaoping Yang, Ting Tao, Minghui Xie, and et al. 2016. "Cholesterol-Modified Amino-Pullulan Nanoparticles as a Drug Carrier: Comparative Study of Cholesterol-Modified Carboxyethyl Pullulan and Pullulan Nanoparticles" Nanomaterials 6, no. 9: 165. https://doi.org/10.3390/nano6090165
APA StyleTao, X., Xie, Y., Zhang, Q., Qiu, X., Yuan, L., Wen, Y., Li, M., Yang, X., Tao, T., Xie, M., Lv, Y., Wang, Q., & Feng, X. (2016). Cholesterol-Modified Amino-Pullulan Nanoparticles as a Drug Carrier: Comparative Study of Cholesterol-Modified Carboxyethyl Pullulan and Pullulan Nanoparticles. Nanomaterials, 6(9), 165. https://doi.org/10.3390/nano6090165





