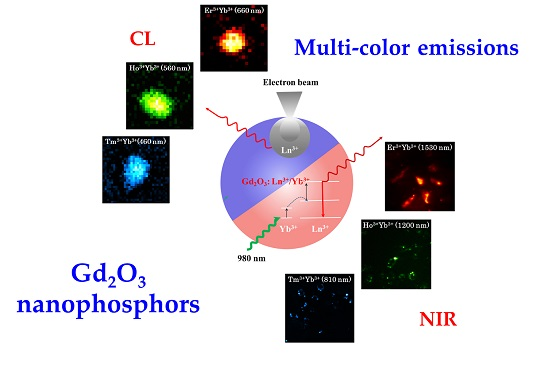
Multispectral Emissions of Lanthanide-Doped Gadolinium Oxide Nanophosphors for Cathodoluminescence and Near-Infrared Upconversion/Downconversion Imaging
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
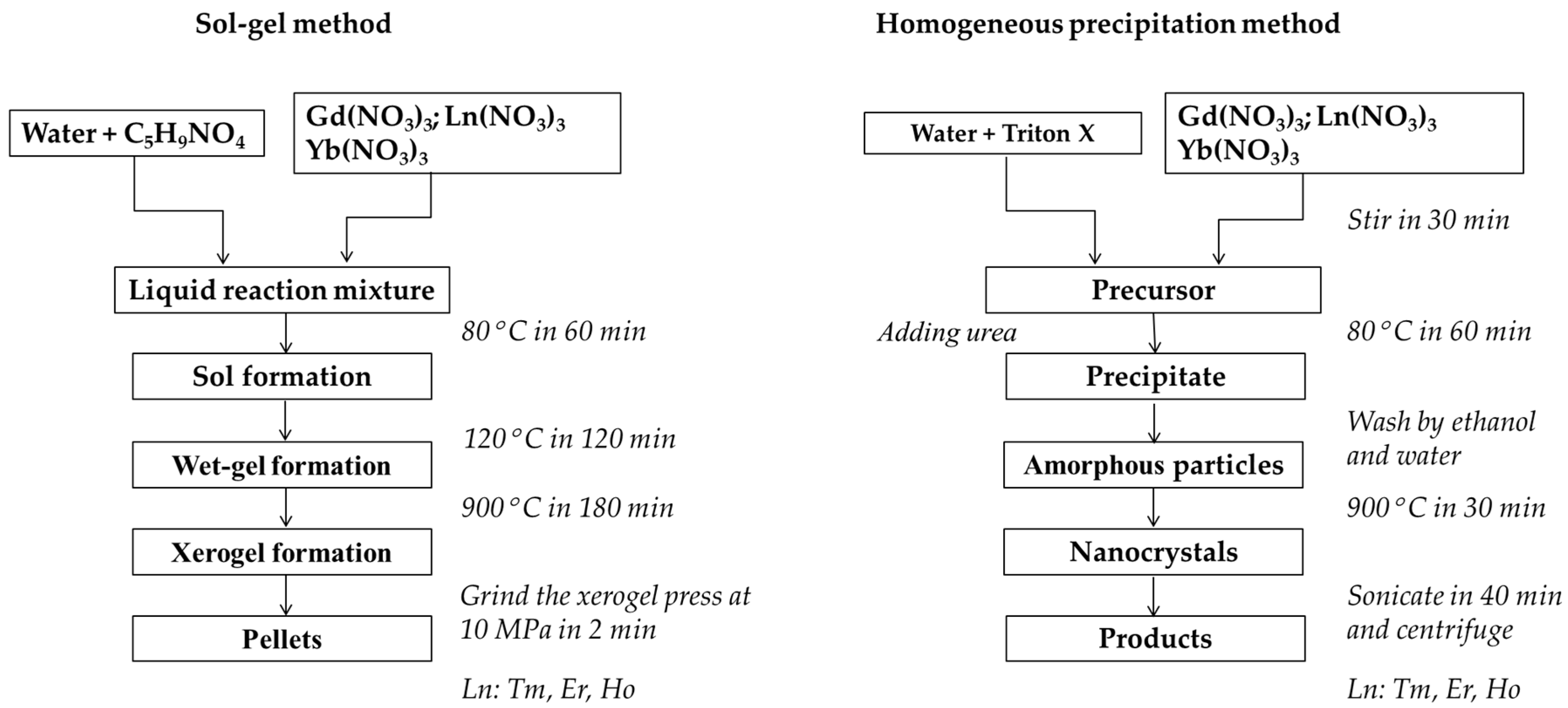
2.2. Synthesis of Nanophosphors
2.3. Sol-Gel Method
2.4. Homogeneous Precipitation (HP) Method
2.5. Characterization
3. Results and Discussion
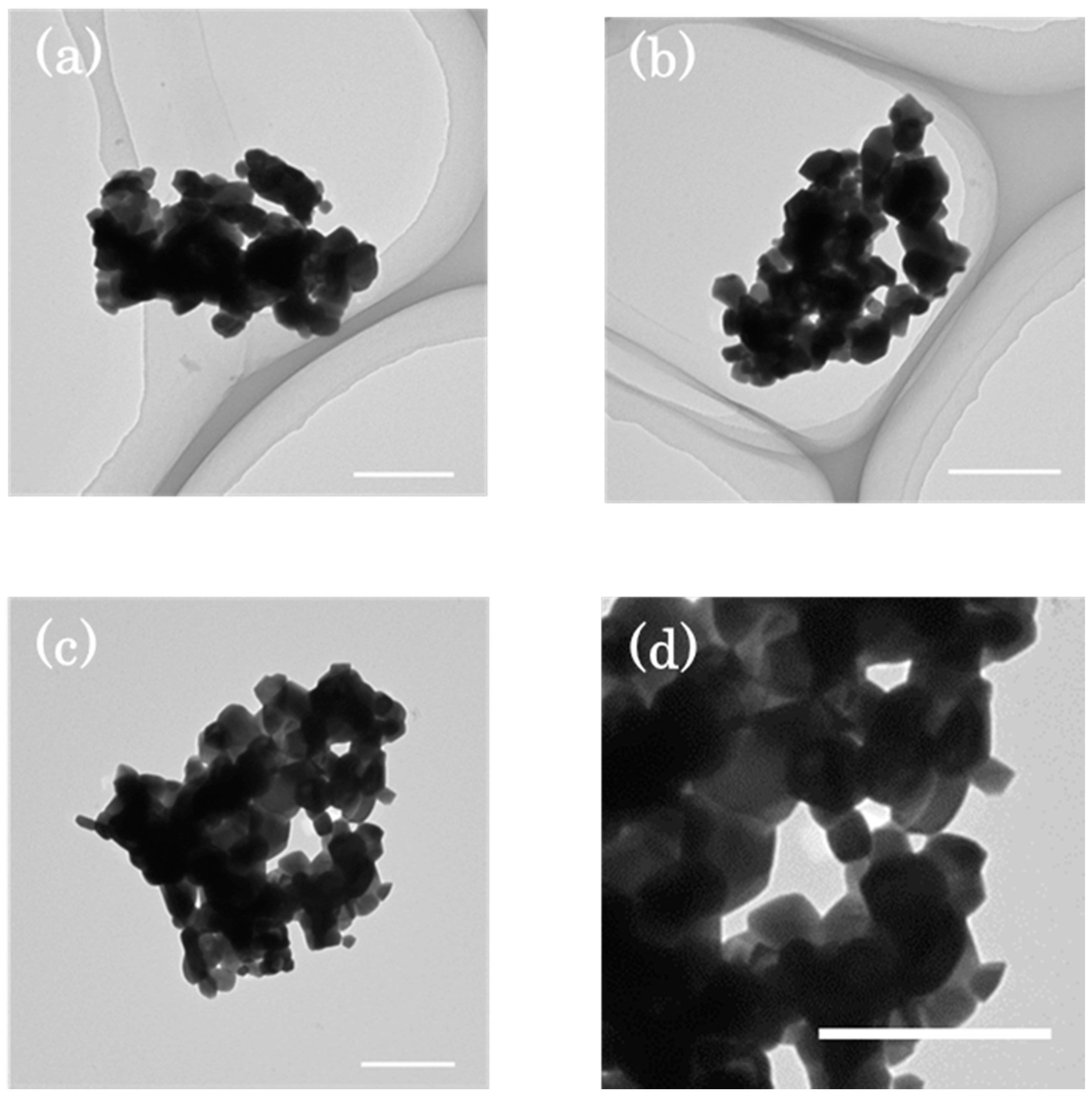
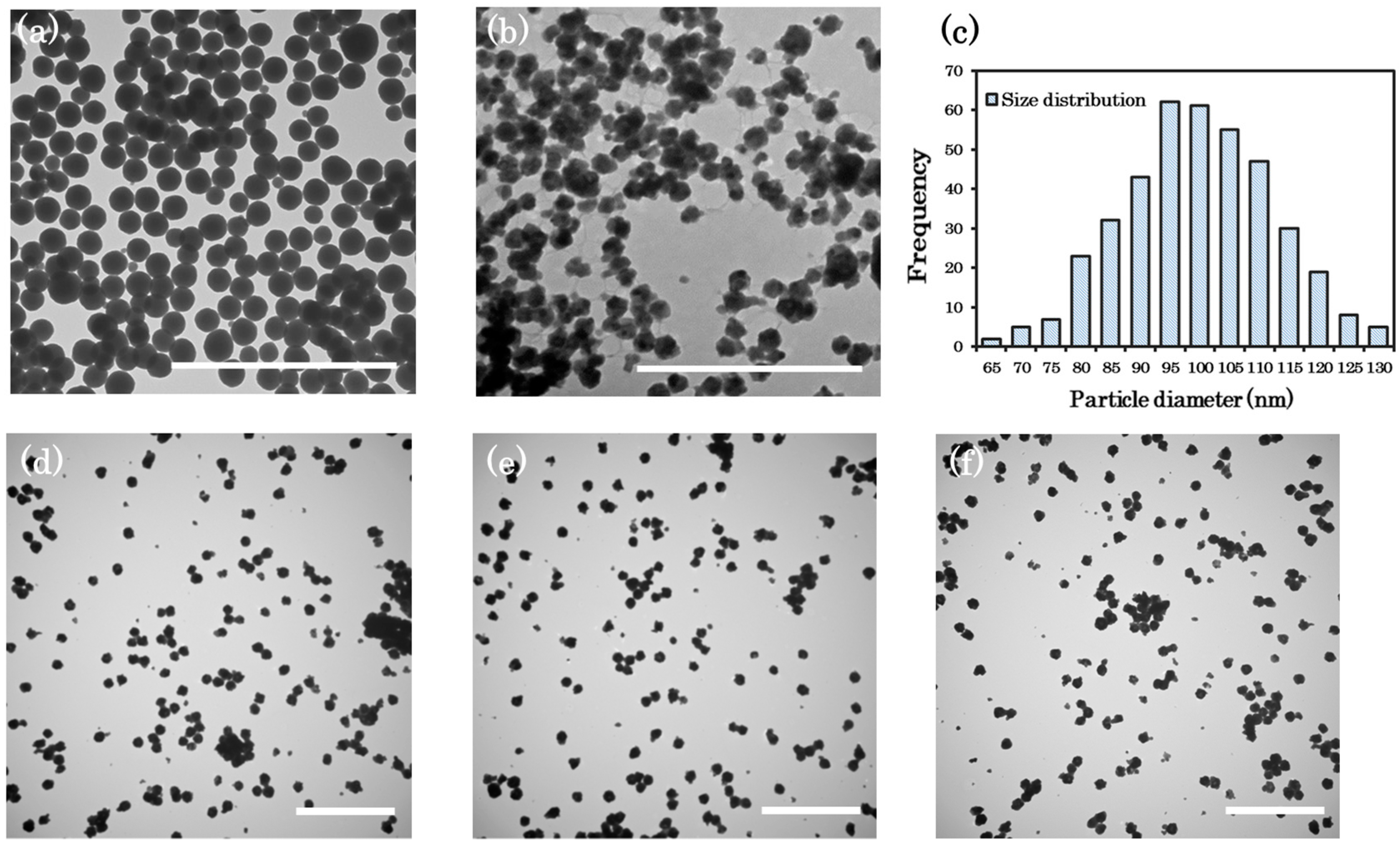
3.1. Morphology of NPPs
3.1.1. The Sol-Gel Method
3.1.2. Homogeneous Precipitation (HP) Method
3.2. Upconverting/Downconverting Luminescence Properties
3.2.1. Mechanism of UC/DC Emissions
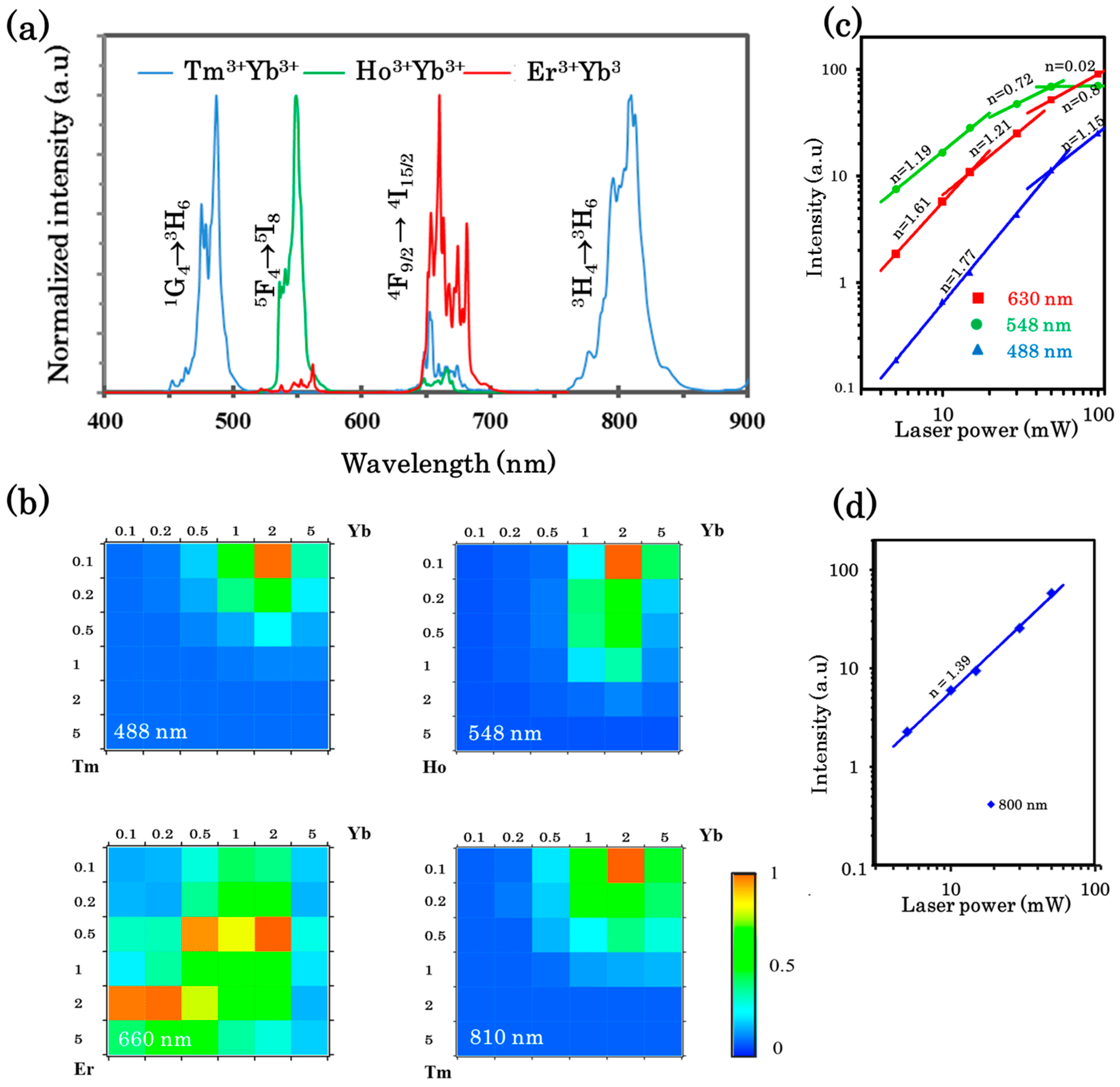
3.2.2. Upconversion of the Luminescence Spectra
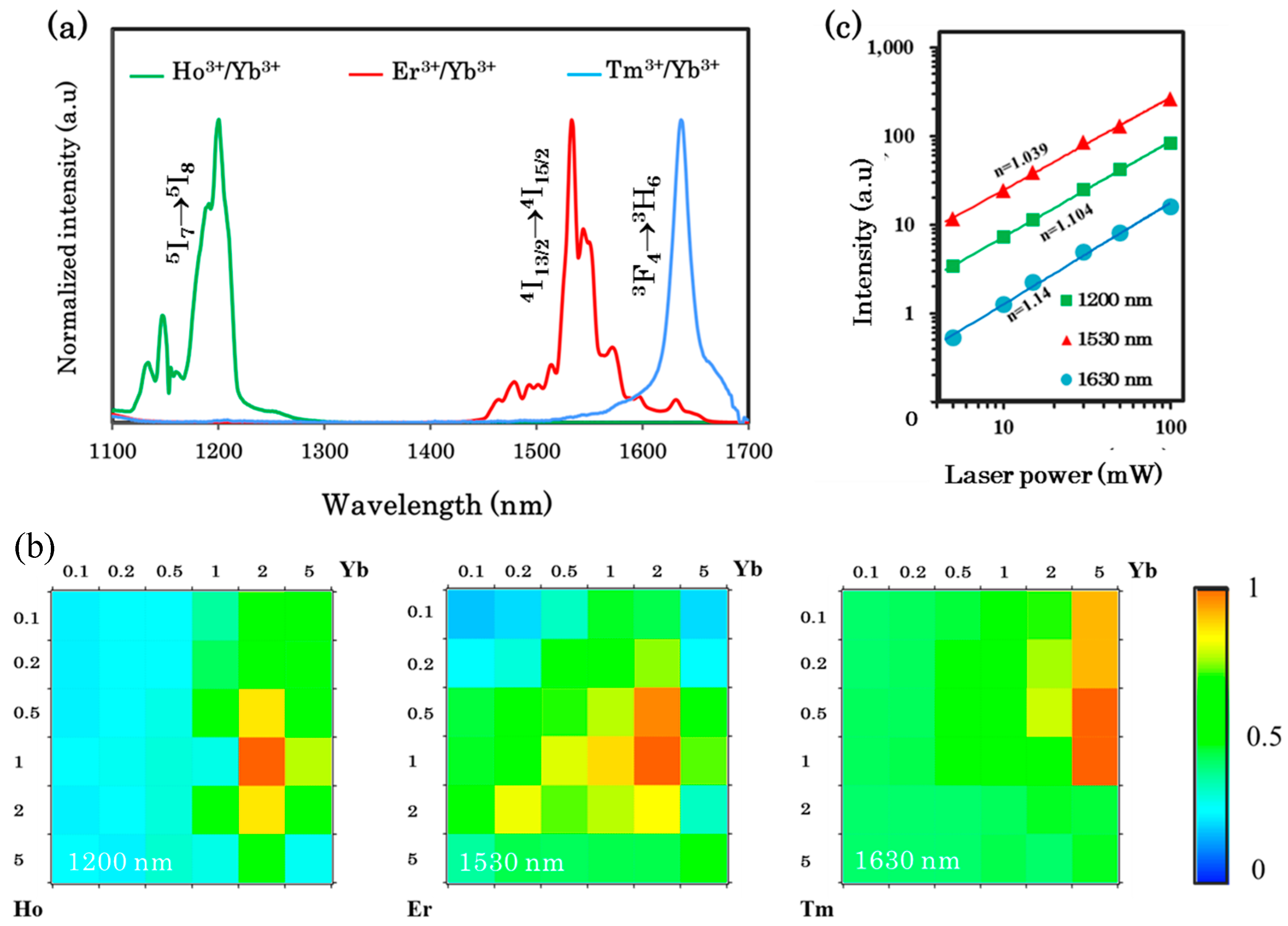
3.2.3. Downconversion of the NIR-Emitting Spectra
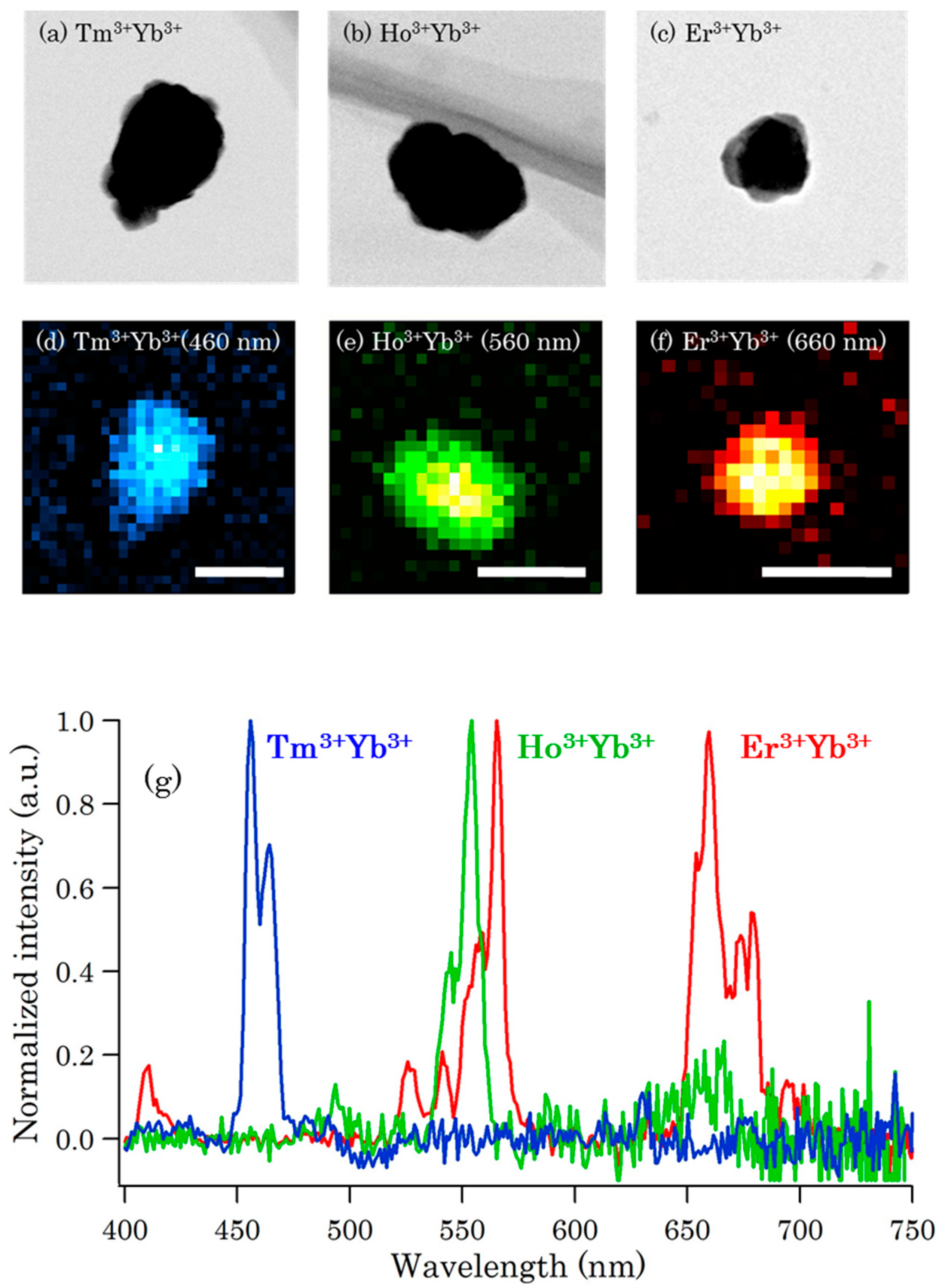
3.2.4. NIR Cellular Imaging
3.3. Cathodoluminescence Images of Nanoparticles
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paeng, J.C.; Lee, D.S. Multimodal molecular imaging in vivo. Open Nucl. Med. J. 2010, 2, 145–152. [Google Scholar] [CrossRef]
- Danhier, P.; De Preter, G.; Magat, J.; Godechal, Q.; Porporato, P.E.; Jordan, B.F.; Feron, O.; Sonveaux, P.; Gallez, B. Multimodal cell tracking of a spontaneous metastasis model: Comparison between MRI, electron paramagnetic resonance and bioluminescence. Contrast Media Mol. Imaging 2014, 9, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Moseley, M.; Donnan, G. Multimodality imaging: Introduction. Stroke 2004, 35, 2632–2634. [Google Scholar] [CrossRef]
- Zaidi, H.; Prasad, R. Advances in multimodality molecular imaging. J. Med. Phys. 2009, 34, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Martí-Bonmatí, L.; Sopena, R.; Bartumeus, P.; Sopena, P. Multimodality imaging techniques. Contrast Media Mol. Imaging 2010, 5, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.B.; Chen, X.Y. Multimodality molecular imaging of tumor angiogenesis. J. Nucl. Med. 2008, 49, 113S–128S. [Google Scholar] [CrossRef] [PubMed]
- Sinusas, A.J. Multimodality cardiovascular molecular imaging: An overview. J. Nucl. Med. 2010, 51, 1S–2S. [Google Scholar] [CrossRef] [PubMed]
- Doubrovin, M.; Serganova, I.; Mayer-Kuckuk, P.; Ponomarev, V.; Blasberg, R.G. Multimodality in vivo molecular-genetic imaging. Bioconjugate Chem. 2004, 15, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Leary, J.F. Nanoparticles for multimodal in vivo imaging in nanomedicine. Int. J. Nanomedicine. 2014, 9, 711–726. [Google Scholar] [PubMed]
- Zurbuchen, M.A.; Lake, M.P.; Kohan, S.A.; Leung, B.; Bouchard, L.S. Nanodiamond landmarks for subcellular multimodal optical and electron imaging. Sci. Rep. 2013, 3, 2668. [Google Scholar] [CrossRef] [PubMed]
- Cortese, K.; Diaspro, A.; Tacchetti, C. Advanced correlative light/electron microscopy: Current methods and new developments using tokuyasu cryosections. Histochemistry 2009, 57, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Salas-Ramirez, K.; Shi, L.; Zhang, L.; Alfano, R.R. Third therapeutic spectral window for deep tissue imaging. Proc. SPIE 2014, 8940, 89400V. [Google Scholar]
- Ntziachristos, V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 2010, 7, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Furukawa, T.; Niioka, H.; Ichimiya, M.; Miyake, J.; Ashida, M.; Araki, T.; Hashimoto, M. Y2O3:Tm,Yb nanophosphors for correlative upconversion luminescence and cathodoluminescence imaging. Micron 2014, 67, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Niioka, H.; Furukawa, T.; Ichimiya, M.; Ashida, M.; Araki, T.; Hashimoto, M. Multicolor cathodoluminescence microscopy for biological imaging with nanophosphors. Appl. Phys. Express 2011, 4, 2–5. [Google Scholar] [CrossRef]
- Furukawa, T.; Niioka, H.; Ichimiya, M.; Nagata, T.; Ashida, M.; Araki, T.; Hashimoto, M. High-resolution microscopy for biological specimens via cathodoluminescence of Eu- and Zn-doped Y2O3nanophosphors. Opt. Express. 2013, 21, 25655–25663. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Somesfalean, G.; Zhang, Z.G.; Sun, Q.; Wang, F.P. Ultraviolet upconversion fluorescence in rare-earth-ion-doped Y2O3 induced by infrared diode laser excitation. Opt. Lett. 2007, 32, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the biological windows: Current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef]
- Van Saders, B.; Al-Baroudi, L.; Tan, M.C.; Riman, R.E. Rare-earth doped particles with tunable infrared emissions for biomedical imaging. Opt. Mater. Express 2013, 3, 566–573. [Google Scholar] [CrossRef]
- Naczynski, D.J.; Tan, M.C.; Zevon, M.; Wall, B.; Kohl, J.; Kulesa, A.; Chen, S.; Roth, C.M.; Riman, R.E.; Moghe, P.V. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat. Commun. 2013, 4, 2199. [Google Scholar] [CrossRef] [PubMed]
- Prodi, L.; Rampazzo, E.; Rastrelli, F.; Speghini, A.; Zaccheronia, N. Imaging agents based on lanthanide doped nanoparticles. Chem. Soc. Rev. 2015, 44, 4922–4952. [Google Scholar] [CrossRef] [PubMed]
- Yacobi, B.G.; Holt, D.B. Cathodoluminescence scanning electron microscopy of semiconductors. J. Appl. Phys. 1986, 59, R1–R24. [Google Scholar] [CrossRef]
- Jakubowicz, A. Theory of cathodoluminescence contrast from localized defects in semiconductors. J. Appl. Phys. 1986, 59, 2205–2209. [Google Scholar] [CrossRef]
- Morrison, I.E.G.; Samilian, A.; Coppo, P.; Ireland, T.G.; Fern, G.R.; Silver, J.; Withnall, R.; O’Toole, P.J. Multicolour correlative imaging using phosphor probes. J. Chem. Biol. 2015, 8, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Zhegalova, N.G.; Wang, S.T.; Akers, W.J.; Berezin, M.Y. Multispectral imaging in the extended near-infrared window based on endogenous chromophores. J. Biomed. Opt. 2013, 18, 101318. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human cranial bone in the spectral range from 800 to 2000 nm. In Proceedings of the SPIE 6163, Saratov Fall Meeting 2005: Optical Technologies in Biophysics and Medicine VII, Saratov, Russia, 27–30 September 2005; Volume 6163.
- Weissleder, R.; Mahmood, U. Molecular imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.A.; Morries, L.D. Near-infrared photonic energy penetration: Can infrared phototherapy effectively reach the human brain. Neuropsychiatr. Dis. Treat. 2015, 11, 2191–2208. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.A.; Pu, Y.; Pratavieira, S.; Budansky, Y.; Alfano, R.R. Deep optical imaging of tissue using the second and third near-infrared spectral windows. J. Biomed. Opt. 2014, 19, 056004. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Furukawa, T.; Niioka, H.; Ichimiya, M.; Sannomiya, T.; Tanaka, N.; Onoshima, D.; Yukawa, H.; Baba, Y.; Ashida, M.; et al. Correlative near-infrared light and cathodoluminescence microscopy using Y2O3:Ln, Yb (Ln = Tm, Er) nanophosphors for multiscale, multicolour bioimaging. Sci. Rep. 2016, 6, 25950. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Venkatachalam, N.; Hyodo, H.; Hattori, A.; Ebina, Y.; Kishimotoa, H.; Soga, K. Upconverting and NIR emitting rare earth based nanostructures for NIR-bioimaging. Nanoscale 2013, 5, 11339–11361. [Google Scholar] [CrossRef] [PubMed]
- Mutelet, B.; Perriat, P.; Ledoux, G.; Aman, D.; Lux, F.; Tillement, O.; Billotey, C.; Janier, M.; Villiers, C.; Bazzi, R.; et al. Suppression of luminescence quenching at the nanometer scale in Gd2O3 doped with Eu3+ or Tb3+: Systematic comparison between nanometric and macroscopic samples of life-time, quantum yield, radiative and non-radiative decay rates. J. Appl. Phys. 2011, 110, 094317. [Google Scholar]
- Meza, O.; Villabona-Leal, E.G.; Diaz-Torres, L.A.; Desirena, H.; Rodríguez-López, J.L.; Pérez, E. Luminescence concentration quenching mechanism in Gd2O3:Eu3+. J. Phys. Chem. A 2014, 118, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Atabaev, T.; Hwang, Y.H.; Kim, H.K. Color-tunable properties of Eu3+- and Dy3+-codoped Y2O3 phosphor particles. Nanoscale Res. Lett. 2012, 7, 556. [Google Scholar] [CrossRef] [PubMed]
- Selvalakshmi, T.; Sellaiyan, S.; Uedono, A.; Bose, A.C. Investigation of defect related photoluminescence property of multicolour emitting Gd2O3:Dy3+ phosphor. RSC Adv. 2014, 4, 34257. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Xu, W.; Kim, S.J.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Park, J.A.; Kim, T.J.; Lee, G.H. Potential dual imaging nanoparticle: Gd2O3 nanoparticle. Sci. Rep. 2015, 5, 8549. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Bony, B.A.; Kim, C.R.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Kim, T.J.; Lee, G.H. Mixed lanthanide oxide nanoparticles as dual imaging agent in biomedicine. Sci. Rep. 2013, 3, 3210. [Google Scholar] [PubMed]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol-gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Nishimori, H.; Tatsumisago, M.; Minami, T. Growth mechanism of large monodispersed silica particles prepared from tetraethoxysilane in the presence of sodium dodecyl sulfate. J. Sol-Gel Sci. Technol. 1997, 9, 25–31. [Google Scholar] [CrossRef]
- Feofilov, S.P.; Kaplyanskii, A.A.; Kulinkin, A.B.; Kutsenko, A.B.; Vasilevskaya, T.N.; Zakharchenya, R.I. Sol-gel synthesis and optical studies of rare earth and transition metal ions doped nanocrystalline aluminum and yttrium oxides. J. Sol-Gel Sci. Technol. 2001, 21, 135–145. [Google Scholar] [CrossRef]
- Štengl, V.; Šubrt, J.; Bezdička, P.; Maříková, M.; Bakardjieva, S. Homogenous precipitation with urea—An easy process for making spherical hydrous metal oxides. Solid State Phenom. 2003, 90–91, 121–126. [Google Scholar] [CrossRef]
- Gavrilovi, T.V.; Jovanovi, D.J.; Smits, K.; Dramićanin, M.D. Multicolor upconversion luminescence of GdVO4:Ln3+/Yb3+ (Ln3+ = Ho3+, Er3+, Tm3+, Ho3+/Er3+/Tm3+) nanorods. Dye Pigment. 2016, 126, 1–7. [Google Scholar] [CrossRef]
- Pollnau, M.; Gameline, D.R.; Lüthi, S.R.; Güdel, H.U.; Hehlen, M.P. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B 2000, 61, 3337–3356. [Google Scholar] [CrossRef]
- Tikhomirov, V.K.; Rodríguez, V.D.; Méndez-Ramos, J.; Castillo, J.D.; Kirilenko, D.; van Tendeloo, G.; Moshchalkov, V.V. Optimizing Er/Yb ratio and content in ErYb co-doped glass-ceramics for enhancement of the up- and down-conversion luminescence. Sol. Energy Mater. Sol. Cells 2012, 100, 209–215. [Google Scholar] [CrossRef]
- Watekar, P.R.; Ju, S.; Han, W.T. 800-nm upconversion emission in Yb-sensitized Tm-doped optical fiber. IEEE Photonics Technol. Lett. 2006, 18, 1609–1611. [Google Scholar] [CrossRef]
- Suo, H.; Guo, C.; Wang, W.; Li, T.; Duan, C.; Yin, M. Mechanism and stability of spectrally pure green up-conversion emission in Yb3+ /Ho3+ co-doped Ba5Gd8Zn4O21 phosphors. Dalt Trans. 2016, 45, 2629–2636. [Google Scholar] [CrossRef] [PubMed]
- Terra, I.A.A.; Borrero-González, L.J.; Figueredo, T.R.; Almeida, J.M.P.; Hernandes, A.C.; Nunes, L.A.O.; Malta, O.L. Down-conversion process in Tb3+Yb3+ co-doped Calibo glasses. J. Lumin. 2012, 132, 1678–1682. [Google Scholar] [CrossRef]
- Maqbool, M.; Kordesch, M.E.; Kayani, A. Enhanced cathodoluminescence from an amorphous AlN:holmium phosphor by co-doped Gd+3 for optical devices applications. J. Opt. Soc. Am. B 2009, 26, 998–1001. [Google Scholar] [CrossRef]
- Lozykowski, H.J.; Jadwisienczak, W.M.; Brown, I. Visible cathodoluminescence of GaN doped with Dy, Er, and Tm. Appl. Phys. Lett. 1999, 74, 1129–1131. [Google Scholar] [CrossRef]
- Arsenev, P.A.; Hassan, H.W.; Chukichev, M.V. Cathodoluminescence of some rare earth ions in GdAlO3 single crystal. Phys. Status Solidi 1975, 28, 1–5. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Xiong, J.; Mariano, A.N.; Fleet, M.E. Rare-earth-element-activated cathodoluminescence in apatite. Can. Mineral. 1997, 35, 979–998. [Google Scholar]
- Myroshnychenko, V.; Nelayah, J.; Adamo, G.; Geuquet, N.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; MacDonald, K.F.; Henrard, L.; Liz-Marzán, L.M.; Zheludev, N.I.; et al. Plasmon spectroscopy and imaging of individual gold nanodecahedra: A combined optical microscopy, cathodoluminescence, and electron energy-loss spectroscopy study. Nano Lett. 2012, 12, 4172–4180. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, Z.; Dijksman, A.; Javaux, C.; Bassoul, P.; Baudrion, A.; Plain, J.; Dubertret, B.; Kociak, M. Cathodoluminescence in a scanning transmission electron microscope: A nanometer-scale counterpart of photoluminescence for the study of II–VI quantum dots. J. Phys. Chem. Lett. 2013, 4, 4090–4094. [Google Scholar] [CrossRef]
- Li, F.; Gu, W.; Wang, H.; Qi, Y.; Deng, Y.; Xiao, N.; Liu, Y.; Xu, Q.; Ye, L. Effect of surface functionalities on relaxometric properties of MR contrast agents based on NaGdF4 nanoparticles. RSC Adv. 2013, 3, 5386–5392. [Google Scholar] [CrossRef]
- Chen, G.; Ohulchanskyy, T.Y.; Liu, S.; Law, W.C.; Wu, F.; Swihart, M.T.; Argen, H.; Prasad, P.N. Core/Shell NaGdF4:Nd3+/NaGdF4 nanocrystals with efficient near-infrared to near-infrared downconversion photoluminescence for bioimaging applications. ACS Nano 2012, 6, 2969–2977. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, X.; Peng, J.; Li, F. Core-Shell lanthanide upconversion nanophosphors as four-modal probes for tumor angiogenesis imaging. ACS Nano 2013, 7, 11290–11300. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Li, Z.; Guthrey, H.; Moseley, J.; Yang, Y.; Wozny, S.; Moutinho, H.; To, B.; Berry, J.J.; Gorman, B.; et al. Mechanisms of electron-beam-induced damage in perovskite thin films revealed by cathodoluminescence spectroscopy. J. Phys. Chem. C 2015, 119, 26904–26911. [Google Scholar] [CrossRef]
- Meyer, J.C.; Eder, F.; Kurasch, S.; Skakalova, V.; Kotakoski, J.; Park, H.J.; Roth, S.; Chuvilin, A.; Eyhusen, S.; Benner, G.; et al. Accurate measurement of electron beam induced displacement cross sections for single-layer graphene. Phys. Rev. Lett. 2012, 108, 196102. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, B.; Kempson, I.; Liu, C.; Hou, Y.; Gao, M. Restructuring and remodeling of NaREF4 nanocrystals by electron irradiation. Small 2014, 10, 4711–4717. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thi Kim Dung, D.; Fukushima, S.; Furukawa, T.; Niioka, H.; Sannomiya, T.; Kobayashi, K.; Yukawa, H.; Baba, Y.; Hashimoto, M.; Miyake, J. Multispectral Emissions of Lanthanide-Doped Gadolinium Oxide Nanophosphors for Cathodoluminescence and Near-Infrared Upconversion/Downconversion Imaging. Nanomaterials 2016, 6, 163. https://doi.org/10.3390/nano6090163
Thi Kim Dung D, Fukushima S, Furukawa T, Niioka H, Sannomiya T, Kobayashi K, Yukawa H, Baba Y, Hashimoto M, Miyake J. Multispectral Emissions of Lanthanide-Doped Gadolinium Oxide Nanophosphors for Cathodoluminescence and Near-Infrared Upconversion/Downconversion Imaging. Nanomaterials. 2016; 6(9):163. https://doi.org/10.3390/nano6090163
Chicago/Turabian StyleThi Kim Dung, Doan, Shoichiro Fukushima, Taichi Furukawa, Hirohiko Niioka, Takumi Sannomiya, Kaori Kobayashi, Hiroshi Yukawa, Yoshinobu Baba, Mamoru Hashimoto, and Jun Miyake. 2016. "Multispectral Emissions of Lanthanide-Doped Gadolinium Oxide Nanophosphors for Cathodoluminescence and Near-Infrared Upconversion/Downconversion Imaging" Nanomaterials 6, no. 9: 163. https://doi.org/10.3390/nano6090163
APA StyleThi Kim Dung, D., Fukushima, S., Furukawa, T., Niioka, H., Sannomiya, T., Kobayashi, K., Yukawa, H., Baba, Y., Hashimoto, M., & Miyake, J. (2016). Multispectral Emissions of Lanthanide-Doped Gadolinium Oxide Nanophosphors for Cathodoluminescence and Near-Infrared Upconversion/Downconversion Imaging. Nanomaterials, 6(9), 163. https://doi.org/10.3390/nano6090163