Table 2 shows the compressive and flexural strengths of ordinary cement paste (CP) and cement paste with CNTs (CNT-CP) at 28 days and 56 days. For carbonated samples (C-CP and C-CNT-CP), the samples experienced 28 days standard curing followed by 28 days of carbonation before testing.
2.1. Effect of CNTs on Mechanical Properties and Microstructure of Cement Paste
From
Table 2, it can be seen that the use of CNTs did not improve the compressive strength of cement paste effectively. The 28-day and 56-day compressive strengths of the CNT-CP samples were 39.4 MPa and 41.5 MPa, respectively, which was higher than those of the CP samples by 2%–3% only. However, the effect of CNTs on the flexural strengths of cement paste is very significant as the 28-day flexural strength increased by 34% (i.e., increased from 5.9 MPa to 7.9 MPa) compared to that of CP samples. The 56-day flexural strength of CP and CNT-CP were 6.2 MPa and 8.1 MPa, respectively.
In order to investigate the underlying reason for this significant improvement in the flexural strength of CNT-CP, the microstructures of CNT-CP were studied by the scanning electron microscope (SEM). From the SEM images shown in
Figure 1, the function of CNTs and their mechanisms in reinforcing the cement paste matrix can be revealed.
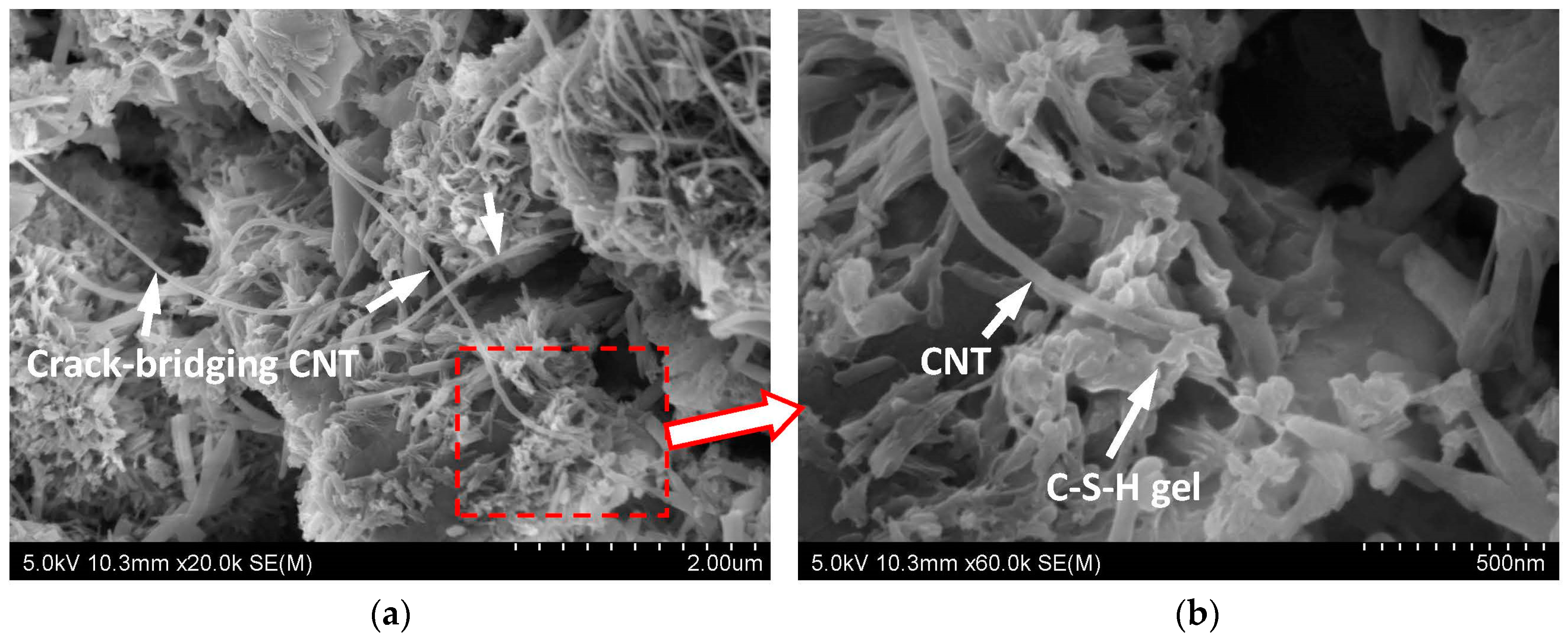
Figure 1a shows the microstructure of the unbroken sample of CNT-CP. Although there were some cracks and pores in the cement paste matrix, the presence of CNTs could span the cracks or pores and connected the parts around the pores. Apparently the primary function of CNTs in cement paste is to reinforce the matrix through crack bridging which is very similar to what has been observed in fiber-reinforced matrixes. From
Figure 1a, it can be seen that CNTs can bridge the micro-sized and nano-sized pores in cement paste. To achieve effective crack bridging by CNTs, a good bonding between the CNTs and cement paste matrix is essential and necessary.
Figure 1b is a close-up of the microstructural photograph from a part of
Figure 1a displaying the bond between CNTs and cement hydration products. From this figure, it can be clearly seen that the C-S-H gel, the main product of cement hydration, can wrap and fix the ends of CNTs firmly. As the diameter of CNTs is of nano-scale (~30 nm), CNTs can play an important role as nucleating agents for C-S-H gel, as reported in References [
1,
17,
33]. Both the crack-bridging mechanism and good bonding between CNTs and the matrix can contribute to a remarkable improvement in the flexural strength of cement paste. Moreover, it is noted that CNTs can reduce the pores in the cement paste, which leads to an increase in the compressive strength of the cement paste.
Figure 2 shows the microstructures of the broken sample after the flexural test. The mechanisms of crack-bridging by CNTs and the pulled-out CNT can be seen in
Figure 2a,b, respectively. The cement hydration product, i.e., C-S-H gel, coated along with the CNT bundles results in a denser microstructure and ultra-strong bonding between CNTs and the matrix. All the above-mentioned functions of CNTs have contributed to the significant improvement in the flexural strength of CNT-CP.
2.2. Synergy Effect of CNT and Carbonation on Mechanical Properties and Microstructure of Cement Paste
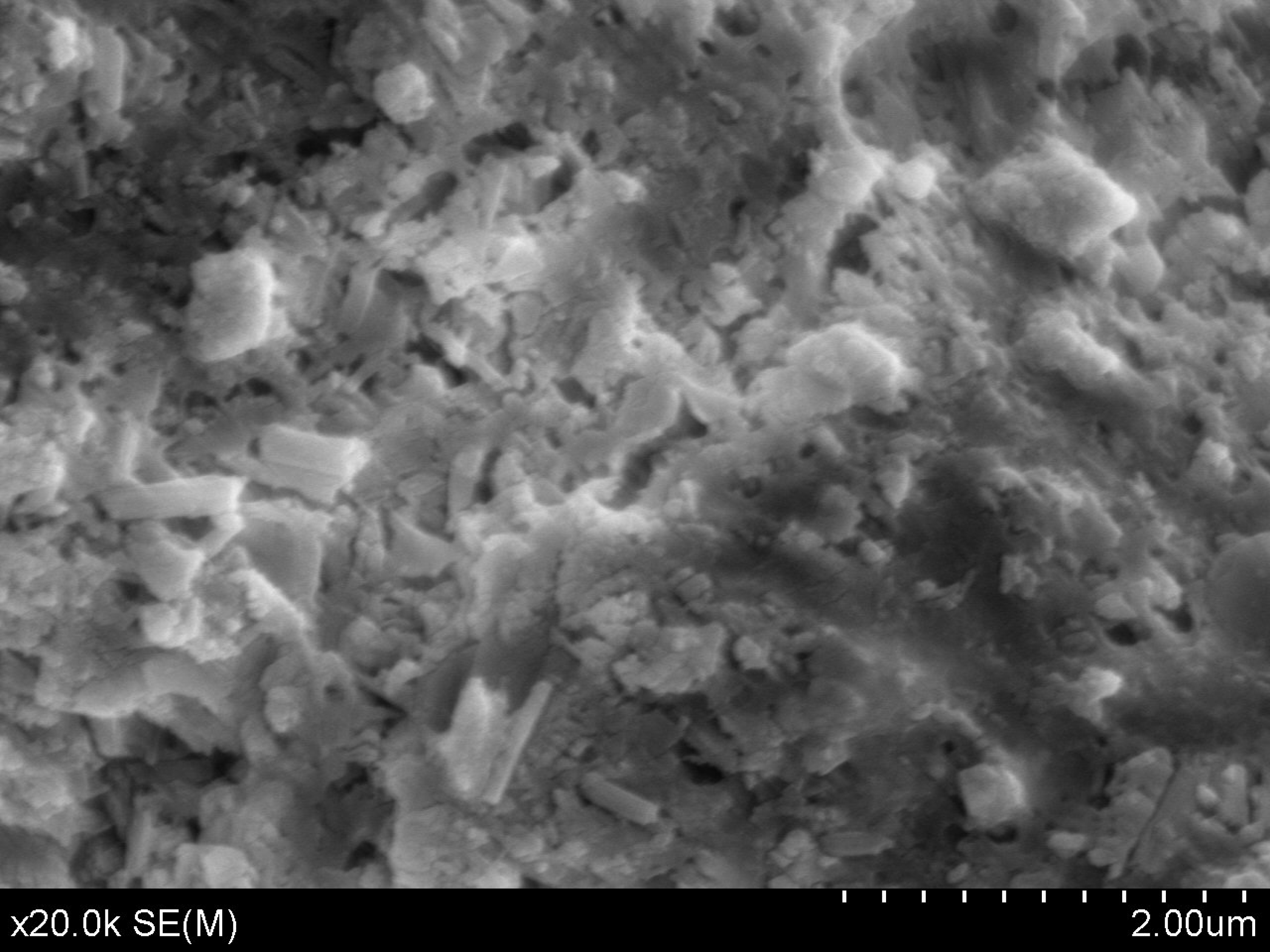
Figure 3 shows the SEM image of the microstructure of a C-CP unbroken sample. Compared with
Figure 1, it is found that the pores in the cement paste have been filled by the carbonation products. Apparently the pores become less interconnected and more tortuous. This finding coincides with that of previous studies and confirms that carbonation can refine the pores of the cement paste matrix [
3,
6]. Due to the carbonation reactions, the porosity of the matrix is lower, resulting in the increase in the compressive strength of the cement-based materials. From
Table 2, it can be seen that the compressive strength of the C-CP after carbonation curing was 44.9 MPa, which is higher than the 56-day compressive strength of CP (i.e., 40.1 MPa) by 12%. In the case of the flexural strength of the cement paste, a similar increase was observed for specimens that underwent carbonation curing, showing an increase of 10% in flexural strength. The results indicated that both the compressive and flexural strengths of cement paste can be improved due to carbonation and the effect of carbonation on the compressive strength is more significant than that on flexural strength. This is ascribed to the fact that the improvement in strength was mainly from the refinement of the pore structures and the compressive strength of the cement paste is more sensitive to the pore structure.
Comparing the strength results of C-CNT-CP with those of CP, it can be clearly seen that the synergy of carbonation and CNTs can improve both the compressive and flexural strengths significantly. Particularly in case of flexural strength, the increase was as high as 55% and the strength increased from 6.2 MPa to 9.6 MPa. As seen in
Table 2, the increase in flexural strength for CNT-CP specimens without carbonation curing was about 31% (i.e., from 6.2 MPa to 8.1 MPa). With carbonation curing, the flexural strength of C-CNT-CP was further enhanced (i.e., from 8.1 MPa to 9.6 MPa) with an additional increase of 19% compared to CNT-CP specimens. In this work, the surfactant (polycarboxylate superplasticizer) and carboxyl group (–COOH) served as crystal control agents to influence the formation of calcium carbonate crystals in carbonated cement-based materials. The significant improvement in flexural strength for C-CNT-CP specimens is mainly ascribed to their microstructural change.
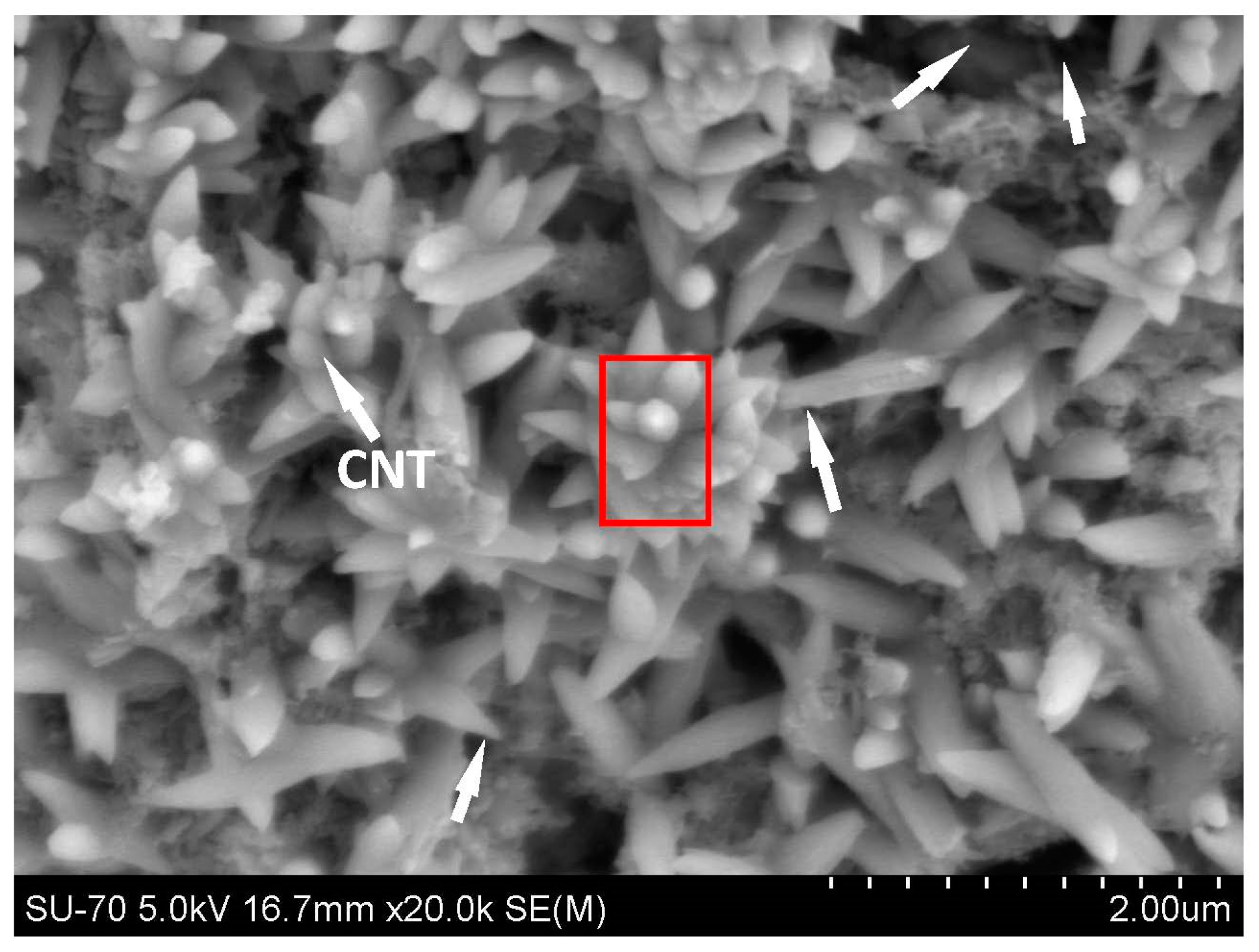
Figure 4 shows the SEM image of the C-CNT-CP unbroken sample after carbonation curing. It can be clearly shown that the microstructure of the C-CNT-CP is totally different from that of the CNT-CP as its microstructure was characterized by lots of spindle-like crystals in the cement paste. Some CNTs can be found among the spindle-like crystals (see
Figure 4). In order to confirm that the spindle-like crystals are calcium carbonate crystals, an energy-dispersive X-ray spectrometer (EDS) was employed, which was utilized to scan the area of the red box as indicated in
Figure 4 for investigating the main elements of the spindle-like crystals. Based on the main elements, the category of the spindle-like crystal can be deduced. The results of the EDS analysis are listed in
Table 3. From the results, it can be known that C (carbon), O (oxygen) and Ca (calcium) are the three main elements of the spindle-like crystals. Therefore, the main composite of the spindle-like crystals should be calcium carbonate (CaCO
3). The lower-content elements of Al (alumina), Si (silica) and S (sulphur) indicated the materials of carbonated/uncarbonated cement hydration products (see Equations (2)–(4)).
CaCO
3 has three different polymorphs: calcite, aragonite and vaterite. Calcium carbonate’s most common and stable form is the hexagonal calcite. The orthorhombic aragonite is less abundant, and the hexagonal vaterite is the least common of the three polymorphs [
34].
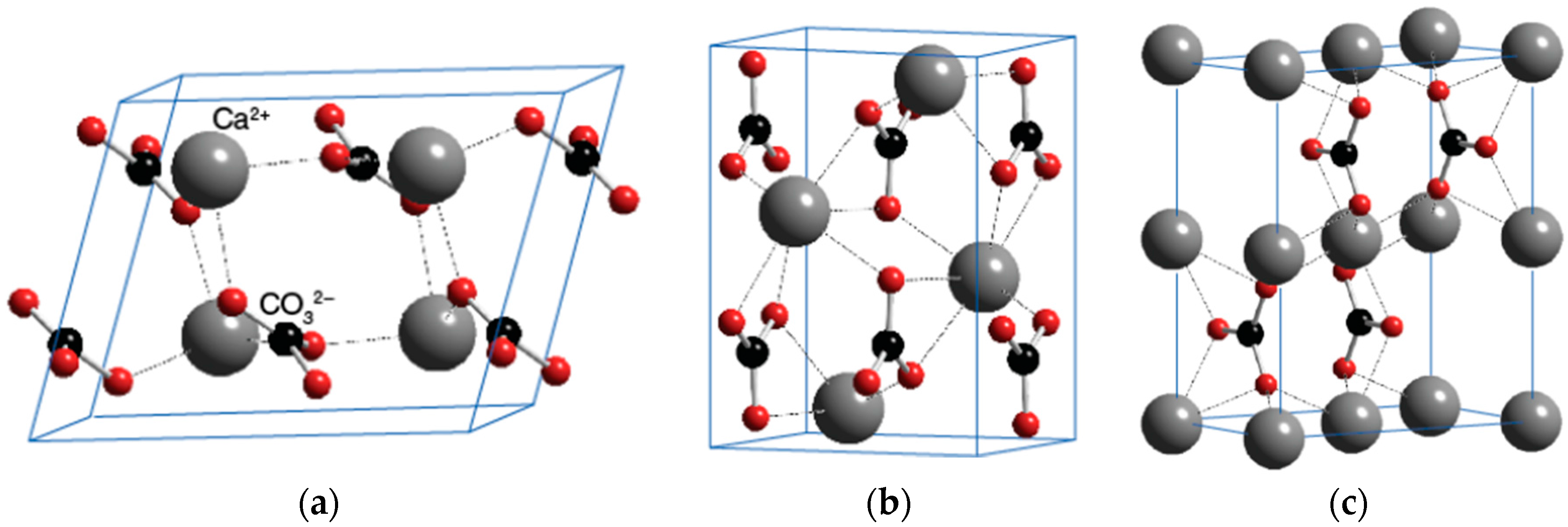
Figure 5 shows the crystal structures of the polymorphs of calcium carbonate. According to the previous studies [
35,
36], the spindle-like crystal of CaCO
3 mainly consists of calcite polymorphs.
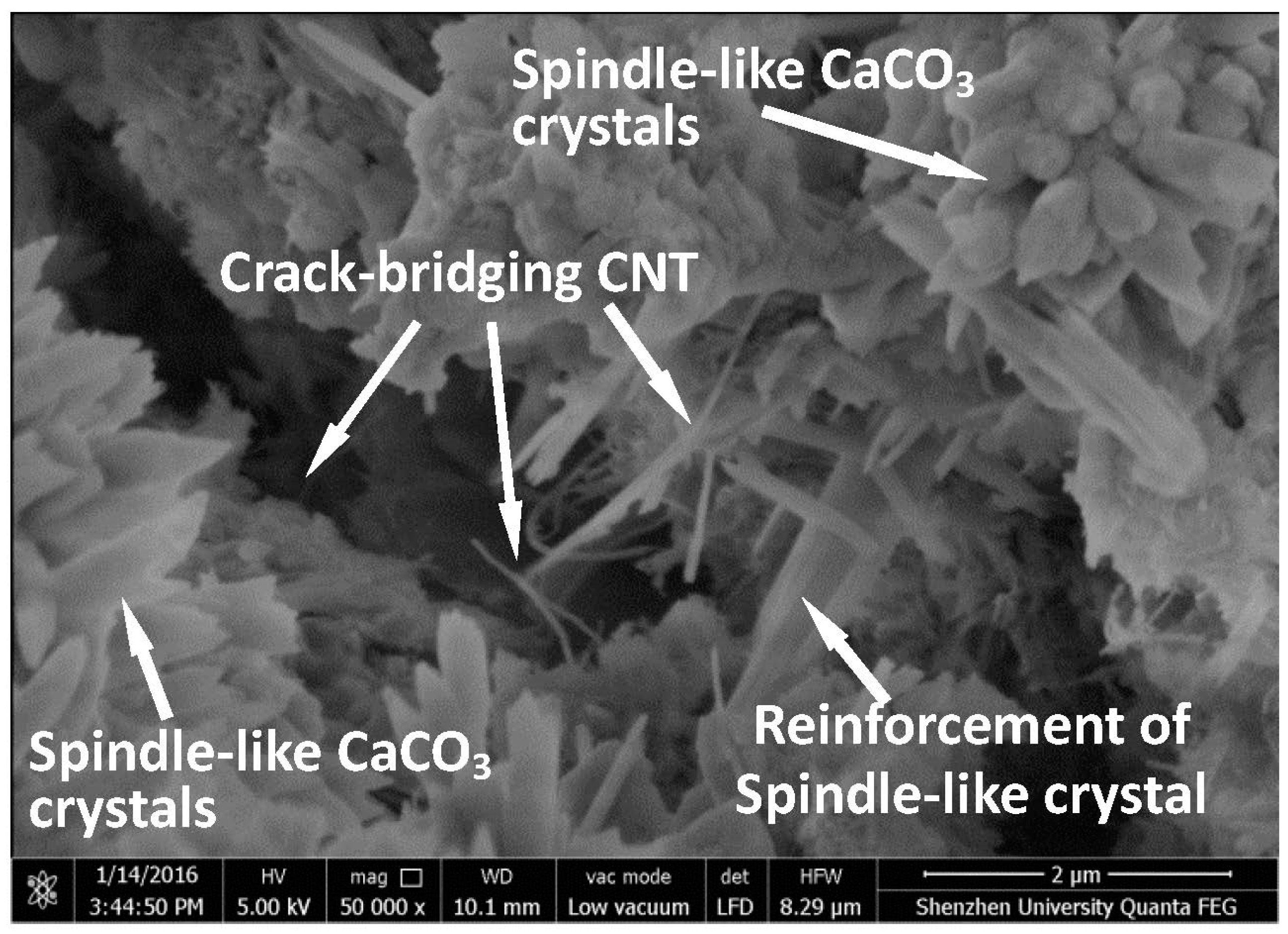
Figure 6 presents the microstructure of the broken C-CNT-CP sample after the flexural test. From this figure, the synergy effect of CNTs and spindle-like CaCO
3 crystals on the flexural strength of cement paste can be clearly seen. The CNTs, as identified earlier, played the role of crack bridging in cement paste. On the other hand, more importantly, the spindle-like CaCO
3 crystals (carbonated crystals) were found to cross the micro-cracks and reinforce the microstructure of the cement paste. The function of spindle-like CaCO
3 crystals is similar to a reinforcing steel bar in concrete. Therefore, the flexural strength of C-CNT-CP was improved dramatically.