1. Introduction
Due to the complexity of tumorigenesis and difficulty of cancer therapy, combinations of chemotherapeutic drugs with other treatment modalities, like nucleic acid, have shown great promise in cancer therapy [
1]. In the past few decades, there has been a growing scientific interest to develop functional drug/gene delivery systems derived from natural resources with good biocompatibility, controllable, and highly efficient delivery capability [
2,
3]. However, it has been difficult to find a stable drug and gene delivery system in blood circulation. The delivery of the drug and gene often show non-specific distribution in the subjects and the iatrogenic immune response to the surrounding normal tissue seriously limited the clinical application of drug/gene combination therapy [
4]. Therefore, it is necessary to design an ideal multifunctional nano-delivery system for co-delivery of the drug and gene together, overcoming the above mentioned problems.
Doxorubicin (DOX) is a potential anticancer drug that commonly used in chemotherapy for cancer treatment. However, the clinical uses of DOX are restricted largely due to limited tissue specificity and serious cardiotoxic effects resulting from the generation of free radicals and lipid peroxidation. In order to reduce the DOX toxicity, a liposomal formulation was used. However, the efficacy of DOX treatment was not enhanced greatly by liposomal delivery. Doxorubicin (DOX) is used widely to treat diverse types of cancer, yet its effectiveness is hampered by the existence of drug-resistant cancer cells [
5]; thus, it is necessary to combine gene therapy with DOX to achieve a synergistic combination therapeutic effect.
Small interfering RNAs (siRNAs) have become the main focus of the recent therapeutic applications. Hence, siRNA therapeutics have demonstrated potential as a more personalized approach in the treatment of many life threatening disease caused by faulty protein expression, such as malignancies and fibrosis [
6]. Vimentin, a major constituent of the intermediate filament (IF) family of proteins, is known to maintain cellular integrity and provide resistance against stress. The increasing of vimentin expression has been reported in diverse epithelial cancers, including breast cancer, prostate cancer, lung cancer, and other types of cancers. In our previous study, we have shown that downregulation of vimentin by Sendai F/HN viroplexes caused vimentin siRNA to inhibit the growth of hepatocellular carcinoma in vitro, which suggests again that vimentin is a potential target for gene therapy [
7]. However, for developing an effective delivery strategy, the feasibility of vimentin targeted therapy needs to be investigated.
Combination therapy with chemotherapeutics and siRNAs has been studied as an alternative way of achieving potentiated anticancer activity. Several investigators have created delivery systems based on liposomes or nanoparticles for co-delivery of anticancer chemotherapeutics and siRNAs. Some of the reported delivery system include: mesoporous silica nanoparticles for deliver Bcl-2 siRNA and DOX to effective treatment of cancer cells [
8] and cationic liposomes for co-delivery of doxorubicin (DOX) and siRNA targeting multi-drug resistance (MDR) protein to boost anticancer effect in lung cancer cells [
9]. Among the various types of delivery systems, the liposomal delivery system has attracted considerable attention. Compared with free chemical drugs, liposomes can help to prolong bioavailability time in circulation, and this help to target MDR-enhanced tumor localization [
10,
11]. Liposomes can also reduce the drug related adverse effects, such as the cardiotoxicity of DOX [
12,
13]. Over two decades, studies have been focused on the development of novel liposomal therapeutic agents that have high encapsulation efficiency and enhanced passive targeting efficiency.
In recent years, many studies have been focused on different targeting delivery systems, such as targeting systems for transferrin receptors [
14], folate receptor targeting delivery system [
15], RGD conjugating drug delivery system [
16], and asialoglycoprotein receptor-mediated hepatocyte targeting [
17]. The asialoglycoprotein receptor (ASGPR) is primarily expressed on hepatocytes and minimally on extrahepatic cells. At this point, to induce liver cancer-specific gene delivery, galactose, or
N-acetylgalactosamine can use as a targeting ligand. Using this ligand for ASGPR-mediated targeting can be manufactured with a variety of therapeutic carriers as target moiety [
18,
19]. This makes it specifically attractive for receptor-mediated drug or gene delivery with minimum concerns of toxicity. ASGPR facilitates internalization by clathrin-mediated endocytosis and exhibits high affinity for carbohydrates, specifically galactose. Popular ligands for ASGPR mediated targeting are galactose-bearing glycoproteins, glycopeptides, and galactose modified polymers and lipids [
20,
21].
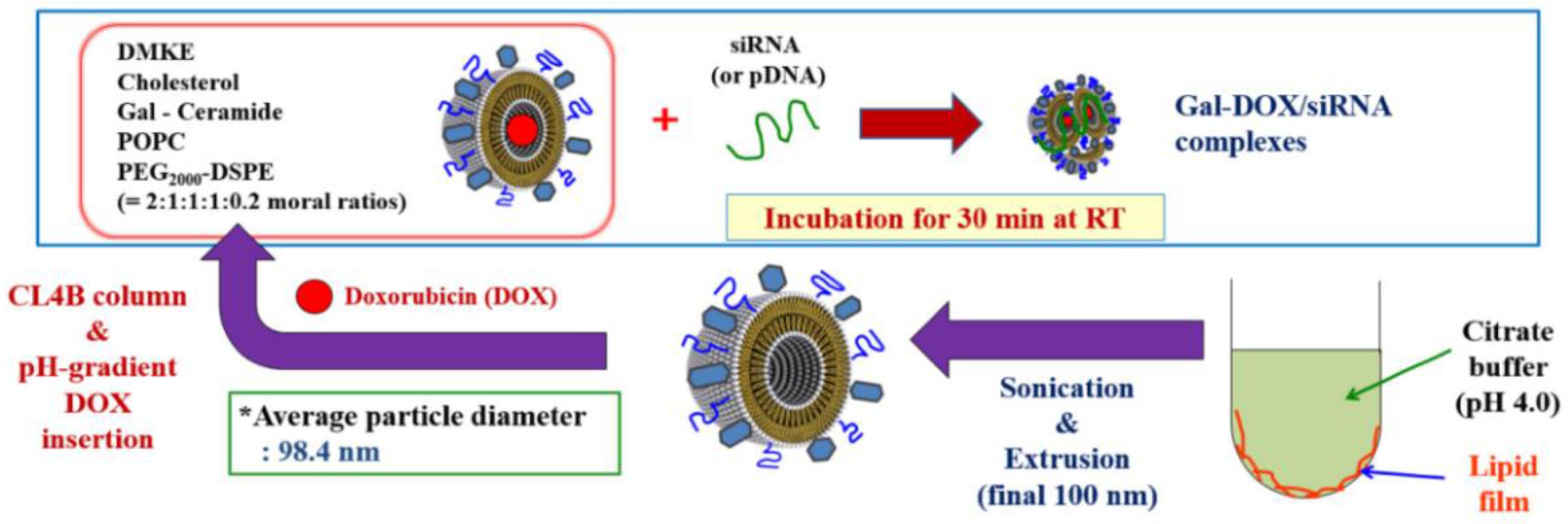
In the present study, asialoglycoprotein receptors (ASGPR) targeting-ligand-based liposomes were prepared by cationic lipid (DMKE), POPC, cholesterol, PEG
2000-DSPE, and galactosylated ceramide to co-deliver DOX and vimentin siRNA, expecting to target hepatic carcinoma sites, enhance transfection efficiency, and realize the optimal combination therapy (as shown in
Scheme 1). These liposomes have shown higher cancer cell cytotoxicity effects triggered by gal- doxorubicin/vimentin siRNA liposome (Gal-DOX/siRNA-L) than each single treatment. In addition, the cellular targeting and in vivo localization of Gal-liposomes ware also observed by fluorescence microscopy and luciferase assay, respectively. Therefore, we believe that the Gal-liposomal particles are a useful hepatic targeting system for co-delivery of drug and gene.
3. Discussion
Vimentin, one of the intermediate filament family proteins, is universally expressed in normal mesenchymal cells to maintain cellular unity. Increasing of vimentin expression in various epithelial cancers has been reported that vimentin in cancer correlates with increased tumor growth, invasion, and poor prognosis [
23,
24]. For that reason of its over-expression in cancers and its role in mediating tumorigenic events, vimentin provide as an available targeted cancer therapy [
25]. Despite these well-characterized correlations, vimentin has not been sufficiently investigated as a new therapeutic target for siRNA. In particular, there are no published studies showing the co-delivery of vimentin siRNA in combination with chemotherapy in vivo.
To our knowledge, our present studies is the first one demonstrating that inhibition of vimentin protein expression by vimentin-specific siRNA could be increasing an induction of apoptosis in tumors together with DOX, and also significantly suppress tumors growth in vivo. In results of co-delivery of DOX and vimentin siRNA by galactosylated liposome, we have showed that it is feasible to use a combined-delivery system to deliver nucleic acid and drug both in vitro and in vivo.
Ligand-mediated targeting for drug delivery systems is one of the most exciting areas because it can improve site-specific drug delivery. Many attempts have been made to deliver drugs specifically to liver cells for effective treatment of liver diseases. Among the various receptors on parenchymal and nonparenchymal cells, the ASGPR is the most promising receptor site because of its high affinity and rapid internalization [
26]. Targeting ASGPR is an attractive strategy for liver-specific delivery due to its exclusive expression by parenchymal hepatocytes [
27]. In order to achieve highly effective hepatic tumor-targeting delivery systems for gene and chemotherapeutic agents, Gal-liposome containing a galactosylated lipid (Gal-ceramide) and a cationic lipid (DMKE) was designed and evaluated for targeting and delivery efficiency.
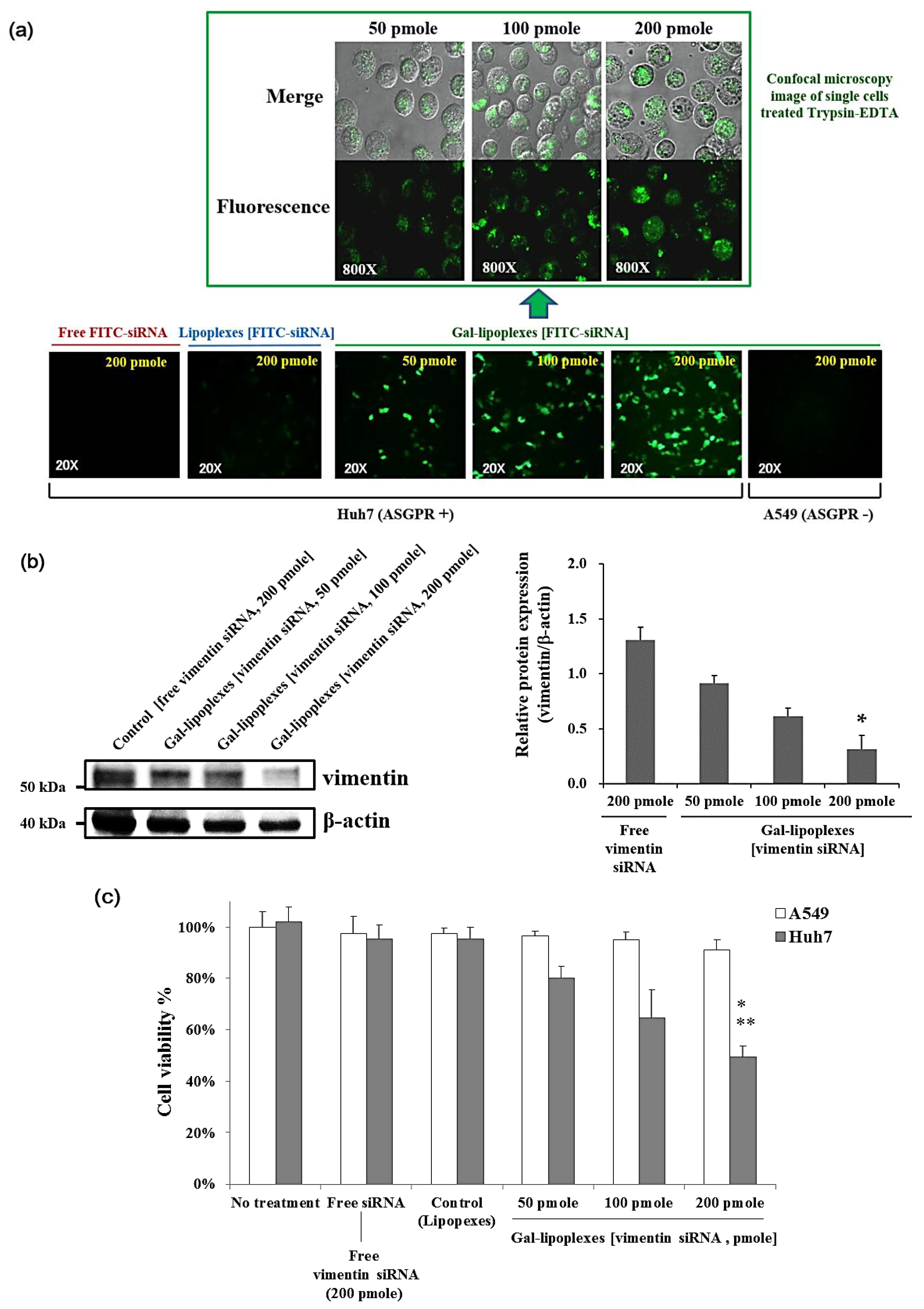
In this study, the hypothesis that the ASGPR targeted Gal-siRNA-L shows better suppression efficacy than free siRNA can be evidenced by the anti-cancer effects and cell uptake effects between these experimental conditions were similar in vitro (
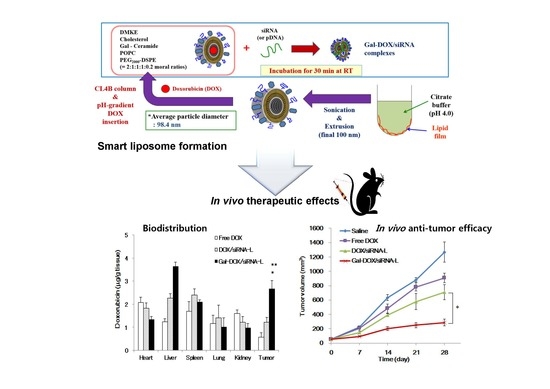
Figure 4). The cytotoxicity of different DOX formations was also studied by MTT assay, which measures mitochondrial function or integrity. DOX proved to be an excellent compound for demonstration of targeted drug delivery. The fluorescence properties of DOX allow for quantitative measurement of biodistribution both by determining the DOX concentration in tissues by its fluorescence intensity at 495 nm measured using fluorescence spectrophotometer (
Figure 6a). The biodistribution study results suggest that Gal-DOX/siRNA-L allows higher DOX concentrations to be reached in both normal liver and hepatic tumor tissue than do DOX/siRNA-L (non-targeted liposomes) and free DOX. We also observed in our study that both the Gal-DOX/siRNA-L and DOX/siRNA-L liposomes concentrations were much higher in livers. This result is consistent with previously published studies showing that the cationic liposomes are preferentially uptaken by the liver. The first possible reason is that the macrophages or Kupffer cells in the liver facilitated the uptake, and consequently stayed the liposomes in hepatocytes [
28]. The second possible cause is that the extensive blood vessel (including capillary) in the liver facilitated the uptake of liposomes by the hepatocytes. This phenomenon may enhance that this delivery system is possibly appropriate for the treatment of hepatocarcinoma. On the other hand, in the heart, Gal-DOX/siRNA-L showed a lower drug concentration than DOX/siRNA-L and free DOX. This result suggests that the galactosylated liposome has selective hepatic tumor targeting.
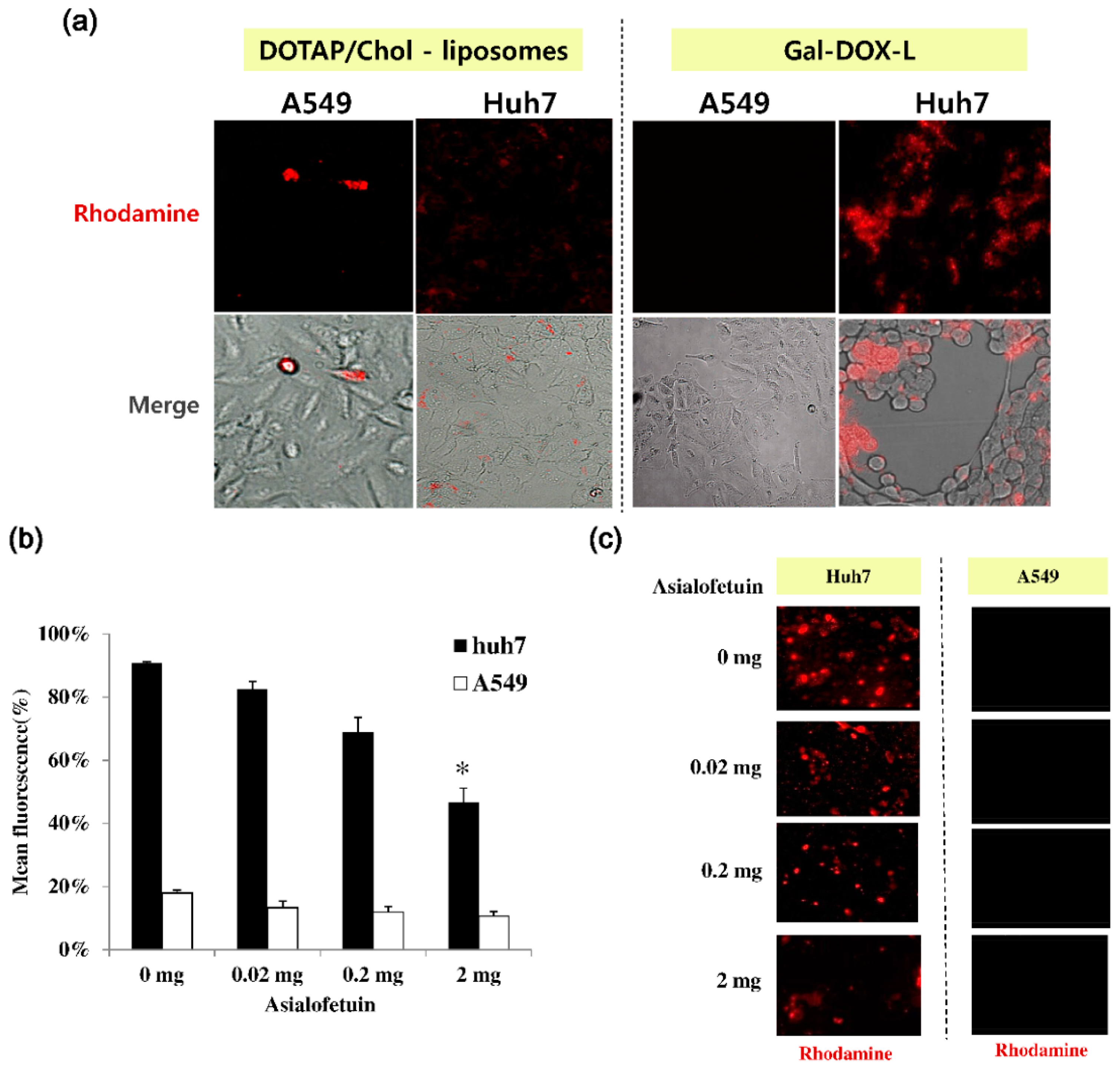
For targeted nano-liposomes, a linked targeting ligand can significantly improve the therapeutic efficacy of chemotherapy or molecular therapy in cancer. Our data showed that the Gal-DOX/siRNA-L could efficiently bind to Huh7 cells overexpressing ASGPR and increase the uptake of DOX and vimentin siRNA, suggesting the effectiveness of ASGPR targeting. On the other hand, the targeting activity of a galactose-conjugated liposome is highly dependent on the asialoglycoprotein receptors (ASGPR), which may limit the application of ASGPR targeting liposome delivery. Importantly, our data demonstrated the rational of co-delivery of DOX and vimentin siRNA since the selection of chemotherapeutics or gene drugs is important for combined therapy. Both in vitro and in vivo results illustrated that these two drugs enhanced effects. Consistent with its higher accumulation in tumor tissue, Gal-DOX/siRNA-L had stronger tumor growth inhibition activity than DOX/siRNA-L and free DOX (
Figure 6b), which may be explained by the following: galactosylation of the liposomes enhanced cellular uptake and internalization of DOX/siRNA into hepatoma cells via ASGPR-mediated pathways; PEGylation of liposomes increased accumulation of DOX in tumor tissue by prolonging the circulation time and reducing uptake by the RES; the small particle size of Gal-DOX/siRNA-L enables easier penetration of the tumor via the enhanced permeation and retention effect.
Insufficient therapeutic efficacy due to low cancer target approaching, and the induction of side effects by non-selectivity into normal cells and the over-dose use of chemotherapeutic agents by drug-resistance still act as a variety of obstacles for successful cancer therapy. In order to overcome these disadvantages, it is to use the combined therapeutic method capable of inducing cancer cell killing by different mechanisms.
At this point, the synergistic or combined effects by co-delivering anti-cancer drugs and siRNAs have been verified by other studies using different delivery system or targeting genes. Chen et al. designed a CD13 targeting (NGR peptide) PEGylated LPD (liposome-polycation-DNA) nanoparticle (LPD-PEG-NGR) to deliver both doxorubicin and c-MYC siRNA, and they reported the enhanced cancer therapeutic effects in tumor mouse model [
29]. Some research groups also applied novel material to deliver chemotherapeutic drugs and siRNA, such as nanostructured lipid carriers (NLCs) [
30,
31]. When these delivery systems were used for co-delivery anti-cancer drugs and siRNA, enhanced anti-cancer effects were achieved as the liposome based drug delivery system dose. Even though our combination system was not examined in drug-resistant cancer cells to demonstrate the superiority of more exact therapeutic effects, we delineate that the combination strategy of gene therapy and chemotherapy elicits increased anti-cancer effects. These studies, together with our study, collectively suggest that the combined effect by co-delivering chemotherapeutic drugs and siRNA to cancer cells is a universal phenomenon and holds promise for future cancer treatments. Our next goal will be focused on the therapeutic effects by combination treatment in drug-resistant cancer cells.
In summary, a novel galactosylated liposomal vehicle modified with galactosylated-ceramide and DMKE lipids was developed for co-delivering chemotherapeutic drugs and siRNA to hepatoma cells. Therefore, this novel galactosylated liposomal formulation is prospective as a targeted carrier for anti-cancer drugs against hepatic tumor and preliminary investigation.
4. Materials and Methods
4.1. Materials
d-Galactosyl ceramide, PEG-DSPE (1,2-distearoyl-
sn-glycero-3-phosphoethanolamine (ammonium salt)), cholesterol, POPC (1-palmitoyl-2-oleoyl-
sn-glycero-3-phosphocholine), rhodamine-conjugated DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine), and DOTAP (dioleoyl-3-trimethylammonium-propane) were purchase from Avanti Polar Lipid, Inc. (Alabaster, AL, USA). The cationic lipid DMKE (
O,
O’-dimyristyl-
N-lysyl glutamate) was synthesized as previously reported [
32]. EX-CyTox that is cell viability and cytotoxicity test reagent is Daeil Lab Service Co., Ltd (Seoul, South Korea). Doxorubicin and CL-4B (agarose beads) were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). FITC-labeled siRNA was purchased from Invitrogen
™ Life Technologies (Grand Island, NY, USA). siRNA against vimentin was obtained from Santa Cruz biotechnology (Dallas, TX, USA).
4.2. Preparation of Galatosylated Liposomes
Cationic liposomes were prepared as described previously with a slight modification [
33]. Briefly, to prepare the galactosylated cationic liposome, DMKE, cholesterol, β-
d-galactosyl ceramide, POPC, and PEG
2000-DSPE were mixed in a molar ratio of 2:1:1:1:0.2 in chloroform:methanol (2:1, v/v). The chloroform and methanol were evaporated under a stream of N
2 gas, and vacuum desiccated for a minimum of 1 h to ensure removal of residual organic solvent. The dried lipid films (1 mg total lipids) were hydrated with 1 mL of citrate buffer (20 mM citiric acid (pH 4.0) and 150 mM NaCl) with a vortex mixer for 5 min. The hydrated lipids (1.0 mg/mL) were sonicated with a bath-type sonicator (Branson Inc. Danburry, CT, USA) three times for 30 s with a 10-s interval, which produced small unilamellar liposomal vesicles. The liposome solution was then extruded through 800-, 400-, 200-, and 100-nm pore size polycarbonate membrane (Merck Millipore, Billerica, MA, USA) to serially decrease the extrusion size.
4.3. Preparation of DOX-Encapsulating Galactosylated Liposomes (Gal-DOX-L) and Determination of DOX Loading Efficiecy
To encapsulate DOX into the liposomes, the pH gradient-driven remote loading method was employed. Briefly, galactosylated liposomes were prepared using the same method described above. The buffer solution containing the liposomes was then exchanged with HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer (pH 7.4) by CL4B column chromatography. To introduce DOX into the liposomes, one milliliter of the liposomal solution (1 mg/mL) was incubated with 150 μL of DOX (2 mg/mL) for 10 min at 60 °C via the difference in pH between the inside and the outside of the liposomes. The concentration of DOX in the Gal-DOX-L calculated by measuring the absorbance at 492 nm and using a standardization curve generated for DOX quantification. For determination of DOX loading efficiency in galactosylated liposome, quantification of DOX within Gal-DOX-L was determined by measuring fluorescence excitation and emission using a plate reader (iMark™ Microplate Absorbance Reader, Bio-Rad) set at 480 and 580 nm after incubation in 1% Triton X-100 solution for 45 min.
4.4. Preparation of Gal-DOX/siRNA-L Complexes
Gal-DOX/siRNA-L complexes were prepared by the charge interaction with different nitrogen to phosphate (N/P) ratios (1, 3, 6, and 9). Briefly, the Gal-DOX/siRNA-L complexes were prepared at an N/P (liposome/siRNA) ratio of 9:1 in RNase free H2O by adding stock solution of Gal-DOX-L into a prepared 5 nmol of siRNA solution. After the samples were gently vortexed, and the solutions were incubated for 30 min at room temperature to facilitate efficient incorporation of siRNA. The loading efficiency of siRNA by Gal-DOX-L was determined by the fluorescence of FITC-labeled siRNA (or ethidium bromide (EB) dye) displacement assay in the agarose gel. Unconjugated siRNA will migrate in the gels, but the complexed siRNA will be stay in the loading wells with liposomes because of the neutral charge of lipoplexes.
4.5. Physicochemical Characterization
The size and zeta-potentials of the liposomes (Gal-DOX-L) and lipoplexes (Gal-DOX/siRNA-L) were measured using a Zetasizer (Nano ZS, Malven, UK). The lipoplexes were prepared by mixing different N/P ratios of cationic liposomes and siRNA. All of the measurements were carried out at 25 °C. Each parameter was checked three times and means and standard deviations were calculated.
Agarose gel retardation studies were used to evaluate the siRNA loading in liposomes. The samples containing 200 nM of FITC-siRNA, with varying N/P ratios in nuclease free water, were applied to a 2% (w/v) agarose gel in TAE buffer. The free siRNA or unconjugated siRNA will migrate in the gels but the complexed siRNA will be stuck in the loading wells with liposomes. The encapsulation efficiency (EE) of siRNA in Gal-DOX/siRNA-L complexes were determined the integrity of the preserved Gal-DOX/siRNA-L compared to free siRNA. The fluorescence intensities measured uncomplexed free FITC-labeled siRNA at varying N/P ratios in the gel by FUSION-SL chemiluminescence analyzer and software (VILBER, Suarlée, Belgium).
4.6. Cell Culture and Assay for Cell Binding of Gal-DOX-L
Huh7 cells, a human hepatoma cell line, and A549 cells, a human lung cancer epithelial cell line, were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 100 U of penicillin, 100 mg of streptomycin, and 10% fetal bovine serum (FBS). To monitor the cell binding of Gal-DOX-L under a fluorescence microscopy, Huh7 cells and A549 cells (1 × 105 cells, each) were seeded into 24-well plates (SPL Life Sciences, Seoul, Korea) in DMEM supplemented with 10% FBS at 37 °C under 5% CO2 for 24 h. After 24 h of incubation, the cells were treated with fresh medium (500 μL) containing DOTAP/cholesterol-liposomes (as a control) or Gal-DOX-Ls (Galactose linked-cationic liposomal doxorubicin) for 15 min in culture media. After the treated cells were washed three times with PBS (phosphate-buffered saline), fresh media was added. These liposomes (containing an amount equivalent to 0.2 μM DOX, each) having the rhodamine-conjugated DOPE lipids (red color) were observed on the cell surface by a fluorescence microscope (JuLi-Smart Fluorescence Cell Imager, NanoEnTek Inc., Seoul, Korea).
4.7. In Vitro Cytotoxicity and Cell Uptake Studies
The in vitro cytotoxicity of DOX- or/and vimentin siRNA-loaded liposomes was evaluated by MTT assay method using EZ-CyTox reagents after 48 h. Briefly, A549 cells (5.0 × 103 cells/100 μL per well) and Huh7 cells (5.0 × 103 cells/100 μL per well) were seeded into 96-well plates in DMEM medium supplemented with 10% v/v fetal bovine serum and with 100 IU of penicillin, 100 μg/mL of streptomycin. Cells were incubated with 100 μL of free DOX (0.2 μM), free vimentin siRNA (200 pmole), Gal-DOX-L [DOX, 0.2 μM], Gal-Lipoplexes [vimentin siRNA, 200 pmole], or Gal-DOX/siRNA-L (containing with DOX concentration of 0.2 μM and vimentin siRNA concentration of 200 pmole) for 2 h at 37 °C. As a control liposome, cells were also treated with liposomal doxorubicin (DOX-L [0.2 μM]) lacking of galactose or lipoplexes lacking of galactose (vimentin siRNA, 200 pmole) under the same conditions. After incubation, the cells were washed three times with PBS and cultured for additional 48 h in 200 μL of fresh medium. After 48 h post-transfection, 10 μL of the Ez-cytox reagent (5 mg/mL) was added to each well, followed by incubation for 4 h at 37 °C. The absorbance was measured at 450 nm using a VersaMax ELISA (enzyme-linked immunosorbent assay) microplate reader (Molecular Devices, Sunnyvale, CA, USA).
The cell uptake for siRNA transfection of galatosylated-cationic liposome devoid of DOX was evaluated in vitro on Huh7 cells expressing asialoglycoprotein surface receptors in comparison to free siRNA. Huh7 cells were treated with galactose conjugated cationic lipoplexes with different FITC-labeled siRNA concentration formations of N/P ratio = 9 for 2 h at 37 °C. Next, to determine the transfection efficacy in the effectiveness of siRNA delivery, the treated cells were observed using a general fluorescence microscope. To evaluate the cellular uptake efficiency of FITC-siRNA, the transfected cells were washed with PBS, and treated with Trypsin-EDTA (0.25%) at 37 °C for 5 min. Then, the harvested cells after wash with PBS were observed FITC-siRNA in cells using a confocal laser scanning microscope (FV-1000, Olympus, Tokyo, Japan).
4.8. Western Blot Analysis
Western blot analyses for the expression of vimentin were performed using standard methods. In brief, Huh7 cell treated vimentin siRNA pallets were lysed in RIPA (Radioimmunoprecipitation assay) lysis and extraction buffer (Thermo Fisher Scientific, Waltham, MA, USA). The cell lysate was centrifuged at 10,000× g for 10 min at 4 °C and then the supernatant was transferred to a fresh 1.5 mL microcentrifuge tube. Protein concentration was determined by the Protein Assay Kit (Pierce Biotechnology, Inc., Rockford, IL, USA) and 20 μg protein samples were loaded per lane of SDS-PAGE gel. These proteins were separated by 10% SDS-PAGE and electrotransferred onto a polyvinylidene difluoride membrane (Merck-Millipore, Billerica, MA, USA). The membrane was blocked with 5% non-fat milk in Tris-buffered saline with Tween 20 (TBST) buffer (20 mM Tris, 150 mM NaCl and 0.1% Tween-20 (pH 7.5)) for 2 h and incubated with the primary antibodies overnight at 4 °C. The dilution of vimentin antibody (mouse monoclonal; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and β-actin antibody (mouse monoclonal; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was 1:1000. After the treatment of primary antibodies, the membranes were washed three times with TBST and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody goat anti-mouse IgG (1:5000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for 1.5 h. The bands were visualized using an ECL Plus Western Reagent (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The FUSION-SL chemiluminescence analyzer system (VILBER, Suarlée, Belgium) was used to capture images of the blots. The intensity of each band was quantified by densitometry and normalized to that controlled β-actin.
4.9. Tissue Distribution and Anti-Tumor Efficacy Study in Vivo
Biodistribution studies were performed on healthy male Balb/c athymic nude mice (eight-weeks of age, 16–18 g) which were purchased from Orient Bio Inc. (Seongnam, Korea). The mice were retained in conformity with the National Institute of Toxicological Research of the Korea Food and Drug Administration guidelines as well as the regulations for the care and use of laboratory animals of the Animal Ethics Committee at Konyang University (No. 0356). All of the animal studies were conducted under protocols approved by the Committee on Use and Care of Animals at Konyang University, Korea. For the tissue distribution assay, Huh7 human hepatoma cells were implanted by subcutaneous inoculation of 3.0 × 10
6 Huh7 cells into the left flank of male athymic nude mice. At the tumor volume of 400 mm
3, randomly dividing the tumor-bearing mice into various three groups (four per group) were injected with free drug (DOX), DOX/siRNA-L, or Gal-DOX/siRNA-L at a single dose of 150 μg/kg vimentin siRNA and/or 5 mg/kg DOX via the tail vein. The mice were sacrificed, and then the tumor and major organs (liver, kidneys, spleen, lungs, and heart) were collected at 4 h after drug administration. Then these tissues were washed using a cool PBS and excess fluid was removed. To measure concentration of DOX content in the tissues, each tissue was determined as described in the literature [
34]. In briefly, 1.5 mL nuclear lysis buffer (10 mM HEPES, 1 mM MgSO
4, 1 mM CaCl
2, pH 7.4) was added to the 0.2–0.3 g piece of tissue samples and then the tissues were homogenized using a Tissue-Tearor homogenizer (BioSpec Products Inc, Bartlesville, OK, USA). The homogenate (100 μL) was treated with 50 μL of 10% (v/v) Triton X-100, 100 mL of DW, and 750 μL acidified isopropanol. The mixture was placed overnight at ‒20 °C, and centrifuged immediately at 14,000 rpm for 10 minutes after thawing. DOX in the supernatant was measured by fluorescence spectrophotometer at fluorescence intensity at 495 nm (Hitachi F-2700, software FL solution, Tokyo, Japan).
For the antitumor activity study, 3.0 × 106 Huh7 cells were injected in the left flank of male athymic nude mice. At the tumor volume of ~55 mm3, liposomes or lipoplexes (DOX/siRNA-L, Gal-DOX-L, Gal-Lipoplexes [siRNA], or Gal-DOX/siRNA-L), free drug, or saline was administrated by a single dose tail vein injection (150 μg/kg vimentin siRNA and/or 5 mg/kg DOX) every seven days. The tumor volumes were calipers and calculated using the following formula: (width)2 × length/2.
4.10. Statistical Analysis
All results are expressed as the mean ± standard deviation (SD) of at least three experiments. Significant differences were determined by t-test using SPSS Statistics Premium 21 software (IBM, Armonk, NY, USA). A p-value of <0.01 and <0.05 were considered to represent a statistically significant difference.