Nano-Biosensor for Monitoring the Neural Differentiation of Stem Cells
Abstract
:1. Introduction
2. Role of Nanotechnology in Developing Biosensors
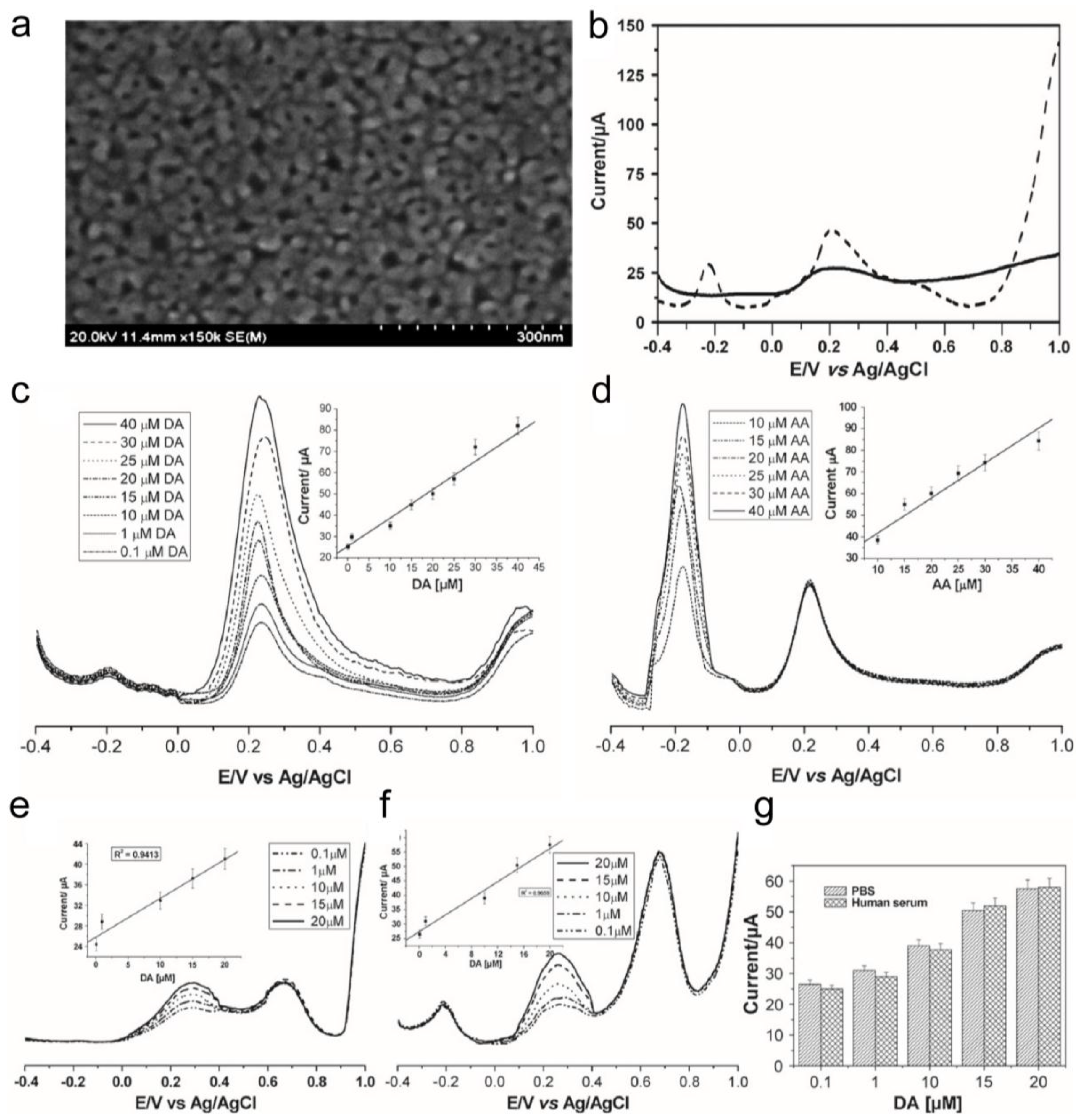
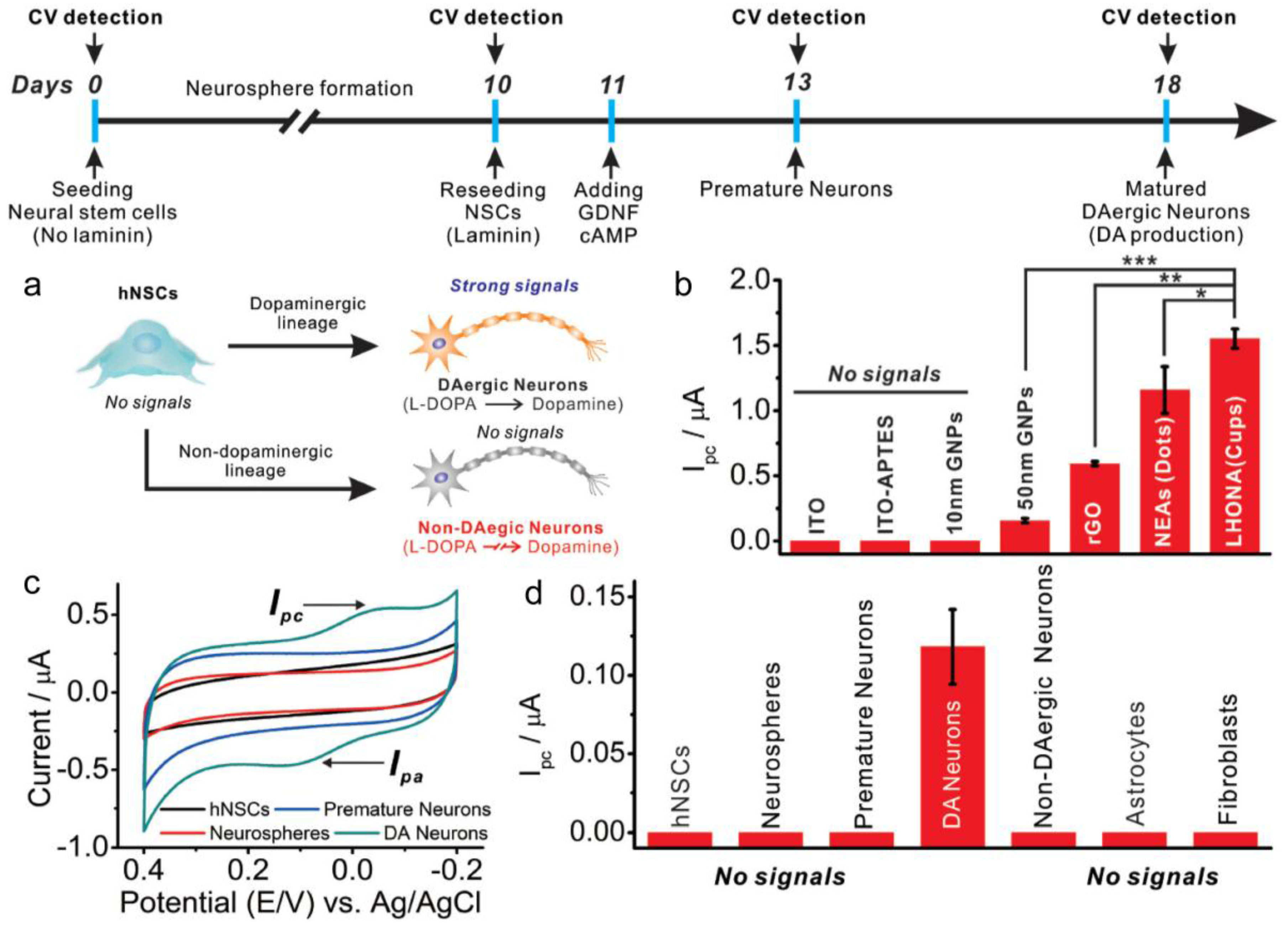
3. Electrochemical and Electrical Detection System to Monitor Stem Cell Differentiation
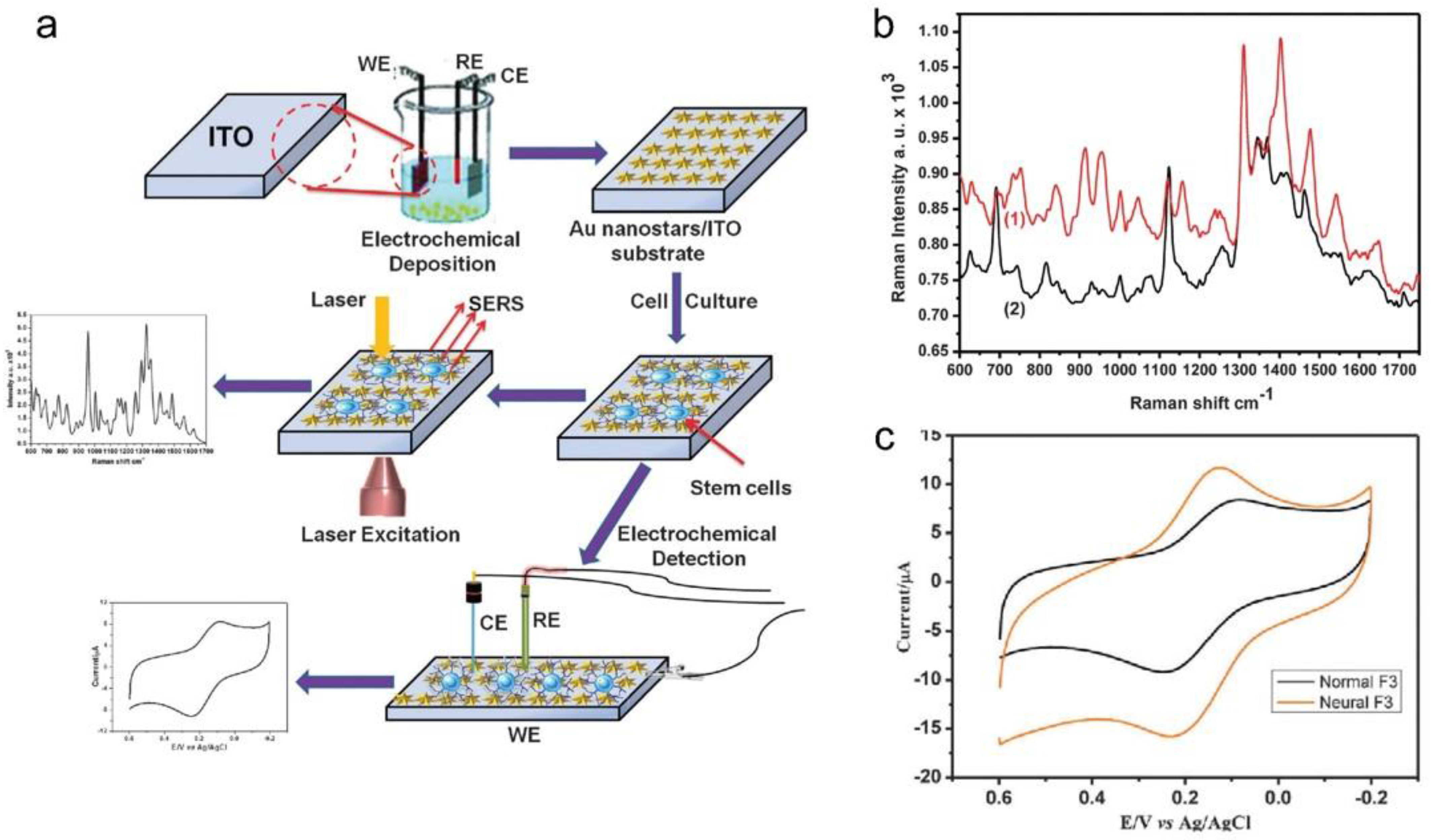
4. Optical Detection System to Monitor Stem Cell Differentiation
5. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jopling, C.; Boue, S.; Belmonte, J.C.I. Dedifferentiation, transdifferentiation and reprogramming: Three routes to regeneration. Nat. Rev. Mol. Cell Biol. 2011, 12, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Maroof, A.M.; Keros, S.; Tyson, J.A.; Ying, S.W.; Ganat, Y.M.; Merkle, F.T.; Liu, B.; Goulburn, A.; Stanley, E.G.; Elefanty, A.G.; et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 2013, 12, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Weissman, I.L. Translating stem and progenitor cell biology to the clinic: Barriers and opportunities. Science 2000, 287, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Shah, S.; Yang, L.; Yin, P.T.; Hossain, M.K.; Conley, B.; Choi, J.-W.; Lee, K.-B. Controlling differentiation of adipose-derived stem cells using combinatorial graphene hybrid-pattern arrays. ACS Nano 2015, 9, 3780–3790. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Chueng, S.T.D.; Yang, L.; Zhang, Y.; Lee, K.B. Multidimensional nanomaterials for the control of stem cell fate. Nano Converg. 2016, 3, 23. [Google Scholar] [CrossRef]
- Daley, G.Q.; Scadden, D.T. Prospects for stem cell-based therapy. Cell 2008, 132, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kurtz, A.; Stamm, C. Mesenchymal stem cells for cardiac cell therapy. Hum. Gene Ther. 2011, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Nadig, R.R. Stem cell therapy-Hype or hope? A review. J. Conserv. Dent. 2009, 12, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Knoepfler, P.S. Deconstructing stem cell tumorigenicity: A roadmap to safe regenerative medicine. Stem Cells 2009, 27, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Fujita, R.; Abeliovich, A. Remodeling neurodegeneration: Somatic cell reprogramming-based models of adult neurological disorders. Neuron 2013, 78, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Wernig, M.; Zhao, J.P.; Pruszak, J.; Hedlund, E.; Fu, D.D.; Soldner, F.; Broccoli, V.; Constantine-Paton, M.; Isacson, O.; Jaenisch, R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2008, 105, 5856–5861. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z.; Martinez-Serrano, A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat. Med. 2004, 10, S42–S50. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E.M.; Lamanna, J.; Boulis, N.M. Stem cell therapy for the spinal cord. Stem Cell Res. Ther. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Benraiss, A.; Goldman, S.A. Cellular therapy and induced neuronal replacement for Huntington’s disease. Neurotherapeutics 2011, 8, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Ganat, Y.M.; Calder, E.L.; Kriks, S.; Nelander, J.; Tu, E.Y.; Jia, F.; Battista, D.; Harrison, N.; Parmar, M.; Tomishima, M.J.; et al. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J. Clin. Investig. 2012, 122, 2928–2939. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Kim, I.G.; Lee, J.Y.; Hong, S.H.; Kim, S.W.; Hwang, T.K.; Oh, S.H.; Lee, J.H.; Ra, J.C.; Lee, J.Y. Therapeutic effect of adipose-derived stem cells and BDNF-immobilized PLGA membrane in a rat model of cavernous nerve injury. J. Sex. Med. 2012, 9, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, S.; Ma, K.; Fu, X.; Han, W.; Sheng, Z. Promising new potential for mesenchymal stem cells derived from human umbilical cord Wharton’s jelly: Sweat gland cell-like differentiative capacity. J. Tissue Eng. Regen. Med. 2012, 6, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Miao, Y.; He, N.; Wu, X.; Li, S. Nanotechnology and biosensors. Biotechnol. Adv. 2004, 22, 505–518. [Google Scholar]
- Holzinger, M.; Goff, A.L.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Oh, B.-K.; Choi, J.-W. Development of a HIV-1 virus detection system based on nanotechnology. Sensors 2015, 15, 9915–9927. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, B.C.; Oh, B.-K.; Choi, J.-W. Rapid and sensitive determination of HIV-1 virus based on surface-enhanced Raman spectroscopy. J. Biomed. Nanotechnol. 2015, 12, 2223–2230. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, K.-B.; Choi, J.-W. 3D graphene oxide-encapsulated gold nanoparticles to detect neural stem cell differentiation. Biomaterials 2013, 34, 8660–8670. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors-Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Alam, M. Dimensionally frustrated diffusion towards fractal adsorbers. Phys. Rev. Lett. 2007, 99, 256101–256104. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, B.K.; Choi, J.W. Electrochemical sensor based on direct electron transfer of HIV-1 virus at Au nanoparticle modified ITO electrode. Biosens. Bioelectron. 2013, 15, 531–535. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Lee, J.-H.; Oh, B.K.; Choi, J.W. 3-D nanoporous gold thin film for the simultaneous electrochemical determination of dopamine and ascorbic acid. Electrochem. Commun. 2010, 12, 1756–1759. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Zhang, S.C. Directed differentiation of dopamine neurons from human pluripotent stem cells. Methods Mol. Biol. 2011, 767, 411–418. [Google Scholar] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, 2nd ed.; Wiley: New York, NY, USA, 2001; p. 452. [Google Scholar]
- Schulz, T.C.; Noggle, S.A.; Palmarini, G.M.; Weiler, D.A.; Lyons, I.G.; Pensa, K.A.; Meedeniya, A.C.; Davidson, B.P.; Lambert, N.A.; Condie, B.G. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells 2004, 22, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yea, C.H.; Chueng, S.D.; Yin, P.T.; Conley, B.; Dardir, K.; Pak, Y.; Jung, G.Y.; Choi, J.W.; Lee, K.B. Large-scale nanoelectrode arrays to monitor the dopaminergic differentiation of human neural stem cells. Adv. Mater. 2015, 27, 6356–6362. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Kimko, H.C.; Cross, J.T.; Abernethy, D.R. Pharmacokinetics and clinical effectiveness of methylphenidate. Clin. Pharmacokinet. 1999, 37, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Wegener, J.; Keese, C.; Giaever, I. ECIS as a non-invasive means to follow the kinetics of cell spreading on artificial surfaces. Exp. Cell Res. 2000, 259, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Gorjup, E.; Thielecke, H. Chip-based time-continuous monitoring of toxic effects on stem cell differentiation. Ann. Anat. 2009, 191, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, C.; Buth, H.; Cho, S.; Impidjati; Thielecke, H. Detection of the osteogenic differentiation of mesenchymal stem cells in 2D and 3D cultures by electrochemical impedance spectroscopy. J. Biotechnol. 2010, 148, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Vinzons, L.U.; Kang, Y.S.; Lai, T.Y. Non-faradaic electrical impedimetric investigation of the interfacial effects of neuronal cell growth and differentiation on silicon nanowire transistors. ACS Appl. Mater. Interfaces 2015, 7, 9866–9878. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.P. Review on impedance detection of cellular responses in micro/nano environment. Micromachines 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Mishra, N.N.; Retterer, S.; Zieziulewicz, T.J.; Isaacson, M.; Szarowski, D.; Mousseau, D.E.; Lawrence, D.A.; Turner, J.N. On-chip micro-biosensor for the detection of human CD4+ cells based on AC impedance and optical analysis. Biosens. Bioelectron. 2005, 21, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Baniukevic, J.; Boyaci, I.H.; Bozkurt, A.G.; Tamer, U.; Ramanavicius, A.; Ramanaviciene, A. Magnetic gold nanoparticles in SERS-based sandwich immunoassay for antigen detection by well oriented antibodies. Biosens. Bioelectron. 2013, 43, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.C.; Sivashanmugan, K.; Liao, J.D.; Yao, C.K.; Peng, H.C. Nanofabricated SERS-active substrates for single-molecule to virus detection in vitro: A review. Biosens. Bioelectron. 2014, 61, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kruss, S.; Landry, P.M.; Ende, E.V.; Lima, B.M.A.; Reue, N.F.; Zhang, J.; Nelson, J.; Mu, B.; Hilmer, A.; Strano, M. Neurotransmitter detection using corona phase molecular recognition on fluorescent single-walled carbon nanotube sensors. J. Am. Chem. Soc. 2014, 136, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Tripp, R.A.; Dluhy, R.A.; Zhao, Y. Novel nanostructures for SERS biosensing. Nano Today 2008, 3, 31–37. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, X.; Yin, J.; Liu, Y.; Dong, Z.; Sun, J.L.; Ma, W. Rapid, controllable growth of silver nanostructured surface-enhanced Raman scattering substrates for red blood cell detection. Sci. Rep. 2016, 6, 24503. [Google Scholar] [CrossRef] [PubMed]
- Schlücker, S. Surface-enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Macias, G.; Alba, M.; Marsal, L.F.; Mihi, A. Surface roughness boosts the SERS performance of imprinted plasmonic architectures. J. Mater. Chem. C 2016, 4, 3970–3975. [Google Scholar] [CrossRef]
- Kudelski, A.; Bukowska, J. The chemical effect in surface enhanced Raman scattering (SERS) for piperidine adsorbed on a silver electrode. Surf. Sci. 1996, 368, 396–400. [Google Scholar] [CrossRef]
- Shanmukh, S.; Jones, L.; Driskell, J.; Zhao, Y.; Dluhy, R.; Tripp, R.A. Rapid and sensitive detection of respiratory virus molecular signatures using a silver nanorod array SERS substrate. Nano Lett. 2006, 6, 2630. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Kim, S.U.; Choi, J.W. Monitoring in vitro neural stem cell differentiation based on surface-enhanced Raman spectroscopy using a gold nanostar array. J. Mater. Chem. C 2015, 3, 3848. [Google Scholar] [CrossRef]
- Yuan, H.; Khory, C.G.; Hwang, H.; Wilson, C.M.; Grant, A.; Vo-Dinh, T. Gold nanostars: Surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Webber, J.P.; Gurney, M.; Mason, M.D.; Tabi, Z.; Clayton, A. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 2015, 6, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Wissleder, R.; Lee, H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef] [PubMed]

| Detection Platform | Advantage | Disadvantage |
|---|---|---|
| Optical (SERS) | Narrow band spectra Rapid signal acquisition time High sensitivity | Requires reliable system (Highly ordered substrate) |
| Optical (LSPR) | High sensitivity Multiple sample analysis | Difficult to distinguish different binding events in sample mixtures |
| Electrochemical (CV/DPV) | Simple to operate Label-free analysis | Not complementary defined where redox signal originates |
| Electrical (ECIS) | Real-time signal acquisition Label-free analysis | Time consuming Undesired signal from environment |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Lee, T.; Choi, J.-W. Nano-Biosensor for Monitoring the Neural Differentiation of Stem Cells. Nanomaterials 2016, 6, 224. https://doi.org/10.3390/nano6120224
Lee J-H, Lee T, Choi J-W. Nano-Biosensor for Monitoring the Neural Differentiation of Stem Cells. Nanomaterials. 2016; 6(12):224. https://doi.org/10.3390/nano6120224
Chicago/Turabian StyleLee, Jin-Ho, Taek Lee, and Jeong-Woo Choi. 2016. "Nano-Biosensor for Monitoring the Neural Differentiation of Stem Cells" Nanomaterials 6, no. 12: 224. https://doi.org/10.3390/nano6120224
APA StyleLee, J.-H., Lee, T., & Choi, J.-W. (2016). Nano-Biosensor for Monitoring the Neural Differentiation of Stem Cells. Nanomaterials, 6(12), 224. https://doi.org/10.3390/nano6120224








