Study on the Efficiency and Mechanism of a Novel Copper-Based Composite Material Activated by Supramolecular Self-Assembly for Degrading Reactive Red 3BS
Abstract
1. Introduction
2. Materials and Methods
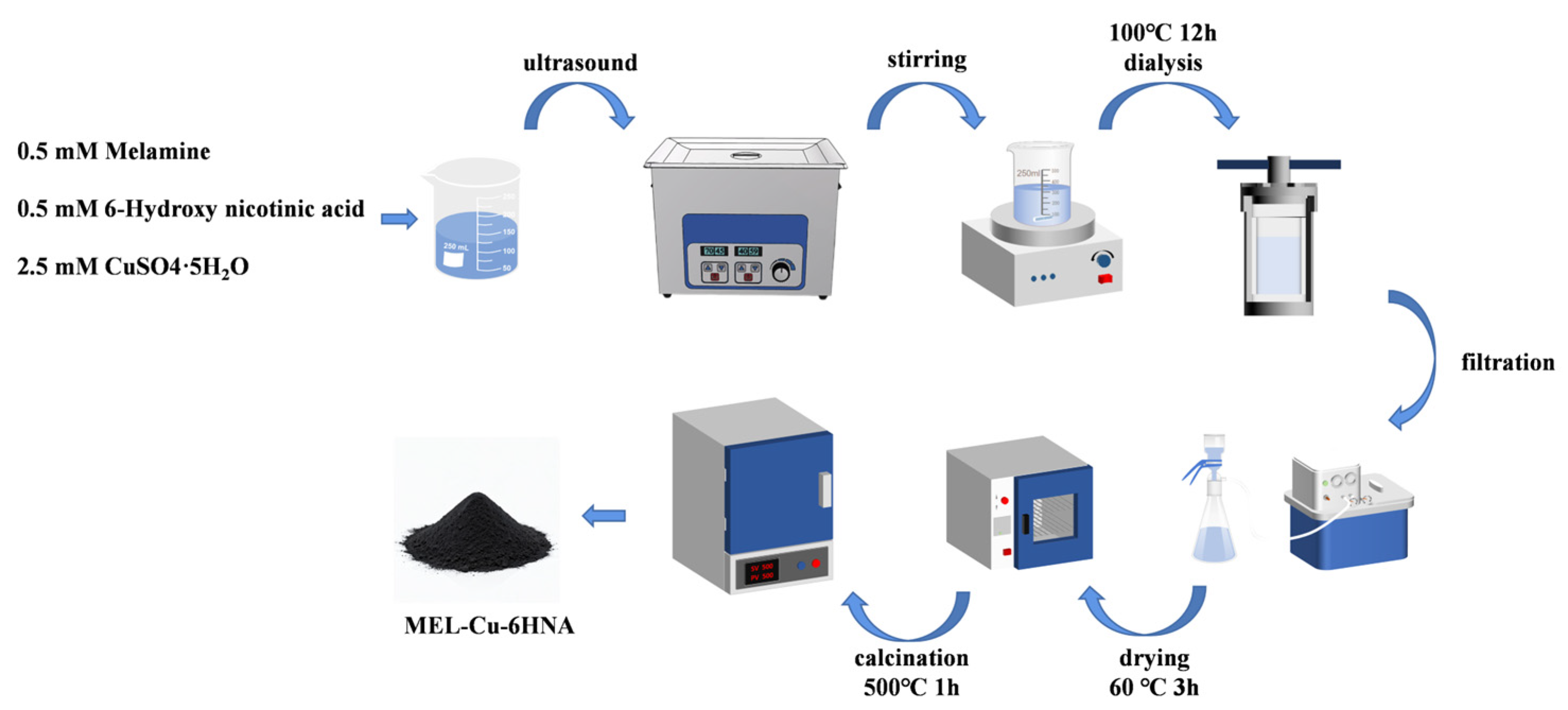
2.1. Preparation of MEL-Cu-6HNA Material
2.2. Characterization of MEL-Cu-6HNA
2.3. Performance Evaluation of MEL-Cu-6HNA for Reactive Red 3BS
3. Research Findings
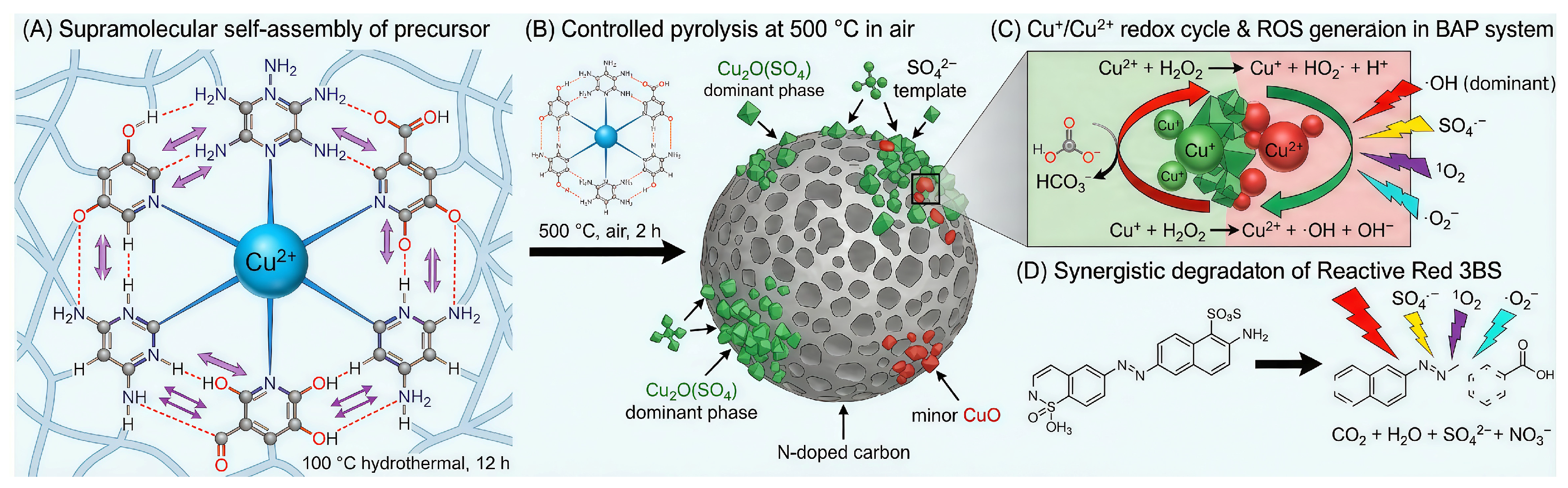
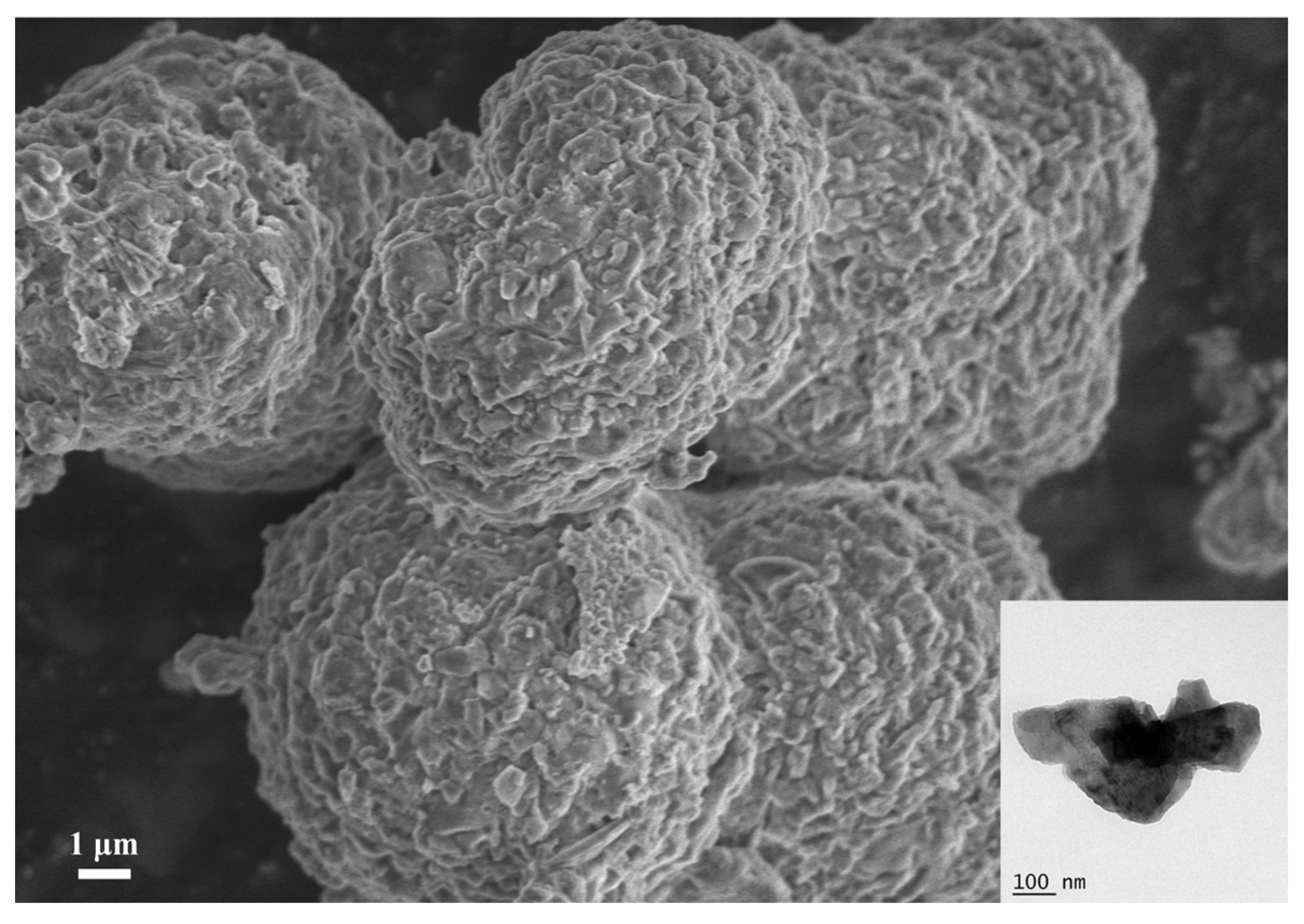
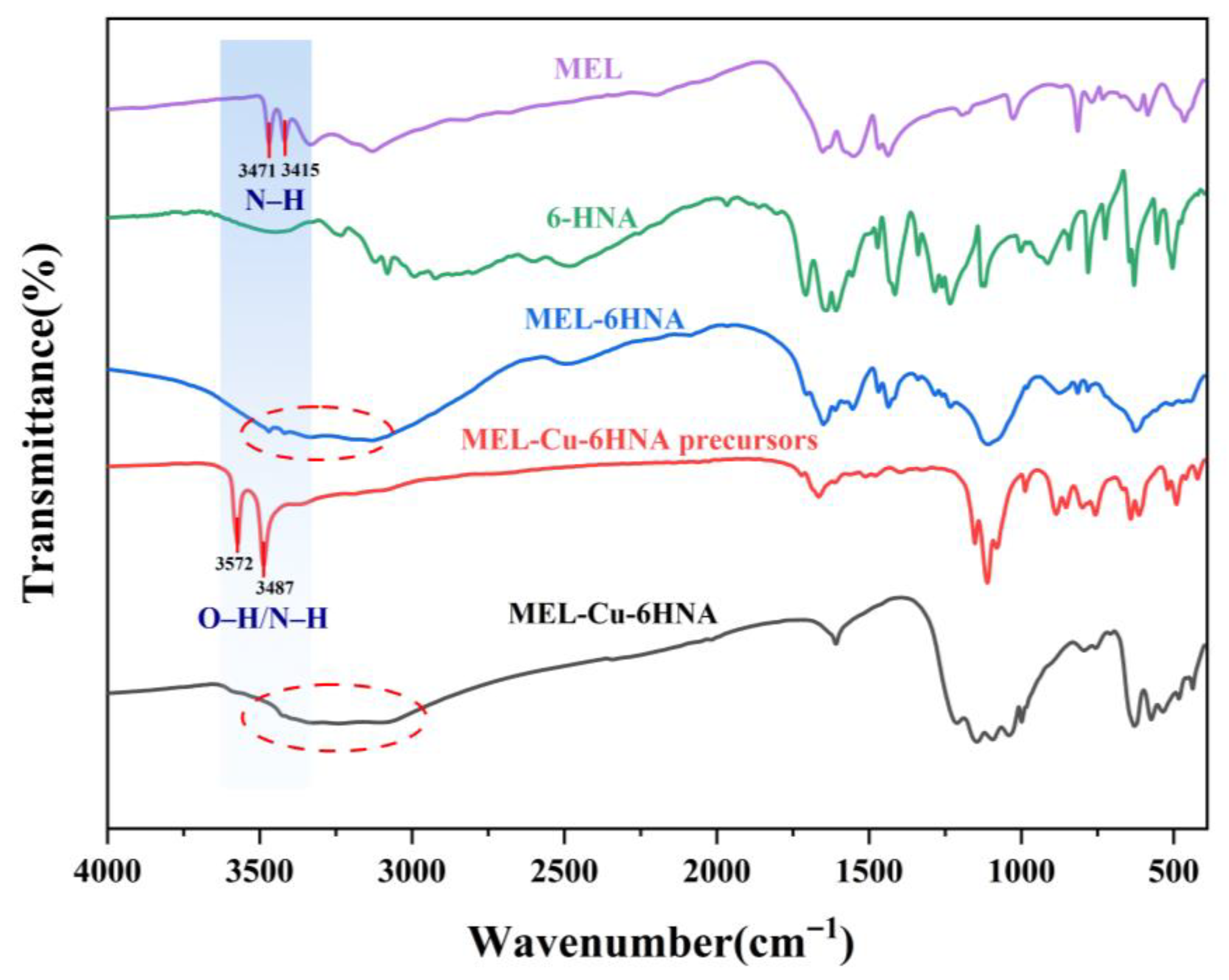
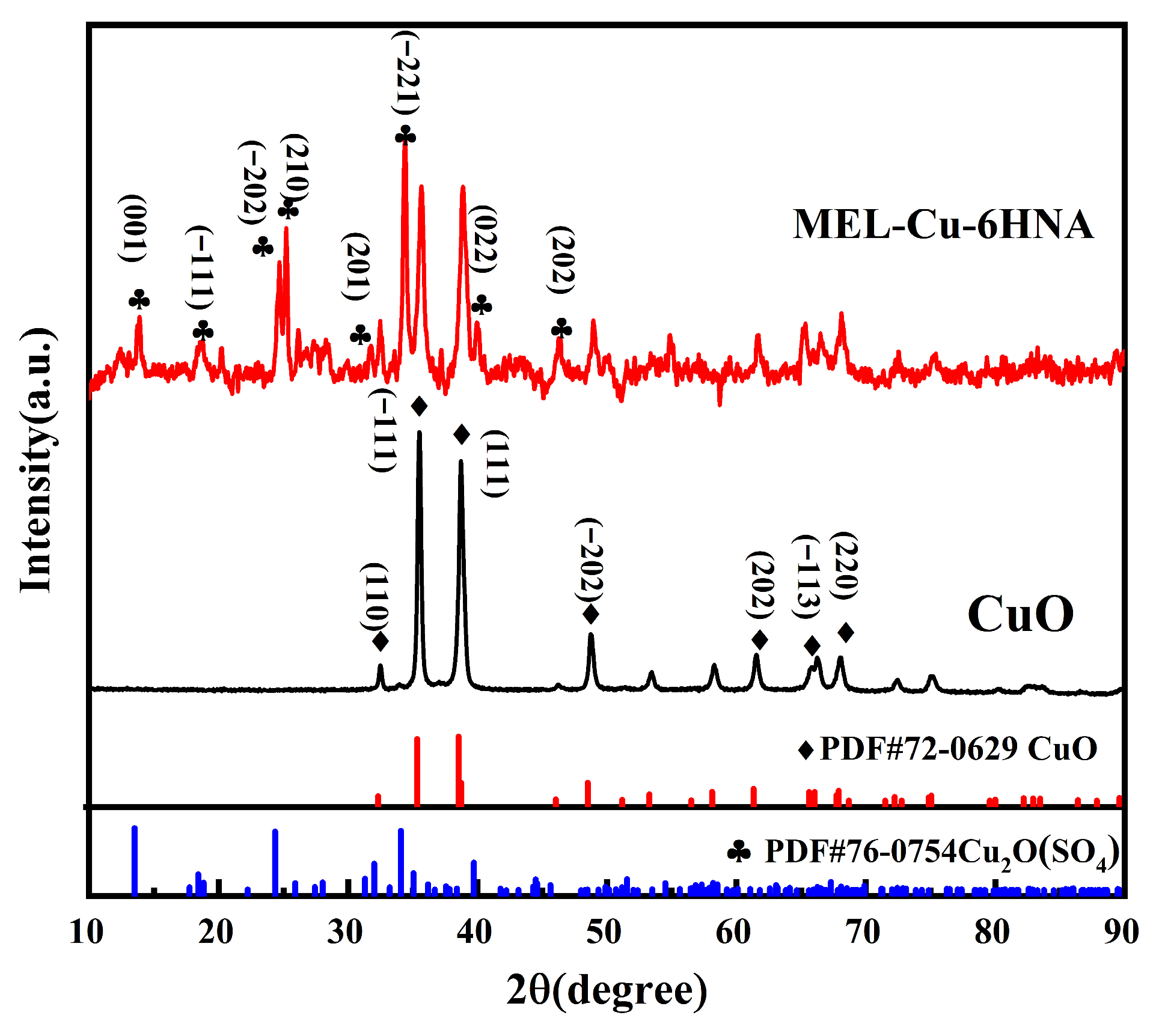
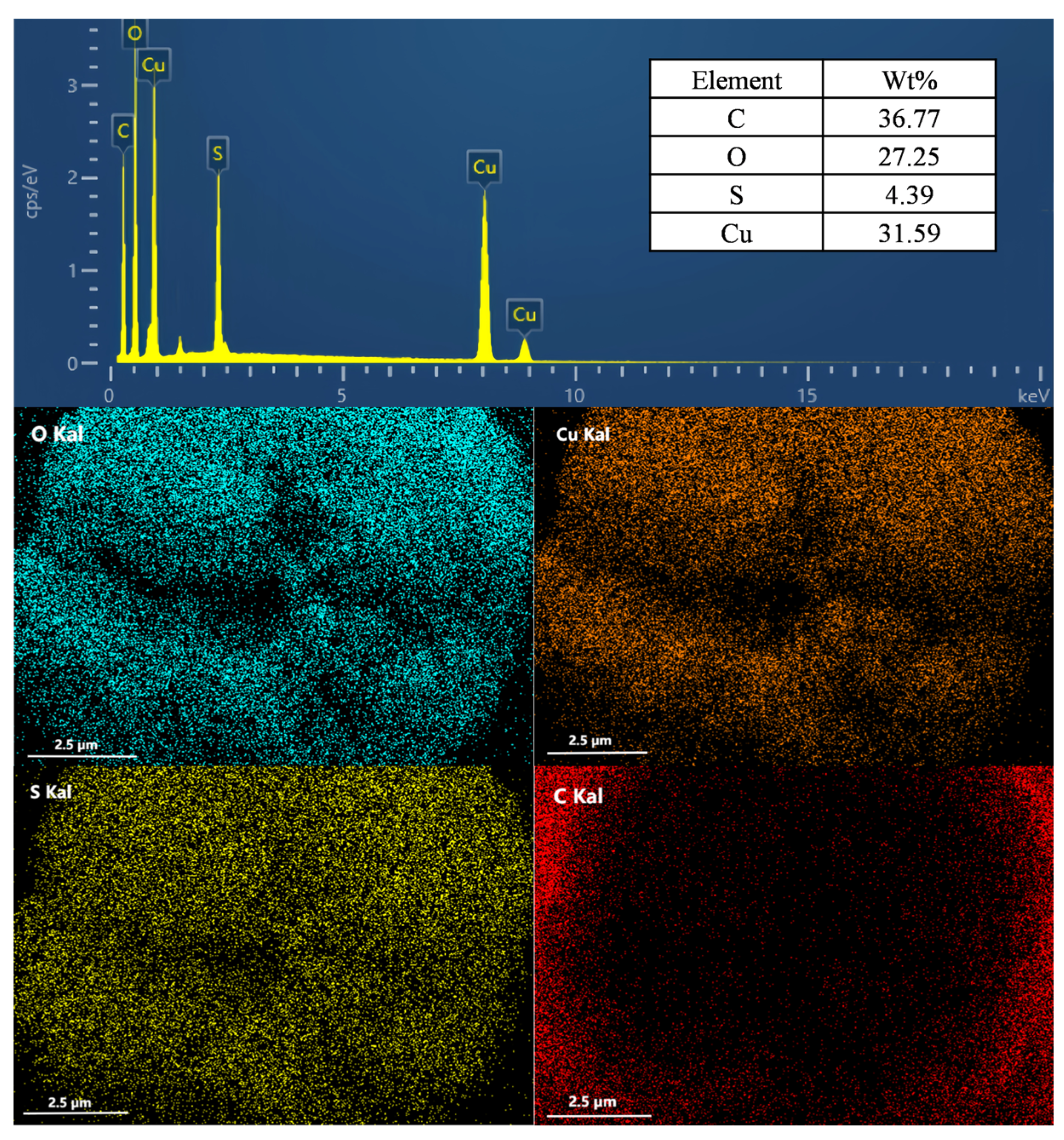
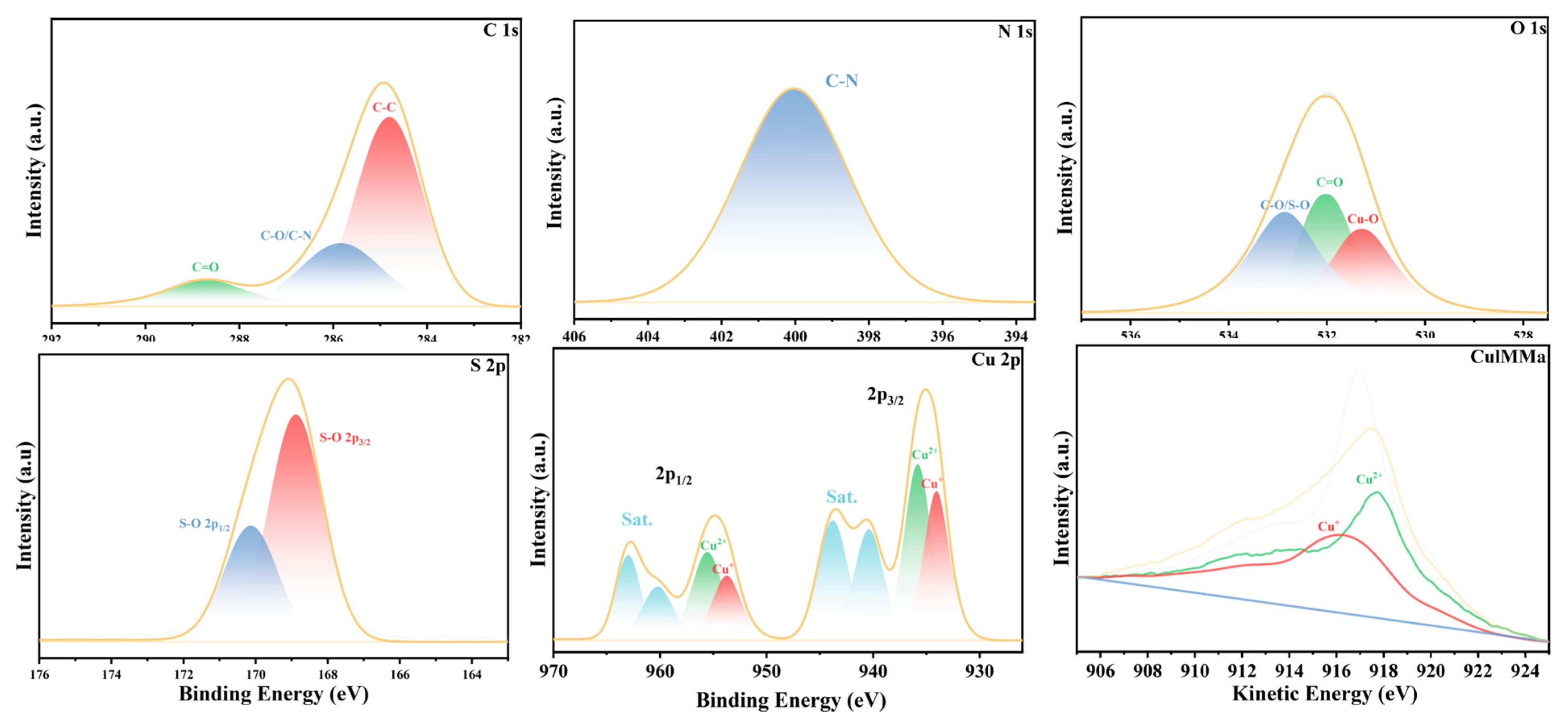
3.1. Characterization of MEL-Cu-6HNA
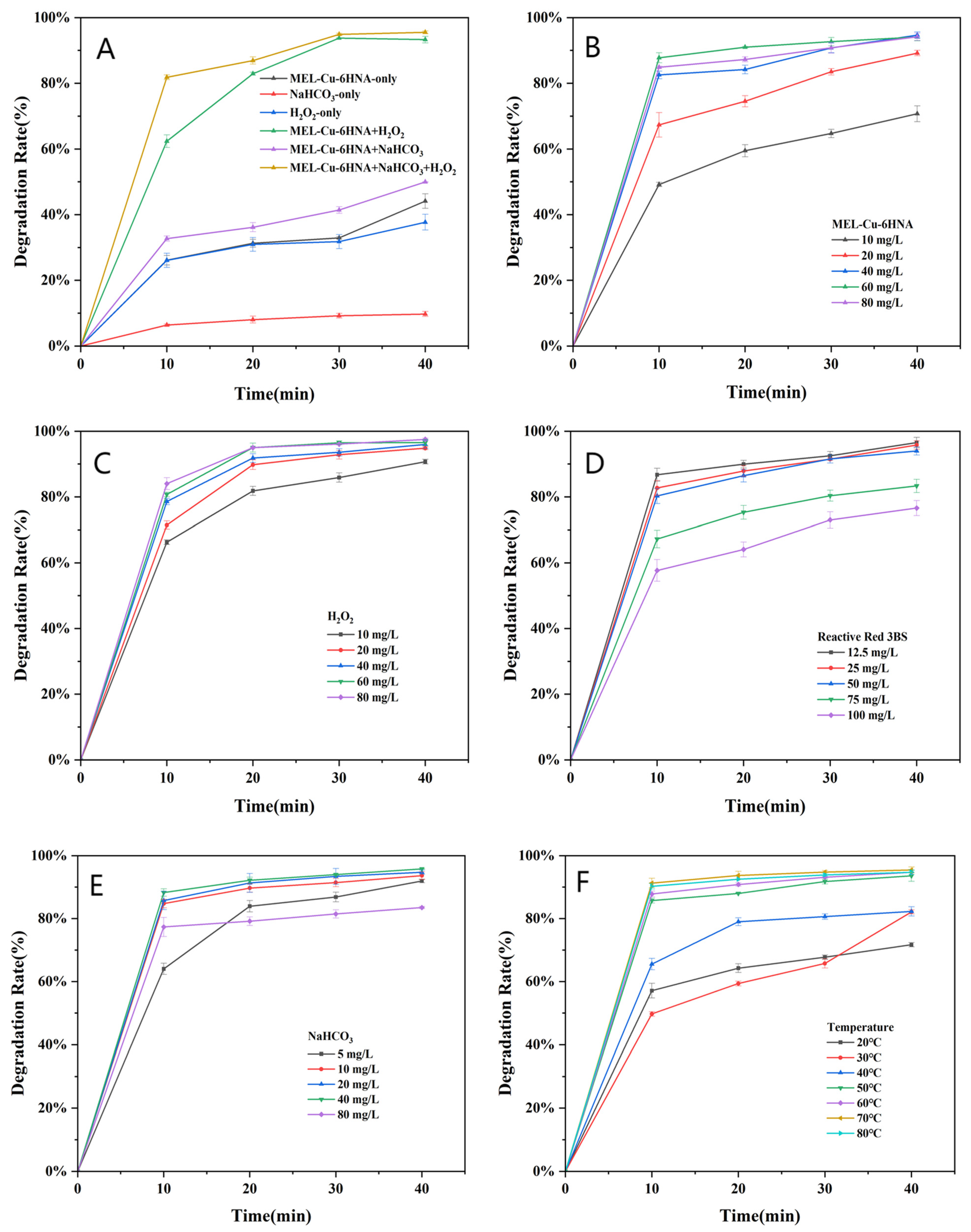
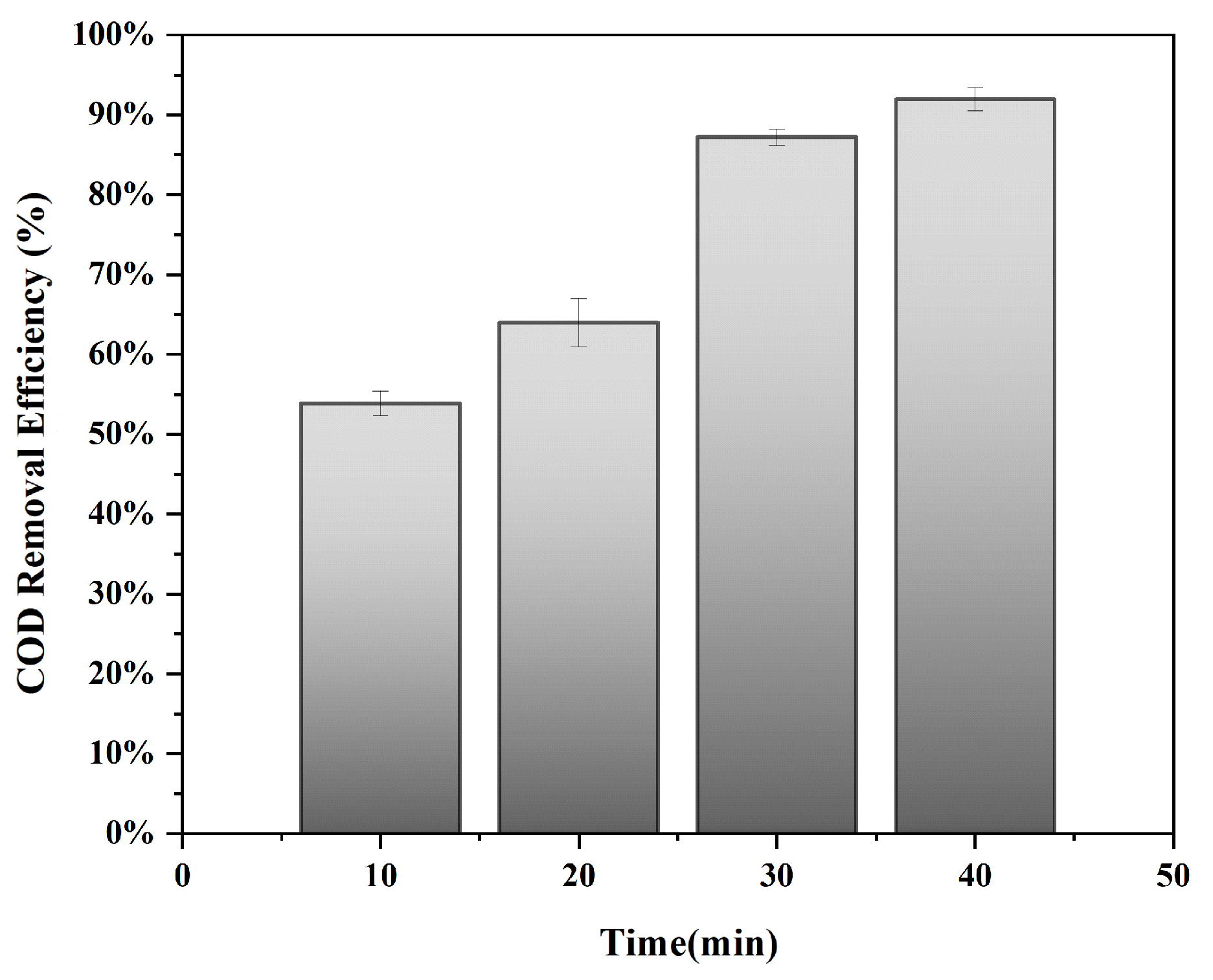
3.2. Catalytic Decolorization Performance Analysis
3.3. Mineralization Capacity of MEL-Cu-6HNA Toward Reactive Red 3BS in the BAP System
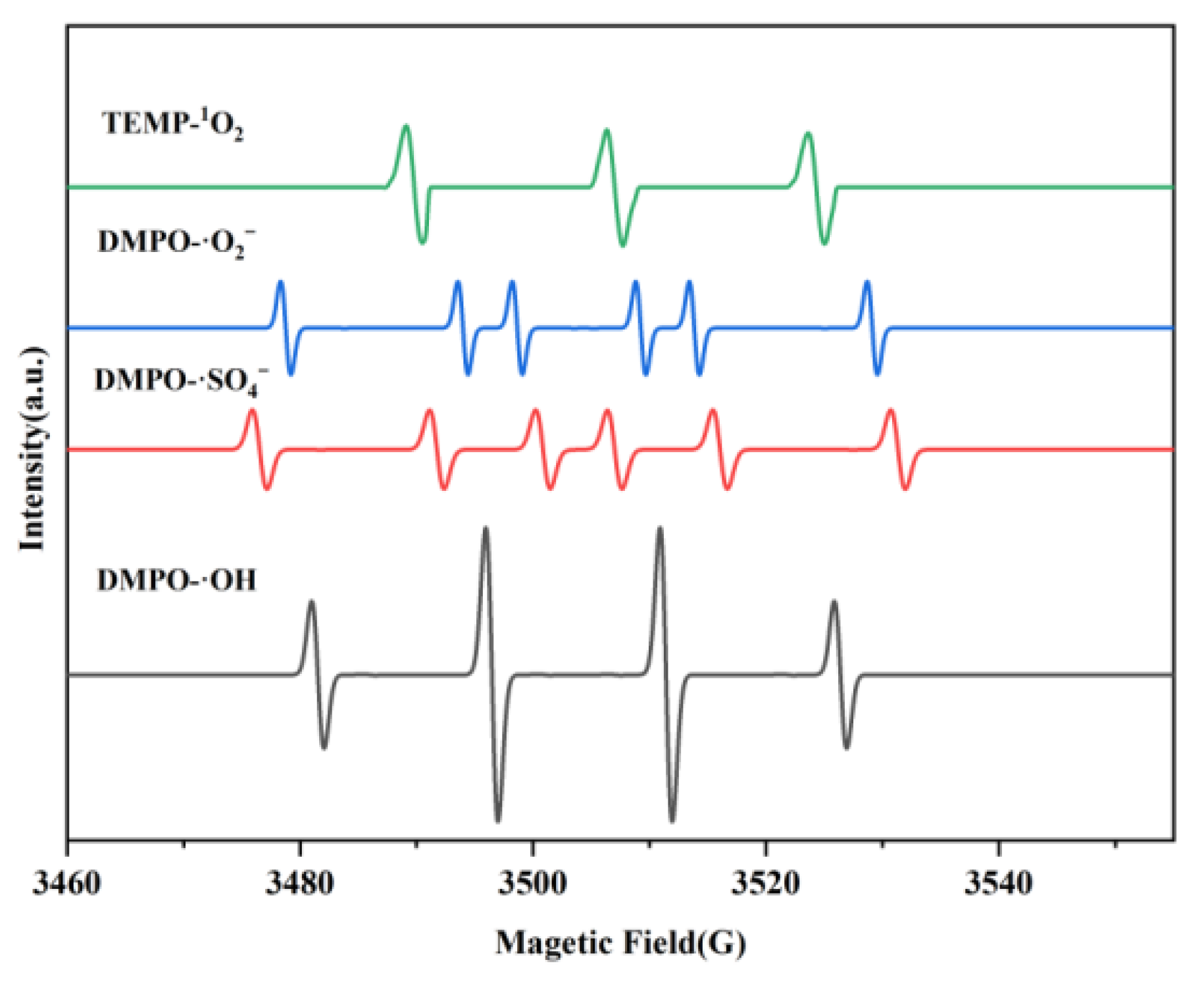
3.4. Analysis of Decolorizing Species Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saha, P.; Rao, K.V.B. Biodegradation of commercial textile reactive dye mixtures by industrial effluent adapted bacterial consortium VITPBC6: A potential technique for treating textile effluents. Biodegradation 2024, 35, 173–193. [Google Scholar] [CrossRef]
- Chowdhury, A.P.; Chinnam, S.; Anantharaju, K.; Kumar, B.S.; Keshavamurthy, K.; Gurushantha, K. Gd3+ Doped CoFe2O4 Coupled with Bismuth Oxybromide for Visible-Light-Driven Removal of Organic Contaminants: Reactive Red 120 and Acid Violet 7 and Its Mechanism Insights. J. Rare Earths 2025, 43, 2166–2176. [Google Scholar] [CrossRef]
- Pandiyarajan, G.; Kesavan, M.P.; Vadivel, R.P.; Arulanandam, X. S Synthesis and Characterization of Microporous Activated Carbons Treated with Sulphuric Acid from Agricultural Waste for Efficient Removal of Reactive Red Dye from Aqueous Solutions. J. Indian Chem. Soc. 2025, 102, 102004. [Google Scholar] [CrossRef]
- Ayadi, I.; Souissi, Y.; Jlassi, I.; Peixoto, F.; Mnif, W. Chemical Synonyms, Molecular Structure and Toxicological Risk Assessment of Synthetic Textile Dyes: A Critical Review. Dev. Drugs 2016, 5, 5–57. [Google Scholar] [CrossRef]
- Othman, N.; Sulaiman, R.N.R.; Rahman, H.A.; Noah, N.F.M.; Jusoh, N.; Idroas, M. Simultaneous Extraction and Enrichment of Reactive Dye Using Green Emulsion Liquid Membrane System. Environ. Technol. 2019, 40, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gao, Y.; Li, N.; Zhou, Y.; Lin, Q.; Jiang, J. Recent Advances on Bicarbonate-Activated Hydrogen Peroxide System in the Field of Water Pollution Control. Chem. Eng. 2021, 408, 1385–8947. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Pérez-Flórez, A.; Arcila, J.S.; Giraldo-Goméz, G.I.; Sanabria-Gonzalez, N.R. Synthesis and Characterization of Co/Al-PILCs for the Oxidation of an Azo Dye Using the Bicarbonate-Activated Hydrogen Peroxide System. Catal. Lett. 2022, 152, 1905–1916. [Google Scholar] [CrossRef]
- Urbina-Suarez, N.A.; Salcedo-Pabón, C.J.; López-Barrera, G.L.; García-Martínez, J.B.; Barajas-Solano, A.F.; Machuca-Martínez, F. Using the Response Surface Methodology to Treat Tannery Wastewater with the Bicarbonate-Peroxide System. ChemEngineering 2023, 7, 62. [Google Scholar] [CrossRef]
- Ariza-Pineda, F.J.; Macías-Quiroga, I.F.; Hinojosa-Zambrano, D.F.; Rivera-Giraldo, J.D.; Ocampo-Serna, D.M.; Sanabria-González, N.R. Treatment of Textile Wastewater Using the Co(II)/NaHCO3/H2O2 Oxidation System. Heliyon 2023, 9, e24058. [Google Scholar] [CrossRef]
- Shi, Y.; Ji, T.; Wei, Y.; Zhang, D.; Yang, S. Gallic Acid-Promoted Fenton-like Catalytic Oxidation: Synergistic Mechanisms Involving the Ligand-Metal (Cu2+/Fe2+)-H2O2 Electron Transfer and d-Band Center Modulation. J. Environ. Chem. Eng. 2025, 13, 119428. [Google Scholar] [CrossRef]
- Mora-Bonilla, K.Y.; Macías-Quiroga, I.F.; Sanabria-González, N.R.; Dávila-Arias, M.T. Bicarbonate-Activated Hydrogen Peroxide for an Azo Dye Degradation: Experimental Design. ChemEngineering 2023, 7, 86. [Google Scholar] [CrossRef]
- Wang, Q.-N.; Duan, R.; Feng, Z.; Zhang, Y.; Luan, P.; Feng, Z.; Wang, J.; Li, C. Understanding the Synergistic Catalysis in Hydrogenation of Carbonyl Groups on Cu-Based Catalysts. ACS Catal. 2024, 14, 1620–1628. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; Phadatare, M.; Lindgren, F.; Brandell, D.; Hahlin, M.; Örtegren, J. Safe and Cost-Effective Synthesis of Porous Silicon Using Urea: Structural, Morphological, and Porosity Analysis. Micropor. Mesopor. Mater. 2025, 397, 113773. [Google Scholar] [CrossRef]
- Hu, G.; Yan, W.; Meng, X.; Liu, Y.; Huang, Y.; Xiao, J.; Zhang, X.; Yan, G.; Guo, Q.; Liang, Y.; et al. Copper Regulated Supramolecular Assembly Based on Aldehyde-Containing Essential Oil with an Improved Management of Plant Diseases. Chem. Eng. J. 2025, 525, 170514. [Google Scholar] [CrossRef]
- Baykov, S.V.; Semenov, A.V.; Presnukhina, S.I.; Tarasenko, M.V.; Shetnev, A.A.; Frontera, A.; Boyarskiy, V.P.; Kukushkin, V.Y. Hybrid 2D Supramolecular Organic Frameworks (SOFs) Assembled by the Cooperative Action of Hydrogen and Halogen Bonding and π–π Stacking Interactions. Int. J. Mol. Sci. 2024, 25, 2062. [Google Scholar] [CrossRef]
- Tandon, S.S.; Bunge, S.D.; Patel, N.; Wang, E.C.; Thompson, L.K. Self-Assembly of Antiferromagnetically-Coupled Copper(II) Supramolecular Architectures with Diverse Structural Complexities. Molecules 2020, 25, 5549. [Google Scholar] [CrossRef]
- Xu, X.; Tang, D.; Cai, J.; Xi, B.; Zhang, Y.; Pi, L.; Mao, X. Heterogeneous activation of peroxymonocarbonate by chalcopyrite (CuFeS2) for efficient degradation of 2,4-dichlorophenol in simulated groundwater. Appl. Catal. B Environ. 2019, 251, 273–282. [Google Scholar] [CrossRef]
- Pi, L.; Yang, N.; Han, W.; Xiao, W.; Wang, D.; Xiong, Y.; Zhou, M.; Hou, H.; Mao, X. Heterogeneous activation of peroxymonocarbonate by Co-Mn oxides for the efficient degradation of chlorophenols in the presence of a naturally occurring level of bicarbonate. Chem. Eng. J. 2018, 334, 1297–1308. [Google Scholar] [CrossRef]
- Jawad, A.; Lu, X.; Chen, Z.; Yin, G. Degradation of Chlorophenols by Supported Co–Mg–Al Layered Double Hydrotalcite with Bicarbonate Activated Hydrogen Peroxide. J. Phys. Chem. A 2014, 118, 10028–10035. [Google Scholar] [CrossRef]
- Alsaaed, F.A.T.; El-Lateef, H.M.A.; Khalaf, M.M.; Mohamed, I.M.A.; Al-Omair, M.A.; Gouda, M. Drug Delivery System Based on Carboxymethyl Cellulose Containing Metal-Organic Framework and Its Evaluation for Antibacterial Activity. Polymers 2022, 14, 3815. [Google Scholar] [CrossRef]
- Direm, A.; Dadda, N.; Falek, W.; Boutobba, Z.; Benali-Cherif, N. Synthesis, Spectroscopic and Structural Analysis of a Melamine Copper Complex. Acta Crystallogr. A Found. Adv. 2014, 70 Pt a1, C1239. [Google Scholar] [CrossRef]
- Kumar, M.R.; Murugadoss, G.; Pirogov, A.N.; Thangamuthu, R. A Facile One Step Synthesis of SnO2/CuO and CuO/SnO2 Nanocomposites: Photocatalytic Application. J. Mater. Sci. Mater. Electron. 2018, 29, 13508–13515. [Google Scholar] [CrossRef]
- Zhang, W.; He, B.; Shang, J.; He, X.; Tian, S.; Liu, Y.; Feng, D.; Chen, M.; Cheng, X. Novel Cu2O(SO4)@NiO Nanocomposite as Peroxymonosulfate Activator for Effective Degradation of Ciprofloxacin. Chem. Eng. J. 2025, 506, 160316. [Google Scholar] [CrossRef]
- Sai, C.D.; Pham, V.T.; Tran, T.T.H.; Vu, T.B.N.; Hoang, T.H.H.; Pham, A.S.; Nguyen, T.M.T.; Duong, T.T.H.; Do, H.H. Construction of Highly Condensed Cu2O/CuO Composites on Cu Sheet and Its Photocatalytic in Photodegradation of Hazardous Colouring Agent Rose Bengal. Mater. Trans. 2023, 64, 2134–2142. [Google Scholar] [CrossRef]
- Chen, K.; Ling, J.-L.; Wu, C.-D. In Situ Generation and Stabilization of Accessible Cu/Cu2O Heterojunctions inside Organic Frameworks for Highly Efficient Catalysis. Angew. Chem. Int. Ed. 2020, 59, 1925. [Google Scholar] [CrossRef] [PubMed]
- Alebachew, N.; Murthy, H.C.A.; Abdissa, B.; Demissie, T.B.; von Eschwege, K.G.; Langner, E.H.G.; Coetsee-Hugo, L. Synthesis and Characterization of CuO@S-Doped g-C3N4 Based Nanocomposites for Binder-Free Sensor Applications. RSC Adv. 2022, 12, 29959–29974. [Google Scholar] [CrossRef] [PubMed]
- Awonusi, B.O.; Li, J.; Li, H.; Wang, Z.; Yang, K.; Zhao, J. In Vitro and In Vivo Studies on Bacteria and Encrustation Resistance of Heparin/Poly-L-Lysine-Cu Nanoparticles Coating Mediated by PDA for Ureteral Stent Application. Regen. Biomater. 2022, 9, rbac047. [Google Scholar] [CrossRef]
- Ciria-Ramos, I.; Navascués, N.; Diaw, F.; Furgeaud, C.; Arenal, R.; Ansón-Casaos, A.; Haro, M.; Juarez-Perez, E.J. Formamidinium Halide Salts as Precursors of Carbon Nitrides. Carbon 2022, 196, 1035–1046. [Google Scholar] [CrossRef]
- Nissar, I.; Hameed, J.; Yousaf, F.; Shah, S.S.; Kim, I.S.; Butt, A.F.; Ullah, S. Sunlight-Driven Efficient and Robust Degradation of Reactive Dyes (Bemacid Red and Violet) Using Carboxylate Complexes as a Photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2026, 729, 138829. [Google Scholar] [CrossRef]
- Ngo, V.N.N.; Pham, N.V.; Ha, T.M.; Luong, T.H.V.; Cao-Luu, N.H.; Nguyen, T.Q.C.; Dang, G.H. Preparation of Fe3O4/ZIF-9 as an Efficient Heterogeneous Nanocatalyst for Brilliant Green Dye Removal via Advanced Oxidation Processes. J. Phys. Chem. Solids 2026, 209, 113265. [Google Scholar] [CrossRef]
- Chai, Y.; Cai, Y.; Guan, Y.; Ai, H.; Yang, Z. Enhanced Degradation of Organic Dye in Aqueous Solutions by Bicarbonate-Activated Hydrogen Peroxide with a Rosin-Based Copper Catalyst. J. Water Process Eng. 2024, 66, 106035. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Chen, J.-B.; Xu, F.; Wang, K.-Q.; Hou, Z.-F.; Huang, T.-Y. Degradation of AO7 with Magnetic Fe3O4-CuO Heterogeneous Catalyzed Sodium Percarbonate System. Environ. Sci. 2020, 41, 1734–1742. [Google Scholar]
- Li, Y.; Buckin, V. State of Oxygen Molecules in Aqueous Supersaturated Solutions. J. Phys. Chem. B 2019, 123, 4025–4043. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, G.; Gong, D.; Zhang, C. Redispersible CuO Nanoparticles: Preparation and Photocatalytic Capacity for the Degradation of Methylene Blue. RSC Adv. 2025, 15, 19023–19033. [Google Scholar] [CrossRef]
- Sun, B.; Li, H.; Li, X.; Liu, X.; Zhang, C.; Xu, H.; Zhao, X.S. Degradation of Organic Dyes over Fenton-Like Cu2O–Cu/C Catalysts. Ind. Eng. Chem. Res. 2018, 57, 14011–14021. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Gong, J.; Wang, J.; Xing, E.; Wang, J.; Zhang, H. Cu/Co Bimetallic Carbon Catalyst as a Highly Efficient Promoter for Reactive Dyes Degradation with PMS. Langmuir 2024, 40, 11039–11048. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, R.; Wang, L.; Luo, D.; Wang, C. Efficient Degradation of Toxic Mixed Dyes through Peroxymonosulfate Activation by Copper/Iron Nanoparticles Loaded on 3D Carbon: Synthesis, Characterizations, and Mechanism. J. Environ. Chem. Eng. 2022, 10, 107606. [Google Scholar] [CrossRef]
- Crivelli, C.I.P.; de Almeida, J.; Lindino, C.A.; de Almeida, L.C.; Rodrigues, C.A.; Bessegato, G.G. Ti–O–Cu Nanotubular Mixed Oxide Grown on a TiCu Alloy as an Efficient Material for Simultaneous Photoelectrocatalytic Oxidation and PMS Activation for Pollutant Degradation. ACS Omega 2024, 9, 47052–47064. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Zhao, X.; Ma, G. Synthesis of Highly Porous Cu2O Catalysts for Efficient Ozone Decomposition. Catalysts 2021, 11, 600. [Google Scholar] [CrossRef]
- Zhang, C.; Chu, H.; Ma, Q.; Chen, Y.; Fan, J. Enhanced Heterogeneous Activation of Peroxymonosulfate by Nitrogen–Sulfur Co-Doped MOFs-Derived Carbon. Appl. Sci. 2023, 13, 3182. [Google Scholar] [CrossRef]
- Moradian, F.; Ramavandi, B.; Jaafarzadeh, N.; Kouhgardi, E. Activation of Periodate Using Ultrasonic Waves and UV Radiation for Landfill Leachate Treatment. Environ. Sci. Pollut. Res. 2022, 29, 90338–90350. [Google Scholar] [CrossRef] [PubMed]
| Catalytic | Catalyst Concentration | Oxidizing Agent | Pollutants | Time (Min) | Decolorization Rate (%) | References |
|---|---|---|---|---|---|---|
| CuO NP | 0.6 mM | H2O2 | Methylene blue, 53.5 μM | 75 | 99.6% | [34] |
| Cu2O–Cu/C | 0.1 M | H2O2 | Methyl Orange, 50 mg/L | 60 | 100% | [35] |
| CuCo-CTs | 0.1 g/L | Peroxymonosulfate | Reactive Violet 5, - | 30 | 90% | [36] |
| Cu/FeNPs@PC | 0.2 g/L | Peroxymonosulfate | Rhodamine B, - | 18 | 98.4% | [37] |
| Cu-Co/C | 0.5 g/L | Peroxymonosulfate | Orange II, 50 mg/L | 30 | 90.4% | [38] |
| MEL-Cu-6HNA | 40 mg/L | NaHCO3/H2O2 | Reactive Res 3BS, 50 mg/L | 30 | 95% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dai, J.; Wang, X.; Chen, B.; Chen, L. Study on the Efficiency and Mechanism of a Novel Copper-Based Composite Material Activated by Supramolecular Self-Assembly for Degrading Reactive Red 3BS. Nanomaterials 2026, 16, 111. https://doi.org/10.3390/nano16020111
Dai J, Wang X, Chen B, Chen L. Study on the Efficiency and Mechanism of a Novel Copper-Based Composite Material Activated by Supramolecular Self-Assembly for Degrading Reactive Red 3BS. Nanomaterials. 2026; 16(2):111. https://doi.org/10.3390/nano16020111
Chicago/Turabian StyleDai, Jiangming, Xinrong Wang, Bo Chen, and Liang Chen. 2026. "Study on the Efficiency and Mechanism of a Novel Copper-Based Composite Material Activated by Supramolecular Self-Assembly for Degrading Reactive Red 3BS" Nanomaterials 16, no. 2: 111. https://doi.org/10.3390/nano16020111
APA StyleDai, J., Wang, X., Chen, B., & Chen, L. (2026). Study on the Efficiency and Mechanism of a Novel Copper-Based Composite Material Activated by Supramolecular Self-Assembly for Degrading Reactive Red 3BS. Nanomaterials, 16(2), 111. https://doi.org/10.3390/nano16020111








