Controlled Capture of Magnetic Nanoparticles from Microfluidic Flows by Ferromagnetic Antidot and Dot Nanostructures
Abstract
1. Introduction
1.1. Applications of Magnetic Nanoparticle Capture
1.2. Magnetophoresis of Magnetic Nanoparticles
1.3. Capture of Magnetic Nanoparticles by Antidot Nanostructures
2. Materials and Methods
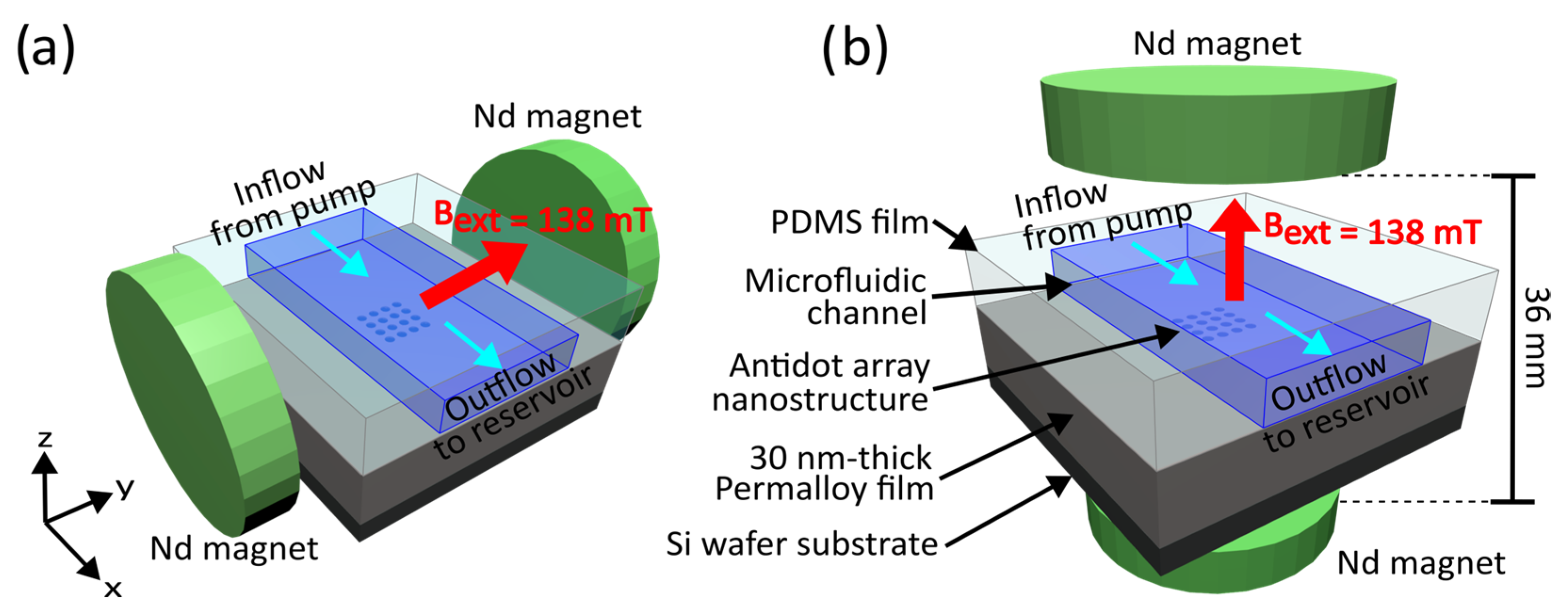
2.1. Fabrication of Ferromagnetic Antidot Nanostructures
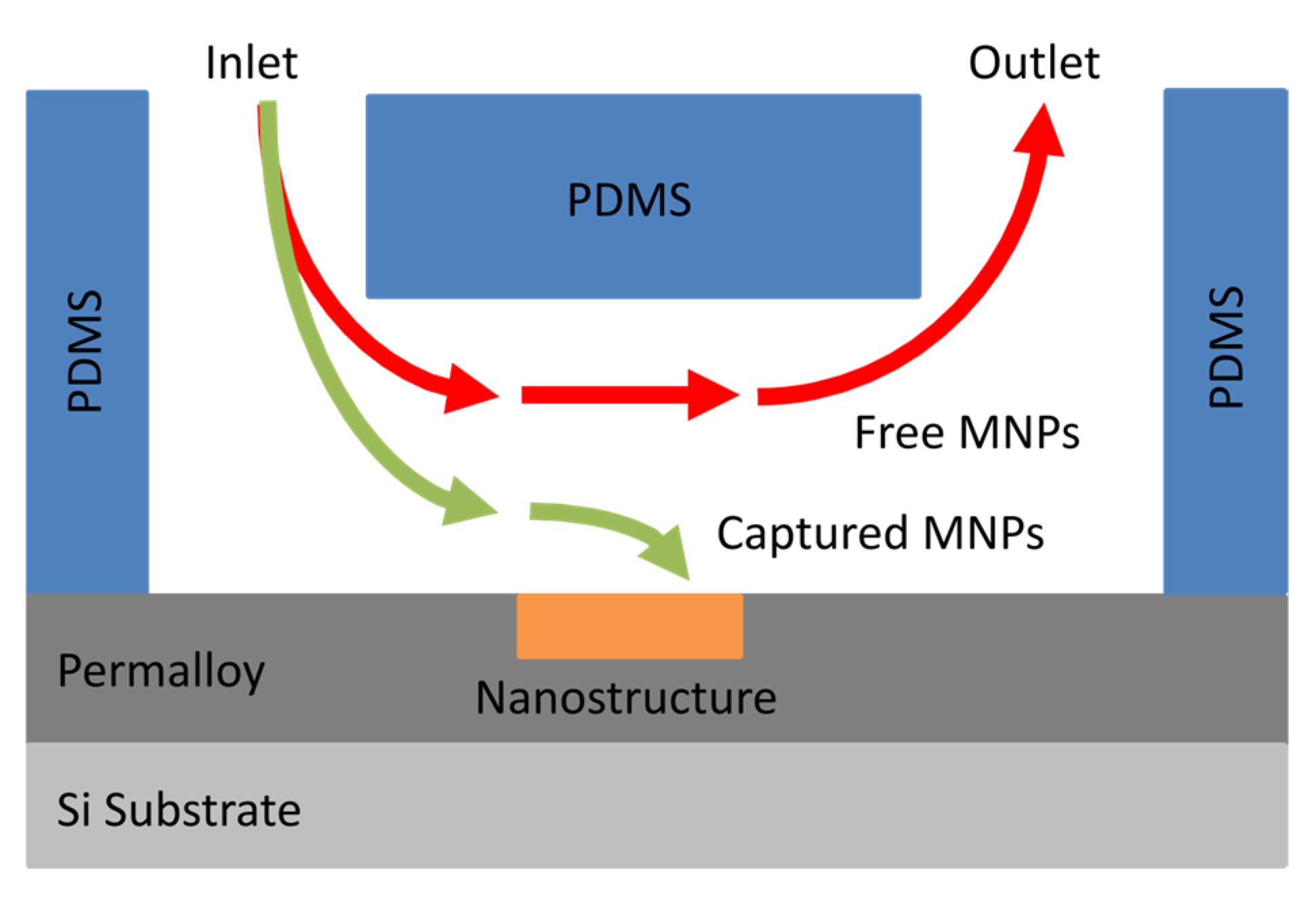
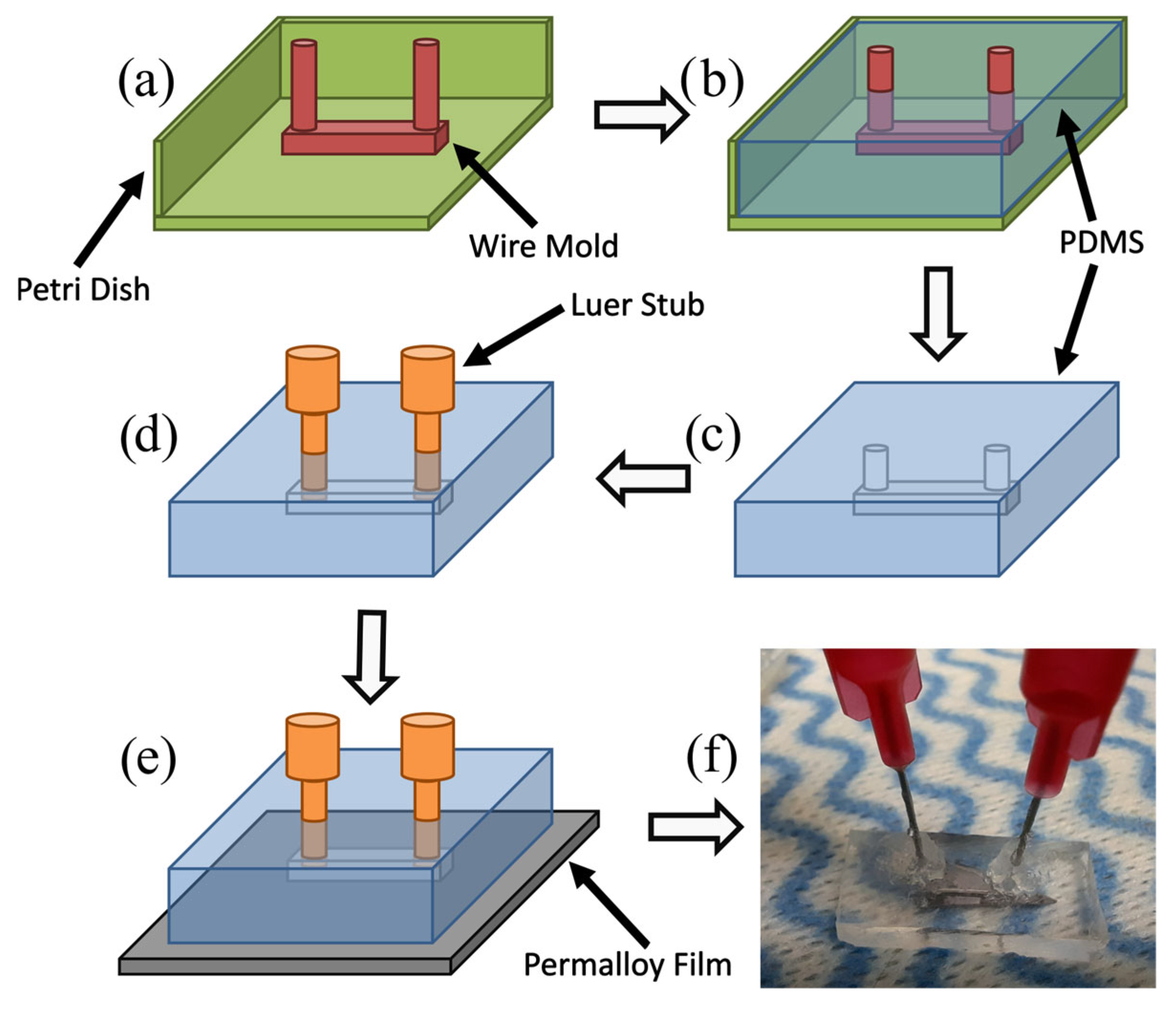
2.2. Fabrication of Microfluidic Channels
2.3. Preparation of Magnetic Nanoparticles
2.4. Capture of Magnetic Nanoparticles
2.5. Analysis of Captured Magnetic Nanoparticles
3. Results
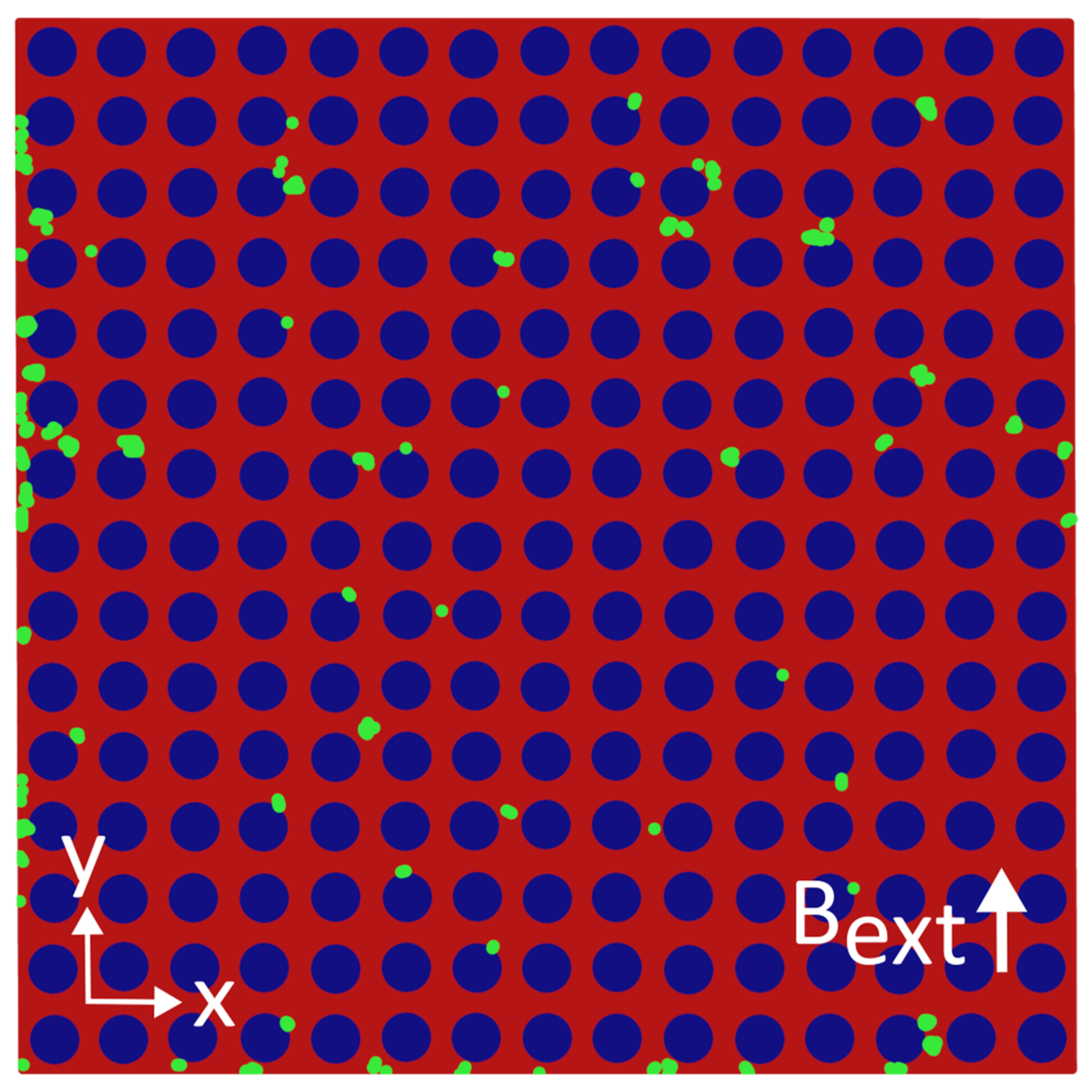
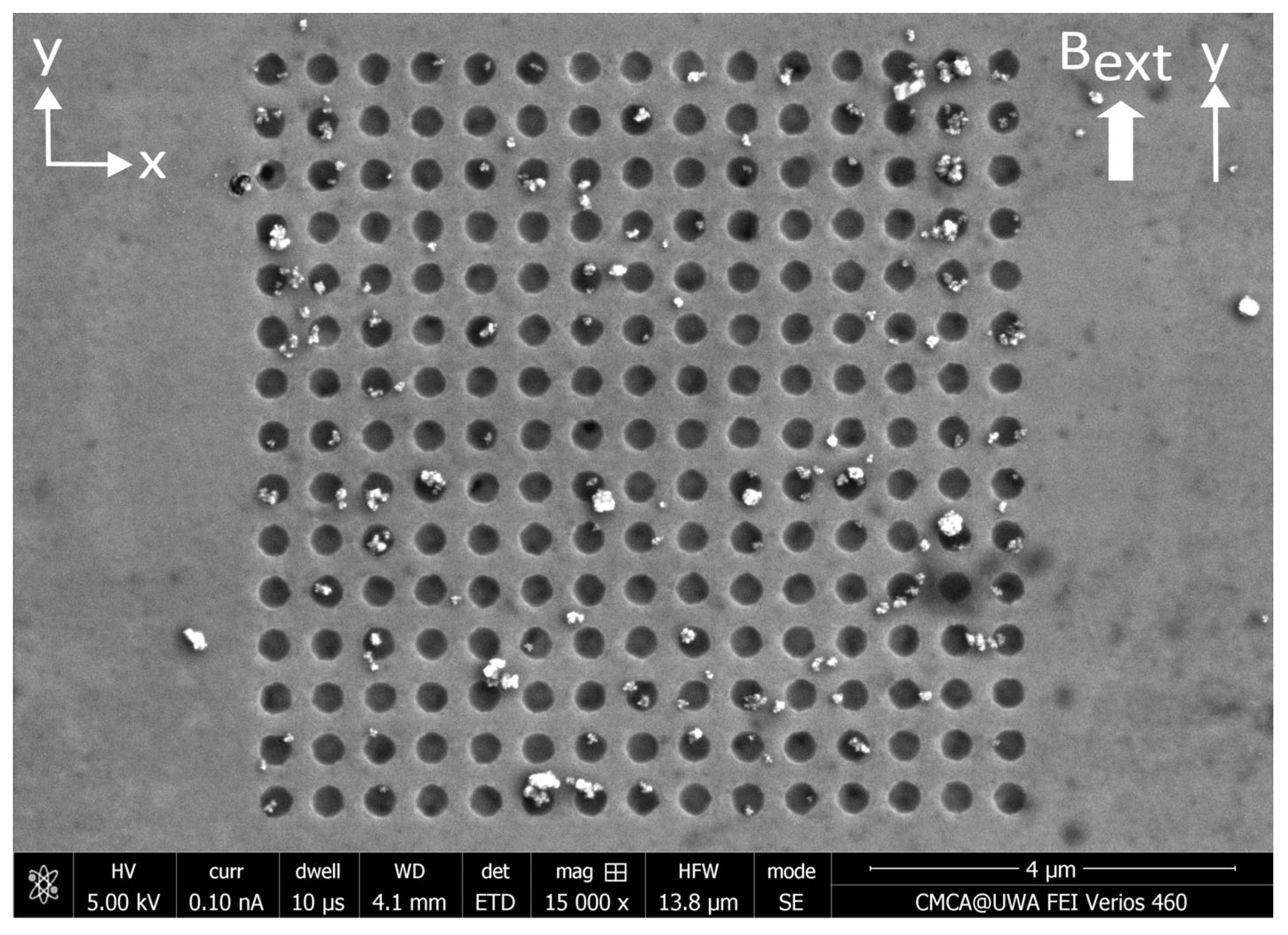
3.1. Results—MNP Capture Without External Magnetic Field
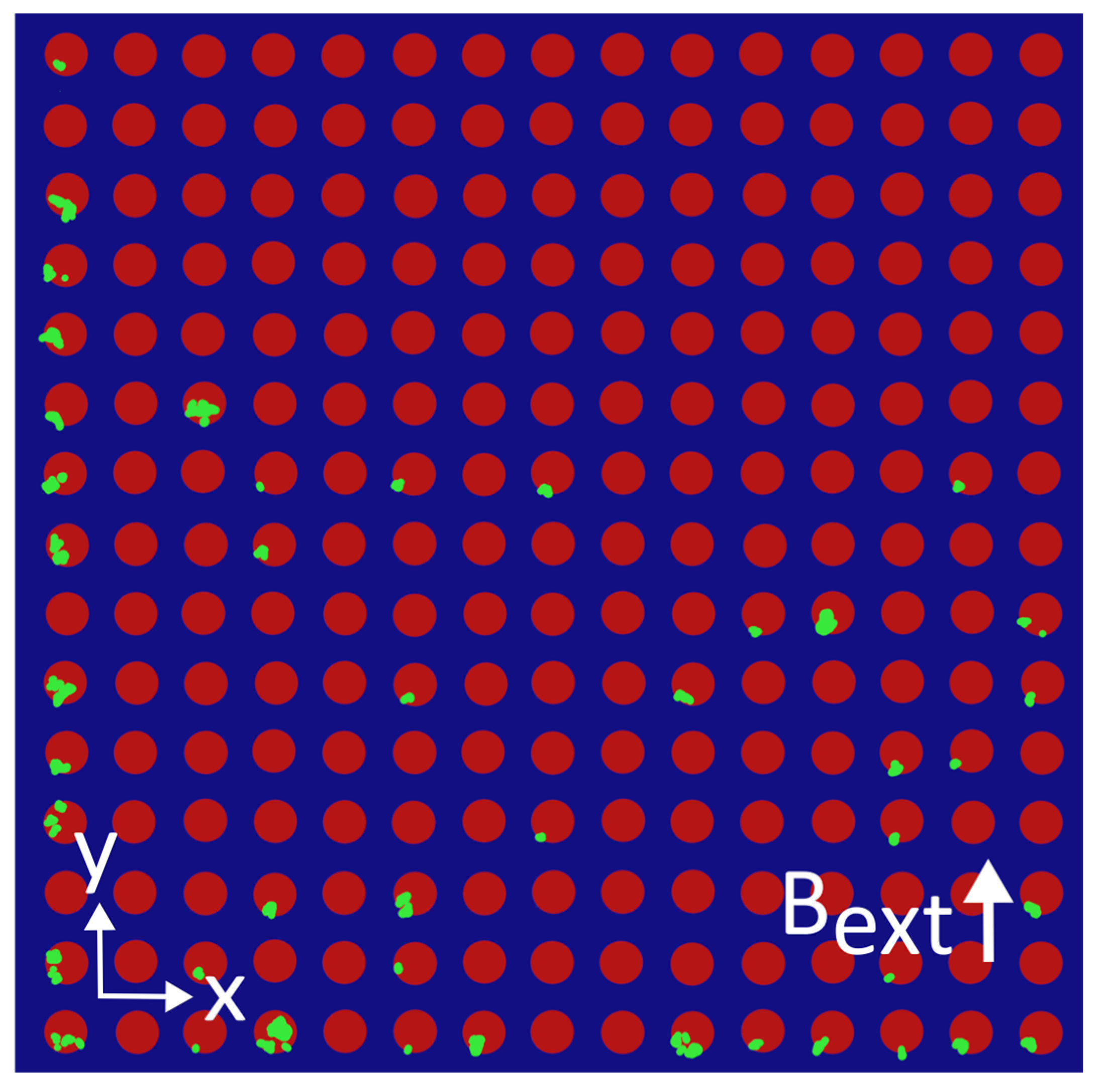
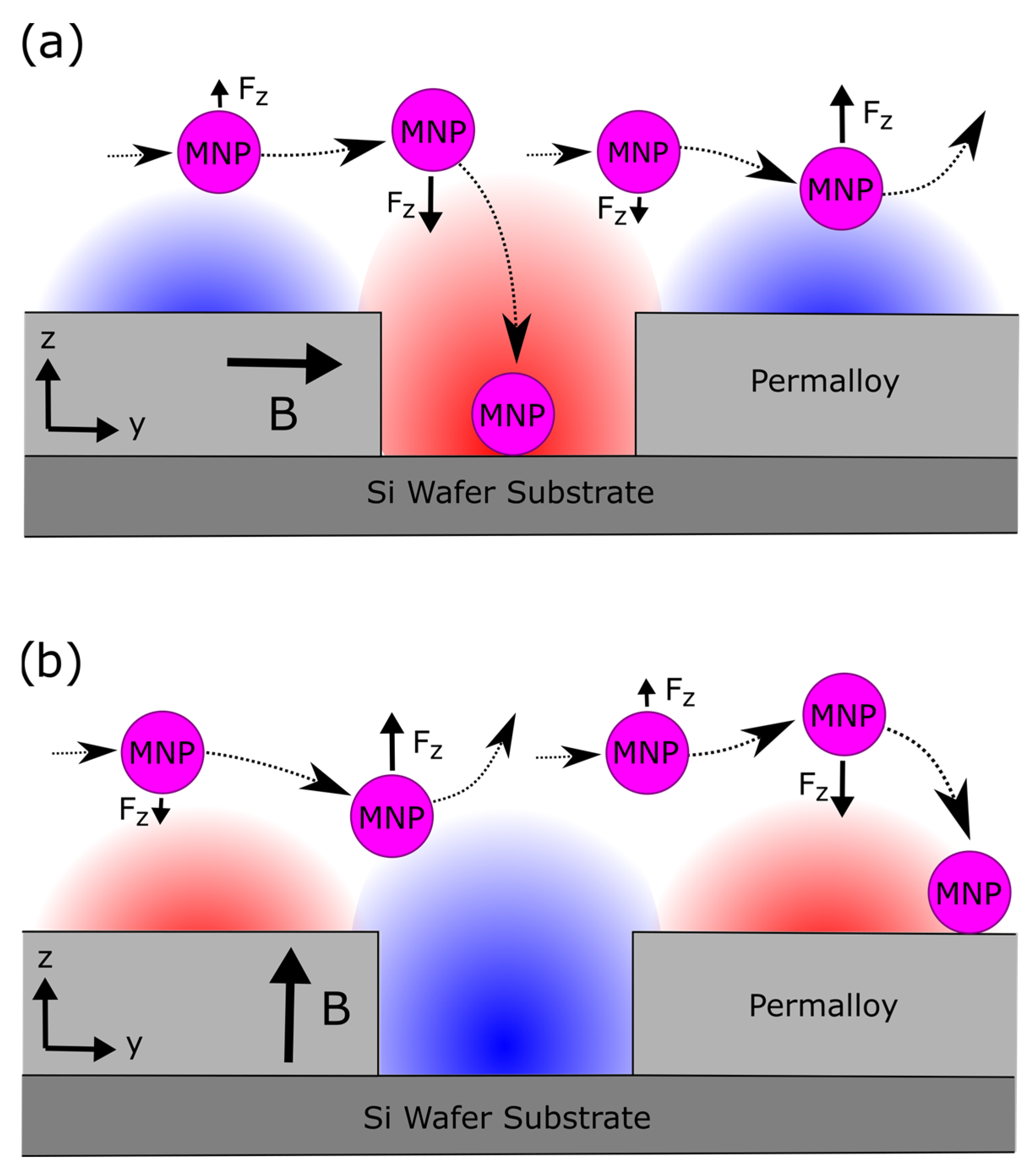
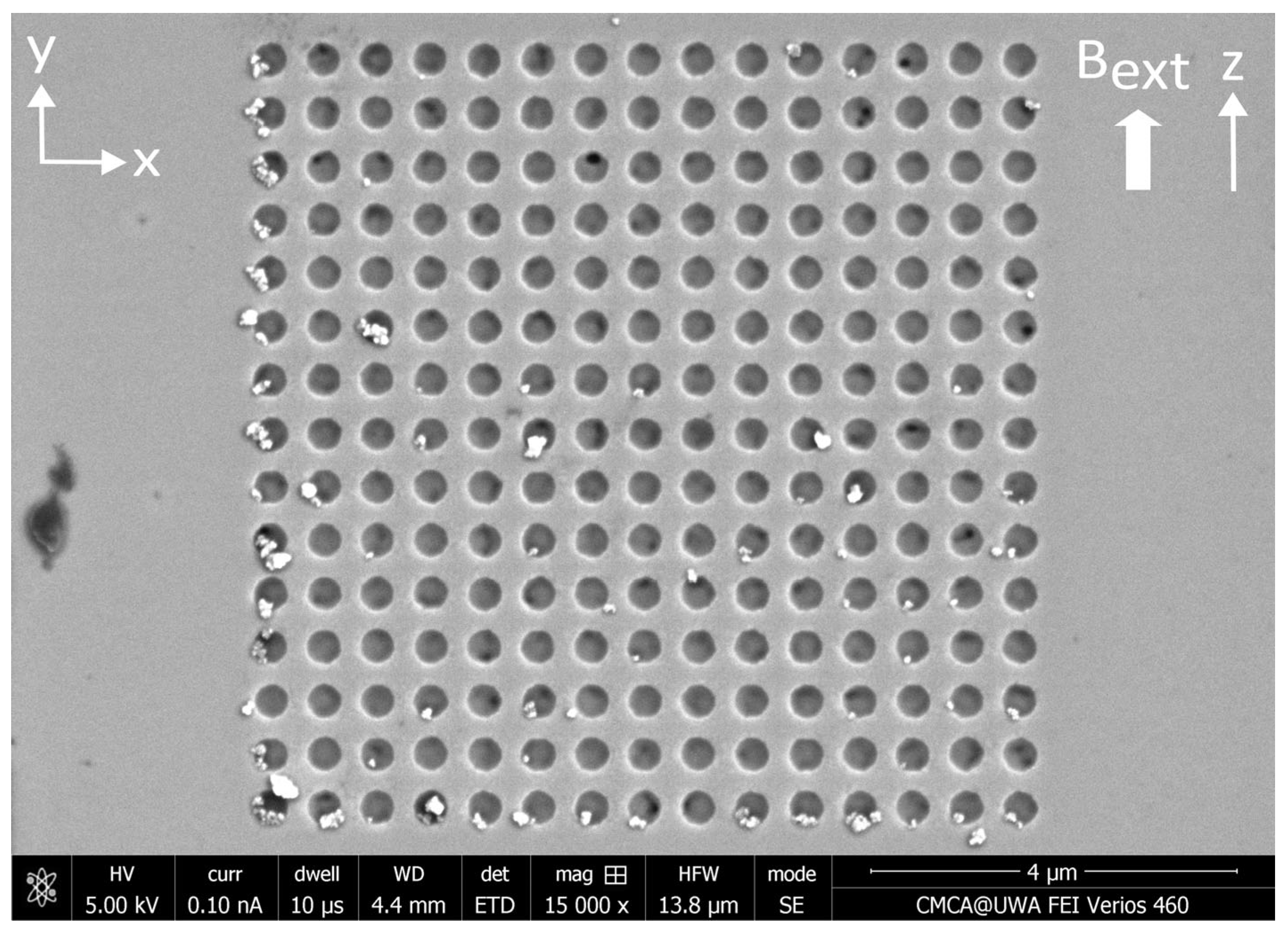
3.2. Results—MNP Capture with External Magnetic Field Parallel to Nanostructure
3.3. Results—MNP Capture with External Magnetic Field Perpendicular to Nanostructure
4. Discussion
4.1. Discussion—MNP Capture Without External Magnetic Field
4.2. Discussion—MNP Capture with External Magnetic Field Parallel to Nanostructure
4.3. Discussion—MNP Capture with External Magnetic Field Perpendicular to Nanostructure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
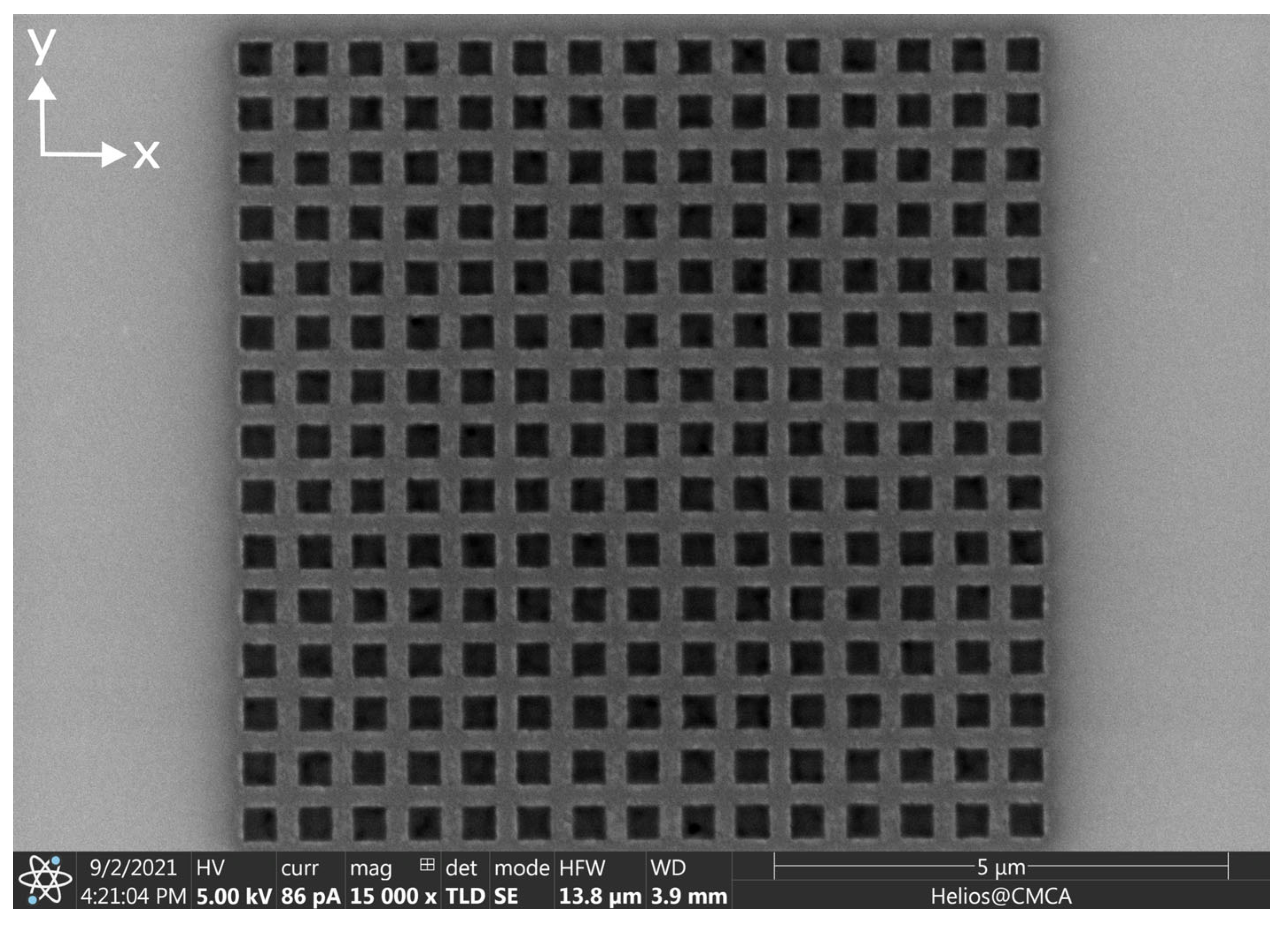
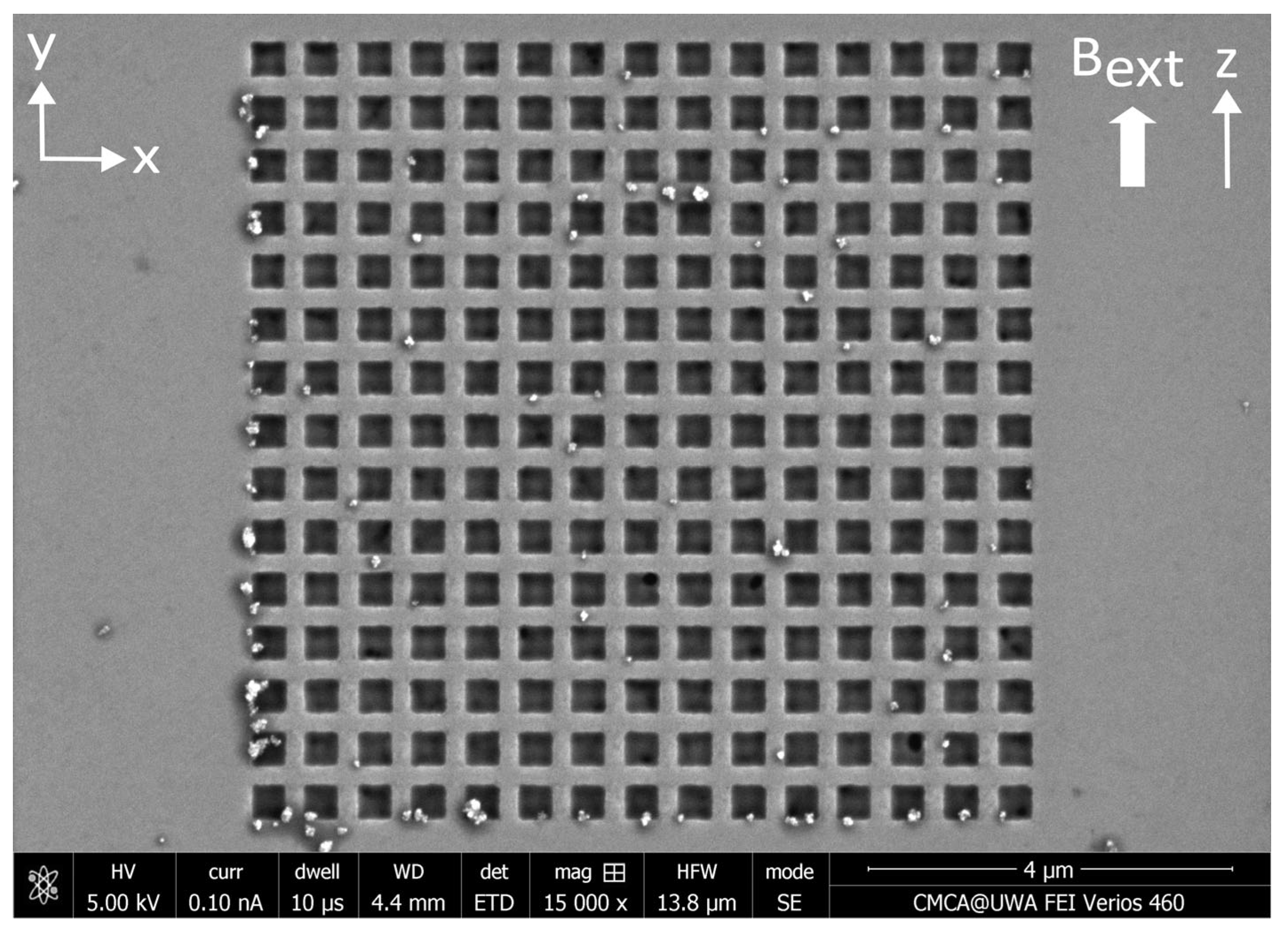
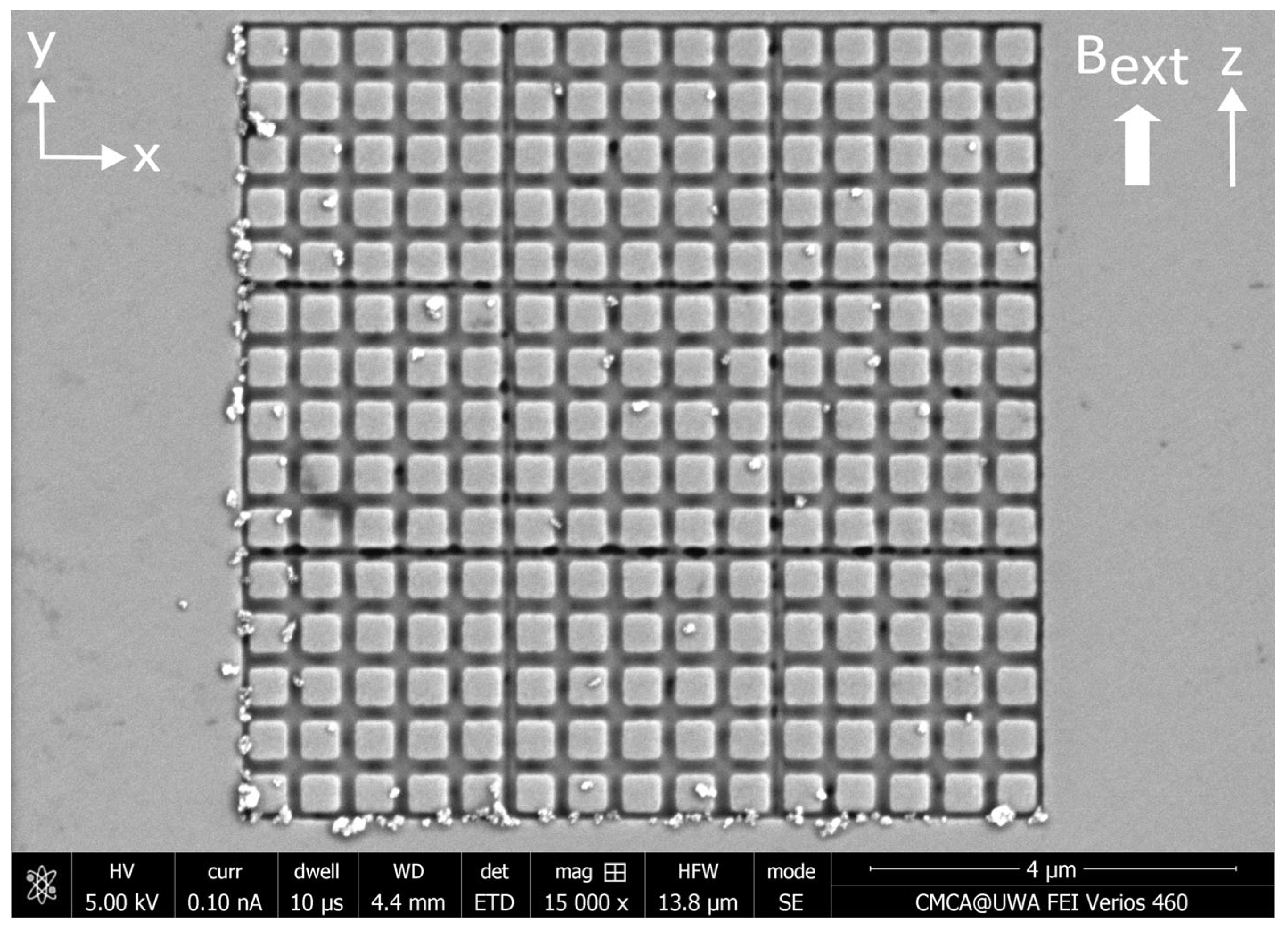
Appendix A. Nanostructures Prior to the Application of MNPs


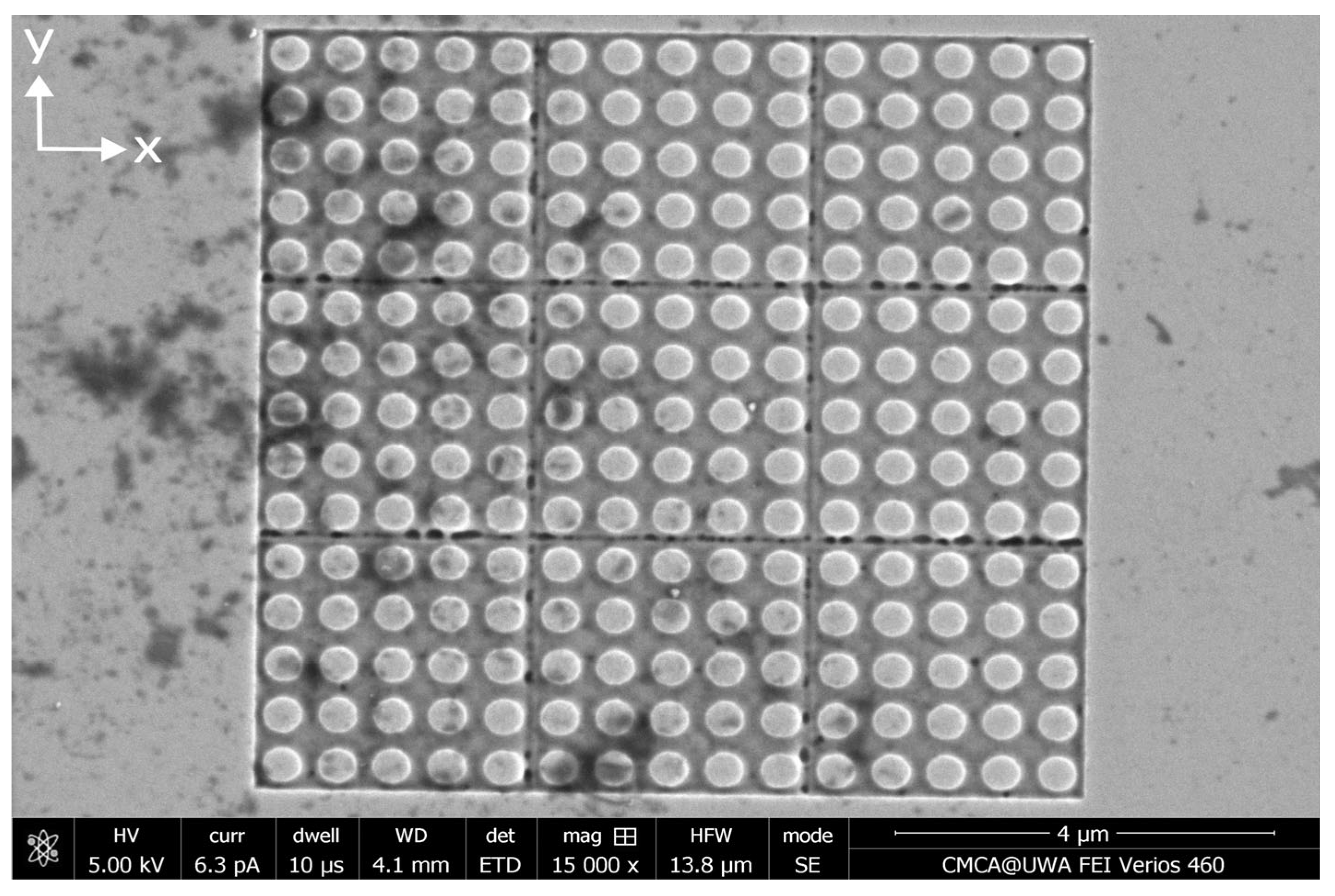
Appendix B. Distributions of MNPs Captured in the Absence of an External Magnetic Field
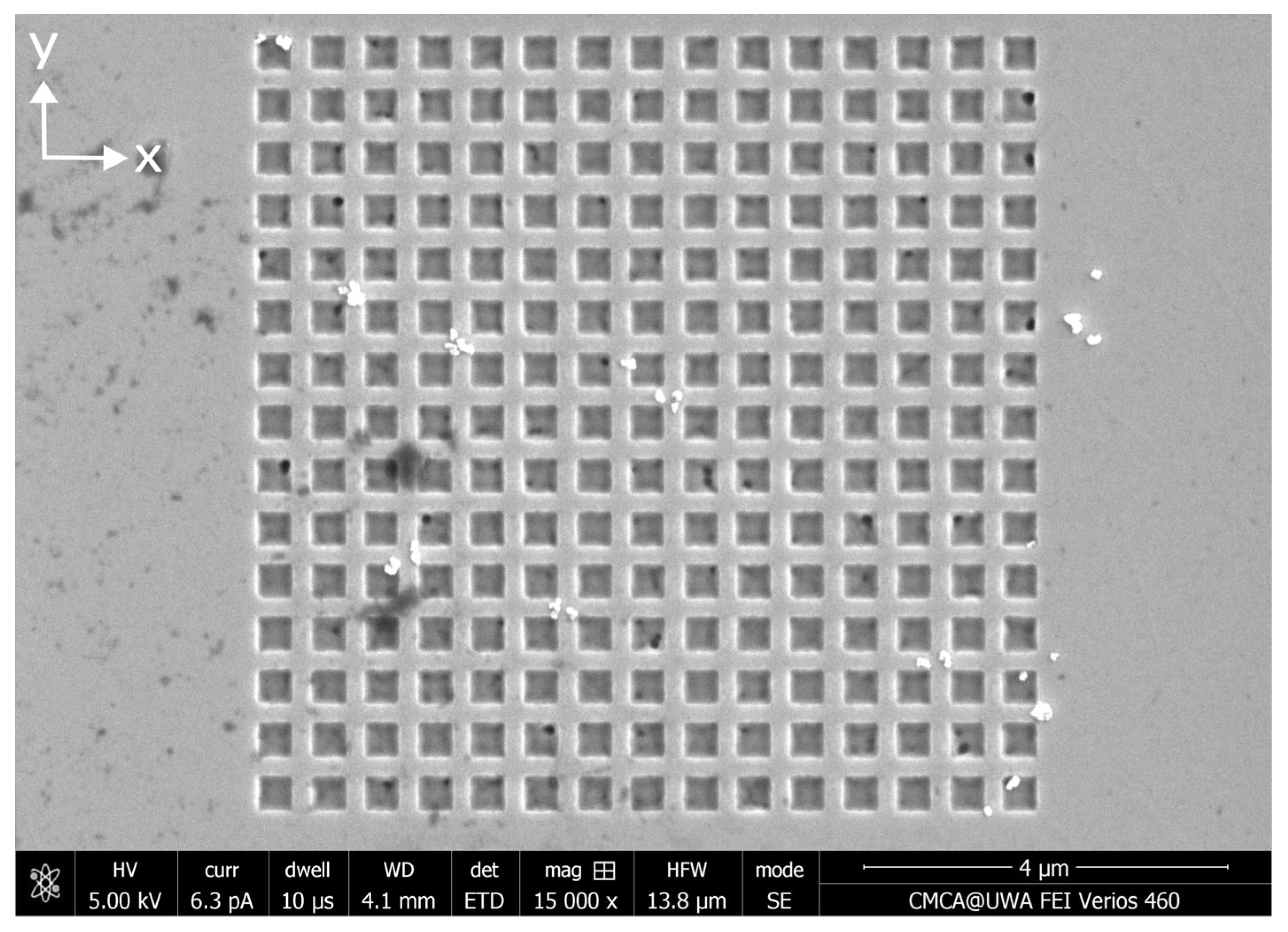
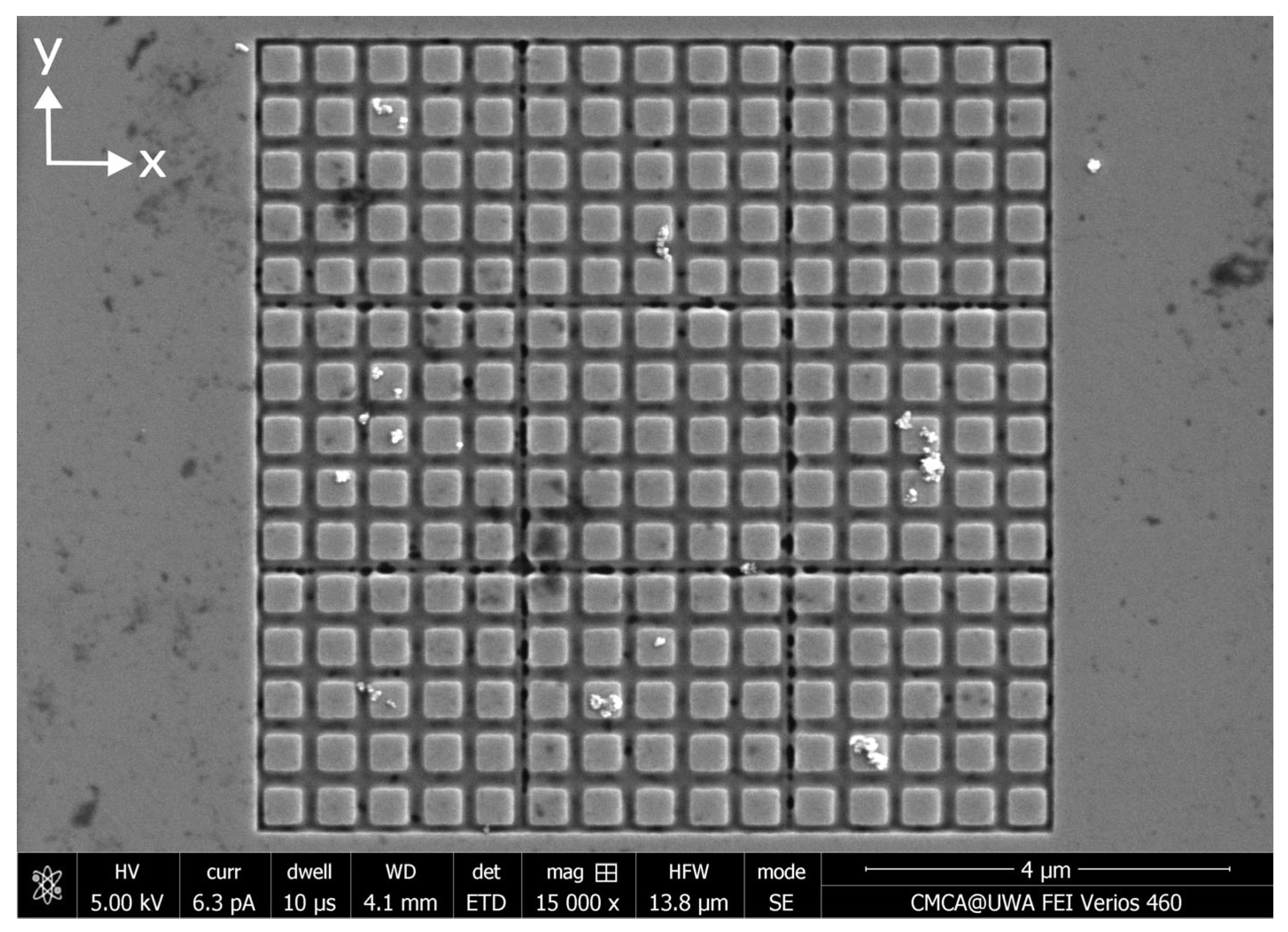
Appendix C. Distributions of MNPs Captured in a Parallel External Magnetic Field
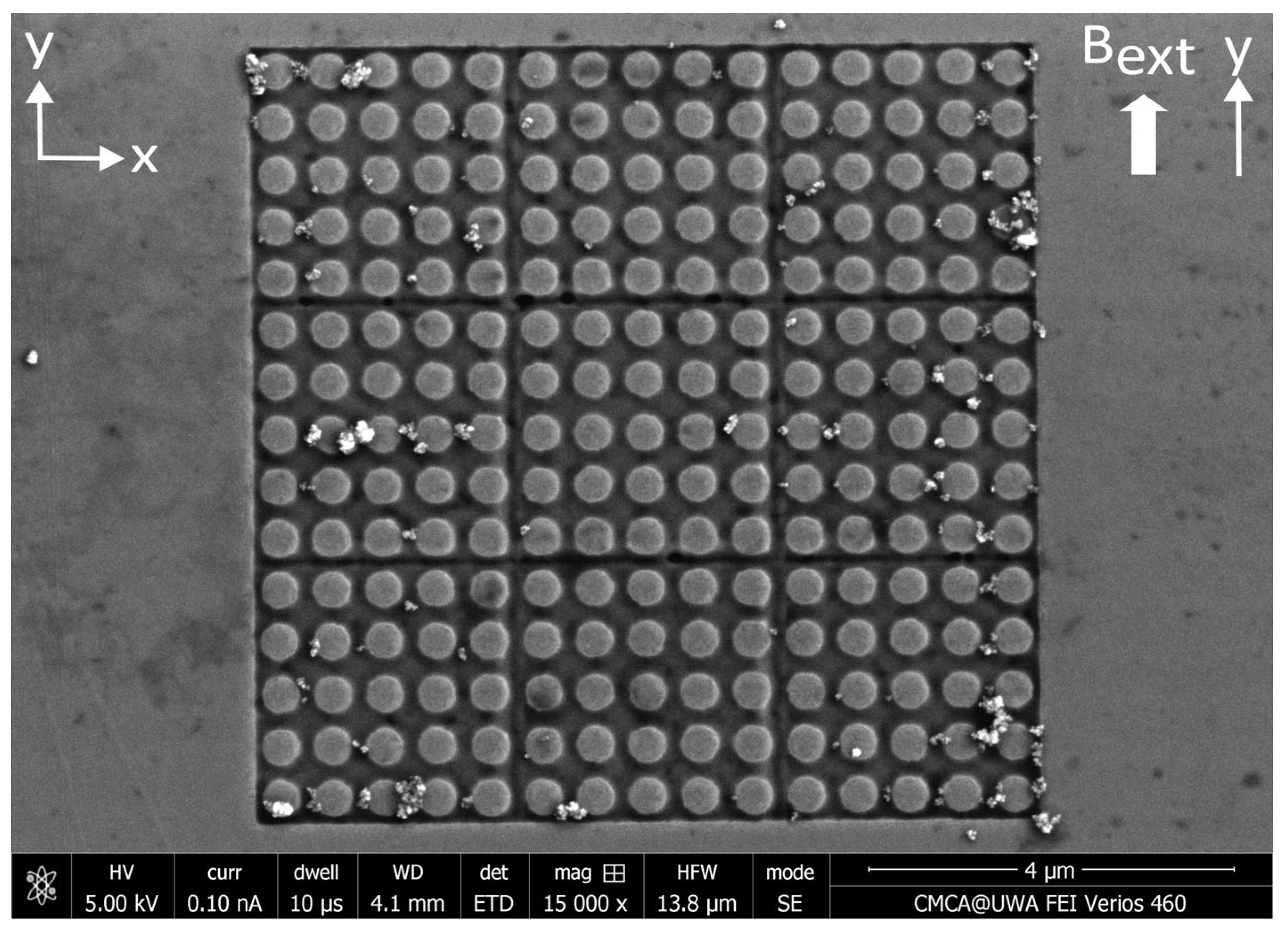
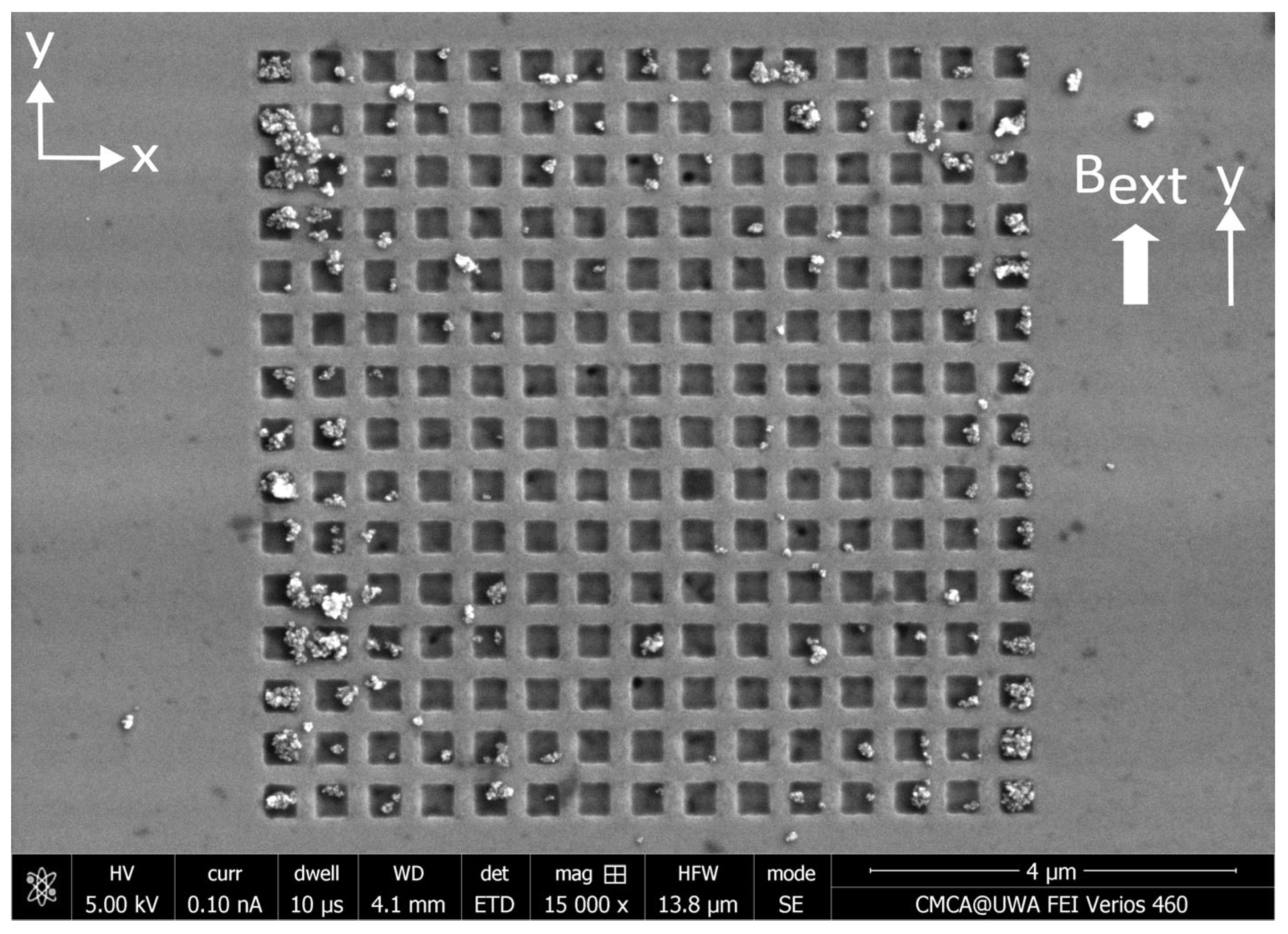
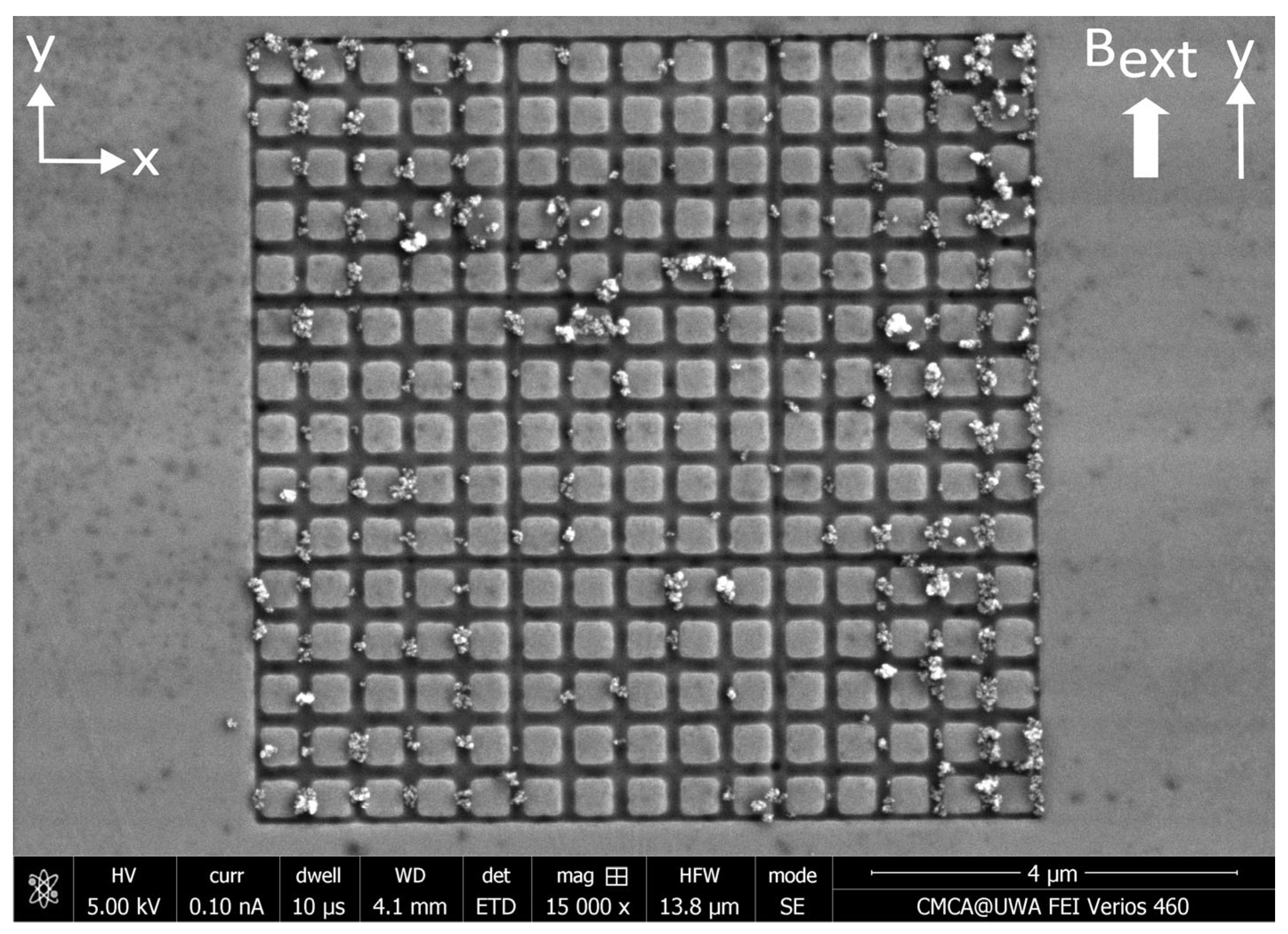
Appendix D. Distributions of MNPs Captured in a Perpendicular External Magnetic Field
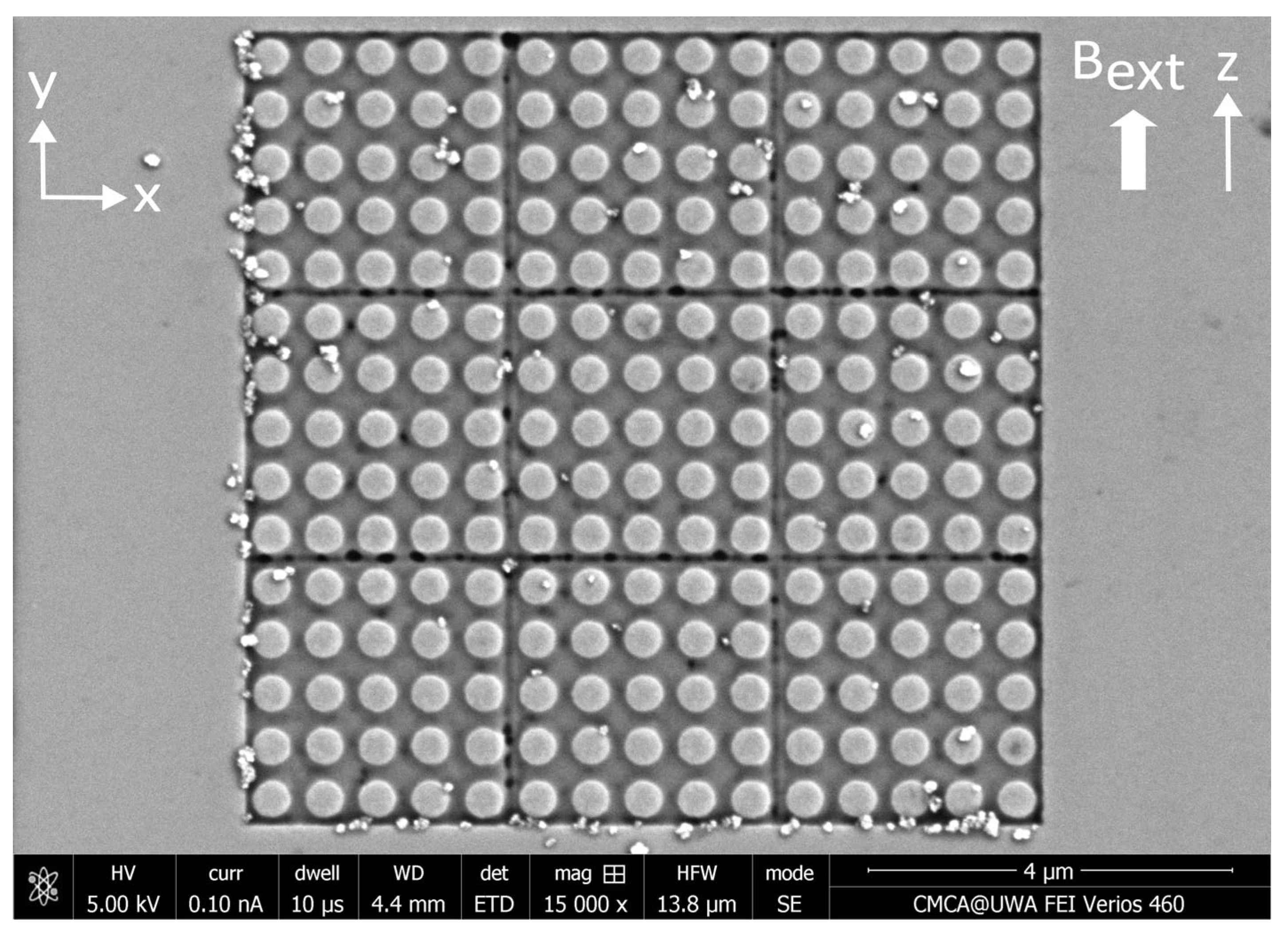
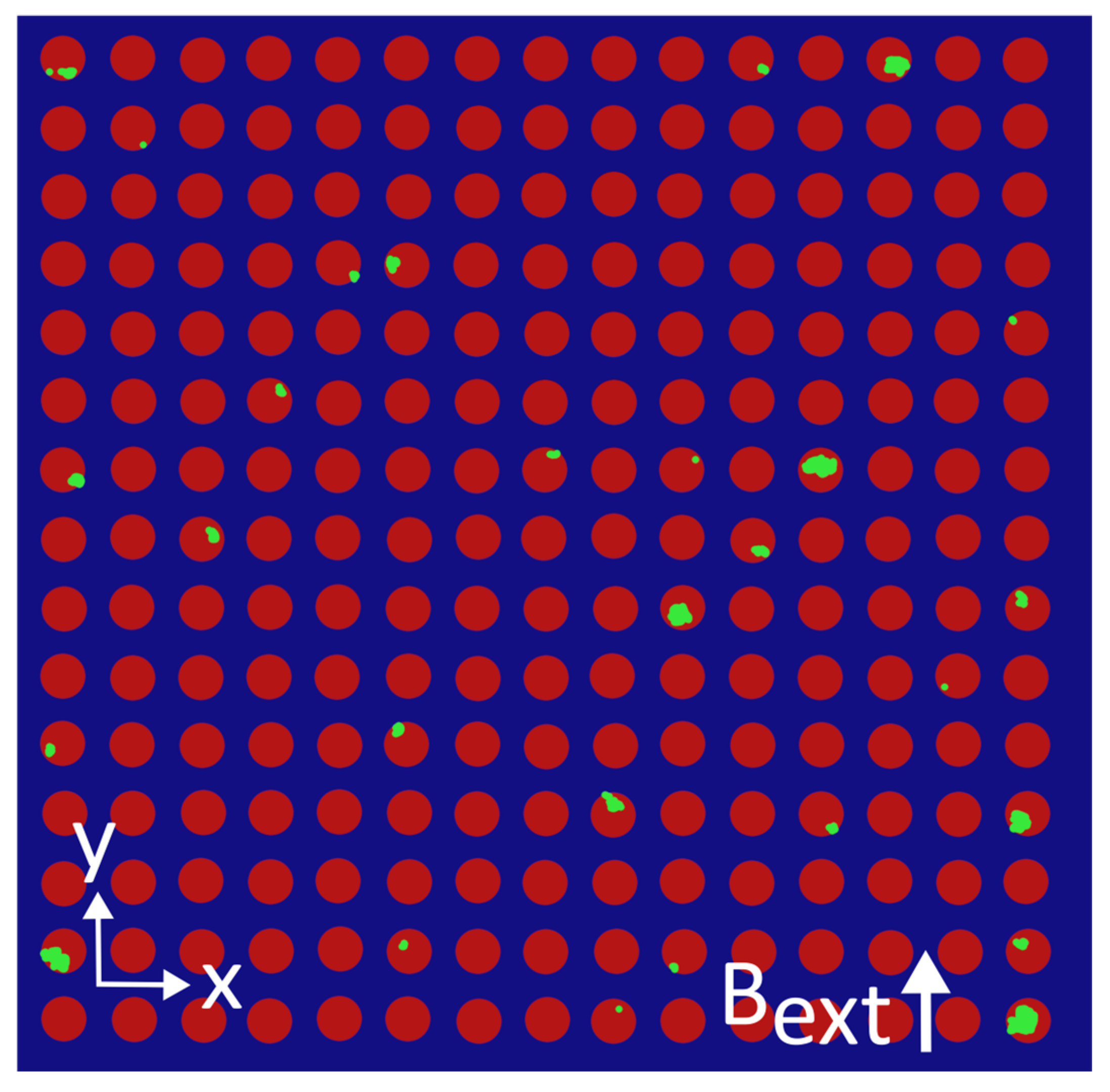
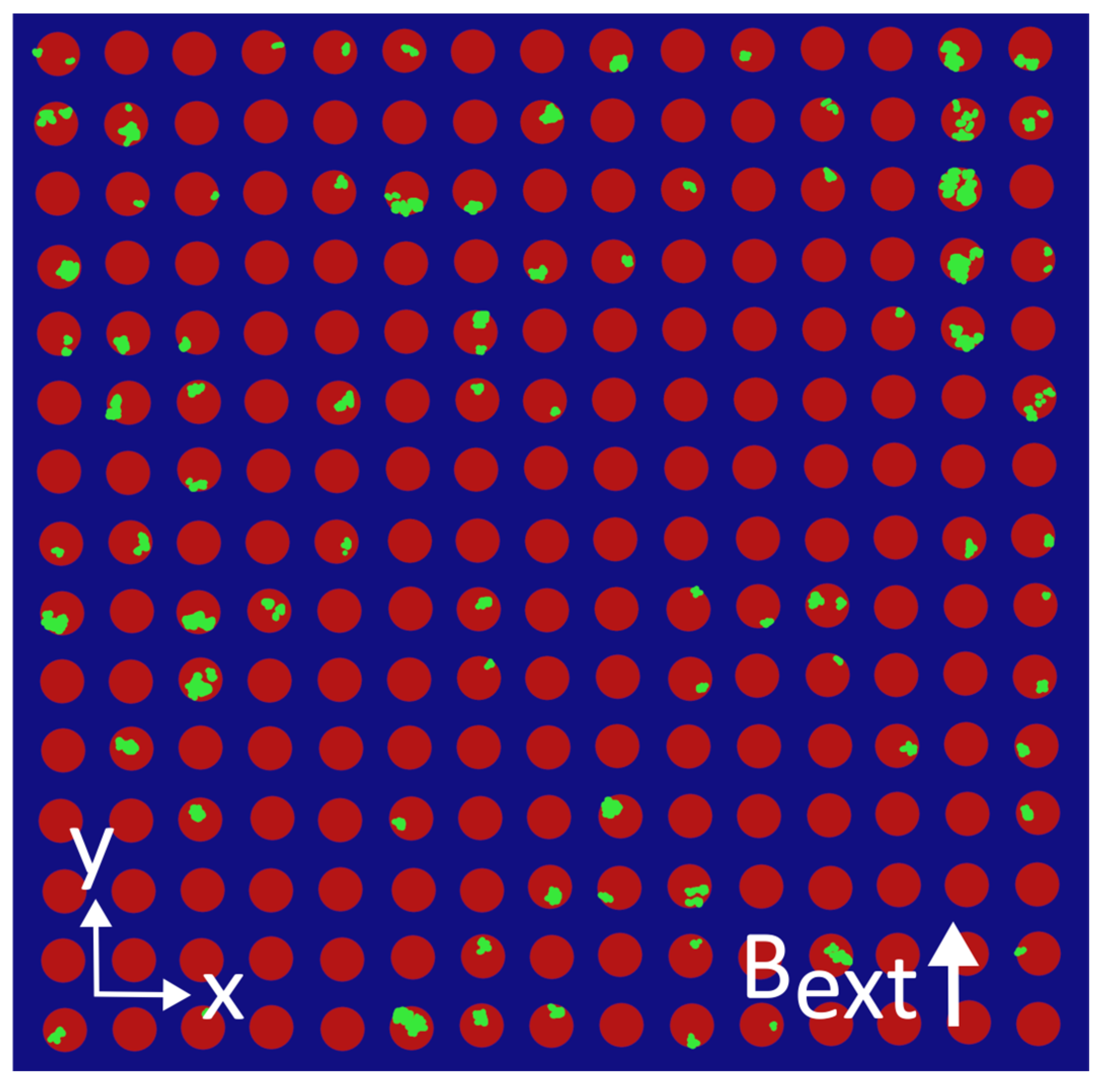
Appendix E. Colorized MNP Distributions


References
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y.K. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Hasany, S.F.; Rehman, A.; Jose, R.; Ahmed, I. Iron Oxide Magnetic Nanoparticles: A Short Review. AIP Conf. Proc. 2012, 1502, 298–321. [Google Scholar]
- Shipway, A.N.; Katz, E.; Willner, I. Nanoparticle Arrays on Surfaces for Electronic, Optical, and Sensor Applications. Chem. Physchem. 2000, 1, 18–52. [Google Scholar] [CrossRef]
- Luo, Y.; Alocilja, E.C. Portable Nuclear Magnetic Resonance Biosensor and Assay for a Highly Sensitive and Rapid Detection of Foodborne Bacteria in Complex Matrices. J. Biol. Eng. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, J.; Voepel, N.; Voß, L.; Rasche, S.; Schubert, M.; Kleines, M.; Krause, H.J.; Shaw, T.M.; Spiegel, H.; Schroeper, F. Development of Fast and Portable Frequency Magnetic Mixing-Based Serological SARS-CoV-2-Specific Antibody Detection Assay. Front. Microbiol. 2021, 12, 643275. [Google Scholar] [CrossRef]
- Xuecheng, S.; Chong, L.; Sun, X.; Lei, C.; Guo, L.; Zhou, Y. Separable Detecting of Escherichia Coli O157H:H7 by a Giant Magneto-Resistance-Based Bio-Sensing System. Sens. Actuators B Chem. 2016, 234, 485–492. [Google Scholar] [CrossRef]
- Su, D.; Wu, K.; Krishna, V.D.; Klein, T.; Liu, J.; Feng, Y.; Perez, A.M.; Cheeran, M.C.J.; Wang, J.P. Detection of Influenza A Virus in Swine Nasal Swab Samples with a Wash-Free Magnetic Bioassay and a Handheld Giant Magnetoresistance Sensing System. Front. Microbiol. 2019, 10, 1077. [Google Scholar] [CrossRef]
- Wu, C.C.; Lin, L.Y.; Lin, L.C.; Huang, H.C.; Yang, Y.F.; Liu, Y.B.; Tsai, M.C.; Gao, Y.L.; Wang, W.C.; Hung, S.W.; et al. Biofunctionalized Magnetic Nanoparticles for in Vitro Labeling and in Vivo Locating Specific Biomolecules. Appl. Phys. Lett. 2008, 92, 142504. [Google Scholar] [CrossRef]
- Wu, K.; Cheeran, M.C.J.; Wang, J.P.; Saha, R.; Su, D.; Krishna, V.D.; Liu, J. Magnetic-Nanosensor-Based Virus and Pathogen Detection Strategies before and during COVID-19. ACS Appl. Nano Mater. 2020, 3, 9560–9580. [Google Scholar] [CrossRef]
- Aytur, T.; Foley, J.; Anwar, M.; Boser, B.; Harris, E.; Beatty, P.R. A Novel Magnetic Bead Bioassay Platform Using a Microchip-Based Sensor for Infectious Disease Diagnosis. J. Immunol. Methods. 2006, 314, 21–29. [Google Scholar] [CrossRef]
- Kokkinis, G.; Cardoso, S.; Keplinger, F.; Giouroudi, I. Microfluidic Platform with Integrated GMR Sensors for Quantification of Cancer Cells. Sens. Actuators B Chem. 2017, 241, 438–445. [Google Scholar] [CrossRef]
- Lee, H.; Shin, T.H.; Cheon, J.; Weissleder, R. Recent Developments in Magnetic Diagnostic Systems. Chem. Rev. 2015, 115, 10690–10724. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, A.D.; Ley, M.W.H.; Flyvbjerg, H.; Hansen, M.F. Configurational Statistics of Magnetic Bead Detection with Magnetoresistive Sensors. PLoS ONE 2015, 10, e0141115. [Google Scholar] [CrossRef] [PubMed]
- Sushruth, M.; Ding, J.; Duczynski, J.; Woodward, R.C.; Begley, R.A.; Fangohr, H.; Fuller, R.O.; Adeyeye, A.O.; Kostylev, M.; Metaxas, P.J. Resonance-Based Detection of Magnetic Nanoparticles and Microbeads Using Nanopatterned Ferromagnets. Phys. Rev. Appl. 2016, 6, 044005. [Google Scholar] [CrossRef]
- Chen, Y.T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef]
- Schotter, J.; Kamp, P.B.; Becker, A.; Pühler, A.; Reiss, G.; Brückl, H. Comparison of a Prototype Magnetoresistive Biosensor to Standard Fluorescent DNA Detection. Biosens. Bioelectron. 2004, 19, 1149–1156. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Saha, R.; Wong, D.; Wang, J.P. Magnetic Particle Spectroscopy-Based Bioassays: Methods, Applications, Advances, and Future Opportunities. J. Phys. D Appl. Phys. 2019, 15, 173001. [Google Scholar] [CrossRef]
- Silverio, V.; López-Martínez, M.J.; Franco, F.; Amaral, M.; Gaspar, J.; Cardoso, S.; Freitas, P.P. On-Chip Magnetic Nanoparticle Manipulation and Trapping for Biomedical Applications. IEEE Trans. Magn. 2017, 53, 5100806. [Google Scholar] [CrossRef]
- Hash, S.; Martinez-Viedma, M.P.; Fung, F.; Han, J.E.; Yang, P.; Wong, C.; Doraisamy, L.; Menon, S.; Lightner, D. Nuclear Magnetic Resonance Biosensor for Rapid Detection of Vibrio Parahaemolyticus. Biomed. J. 2019, 42, 187–192. [Google Scholar] [CrossRef]
- Clarke, J.; Lee, Y.H.; Schneiderman, J. Focus on SQUIDs in Biomagnetism. Supercond. Sci. Technol. 2018, 31, 080201. [Google Scholar] [CrossRef]
- Cardoso, S.; Leitao, D.C.; Dias, T.M.; Valadeiro, J.; Silva, M.D.; Chicharo, A.; Silverio, V.; Gaspar, J.; Freitas, P.P. Challenges and Trends in Magnetic Sensor Integration with Microfluidics for Biomedical Applications. J. Phys. D Appl. Phys. 2017, 50, 213001. [Google Scholar] [CrossRef]
- Giouroudi, I.; Kokkinis, G. Recent Advances in Magnetic Microfluidic Biosensors. Nanomaterials 2017, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Little, C.A.E.; Pellegrino, J.; Russek, S.E. Microfluidic Platform for Magnetic Nanoparticle Trapping and Detection. IEEE Trans. Magn. 2013, 49, 3402–3405. [Google Scholar] [CrossRef]
- Munir, A.; Wang, J.; Zhou, H.S. Dynamics of Capturing Process of Multiple Magnetic Nanoparticles in a Flow through Microfluidic Bioseparation System. IET Nanobiotechnol. 2009, 3, 55–64. [Google Scholar] [CrossRef]
- Tian, W.-C.; Finehout, E. (Eds.) Microfluidics for Biological Applications; Springer Science+Business Media LLC: New York, NY, USA, 2008; ISBN 978-0-387-09479-3. [Google Scholar]
- Zhi, X.; Deng, M.; Yang, H.; Gao, G.; Wang, K.; Fu, H.; Zhang, Y.; Chen, D.; Cui, D. A Novel HBV Genotypes Detecting System Combined with Microfluidic Chip, Loop-Mediated Isothermal Amplification and GMR Sensors. Biosens. Bioelectron. 2014, 54, 372–377. [Google Scholar] [CrossRef]
- Metaxas, P.J.; Sushruth, M.; Begley, R.A.; Ding, J.; Woodward, R.C.; Maksymov, I.S.; Albert, M.; Wang, W.; Fangohr, H.; Adeyeye, A.O.; et al. Sensing Magnetic Nanoparticles Using Nano-Confined Ferromagnetic Resonances in a Magnonic Crystal. Appl. Phys. Lett. 2015, 106, 2392. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, K.; Xia, F.; Atwal, R.; Wang, H.; Ahmed, S.U.; Cardarelli, L.; Lui, I.; Duong, B.; Wang, Z.; et al. Phage-Based Profiling of Rare Single Cells Using Nanoparticle-Directed Capture. ACS Nano. 2021, 15, 19202–19210. [Google Scholar] [CrossRef]
- Wilson, R.J.; Hu, W.; Fu, C.W.P.; Koh, A.L.; Gaster, R.S.; Earhart, C.M.; Fu, A.; Heilshorn, S.C.; Sinclair, R.; Wang, S.X. Formation and Properties of Magnetic Chains for 100 Nm Nanoparticles Used in Separations of Molecules and Cells. J. Magn. Magn. Mater. 2009, 321, 1452–1458. [Google Scholar] [CrossRef]
- Zarutskaya, T.; Shapiro, M. Capture of Nanoparticles by Magnetic Filters. J. Aerosol. Sci. 2000, 31, 907–921. [Google Scholar] [CrossRef]
- Chuan Lim, E.W.; Feng, R. Agglomeration of Magnetic Nanoparticles. J. Chem. Phys. 2012, 136, 7865. [Google Scholar] [CrossRef]
- Lim, J.; Lanni, C.; Evarts, E.R.; Lanni, F.; Tilton, R.D.; Majetich, S.A. Magnetophoresis of Nanoparticles. ACS Nano 2011, 5, 217–226. [Google Scholar] [CrossRef]
- Shih, P.H.; Shiu, J.Y.; Lin, P.C.; Lin, C.C.; Veres, T.; Chen, P. On Chip Sorting of Bacterial Cells Using Sugar-Encapsulated Magnetic Nanoparticles. J. Appl. Phys. 2008, 103, 4720. [Google Scholar] [CrossRef]
- Suwa, M.; Watarai, H. Magnetoanalysis of Micro/Nanoparticles: A Review. Anal. Chim. Acta 2011, 690, 137–147. [Google Scholar] [CrossRef]
- Ferraro, D.; Champ, J.; Teste, B.; Serra, M.; Malaquin, L.; Viovy, J.L.; De Cremoux, P.; Descroix, S. Microfluidic Platform Combining Droplets and Magnetic Tweezers: Application to HER2 Expression in Cancer Diagnosis. Sci. Rep. 2016, 6, srep25540. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.F.; Miranda, D.; Botelho, G.; Minas, G.; Lanceros-Méndez, S. Highly Effective Clean-up of Magnetic Nanoparticles Using Microfluidic Technology. Sens. Actuators B Chem. 2018, 255, 2384–2391. [Google Scholar] [CrossRef]
- Najafpoor, A.; Norouzian-Ostad, R.; Alidadi, H.; Rohani-Bastami, T.; Davoudi, M.; Barjasteh-Askari, F.; Zanganeh, J. Effect of Magnetic Nanoparticles and Silver-Loaded Magnetic Nanoparticles on Advanced Wastewater Treatment and Disinfection. J. Mol. Liq. 2020, 303, 112640. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Z.; Jia, S.; Zhang, P. The Force Analysis for Superparamagnetic Nanoparticles-Based Gene Delivery in an Oscillating Magnetic Field. J. Magn. Magn. Mater. 2017, 427, 85–89. [Google Scholar] [CrossRef]
- Munaz, A.; Shiddiky, M.J.A.; Nguyen, N.T. Recent Advances and Current Challenges in Magnetophoresis Based Micro Magnetofluidics. Biomicrofluidics 2018, 12, 5388. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Z.; Chen, S.; Jia, S. Experimental and Numerical Analysis of the Magnetophoresis of Magnetic Nanoparticles under the Influence of Cylindrical Permanent Magnet. J. Magn. Magn. Mater. 2019, 475, 703–714. [Google Scholar] [CrossRef]
- Teste, B.; Jamond, N.; Ferraro, D.; Viovy, J.L.; Malaquin, L. Selective Handling of Droplets in a Microfluidic Device Using Magnetic Rails. Microfluid. Nanofluidics 2015, 19, 141–153. [Google Scholar] [CrossRef]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the Coffee-Ring Effect by Shape-Dependent Capillary Interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef]
- Dowling, R.; Kostylev, M. Enhancing the Capture of Magnetic Nanoparticles Inside of Ferromagnetic Nanostructures Using External Magnetic Fields. arXix 2023, arXiv:2308.12321. [Google Scholar] [CrossRef]
- Vansteenkiste, A.; De Wiele, B. Van MUMAX: A New High-Performance Micromagnetic Simulation Tool. J. Magn. Magn. Mater. 2011, 323, 2585–2591. [Google Scholar] [CrossRef]
- Vansteenkiste, A.; Leliaert, J.; Dvornik, M.; Helsen, M.; Garcia-Sanchez, F.; Van Waeyenberge, B. The Design and Verification of MuMax3. AIP Adv. 2014, 4, 9186. [Google Scholar] [CrossRef]
- Ezzaier, H.; Alves Marins, J.; Schaub, S.; Amara, B.H.; Kuzhir, P. Capture of Magnetic Nanoparticles on Ordered Magnetizable Arrays: A Parametric Study. J. Magn. Magn. Mater. 2018, 459, 350–355. [Google Scholar] [CrossRef]
- Bruchhaus, L.; Mazarov, P.; Bischoff, L.; Gierak, J.; Wieck, A.D.; Hövel, H. Comparison of Technologies for Nano Device Prototyping with a Special Focus on Ion Beams: A Review. Appl. Phys. Rev. 2017, 4, 2262. [Google Scholar] [CrossRef]
- Gierak, J.; Madouri, A.; Biance, A.L.; Bourhis, E.; Patriarche, G.; Ulysse, C.; Lucot, D.; Lafosse, X.; Auvray, L.; Bruchhaus, L.; et al. Sub-5 Nm FIB Direct Patterning of Nanodevices. Microelectron. Eng. 2007, 84, 779–783. [Google Scholar] [CrossRef]
- Joshi-Imre, A.; Bauerdick, S. Direct-Write Ion Beam Lithography. J. Nanotechnol. 2014, 2014, 170415. [Google Scholar] [CrossRef]
- Khizroev, S.; Litvinov, D. Focused-Ion-Beam-Based Rapid Prototyping of Nanoscale Magnetic Devices. Nanotechnology 2004, 15, R7. [Google Scholar] [CrossRef]
- Orloff, J. High-Resolution Focused Ion Beams. Rev. Sci. Instrum. 1993, 64, 1105–1130. [Google Scholar] [CrossRef]
- Yao, N. Focused Ion Beam Systems—A Multifunctional Tool for Nanotechnology. In Handbook of Microscopy for Nanotechnology; Yao, N., Wang, Z.L., Eds.; Kluwer Academic Publishers: Norwell, MA, USA, 2005; pp. 247–286. ISBN 1-4020-8003-4. [Google Scholar]
- Gierak, J.; Mailly, D.; Hawkes, P.; Jede, R.; Bruchhaus, L.; Bardotti, L.; Frével, B.; Mélinon, P.; Perez, A.; Hyndman, R.; et al. Exploration of the Ultimate Patterning Potential Achievable with High Resolution Focused Ion Beams. Appl. Phys. A Mater. Sci. Process. 2005, 80, 187–194. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer Microfabrication Methods for Microfluidic Analytical Applications. Electrophoresis 2000, 21, 12–26. [Google Scholar] [CrossRef]
- Mcdonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of Microfluidic Systems in Poly(Dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Mulunfeh, M.; Issadore, D. A Multi-Scale PDMS Fabrication Strategy to Bridge the Size Mismatch between Integrated Circuits and Microfluidics. Lab Chip 2014, 14, 4552–4558. [Google Scholar] [CrossRef] [PubMed]
- Taib, N.I.; Woodward, R.C.; Pierre, T.G.S.; Iyer, K.S. Chain Formation of PNIPAM-Coated Magnetic Nanoparticles in an External Magnetic Field and the Effect of Temperature. IEEE Trans. Magn. 2022, 58, 2951. [Google Scholar] [CrossRef]
- Ku, J.Y.; Aruguete, D.M.; Alivisatos, A.P.; Geissler, P.L. Self-Assembly of Magnetic Nanoparticles in Evaporating Solution. J. Am. Chem. Soc. 2011, 133, 838–848. [Google Scholar] [CrossRef]
- Tsouris, C.; Scott, T.C. Flocculation of Paramagnetic Nanoparticles in a Magnetic Field. J. Colloid. Interface Sci. 1995, 171, 319–330. [Google Scholar] [CrossRef]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-Free Detection and Molecular Profiling of Exosomes with a Nano-Plasmonic Sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef]




| Nanostructure Geometry | (%) | (%) | ||||
|---|---|---|---|---|---|---|
| Circle Antidots | 152 | 321 | 12.88 | 7.82 | 62.2 | 70.8 |
| Circle Dots | 7 | 6 | 0.19 | 0.62 | 23.5 | 21.3 |
| Square Antidots | 50 | 195 | 3.13 | 7.43 | 29.6 | 55.9 |
| Square Dots | 191 | 354 | 7.28 | 21.07 | 25.7 | 35.7 |
| Nanostructure Geometry | (%) | (%) | ||||
|---|---|---|---|---|---|---|
| Circle Antidots | 474 | 389 | 46.52 | 9.48 | 83.1 | 70.5 |
| Circle Dots | 363 | 120 | 9.73 | 12.43 | 43.9 | 44.1 |
| Square Antidots | 1095 | 493 | 72.04 | 18.78 | 79.3 | 63.9 |
| Square Dots | 1204 | 421 | 48.16 | 25.06 | 65.8 | 47.7 |
| Nanostructure Geometry | (%) | |||||
|---|---|---|---|---|---|---|
| Circle Antidots | 254 | 277 | 22.56 | 6.75 | 77.0 | 24.1 |
| Circle Dots | 323 | 400 | 7.87 | 41.44 | 16.0 | 7.5 |
| Square Antidots | 173 | 268 | 10.3 | 10.21 | 50.2 | 28.8 |
| Square Dots | 206 | 295 | 7.85 | 17.56 | 30.9 | 20.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dowling, R.; Kostylev, M. Controlled Capture of Magnetic Nanoparticles from Microfluidic Flows by Ferromagnetic Antidot and Dot Nanostructures. Nanomaterials 2025, 15, 132. https://doi.org/10.3390/nano15020132
Dowling R, Kostylev M. Controlled Capture of Magnetic Nanoparticles from Microfluidic Flows by Ferromagnetic Antidot and Dot Nanostructures. Nanomaterials. 2025; 15(2):132. https://doi.org/10.3390/nano15020132
Chicago/Turabian StyleDowling, Reyne, and Mikhail Kostylev. 2025. "Controlled Capture of Magnetic Nanoparticles from Microfluidic Flows by Ferromagnetic Antidot and Dot Nanostructures" Nanomaterials 15, no. 2: 132. https://doi.org/10.3390/nano15020132
APA StyleDowling, R., & Kostylev, M. (2025). Controlled Capture of Magnetic Nanoparticles from Microfluidic Flows by Ferromagnetic Antidot and Dot Nanostructures. Nanomaterials, 15(2), 132. https://doi.org/10.3390/nano15020132






