Local Structural Changes in High-Alumina, Low-Lithium Glass-Ceramics During Crystallization
Abstract
1. Introduction
2. Experimental
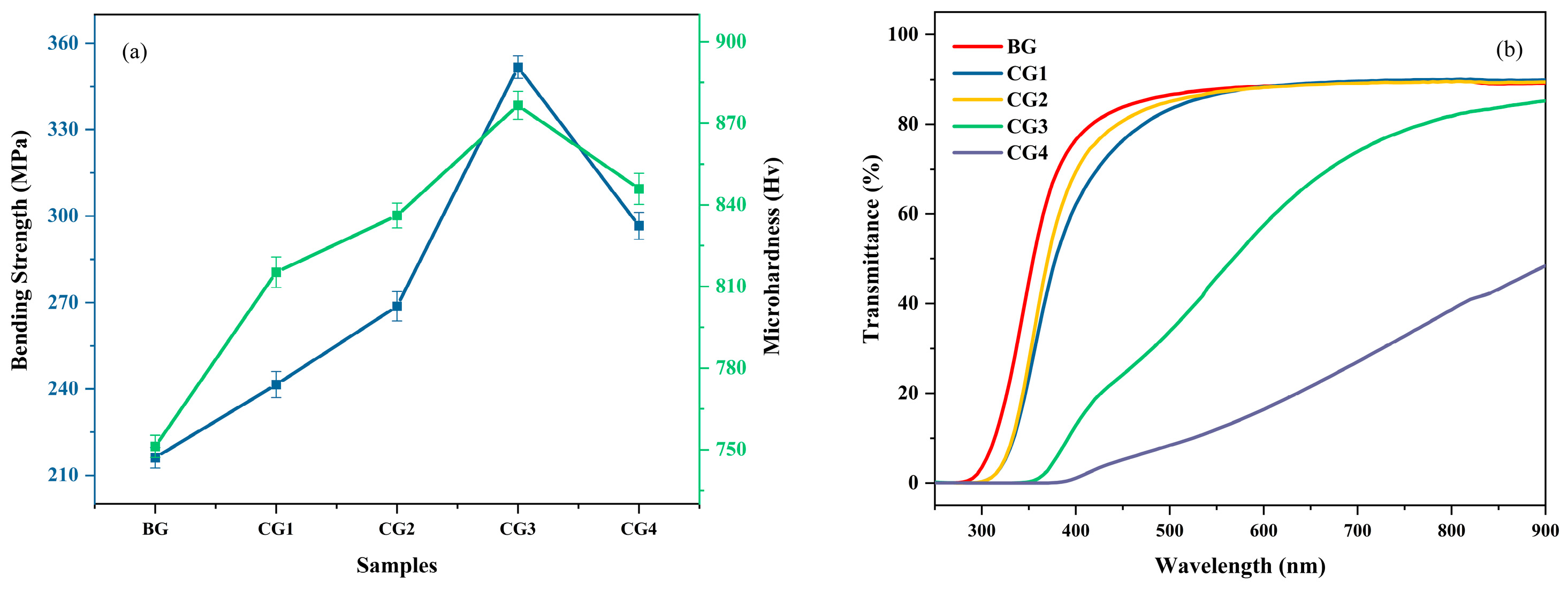
3. Results and Discussion
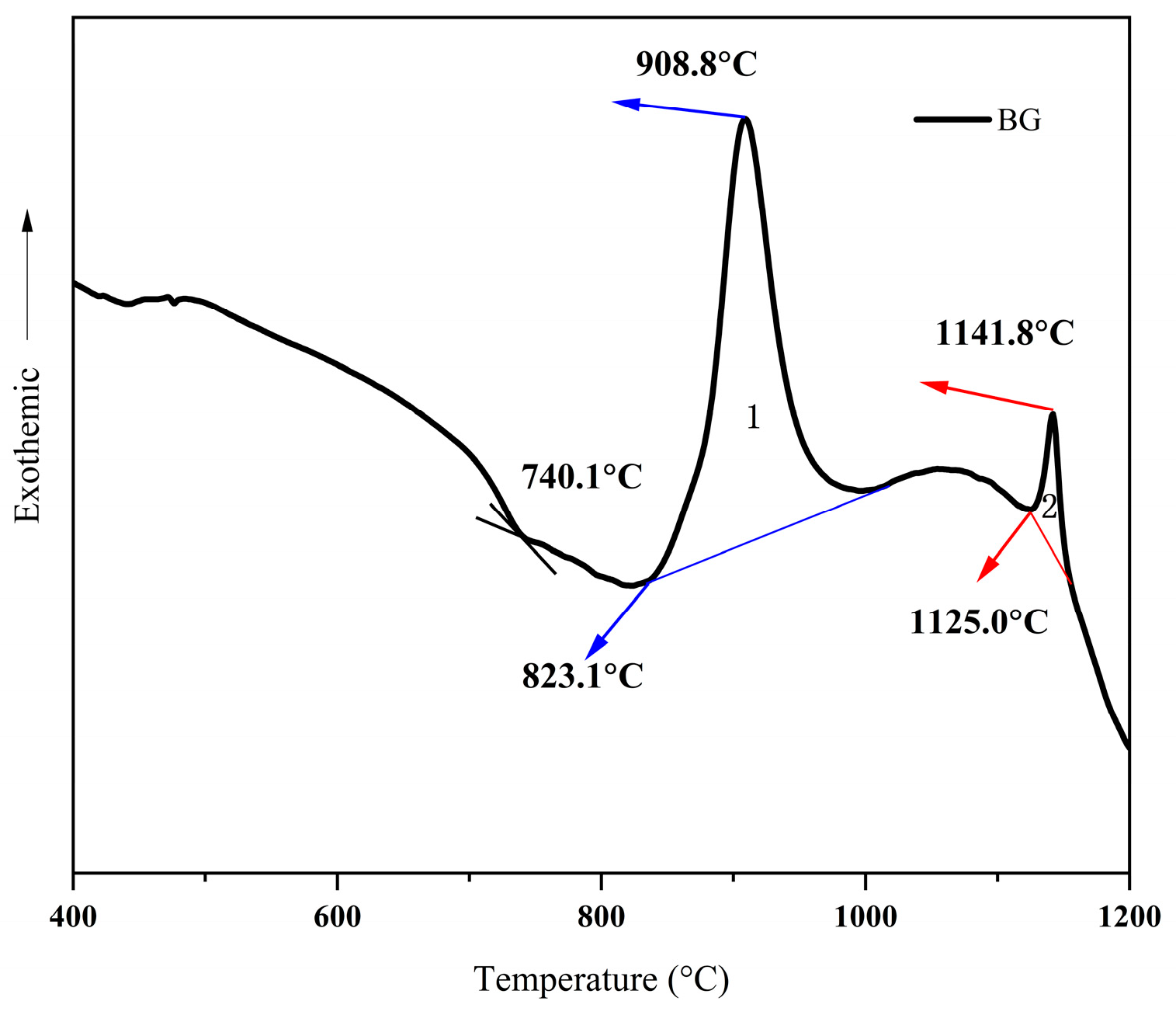
3.1. Differential Scanning Calorimetry (DSC)
3.2. HT-XRD
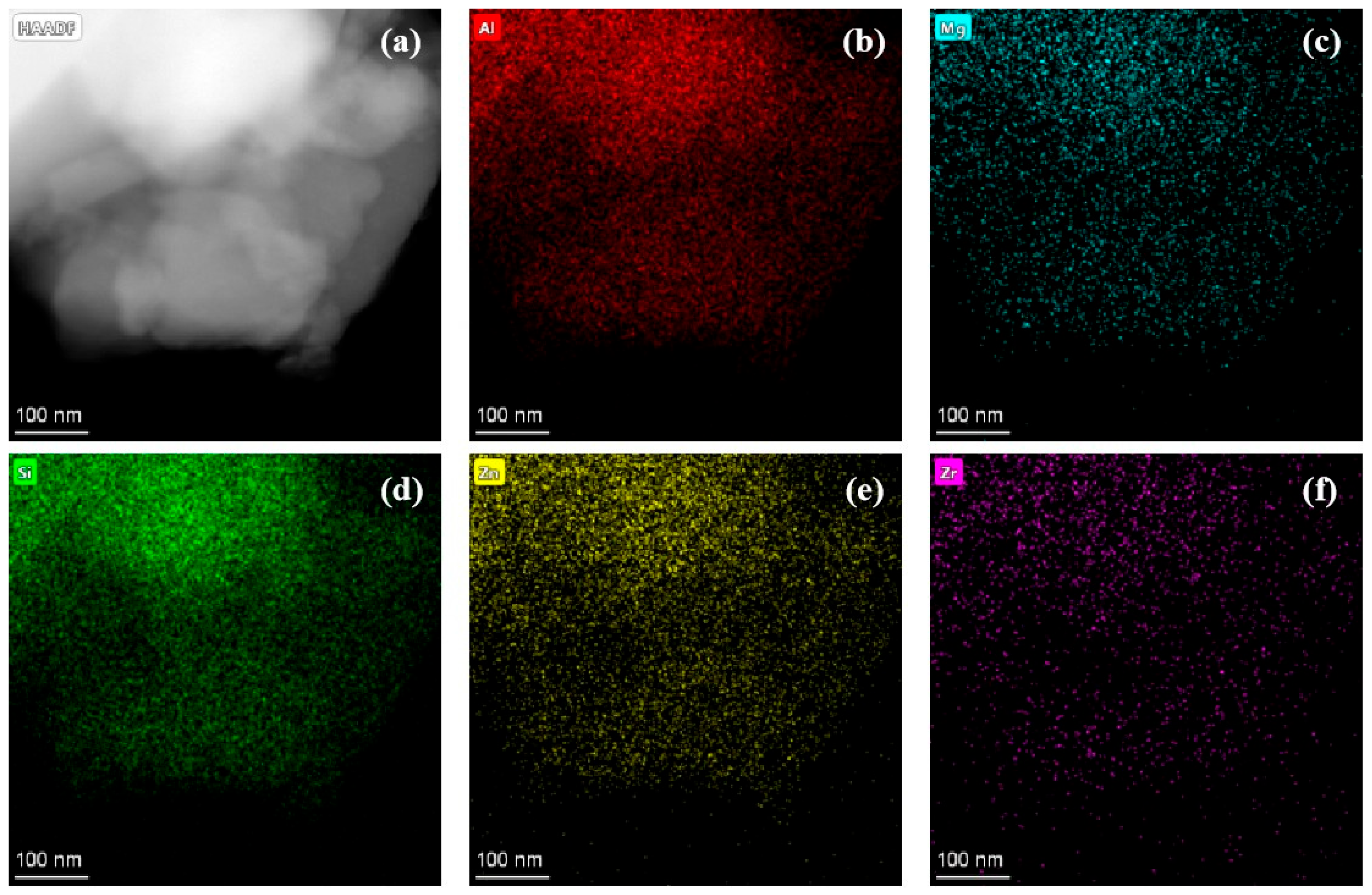
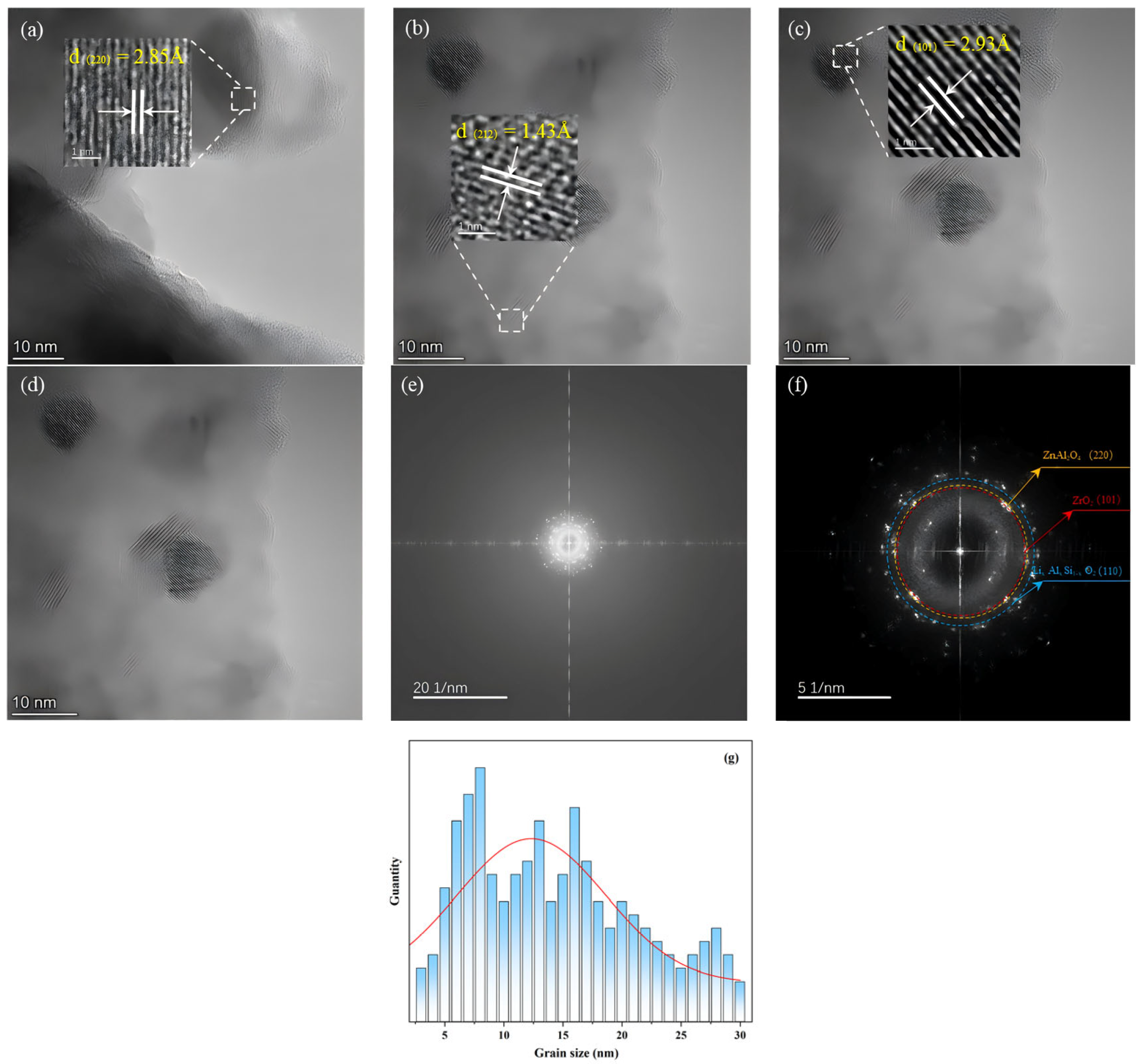
3.3. TEM-EDS
3.4. Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, G.; Wu, Y.; Yang, J.; Qian, J.; Gong, F.; Zheng, Q.; Zhang, D.; Wang, M. Transparent aluminosilicate glass-ceramics containing ZrO2 nanocrystals: Role of Al2O3/Li2O in glass structure and mechanical properties. J. Alloys Compd. 2025, 1018, 179257. [Google Scholar] [CrossRef]
- Heydari, F.; Mirkazemi, S.M.; Yekta, B.E.; Afghahi, S.S.S. Low-temperature spark plasma sintering and heat treatment of 70 wt% Si3N4—30 wt% (BaO-Al2O3-SiO2-ZrO2) glass-ceramic composites: Microstructure, mechanical, and dielectric properties. J. Mater. Res. Technol. 2025, 37, 133–145. [Google Scholar] [CrossRef]
- Neuville, D.R. From Glass to Crystal: Nucleation, Growth and Phase Separation: From Research to Applications; EDP Sciences: Les Ulis, France, 2017. [Google Scholar]
- Ketkova, L.A.; Velmuzhov, A.P.; Sukhanov, M.V.; Stepanov, B. Transparent IR glass ceramics: Requirements for the dispersed structure and for the methods of its control. J. Eur. Ceram. Soc. 2021, 41, 7852–7861. [Google Scholar] [CrossRef]
- Chen, D.; Xiang, W.; Liang, X.; Zhong, J.; Yu, H.; Ding, M.; Lu, H.; Ji, Z. Advances in transparent glass-ceramic phosphors for white light-emitting diodes-A review. J. Eur. Ceram. Soc. 2015, 35, 859–869. [Google Scholar] [CrossRef]
- Akinribide, O.J.; Mekgwe, G.N.; Akinwamide, S.O.; Gamaoun, F.; Abeykoon, C.; Johnson, O.T.; Olubambi, P.A. A review on optical properties and application of transparent ceramics. J. Mater. Res. Technol. 2022, 21, 712–738. [Google Scholar] [CrossRef]
- Kenzhina, I.E.; Kozlovskiy, A.L.; Begentayev, M.; Blynskiy, P.; Tolenova, A.; Popov, A.I. Study of Phase Transformations in ZrO2 Ceramics Stabilized by Y2O3 and Their Role in Changing Strength Characteristics and Heat Resistance. Sustainability 2025, 17, 4284. [Google Scholar] [CrossRef]
- Qi, S.; Porotnikova, N.M.; Ananyev, M.V.; Kuzmin, A.V.; Eremin, V.A.; Pankratov, A.A.; Molchanova, N.G.; Reznitskikh, O.G.; Farlenkov, A.S.; Vovkotrub, E.G.; et al. High-temperature glassy-ceramic sealants SiO2–Al2O3–BaO–MgO and SiO2–Al2O3–ZrO2–CaO–Na2O for solid oxide electrochemical devices. Trans. Nonferrous Met. Soc. China 2016, 26, 2916–2924. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, K.; Huang, Z.; Zhang, H.; Xie, H.; He, F.; Duo, S.; Shi, J. Optimizing damage resistance and structural evolution in CaO-modified Li2O-Al2O3-B2O3-TiO2-P2O5 glass-ceramics. Ceram. Int. 2024, 50, 50635–50647. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, M.; Liu, G.; Yuan, J.; Tian, P.; Peng, Z. The effects of B2O3 on the structure, phase separation and crystallization of lithium aluminosilicate glass-ceramics with Y2O3 addition. Ceram. Int. 2025, 51, 6515–6524. [Google Scholar] [CrossRef]
- Huang, X.; Sun, Z.; Fang, J.; Deng, S.; Zhao, W.; Li, P.; Yan, B.; Guo, H.; Song, Q. Effect of sintering behavior and phase evolution on glass-ceramics entirely derived from ferrochrome slag and fluorite tailings. Ceram. Int. 2024, 50, 48724–48735. [Google Scholar] [CrossRef]
- Simkins, K.; Greiner, L.; Nascimento, M.L.F.; Bragatto, C.; Misture, S.T.; Wilkinson, C. Approximating nucleation rates of glass ceramics using in-situ X-ray diffraction. Materialia 2024, 38, 102239. [Google Scholar] [CrossRef]
- Mendez-Gonzalez, Y.; Pelaiz-Barranco, A.; Curcio, A.L.; Rodrigues, A.D.; Guerra, J.D.S. Raman spectroscopy study of the La-modified (Bi0.5Na0.5)0.92Ba0.08TiO3 lead-free ceramic system. J. Raman Spectrosc. 2019, 50, 1044–1050. [Google Scholar] [CrossRef]
- Enríquez, E.; Fuertes, V.; Cabrera, M.J.; Seores, J.; Muñoz, D.; Galiana, B.; Fernández, J. Study of the crystallization in fast sintered Na-rich plagioclase glass-ceramic. Ceram. Int. 2019, 45, 8899–8907. [Google Scholar] [CrossRef]
- Colomban, P.; Schreiber, H.D. Raman signature modification induced by copper nanoparticles in silicate glass. J. Raman Spectrosc. 2005, 36, 884–890. [Google Scholar] [CrossRef]
- Sundararaman, S.; Huang, L.P.; Ispas, S.; Kob, W. New interaction potentials for alkali and alkaline-earth aluminosilicate glasses. J. Chem. Phys. 2019, 151, 154505. [Google Scholar] [CrossRef]
- Garces, H.F.; Senturk, B.S.; Padture, N.P. In situ Raman spectroscopy studies of high-temperature degradation of thermal barrier coatings by molten silicate deposits. Scr. Mater. 2014, 76, 29–32. [Google Scholar] [CrossRef]
- Yoshiya, M.; Tanaka, I.; Adachi, H. Energetical role of modeled intergranular glassy film in Si3N4-SiO2 ceramics. Acta Mater. 2000, 48, 4641–4645. [Google Scholar] [CrossRef]
- Lu, Y.; Xie, J.; Guo, Y.; Liu, C. Transparent magnesium aluminosilicate glass-ceramics with high content of ZrO2. J. Eur. Ceram. Soc. 2024, 44, 116721. [Google Scholar] [CrossRef]
- Shen, H.; Liu, B.; Liu, Y.; Zhang, J.; Liu, J.; Zhang, S. Phase transition during nucleation process in calcium aluminate glass-ceramics manufactured from secondary aluminum dross. J. Alloys Compd. 2022, 911, 165010. [Google Scholar] [CrossRef]
- Zhou, H.; Persson, C.; Donzel-Gargand, O.; Engqvist, H.; Xia, W. Structural Si3N4-SiO2 glass ceramics with bioactive and anti-bacterial properties. J. Eur. Ceram. Soc. 2024, 44, 4260–4271. [Google Scholar] [CrossRef]
- Wei, S.G.; Jiang, H.; Dong, C.; Xiong, C.; Hao, H.; Ma, Y.; Li, M. Effects of Al2O3 on crystallization, microstructure, and properties of zinc spinel glass-ceramics. Ceram. Int. 2025, 51, 23244–23254. [Google Scholar] [CrossRef]
- Ren, B.; Hao, H.; Liu, Y.; Heng, J.Y.; Gosiamemang, T.; Wang, T.; Jiang, H.; Dong, C.; Xiong, C.; Wang, N.; et al. High-alumina and low-lithium glass-ceramics modulated by heat treatment and its structural evolution mechanism. J. Eur. Ceram. Soc. 2024, 44, 116723. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Yu, Y.; Bai, H.; Zhang, Z.; Huang, Y. Transparent MgO-Al2O3-SiO2 glass-ceramics prepared with ZrO2 and SnO2 as nucleating agents. J. Non-Cryst. Solids 2022, 588, 121585. [Google Scholar] [CrossRef]
- Ivanov, N.P.; Shichalin, O.O.; Trigub, A.L.; Rastorguev, V.; Nekrasova, N.; Provatorova, V.; Gnilyak, E.; Zaikova, A.; Rinchinova, V.; Lembikov, A.; et al. Optimization of Zn/Al cationic ratio in Zn-Al-LDH for efficient U(VI) adsorption. J. Water Process Eng. 2025, 69, 106735. [Google Scholar] [CrossRef]
- Buravlev, I.Y.; Vornovskikh, A.A.; Shichalin, O.O.; Lembikov, A.; Simonenko, T.; Seroshtan, A.; Buravleva, A.; Kosyanov, D.Y.; Papynov, E. Reactive spark plasma synthesis of Mo2C/Mo3Co3C ceramic for heterostructured electrodes used for hydrogen energy technology. Ceram. Int. 2024, 50, 14445–14457. [Google Scholar] [CrossRef]
- Kleebusch, E.; Patzig, C.; Krause, M.; Hu, Y.; Höche, T.; Rüssel, C. The effect of TiO2 on nucleation and crystallization of a Li2O-Al2O3-SiO2 glass investigated by XANES and STEM. Sci. Rep. 2018, 8, 2929. [Google Scholar] [CrossRef] [PubMed]
- Kleebusch, E.; Rüssel, C.; Patzig, C.; Höche, T. Evidence of epitaxial growth of high-quartz solid solution on ZrTiO4 nuclei in a Li2O-Al2O3-SiO2 glass. J. Alloys Compd. 2018, 748, 73–79. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, W.; Pu, S.; Chen, Y.; Shen, Y.; Han, Y.; Ran, X. Joining MgAl2O4 ceramics using ZnO–Al2O3–SiO2 glass ceramic with precipitated ZnAl2O4. Ceram. Int. 2023, 49, 32835–32842. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, X.; Ye, Z.; Tong, X.; Chen, Z.; Xia, Q.; Wang, T.; Jin, Y.; Li, Y.; Lin, J.; et al. β-spodumene-doped lithium disilicate glass-ceramics via additive manufacturing for dental applications. J. Mater. Sci. Technol. 2025, 247, 289–301. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Z.; Yang, G.; Sun, M.; Li, X.; Wang, Z. The structure and physical properties of peraluminous Li2O-Al2O3-SiO2 transparent glass-ceramics free of nucleating agents. J. Non-Cryst. Solids 2025, 653, 123427. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D. Solubility mechanisms of carbon-dioxide in silicate melts: A Raman spectroscopic study. Am. Mineral. 1980, 65, 885–899. [Google Scholar]
- Mysen, B.O.; Richet, P. Silicate Glasses and Melts: Properties and Structure; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Utegulov, Z.N.; Eastman, M.A.; Prabakar, S.; Mueller, K.T.; Hamad, A.Y.; Wicksted, J.P.; Dixon, G.S. Structural characterization of Eu2O3-MgO-Na2O-Al2O3-SiO2 glasses with varying Eu2O3 content: Raman and NMR studies. J. Non-Cryst. Solids 2003, 315, 43–53. [Google Scholar] [CrossRef]
- Colomban, P.; Tournie, A.; Bellot-Gurlet, L. Raman identification of glassy silicates used in ceramics, glass and jewellery: A tentative differentiation guide. J. Raman Spectrosc. 2006, 37, 841–852. [Google Scholar] [CrossRef]
- Chung, S.Y.; Kim, Y.M.; Kim, J.G.; Kim, Y.-J. Multiphase transformation and Ostwald’s rule of stages during crystallization of a metal phosphate. Nat. Phys. 2009, 5, 68–73. [Google Scholar] [CrossRef]
- Ficheux, M.; Burov, E.; Aquilanti, G.; Trcera, N.; Montouillout, V.; Cormier, L. Structural evolution of high zirconia aluminosilicate glasses. J. Non-Cryst. Solids 2020, 539, 120050. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Perea, D.E.; Wirth, M.G.; Ryan, J.V.; Youngman, R.E.; Rezikyan, A.; Fahey, A.J.; Schreiber, D.K. Nanoscale microstructure and chemistry of transparent gahnite glass-ceramics revealed by atom probe tomography. Scr. Mater. 2021, 203, 114110. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, S.; Wu, N.; OuYang, S. Raman spectroscopic study of irregular network in the process of glass conversion to CaO-MgO-Al2O3-SiO2 glass-ceramics. J. Non-Cryst. Solids 2021, 563, 120701. [Google Scholar] [CrossRef]
- Lenaz, D.; Lughi, V. Raman study of MgCr2O4-Fe2+Cr2O4 and MgCr2O4-MgFe3+2O4 synthetic series: The effects of Fe2+ and Fe3+ on Raman shift. Phys. Chem. Miner. 2013, 40, 491–498. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhang, X.Y. Glass Chemistry; Chemical Industry Press: Beijing, China, 2014. [Google Scholar]
- Tian, Y.L.; Sun, S.B. New Glass Technology; China Light Industry Press: Beijing, China, 2011. [Google Scholar]
- Liu, Y.; Ren, B.; Huang, X.; Jiang, H.; Dong, C.; Xiong, C.; Wang, T.; Wang, N.; Hao, H. Effect of nucleating agents on structure and crystallization behavior of high-aluminum glass-ceramics system. J. Eur. Ceram. Soc. 2024, 44, 116692. [Google Scholar] [CrossRef]




| wt% | SiO2 | Al2O3 | ZnO | MgO | ZrO2 | Li2O | Na2O |
|---|---|---|---|---|---|---|---|
| BG | 40.70 ± 0.05 | 33.90 ± 0.03 | 11.20 ± 0.03 | 5.50 ± 0.03 | 4.5 ± 0.02 | 2.70 + 0.02 | 1.5 ± 0.02 |
| Qn | Bands (cm−1) | Recommended Assignments | Structural Unit |
|---|---|---|---|
| Q0 | 870 | The Si-O band stretching vibration in fully non-bridging oxygen tetrahedra | [SiO4]4− |
| Q1 | 905 | the Si-O band stretching vibrations in tetrahedra with three non-bridge oxygen per tetrahedron | [Si2O7]6− |
| Q2 | 960 | the Si-O band stretching vibrations in tetrahedra with two non-bridge oxygen per tetrahedro | [SiO3]2− |
| Q3 | 1015 | the Si-O band stretching vibrations in tetrahedra with one non-bridge oxygen per tetrahedron | [Si2O5]2− |
| Q4 | 1060 | the Si-O band stretching vibration in fully polymerized units | SiO2 |
| BG | HG | CG1 | CG2 | CG3 | CG4 | |
|---|---|---|---|---|---|---|
| SQ0 | 13.87 ± 3% | 13.05 ± 3% | 7.97 ± 3% | 6.14 ± 3% | 5.99 ± 3% | 0.00% |
| SQ1 | 20.25 ± 3% | 19.19 ± 3% | 19.15 ± 3% | 17.78 ± 3% | 16.29 ± 3% | 10.01 ± 3% |
| SQ2 | 22.78 ± 3% | 22.76 ± 3% | 24.67 ± 3% | 24.82 ± 3% | 25.92 ± 3% | 31.16 ± 3% |
| SQ3 | 32.60 ± 3% | 33.32 ± 3% | 33.90 ± 3% | 34.95 ± 3% | 35.45 ± 3% | 41.16 ± 3% |
| SQ4 | 10.51 ± 3% | 11.67 ± 3% | 15.20 ± 3% | 16.32 ± 3% | 16.35 ± 3% | 17.79 ± 3% |
| NBO/(NBO + BO) | 0.94 ± 0.08 | 0.90 ± 0.08 | 0.76 ± 0.09 | 0.68 ± 0.10 | 0.67 ± 0.10 | 0.50 ± 0.10 |
| NBO/tetrahedron | 1.94 ± 0.09 | 1.89 ± 0.09 | 1.73 ± 0.10 | 1.62 ± 0.11 | 1.60 ± 0.11 | 1.34 ± 0.12 |
| bridges/tetrahedron | 2.06 ± 0.09 | 2.11 ± 0.09 | 2.27 ± 0.10 | 2.38 ± 0.11 | 2.40 ± 0.11 | 2.66 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Pan, Y.; Wei, S.; Ma, Y.; Dong, C.; Hao, H.; Jiang, H. Local Structural Changes in High-Alumina, Low-Lithium Glass-Ceramics During Crystallization. Nanomaterials 2025, 15, 1449. https://doi.org/10.3390/nano15181449
Li M, Pan Y, Wei S, Ma Y, Dong C, Hao H, Jiang H. Local Structural Changes in High-Alumina, Low-Lithium Glass-Ceramics During Crystallization. Nanomaterials. 2025; 15(18):1449. https://doi.org/10.3390/nano15181449
Chicago/Turabian StyleLi, Minghan, Yan Pan, Shuguang Wei, Yanping Ma, Chuang Dong, Hongxun Hao, and Hong Jiang. 2025. "Local Structural Changes in High-Alumina, Low-Lithium Glass-Ceramics During Crystallization" Nanomaterials 15, no. 18: 1449. https://doi.org/10.3390/nano15181449
APA StyleLi, M., Pan, Y., Wei, S., Ma, Y., Dong, C., Hao, H., & Jiang, H. (2025). Local Structural Changes in High-Alumina, Low-Lithium Glass-Ceramics During Crystallization. Nanomaterials, 15(18), 1449. https://doi.org/10.3390/nano15181449








