Advances in Magnetic Nanocomposite Adsorbents for Water Remediation: Design, Performance, and Challenges
Abstract
1. Introduction
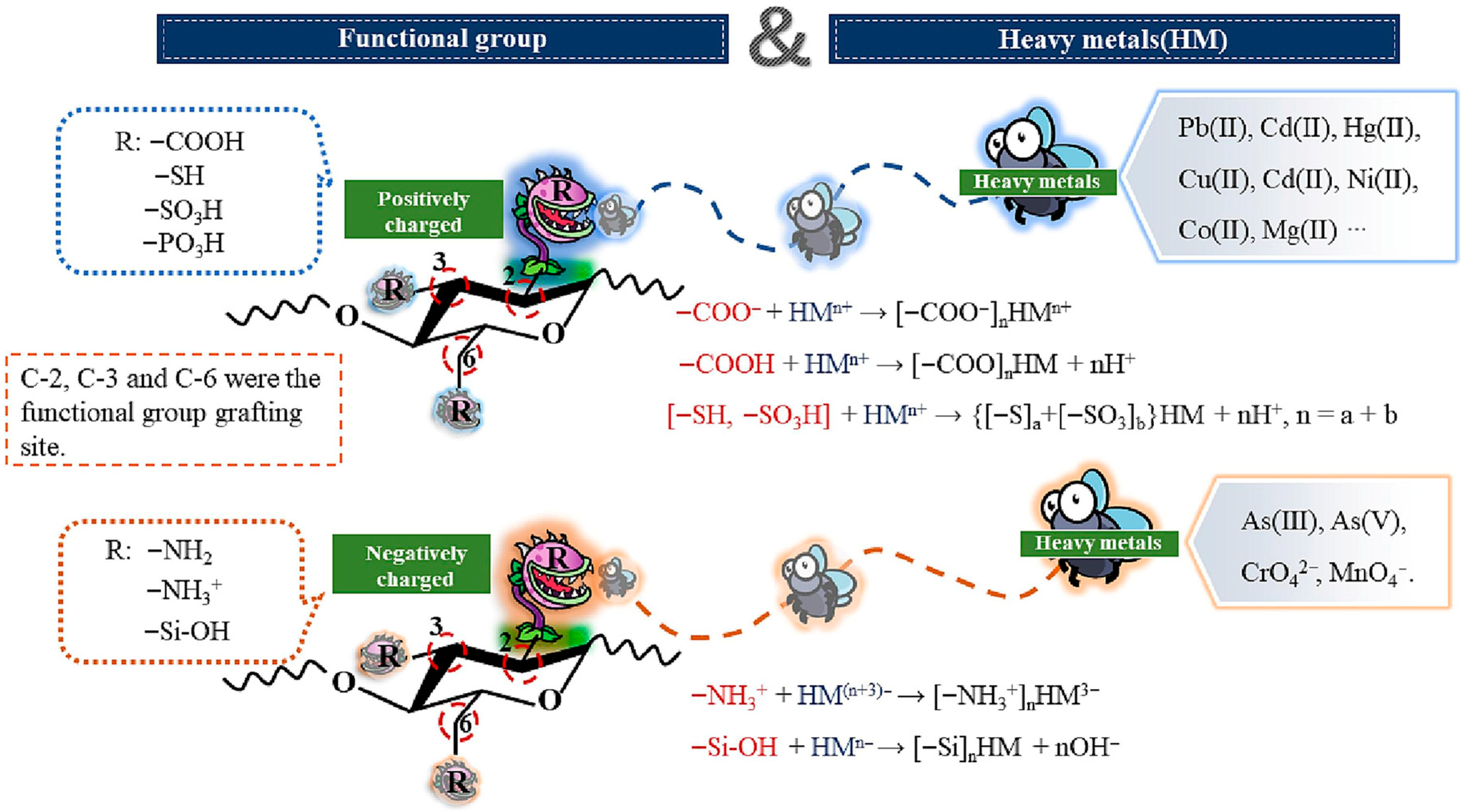
2. Synergistic Mechanisms in Magnetic Adsorbents
3. Magnetic Carbon-Based Composites
3.1. Activated Carbon
3.2. Carbon Nanotubes

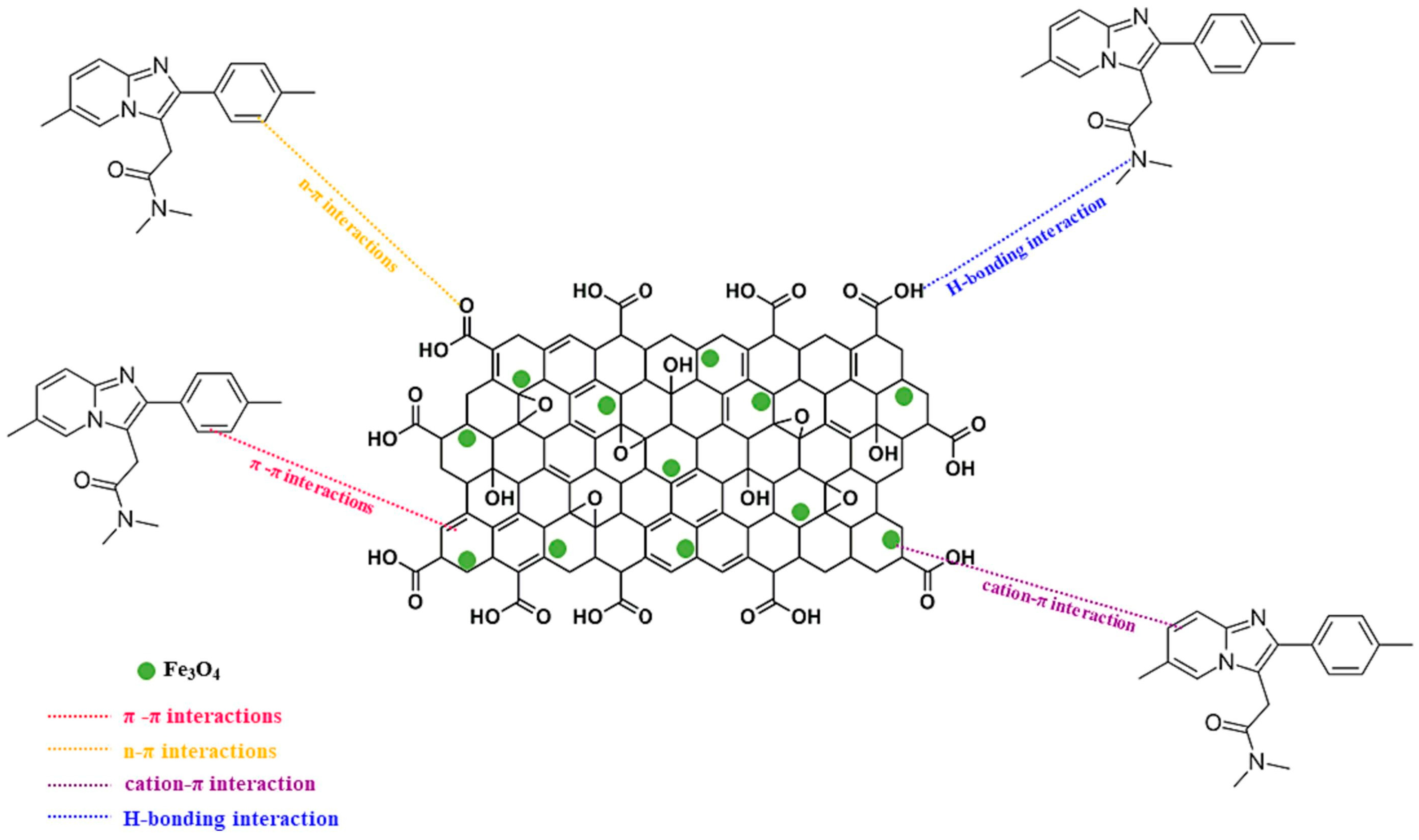
3.3. Graphene Oxide
4. Magnetic Composites Based on Natural Inorganic Mineral Materials
4.1. Montmorillonite
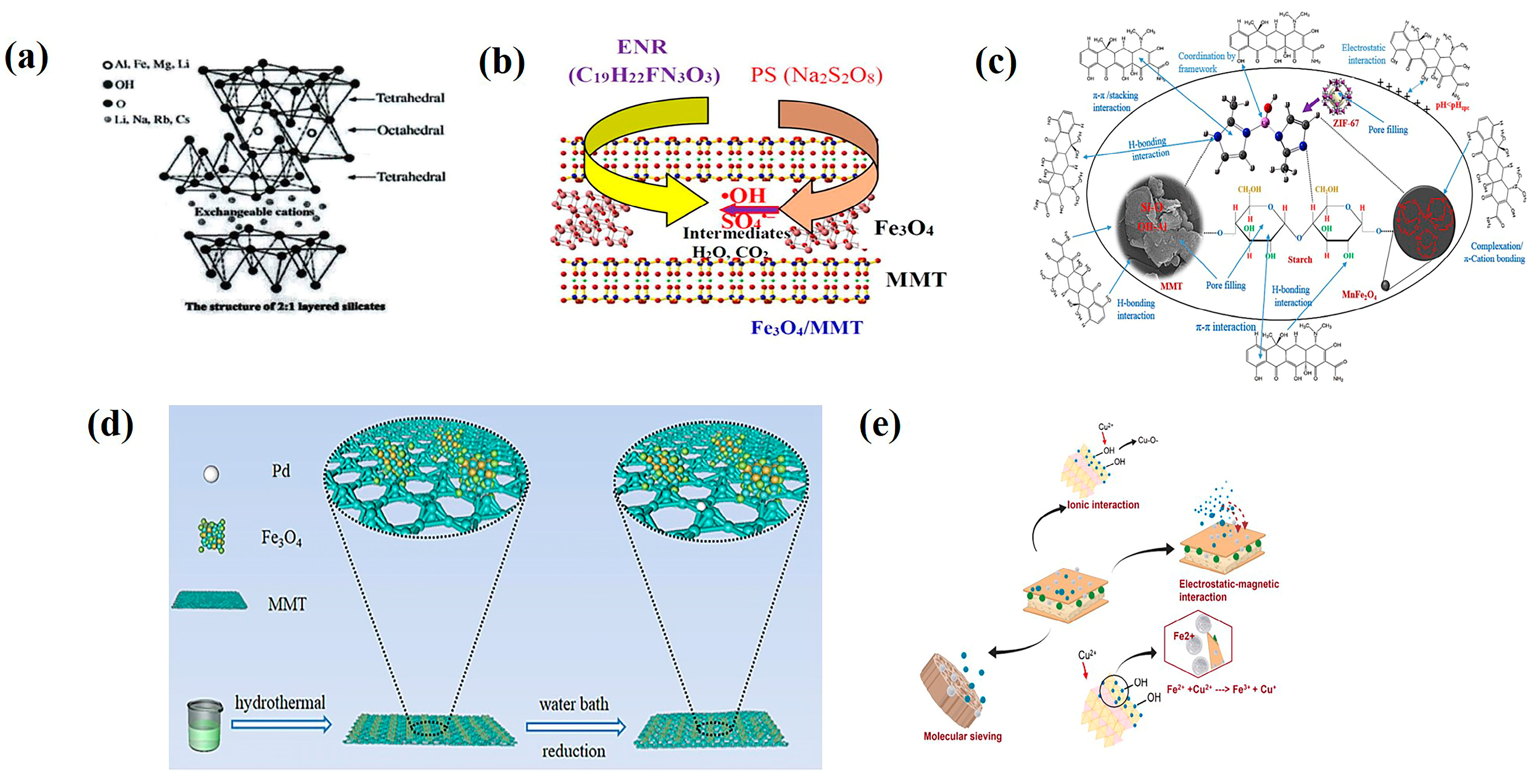
4.2. Sepiolite
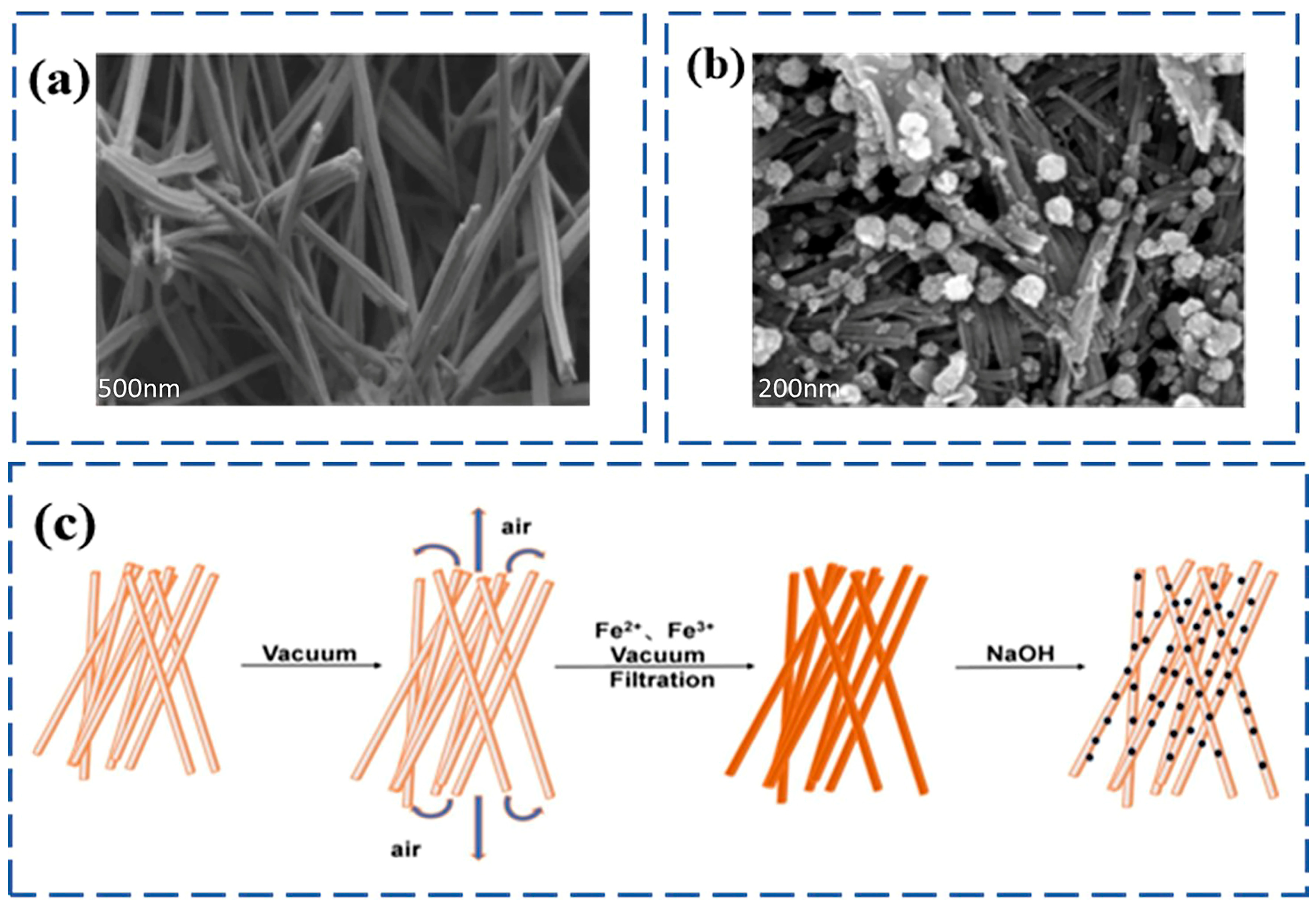
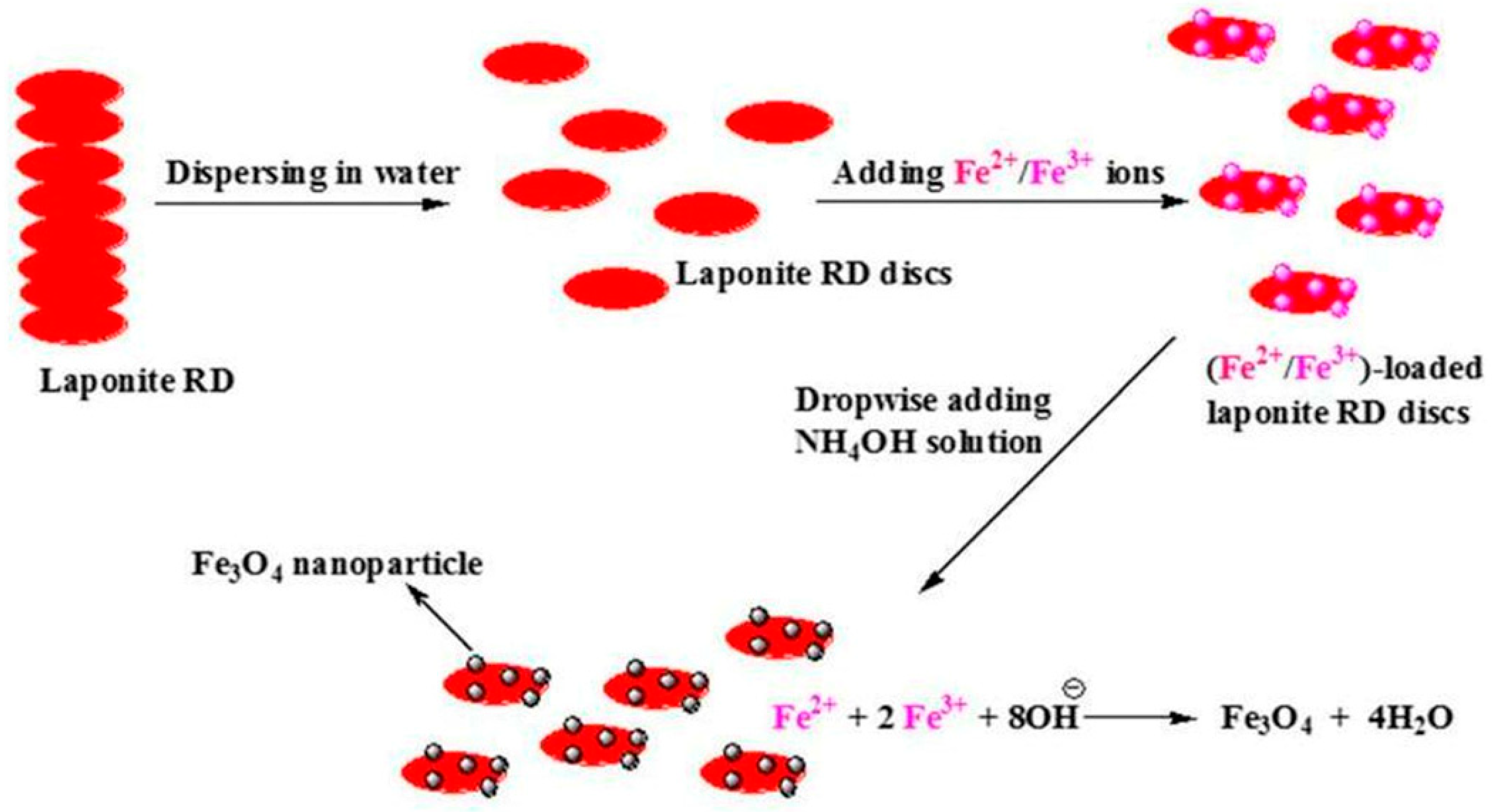
4.3. Laponite
5. Magnetic Composites Based on Natural Polymer Materials
5.1. Cellulose
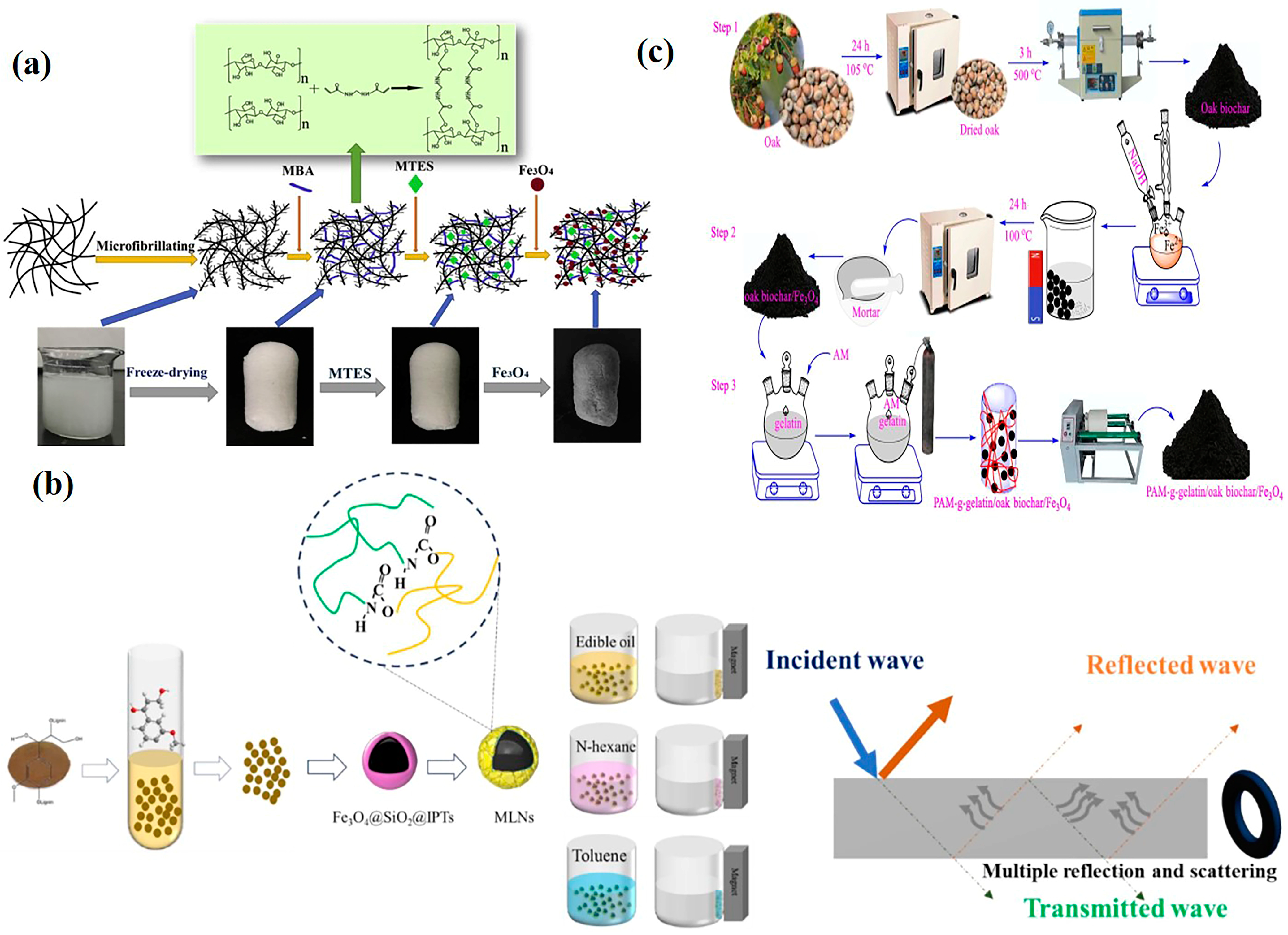
- Chemical Crosslinking: Utilizes crosslinking agents to anchor magnetic particles onto the cellulose surface. Liu et al. [88] used glutaraldehyde as a crosslinker to prepare polyethyleneimine-modified magnetic sugarcane bagasse cellulose film. This film material achieved 92.63% removal efficiency for ibuprofen, demonstrating high hydrophilicity, thermal stability, non-toxicity, and maintained ~96% removal efficiency after 17 cycles. He et al. [89] employed N, N′-methylenebisacrylamide (MBA) as a crosslinker to prepare magnetic and hydrophobic cellulose Fe3O4 aerogels (Figure 8a). The aerogel retained its original fluffy and porous structure, exhibiting excellent superhydrophobicity and superparamagnetism. After 10 adsorption–recovery cycles for oil, the aerogel maintained a high oil adsorption rate. However, the use of crosslinking agents might block cellulose pores, reducing specific surface area.
- Physical Coating: Combines pre-synthesized magnetic particles with cellulose via ultrasonic dispersion or electrostatic adsorption. For example, Barzegarzadeh et al. [90] compared magnetic cellulose/Al-MOF prepared with and without ultrasound, finding they followed the Freundlich and Langmuir models, respectively. Ultrasound increased the adsorption capacity for doxorubicin from 96 mg/g to 108 mg/g through cavitation effects. This method avoids complex chemical modification but suffers from weaker physical binding strength, potentially leading to particle detachment.
5.2. Lignin
5.3. Gelatin
6. Conclusions
- The integration of magnetic components with carriers induces synergistic enhancement in the composites. The carriers provide a large specific surface area and active adsorption sites, while the magnetic components confer magnetic responsiveness. The chemical bonding, electrostatic self-assembly, and spatial confinement between them significantly improve the structural stability of the composites and their efficiency in capturing pollutants.
- The preparation process substantially influences the performance of magnetic composites. Techniques such as hydrothermal synthesis, co-precipitation, in situ synthesis, and auxiliary methods like microwave/ball milling each possess distinct advantages and disadvantages. Furthermore, different types of carrier materials are suited to specific magnetic loading strategies.
- However, the field still faces significant challenges:
- Unclear selective adsorption mechanisms hinder effective performance in multi-pollutant systems due to competitive adsorption.
- Prominent bottlenecks in scalable production manifest as high energy consumption and poor batch-to-batch reproducibility.
- Absence of comprehensive LCA neglects the environmental costs associated with raw material extraction, synthesis processes, and disposal of spent adsorbents.
7. Future Perspectives
- Enhance targeted adsorption capabilities in complex systems through molecular imprinting technology and biomimetic surface modification.
- Develop green mining practices for mineral resources and synthesis routes utilizing biomass-derived carriers to achieve sustainable large-scale production.
Funding
Conflicts of Interest
References
- Dube, A.; Malode, S.J.; Alshehri, M.A.; Shetti, N.P. Electrochemical water treatment: Review of different approaches. J. Environ. Manag. 2025, 373, 123911. [Google Scholar] [CrossRef]
- Li, S.; Gong, Y.; Yang, Y.; He, C.; Hu, L.; Zhu, L.; Sun, L.; Shu, D. Recyclable CNTs/Fe3O4 magnetic nanocomposites as adsorbents to remove bisphenol A from water and their regeneration. Chem. Eng. J. 2015, 260, 231–239. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, J.; Dai, R.; Wang, Z. Selective removal of organic matters from high-salinity chemical industrial wastewater: Ultrafiltration or nanofiltration? Water Res. 2025, 282, 123762. [Google Scholar] [CrossRef]
- Xiao, Z.; Pan, Y.; Zhao, Q.; Yang, J.; Wang, H.; Yu, L.; Ding, X.; Huang, J. Surface cationization of cellullose via Fenton oxidation for remedying dye contaminants: Adsorption performance and mechanism. Int. J. Biol. Macromol. 2025, 312, 144040. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Shi, J.; Wang, Y.; Tong, H.; Zhu, Y.; Xu, L.; Wang, Y.; Zhang, B.; Tao, Y.; Dai, X.; et al. Applications of functionalized magnetic biochar in environmental remediation: A review. J. Hazard. Mater. 2022, 434, 128841. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, T.; Hao, L.; Guo, Y.; Liu, W.; Guo, L.; Wang, C.; Wang, Z.; Wu, Q. Advances in magnetic porous organic frameworks for analysis and adsorption applications. Trends Anal. Chem. 2020, 132, 116048. [Google Scholar] [CrossRef]
- Shi, S.; Xu, C.; Wang, X.; Xie, Y.; Wang, Y.; Dong, Q.; Zhu, L.; Zhang, G.; Xu, D. Electrospinning fabrication of flexible Fe3O4 fibers by sol-gel method with high saturation magnetization for heavy metal adsorption—ScienceDirect. Mater. Des. 2020, 186, 108298. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Chen, M.; Gu, H.; Rapole, S.B.; Pallavkar, S.; Ho, T.C.; Hopper, J.; Guo, Z. Magnetic nanocomposites for environmental remediation. Adv. Powder Technol. 2013, 24, 459–467. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, X.; Zhao, R.; Fu, M.; Wang, M.; Chen, Z.; Zhang, C.; Li, X. Enhanced antibiotic removal via magnetic Ni@ZIF-8 composite nanotubes: Synergistic adsorption, facile recovery and adsorption mechanism. Sep. Purif. Technol. 2025, 363, 131988. [Google Scholar] [CrossRef]
- Pan, M.; Shi, X.; Lyu, F.; Wendt, B.L.L.; Zheng, X.; Tang, S.K.Y. Encapsulation of single nanoparticle in fast-evaporating micro-droplets prevents particle agglomeration in nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 26602–26609. [Google Scholar] [CrossRef]
- Guo, T.; Bulin, C.; Ma, Z.; Li, B.; Zhang, Y.; Zhang, B.; Xing, R.; Ge, X. Mechanism of Cd(II) and Cu(II) Adsorption onto Few-Layered Magnetic Graphene Oxide as an Efficient Adsorbent. ACS Omega 2021, 6, 16535–16545. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.; Zhong, F.; Zhang, H.; Wei, S.; Dong, Y.; Chen, S.; Cao, F.; Zuo, L.; Xu, J. Investigation of the adsorption pattern and mechanism of enrofloxacin on montmorillonite and activated carbon surfaces: Effect of Cu(II) complexation. Surf. Interfaces 2025, 60, 106012. [Google Scholar] [CrossRef]
- Han, Y.; Tao, J.; Zhou, W. Enhanced dispersion and surface hydroxyl density of amorphous MnO2 by sepiolite for efficient adsorption and degradation of tetracycline. Ceram. Int. 2025, 51, 16310–16322. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, L.; Zhang, H.; Ren, J.; Liang, T.; Lv, X.; Cheng, C.; Yu, H. Efficient adsorption of cationic dyes by a novel honeycomb-like porous hydrogel with ultrahigh mechanical property. Int. J. Biol. Macromol. 2024, 278, 134457. [Google Scholar] [CrossRef]
- Tan, Z.; Mao, J.; Hu, Y.; Xue, F.; Yang, Y.; Wang, L.; Zhu, H.; He, H. Fabrication of biomimetic giant waterlily cellulosic adsorption-catalytic material for efficient water purification. Appl. Catal. B Environ. Energy 2025, 366, 125063. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, W.; Zhao, W.; Niu, L.; Li, W. Rational design of a starch/whey protein isolate/caffeic acid ternary system to alleviate gel deterioration during freeze-thaw cycles. Carbohydr. Polym. 2025, 352, 123221. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhang, L.; Zhu, X.; Zhu, X. The preparation of activated carbon from walnut shell bio-oil distillation residues. Carbon 2020, 158, 930–931. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, G.; Liu, S.; Li, M.; He, J.; Wen, X. Activated carbon microspheres with high surface area for efficient organic contaminants removal. Colloids Surf. A Physicochem. Eng. Asp. 2023, 669, 131479. [Google Scholar] [CrossRef]
- Peng, Y.; Azeem, M.; Li, R.; Xing, L.; Li, Y.; Zhang, Y.; Guo, Z.; Wang, Q.; Ngo, H.H.; Qu, G.; et al. Zirconium hydroxide nanoparticle encapsulated magnetic biochar composite derived from rice residue: Application for As(III) and As(V) polluted water purification. J. Hazard. Mater. 2022, 423, 127081. [Google Scholar] [CrossRef]
- Feng, Z.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review. Sci. Total Environ. 2021, 765, 142673. [Google Scholar] [CrossRef]
- Wu, Q.; Ye, X.; Lv, Y.; Pei, R.; Wu, M.; Liu, M. Lignin-based magnetic activated carbon for p-arsanilic acid removal: Applications and absorption mechanisms. Chemosphere 2020, 258, 127276. [Google Scholar] [CrossRef]
- Cai, W.; Wei, J.; Li, Z.; Liu, Y.; Zhou, J.; Han, B. Preparation of amino-functionalized magnetic biochar with excellent adsorption performance for Cr(VI) by a mild one-step hydrothermal method from peanut hull. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 102–111. [Google Scholar] [CrossRef]
- Chen, T.; Quan, X.; Ji, Z.; Li, X.; Pei, Y. Synthesis and characterization of a novel magnetic calcium-rich nanocomposite and its remediation behaviour for As(III) and Pb(II) co-contamination in aqueous systems. Sci. Total Environ. 2020, 706, 135122. [Google Scholar] [CrossRef]
- Lv, M.; Li, D.; Zhang, Z.; Logan, B.E.; Liu, G.; Sun, M.; Dai, C.; Feng, Y. Unveiling the correlation of Fe3O4 fractions upon the adsorption behavior of sulfamethoxazole on magnetic activated carbon. Sci. Total Environ. 2021, 757, 143717. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.; Dang, J.; Wang, W.; Wang, X.; Wu, M.; Hu, J. Study on the adsorption performance of amoxicillin by hydrothermally modified coconut shell activated carbon. J. Chem. Technol. Biotechnol. 2024, 99, 1191–1200. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; He, Y.; An, X.; Fan, D.; Wu, Z. Microwave-assisted catalytic pyrolysis of apple wood to produce biochar: Co-pyrolysis behavior, pyrolysis kinetics analysis and evaluation of microbial carriers. Bioresour. Technol. 2021, 320, 124345. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Tong, J.; Shao, X.; Chen, R.; Yang, Q.; Li, F.; Xue, B.; Li, G.; Han, Y.; et al. Synthesis of hickory biochar via one-step acidic ball milling: Characteristics and titan yellow adsorption. J. Clean. Prod. 2022, 338, 130575. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, B.; Tong, H.; Liu, Y.; Wang, S.; Wei, S.; Wang, L.; Wang, Y.; Zhang, Y. High-efficiency decontamination of Pb(II) and tetracycline in contaminated water using ball-milled magnetic bone derived biochar. J. Clean. Prod. 2023, 385, 135683. [Google Scholar] [CrossRef]
- Nasrullah, A.; Khan, A.S.; Bhat, A.H.; Din, I.U.; Inayat, A.; Muhammad, N.; Bakhsh, E.M.; Khan, S.B. Effect of short time ball milling on physicochemical and adsorption performance of activated carbon prepared from mangosteen peel waste. Renew. Energy 2020, 168, 723–733. [Google Scholar] [CrossRef]
- Li, Y.; Zimmerman, A.R.; He, F.; Chen, J.; Han, L.; Chen, H.; Hu, X.; Gao, B. Solvent-free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue. Sci. Total Environ. 2020, 722, 137972. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, X.; Zhao, S.; Du, Q.; Pi, X.; Jing, Z.; Jin, Y. Polydopamine coating for enhanced electrostatic adsorption of methylene blue by multiwalled carbon nanotubes in alkaline environments. J. Colloid Interface Sci. 2024, 675, 263–274. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J. Exploring the potential of cellulose benzoate adsorbents modified with carbon nanotubes and magnetic carbon nanotubes for microplastic removal from water. Chem. Eng. J. 2023, 469, 143910. [Google Scholar] [CrossRef]
- Younes, H.; Rahman, M.M.; Ni, G.; Ghaferi, A.A.; Rub, R.A.A.; Bsoul, I. Investigation of magnetic properties of γ-Fe2O3 NP-Decorated carbon nanostructured mats. JOM 2019, 71, 3142–3150. [Google Scholar] [CrossRef]
- Zhao, W.; Tian, Y.; Chu, X.; Cui, L.; Zhang, H.; Li, M.; Zhao, P. Preparation and characteristics of a magnetic carbon nanotube adsorbent: Its efficient adsorption and recoverable performances. Sep. Purif. Technol. 2021, 257, 117917. [Google Scholar] [CrossRef]
- Gao, H.; Han, X.; Wang, R.; Zhu, K.; Han, R. Adsorption and catalytic degradation of bisphenol A and p-chlorophenol by magnetic carbon nanotubes. Environ. Res. 2023, 231, 116314. [Google Scholar] [CrossRef]
- Liu, R.; Qiao, Y.; Xu, Y.; Ma, X.; Li, Z. A facile controlled in-situ synthesis of monodisperse magnetic carbon nanotubes nanocomposites using water-ethylene glycol mixed solvents. J. Alloys Compd. 2016, 657, 138–143. [Google Scholar] [CrossRef]
- Morales-Cid, G.; Fekete, A.; Simonet, B.M.; Lehmann, R.; Cárdenas, S.; Zhang, X.; Valcárcel, M.; Schmitt-Kopplin, P. In situ synthesis of magnetic multiwalled carbon nanotube composites for the clean-up of (fluoro) quinolones from human plasma prior to ultrahigh pressure liquid chromatography analysis. Anal. Chem. 2010, 82, 2743–2752. [Google Scholar] [CrossRef]
- Cheng, S.; Zeng, X.; Liu, P. One-step synthesis of magnetic N-doped carbon nanotubes derived from waste plastics for effective Cr(VI) removal. Arab. J. Chem. 2024, 17, 105956. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H. Synthesis, characterization and life cycle assessment of carbon nanospheres from waste tires pyrolysis over ferrocene catalyst. J. Environ. Chem. Eng. 2020, 8, 103669. [Google Scholar] [CrossRef]
- Cheng, Z.; Lyu, H.; Shen, B.; Tian, J.; Sun, Y.; Wu, C. Removal of antimonite (Sb(III)) from aqueous solution using a magnetic iron-modified carbon nanotubes (CNTs) composite: Experimental observations and governing mechanisms. Chemosphere 2022, 288, 132581. [Google Scholar] [CrossRef]
- Travessa, D.N.; Rocha, G.V.B.; Cardoso, K.R.; Lieblich, M. Carbon Nanotube-Reinforced Aluminum Matrix Composites Produced by High-Energy Ball Milling. J. Mater. Eng. Perform. 2017, 26, 2998–3006. [Google Scholar] [CrossRef]
- Ma, F.; Tang, F.; Yang, B.; Guo, Q.; Li, P.; Yu, L. Vitamin C-reduced graphene oxide/Fe3O4 composite for simultaneous removal of aflatoxin B1 and benzo(a)pyrene in vegetable oils. LWT Food Sci. Technol. 2024, 203, 116342. [Google Scholar] [CrossRef]
- Barbieri, I.A.; Oliveira, M.L.S.; Bruckmann, F.S.; Salles, T.R.; Zancanaro, L.V.; Silva, L.F.; Dotto, G.L.; Lima, E.C.; Naushad, M.; Rhoden, C.R.D. Effective removal of hypnotic drug from the aqueous medium through adsorption on graphene oxide magnetic derivatives. J. Mol. Liq. 2024, 393, 123657. [Google Scholar] [CrossRef]
- Konuk, H.H.; Alp, E.; Ozaydin, Z.; Koyuncu, D.D.E.; Arbag, H. Effects of Iron Ion Ratios on the Synthesis and Adsorption Capacity of the Magnetic Graphene Oxide Nanomaterials. Arab. J. Sci. Eng. 2025, 50, 4137–4150. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, S.; E, T. Magnetic graphene oxide prepared via ammonia coprecipitation method: The effects of preserved functional groups on adsorption property. Inorg. Chem. Commun. 2021, 128, 108603. [Google Scholar] [CrossRef]
- Bulin, C.; Zhang, Y.; Li, B.; Zhang, B. Removal performance of aqueous Co(II) by magnetic graphene oxide and adsorption mechanism. J. Phys. Chem. Solids 2020, 144, 109483. [Google Scholar] [CrossRef]
- Tran, V.T.; Vu, D.T. Influence of temperature on structure, morphology, and magnetic property of graphene-MnFe2O4 nanocomposites synthesized by a combined hydrothermal/co-precipitation method. Appl. Phys. A 2018, 124, 675. [Google Scholar] [CrossRef]
- Gao, M.; Wang, Z.; Yang, C.; Ning, J.; Zhou, Z.; Li, G. Novel magnetic graphene oxide decorated with persimmon tannins for efficient adsorption of malachite green from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 566, 48–57. [Google Scholar] [CrossRef]
- Zourou, A.; Ntziouni, A.; Adamopoulos, N.D.; Roman, T.; Zhang, F.; Terrones, M.; Kordatos, K.V. Graphene oxide-MnFe2O4 nanohybrid material as an adsorbent of Congo red dye. J. Phys. Chem. Solids 2023, 181, 111490. [Google Scholar] [CrossRef]
- Lu, T.M.T.; Le, T.T.; Nguyen, D.H.; Che, Q.C.; Nguyen, M.D.; Dinh, N.T.; Nguyen, T.S.; Doan, T.Y.O.; Mai, T.P.; Nguyen, H.H. Comparison of in-situ and ex-situ methods for synthesis of iron magnetic nanoparticles-doped graphene oxide: Characterization, adsorption capacity, and Fenton catalytic efficiency. FlatChem 2022, 33, 100365. [Google Scholar] [CrossRef]
- Yuwen, C.; Liu, B.; Zhou, B.; Tian, S.; Zhang, L. Structure and properties of graphene oxide during the synthesis process at fixed temperatures. Ceram. Int. 2021, 47, 17487–17493. [Google Scholar] [CrossRef]
- Donga, C.; Mishra, S.B.; Ndlovu, L.N.; Abd-El-Aziz, A.S.; Kuvarega, A.T.; Mishra, A.K. Magnetic Magnetite-Graphene Oxide (Fe3O4-GO) Nanocomposites for Removal of Dyes from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2024, 34, 4192–4202. [Google Scholar] [CrossRef]
- Younes, H.; Kuang, X.; Luo, D.; DeVries, B.; Rahman, M.M.; Hong, H. Magnetic-field-assisted DLP stereolithography for controlled production of highly aligned 3D printed polymer-Fe3O4@graphene nanocomposites. Mater. Res. Bull. 2022, 154, 111938. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, Y.; Wang, P.; Wang, Q.; Wan, S.; Huang, S.; Su, R.; Song, Y.; Wang, Y. Enhanced removal of Co(II) and Ni(II) from high-salinity aqueous solution using reductive self-assembly of three-dimensional magnetic fungal hyphal/graphene oxide nanofibers. Sci. Total. Environ. 2021, 756, 143871. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.; Chen, Y.; Feng, W.; Tang, Z.; Pan, D.; Wu, W.; Lin, M. Magnetic graphene oxide for rapid capture and efficient separation of cobalt from radioactive wastewater. Desalination 2025, 607, 118803. [Google Scholar] [CrossRef]
- Tarekegn, M.M.; Balakrishnan, R.M.; Hiruy, A.M.; Dekebo, A.H. Removal of methylene blue dye using nano zerovalent iron, nanoclay and iron impregnated nanoclay—A comparative study. RSC Adv. 2021, 11, 30109–30131. [Google Scholar] [CrossRef]
- Rosado, A.; Borrás, A.; Turull, M.; Díez, S.; Ruano, D.; Ayllón, J.A.; López-Periago, A.M.; Domingo, C. Novel zirconium-based MOF@rGO composite aerogel: Towards remediation of Hg(II) polluted water bodies to reach safe drinking limits. Chem. Eng. J. 2025, 510, 161824. [Google Scholar] [CrossRef]
- Liu, D.; Dong, C.; Zhong, J.; Ren, S.; Chen, Y.; Qiu, T. Facile preparation of chitosan modified magnetic kaolin by one-pot coprecipitation method for efficient removal of methyl orange. Carbohydr. Polym. 2020, 245, 116572. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ding, J.; Wang, W.; Zong, L.; Xu, J.; Wang, A. Magnetic nano-hybrids adsorbents formulated from acidic leachates of clay minerals. J. Clean. Prod. 2020, 256, 120383. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Qiao, S.; Zhou, J. Magnetic Fe0/iron oxide-coated diatomite as a highly efficient adsorbent for recovering phosphorus from water. Chem. Eng. J. 2021, 412, 128696. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Li, Y.; Liu, Y.; Chen, Y.; Wu, Y.; Zhang, J.; Li, H.; Peng, Z.; Xu, R.; et al. Adsorption of Pb(II) by tourmaline-montmorillonite composite in aqueous phase. J. Colloid Interface Sci. 2020, 575, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Niroumand, J.S.; Peighambardoust, S.J.; Mohammadi, R. Tetracycline decontamination from aqueous media using nanocomposite adsorbent based on starch-containing magnetic montmorillonite modified by ZIF-67. Int. J. Biol. Macromol. 2024, 259, 129263. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Li, T.; Ai, B.; Yang, S.; Fu, J.; He, Q.; Yu, G.; Deng, S. Highly efficient removal of enrofloxacin by magnetic montmorillonite via adsorption and persulfate oxidation. Chem. Eng. J. 2019, 360, 1119–1127. [Google Scholar] [CrossRef]
- Hou, S.; Gao, Y.; Song, P.; Zhao, J.; Gong, B.; Zhu, X.; Liu, Q. Synthesis of Pd-modified Fe3O4 loaded on montmorillonite catalyst for photocatalytic degradation of tetracycline. Inorg. Chem. Commun. 2024, 159, 111745. [Google Scholar] [CrossRef]
- Fatimah, I.; Citradewi, P.W.; Fadillah, G.; Sahroni, I.; Purwiandono, G.; Dong, R. Enhanced performance of magnetic montmorillonite nanocomposite as adsorbent for Cu(II) by hydrothermal synthesis. J. Environ. Chem. Eng. 2021, 9, 104968. [Google Scholar] [CrossRef]
- Irawan, C.; Nata, I.F.; Lee, C. Removal of Pb(II) and As(V) using magnetic nanoparticles coated montmorillonite via one-pot solvothermal reaction as adsorbent. J. Environ. Chem. Eng. 2019, 7, 103000. [Google Scholar] [CrossRef]
- Yang, F.; Wang, A. Recent researches on antimicrobial nanocomposite and hybrid materials based on sepiolite and palygorskite. Appl. Clay Sci. 2022, 219, 106454. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, S.; Zhao, J.; Ma, J.; Wu, H.; Sun, H.; Cheng, S. Experimental study on removal of microplastics from aqueous solution by magnetic force effect on the magnetic sepiolite. Sep. Purif. Technol. 2022, 288, 120564. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, F.; Wang, Y.; Zhong, Y.; Qiu, Y.; Deng, L.; Wu, R.; Huang, Z.; Deng, Q.; Tong, C.; et al. Magnetic Prussian Blue nanosorbent loading on sepiolite for specific removal of Tl(I). J. Alloys Compd. 2024, 972, 172741. [Google Scholar] [CrossRef]
- Kang, S.; Yi, B.; Nie, X.; Wan, Q.; Yang, H. Boosting adsorption of ciprofloxacin on Fe3O4 nanoparticles modified sepiolite composite synthesized via a vacuum-filtration assisted coprecipitation strategy. J. Water Process Eng. 2024, 66, 106038. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, X.; Lan, X.; Yin, Y.; Chang, X. Effect of sepiolite as a new greener additive on the microstructure and nanomechanical properties of cement-based materials. Constr. Build. Mater. 2025, 491, 142780. [Google Scholar] [CrossRef]
- Su, G.; Liu, L.; Kuang, Q.; Liu, X.; Dong, W.; Niu, M.; Tang, A.; Xue, J. Enhanced visible-light photocatalytic activity and recyclability of magnetic core-shell Fe3O4@SiO2@BiFeO3-sepiolite microspheres for organic pollutants degradation. J. Mol. Liq. 2021, 335, 116566. [Google Scholar] [CrossRef]
- Deng, S.; Ren, B.; Cheng, S.; Hou, B.; Deng, R.; Zhu, G. Study on the adsorption performance of carbon-magnetic modified sepiolite nanocomposite for Sb(V), Cd(II), Pb(II), and Zn(II): Optimal conditions, mechanisms, and practical applications in mining areas. J. Hazard. Mater. 2025, 487, 137129. [Google Scholar] [CrossRef]
- Xu, J.; He, J.; Zhu, L.; Guo, S.; Chen, H. A novel utilization of raw sepiolite: Preparation of magnetic adsorbent directly based on sol-gel for adsorption of Pb(II). Environ. Sci. Pollut. Res. 2022, 29, 77448–77461. [Google Scholar] [CrossRef]
- Li, F.; Dai, Y.; Gong, M.; Yu, T.; Chen, X. Synthesis, characterization of magnetic-sepiolite supported with TiO2, and the photocatalytic performance over Cr(VI) and 2,4-dichlorophenol co-existed wastewater. J. Alloys Compd. 2015, 638, 435–442. [Google Scholar] [CrossRef]
- Gonzalez-Pujana, A.; Igartua, M.; Hernandez, R.M.; Santos-Vizcaino, E. Laponite nanoclays for the sustained delivery of therapeutic proteins. Eur. J. Pharm. Sci. 2024, 201, 106858. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Soleymani, M.; Sabzi, M.; Azimi, H.; Atlasi, Z. Novel magnetic polyvinyl alcohol/laponite RD nanocomposite hydrogels for efficient removal of methylene blue. J. Environ. Chem. Eng. 2017, 5, 2617–2630. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Mousanezhad, S.; Hosseinzadeh, H.; Darvishi, F.; Sabzi, M. Magnetic hydrogel beads based on PVA/sodium alginate/laponite RD and studying their BSA adsorption. Carbohydr. Polym. 2016, 147, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Abasiyan, S.M.A.; Mahdavinia, G.R. Polyvinyl alcohol-based nanocomposite hydrogels containing magnetic laponite RD to remove cadmium. Environ. Sci. Pollut. Res. 2018, 25, 14977–14988. [Google Scholar] [CrossRef]
- Rashidi, S.; Ghorbani-Kalhor, E.; Rashidzadeh, B.; Abolhasani, J. Comparative adsorption of Cu(II), Hg(II), Co(II), and Ni(II) ions on novel magnetic chitosan/cellulose/laponite RD nanocomposite hydrogel. Int. J. Environ. Anal. Chem. 2024, 104, 3098–3121. [Google Scholar] [CrossRef]
- Li, X.; Cui, W.; Jiang, W.; Yuan, R.; Liang, R.; Qiu, J. Bi-functional natural polymers for highly efficient adsorption and reduction of gold. Chem. Eng. J. 2021, 422, 130577. [Google Scholar] [CrossRef]
- Sun, X.; Liu, B.; Jing, Z.; Wang, H. Preparation and adsorption property of xylan/poly(acrylic acid) magnetic nanocomposite hydrogel adsorbent. Carbohydr. Polym. 2015, 118, 16–23. [Google Scholar] [CrossRef]
- Haniffa, M.A.C.M.; Ching, Y.C.; Illias, H.A.; Munawar, K.; Ibrahim, S.; Nguyen, D.H.; Chuah, C.H. Cellulose supported promising magnetic sorbents for magnetic solid-phase extraction: A review. Carbohydr. Polym. 2021, 253, 117245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wu, S.; Zhou, J. Preparation and modification of nanocellulose and its application to heavy metal adsorption: A review. Int. J. Biol. Macromol. 2023, 236, 123916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, J.; Zhao, N.; Wang, Y.; Zheng, W.; Guo, Y.; Pan, Q.; Li, S. Accessing renewable magnetic cellulose nanofiber adsorbent to enhance separation efficiency for adsorption and recovery of Cd2+. Int. J. Biol. Macromol. 2025, 296, 139765. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.I.A.; Baca, J.A.D.; Fatehi, P. One-pot synthesis of magnetic cellulose nanocrystal and its post-functionalization for doxycycline adsorption. Carbohydr. Polym. 2023, 308, 120619. [Google Scholar] [CrossRef]
- Shruthi, S.; Vishalakshi, B. Development of banana pseudo stem cellulose fiber based magnetic nanocomposite as an adsorbent for dye removal. Int. J. Biol. Macromol. 2024, 278, 134877. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, Y.; Li, M.; Li, W.; Li, K. Polyethyleneimine-functionalized magnetic sugarcane bagasse cellulose film for the efficient adsorption of ibuprofen. Int. J. Biol. Macromol. 2024, 265, 130969. [Google Scholar] [CrossRef]
- He, X.; Chen, T.; Jiang, T.; Wang, C.; Luan, Y.; Liu, P.; Liu, Z. Preparation and adsorption properties of magnetic hydrophobic cellulose aerogels based on refined fibers. Carbohydr. Polym. 2021, 260, 117790. [Google Scholar] [CrossRef]
- Barzegarzadeh, M.; Hazrati, A.; Amini-Fazl, M.S. Cellulose extraction from corn husk for cellulose-based bionanocomposite preparation with remarkable adsorption capacity for doxorubicin drug: Emphasis on effects ultrasonic waves. Int. J. Biol. Macromol. 2025, 307, 141875. [Google Scholar] [CrossRef]
- Guo, F.; Hu, X.; Zhang, X.; Chen, Y.; Hou, J. Study on soil heavy metal contamination and its remediation using lignin-based adsorbents: A review. Environ. Technol. Innov. 2025, 37, 103958. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, Y.; Zhang, W.; Zhao, R.; Ru, Y.; Liu, T. One-pot method to prepare lignin-based magnetic biosorbents for bioadsorption of heavy metal ions. Ind. Crops Prod. 2022, 176, 114387. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.; Dai, K.; Xiang, H.; Kou, J.; Guo, H.; Ying, H.; Chen, X.; Qu, J. Selective adsorption of anionic dyes by a macropore magnetic lignin-chitosan adsorbent. Int. J. Biol. Macromol. 2024, 269, 131955. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cui, Y.; Zhou, S.; Ye, J.; Sun, J.; Liu, X. Rapid adsorption of dyes from aqueous solutions by modified lignin derived superparamagnetic composites. J. Mol. Struct. 2022, 1261, 132954. [Google Scholar] [CrossRef]
- Bai, Y.; Han, Y.; Ma, Z.; Guo, Y.; Wang, X.; Sun, D. Magnetic composite particles coated with multi-layered lignin universally utilizing for oil–water separation and electromagnetic wave absorption. Sep. Purif. Technol. 2025, 362, 131690. [Google Scholar] [CrossRef]
- Yun, H.; Park, S.; Bang, J.; Kim, J.; Jung, S.; Won, S.; Kim, S.; Lim, H.; Kim, S.; Choi, I.; et al. Lignin-derived carbon flake sorbent for efficient oil-water separation. Int. J. Biol. Macromol. 2025, 308, 142618. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Qu, J.; Xie, S.; Zhao, T.; Liu, F.; Liu, C. Facile fabrication of green and sustainable gelatin-based aerogels for marine oil spill recovery. J. Water Process Eng. 2024, 68, 106383. [Google Scholar] [CrossRef]
- Huang, Y.; Lapanje, A.; Parakhonskiy, B.; Skirtach, A.G. Versatile and durable polyvinyl alcohol/alginate/gelatin/quaternary ammonium chitosan/Fe3O4 particles hybrid hydrogel beads: Adsorption capabilities for cleaning pollutants. Int. J. Biol. Macromol. 2024, 280, 135729. [Google Scholar] [CrossRef]
- Cui, C.; Qiao, W.; Li, D.; Wang, L. Dual cross-linked magnetic gelatin/carboxymethyl cellulose cryogels for enhanced Congo red adsorption: Experimental studies and machine learning modelling. J. Colloid Interface Sci. 2025, 678, 619–635. [Google Scholar] [CrossRef]
- Anulekshmi, P.S.; Nithya, K.; Kumar, P.S.; Sathish, A.; M, P.; Rekha, E.; Cheruvally, A.S.; Rangasamy, G. Design of biocompatible gelatin hydrogels reinforced with magnetite nanoparticles: Effective removal of chromium from water environment. Environ. Res. 2024, 260, 119768. [Google Scholar] [CrossRef]
- Yao, L.; Chi, T.; Huang, A.; Wang, L. Design and application of a polyacrylamide-grafted gelatin/biochar/Fe3O4 magnetic coagulant for microcystin-LR and turbidity co-removal: A case study with Yangtze River water. Int. J. Biol. Macromol. 2025, 311, 143349. [Google Scholar] [CrossRef] [PubMed]
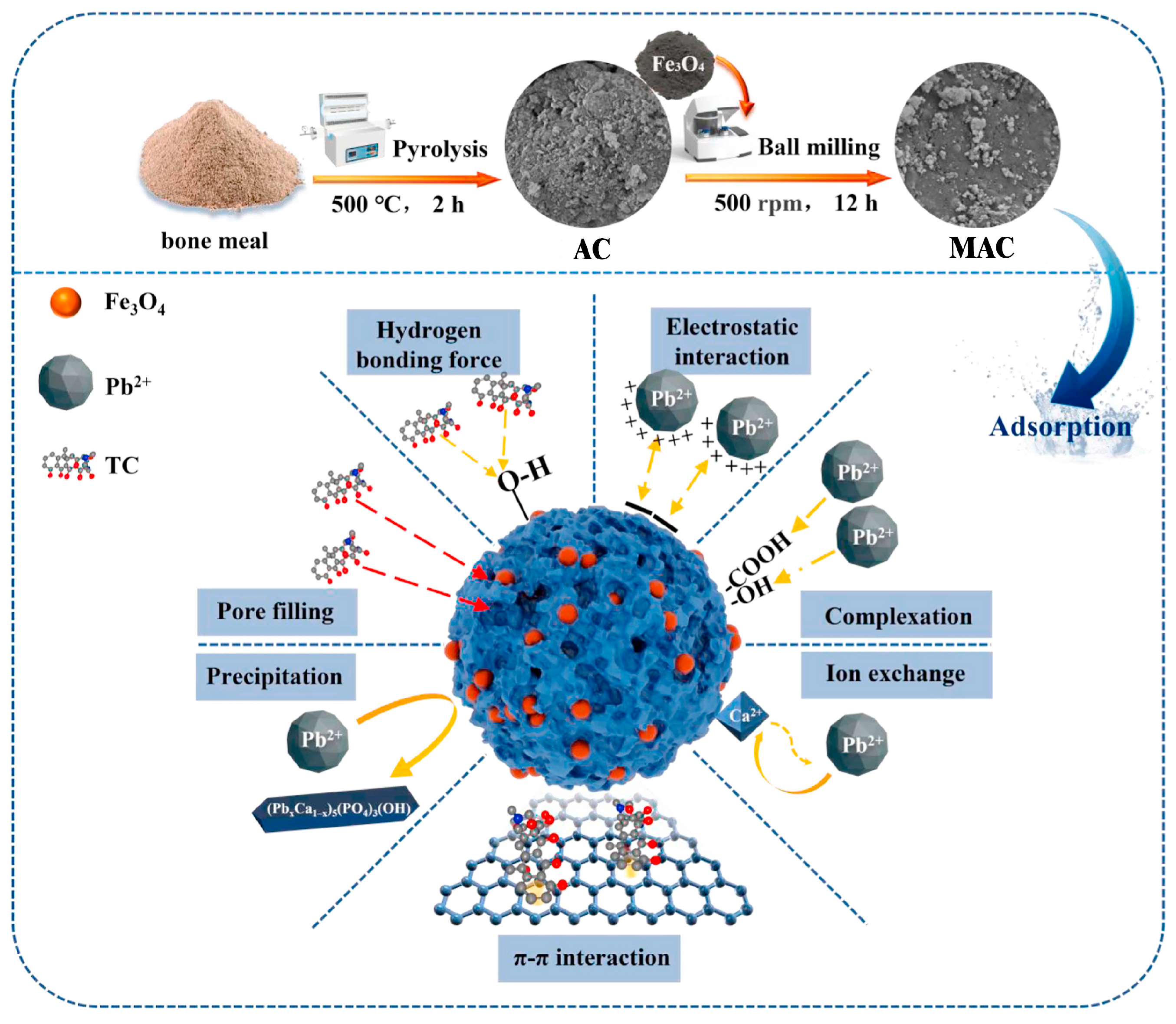
| Carrier Category | Key Advantages | Main Limitations | Typical Target Pollutants |
|---|---|---|---|
| Carbon-based materials | high specific surface area rich porosity strong modifiability | relatively high cost synthesis may involve toxic chemicals | dyes antibiotics heavy metals |
| Inorganic mineral materials | low cost abundant reserves high ion exchange capacity | poor regenerability performance susceptible to environmental pH | heavy metal ions cationic dyes |
| Natural polymer materials | renewable biodegradable abundant functional groups | low mechanical strength poor stability in complex water matrices | various heavy metals and organic pollutants |
| Carrier Category | Typical Carriers | Core Synergistic Mechanisms | Key Influencing Factors | Performance Enhancement Effect |
|---|---|---|---|---|
| Carbon-based materials | Activated carbon Carbon nanotubes Graphene oxide | π-π stacking/electrostatic attraction Magnetic particle-oxygen functional group H-bond networks Fe2+/Fe3+ reduction effects | Graphitization degree, functional group density, magnetic particle loading position | Increased adsorption capacity |
| Inorganic mineral materials | Montmorillonite Sepiolite Laponite | Cation exchange fixation of Fe2+/Fe3+ Binding with surface silanol groups Interlayer confinement inhibiting particle growth | Layer charge density, cation exchange capacity, mineral pretreatment | Selective adsorption of heavy metal ions |
| Natural polymer materials | Cellulose Lignin Gelatin | Functional group coordination bonding π-π interactions via lignin aromatic structures Polymer network encapsulation prevents detachment | Functional group density, crosslinking degree, molecular weight, magnetic particle surface modification | Enhanced anti-salinity interference capability |
| Synthesis Method | Key Process Conditions | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Impregnation-Pyrolysis | 300–1000 °C, inert atmosphere | Simultaneous carbonization and magnetization, stable material properties | High energy consumption, may generate harmful gases, pore collapse | [20,21,22,23] |
| Hydrothermal Synthesis | 100–300 °C, autoclave | Mild reaction conditions, controllable morphology | Long reaction time, sensitive to parameters, sometimes poor magnetic stability | [24,25,26] |
| Mechanical Ball Milling | Room temperature, mechanical force | Simple operation, green and economical, potential for scalability | May cause structural damage, potentially weak interfacial bonding | [27,28,29,30] |
| Mineral Carrier | Typical Composite | Adsorption Capacity (Example) | Adsorption Mechanism | Practical Application Challenges |
|---|---|---|---|---|
| Montmorillonite | Fe3O4/MMT | TC: ~285 mg/g [58] | ion exchange surface complexation catalytic degradation | Layered structure easily damaged under high pressure, Fe2+ prone to oxidation |
| Sepiolite | Fe3O4/SEP | CIP: ~100 mg/g [66] | complexation with surface groups channel capture | Fibrous structure prone to pore blockage, performance varies with mineral purity |
| Laponite | PVA/Laponite/Fe3O4 | Cd2+: ~90 mg/g [75] | interlayer confinement electrostatic attraction | High cost, weak resistance to ionic interference, difficult regeneration |
| Polymer Matrix | Functionalization Strategy | Effect | New Functionality Introduced | References |
|---|---|---|---|---|
| Cellulose | Grafting polyethyleneimine (PEI) | Significantly enhanced adsorption capacity for ibuprofen | Introduces amine groups for higher affinity to specific pollutants | [88] |
| Lignin | Acetylation modification | Rapid adsorption capacity for both cationic and anionic dyes | Modifies hydrophilicity/hydrophobicity, enhances structural stability | [94] |
| Gelatin | Blending with alginate/PVA | Forms robust hydrogel beads for easy separation | Improves mechanical properties, enables multi-network synergistic adsorption | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.; Sun, C.; Sun, K.; Chen, D.; Xu, L.; Han, S.; Li, X. Advances in Magnetic Nanocomposite Adsorbents for Water Remediation: Design, Performance, and Challenges. Nanomaterials 2025, 15, 1425. https://doi.org/10.3390/nano15181425
Yan M, Sun C, Sun K, Chen D, Xu L, Han S, Li X. Advances in Magnetic Nanocomposite Adsorbents for Water Remediation: Design, Performance, and Challenges. Nanomaterials. 2025; 15(18):1425. https://doi.org/10.3390/nano15181425
Chicago/Turabian StyleYan, Mingyu, Chao Sun, Keying Sun, Derui Chen, Longbin Xu, Shunyu Han, and Xinyu Li. 2025. "Advances in Magnetic Nanocomposite Adsorbents for Water Remediation: Design, Performance, and Challenges" Nanomaterials 15, no. 18: 1425. https://doi.org/10.3390/nano15181425
APA StyleYan, M., Sun, C., Sun, K., Chen, D., Xu, L., Han, S., & Li, X. (2025). Advances in Magnetic Nanocomposite Adsorbents for Water Remediation: Design, Performance, and Challenges. Nanomaterials, 15(18), 1425. https://doi.org/10.3390/nano15181425








