Nanotechnology for Biomedical Applications: Synthesis and Properties of Ti-Based Nanocomposites
Abstract
1. Introduction
| Biomaterial (wt. %) | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) | Elastic Modulus (GPa) | Density (g/cm3) |
|---|---|---|---|---|---|
| Natural bone | 35–283 | – | 1.07–2.10 | 5–23 | 1.85 |
| α-type cp-Ti ann. | 240 | 170 | 24 | 102.7 | 4.5 |
| α + β-type Ti6Al4V ELI ann. | 860–965 | 795–875 | 10–15 | 101–110 | 4.42 |
| β-type Ti-15Mo ann. | 874 | 544 | 21 | 78 | 4.95 |
| Pure Mg | 100.47 | 29.88 | 7.43 | 1.86 | 1.7 |
| Mg-5Zn | 194.59 | 75.60 | 8.50 | 36.47 | 1.735 |
| 316L stainless steel | 580–1300 | 220–1200 | 50–60 | 190 | 7.9 |
| Co-Cr-Mo alloy | 650–1900 | 450–1600 | 5–20 | 210–253 | 8.3 |
| Hydrogel sodium alginate PYG/A70 | – | – | – | 4.93 | 1.0 |
| PMMA | 8.16 | – | 1.04 | 7.85 | 1.17–1.20 |
| PMMA/0.3 MWCNTs hybrid composite | 30.99 | – | 4.72 | 6.57 | – |
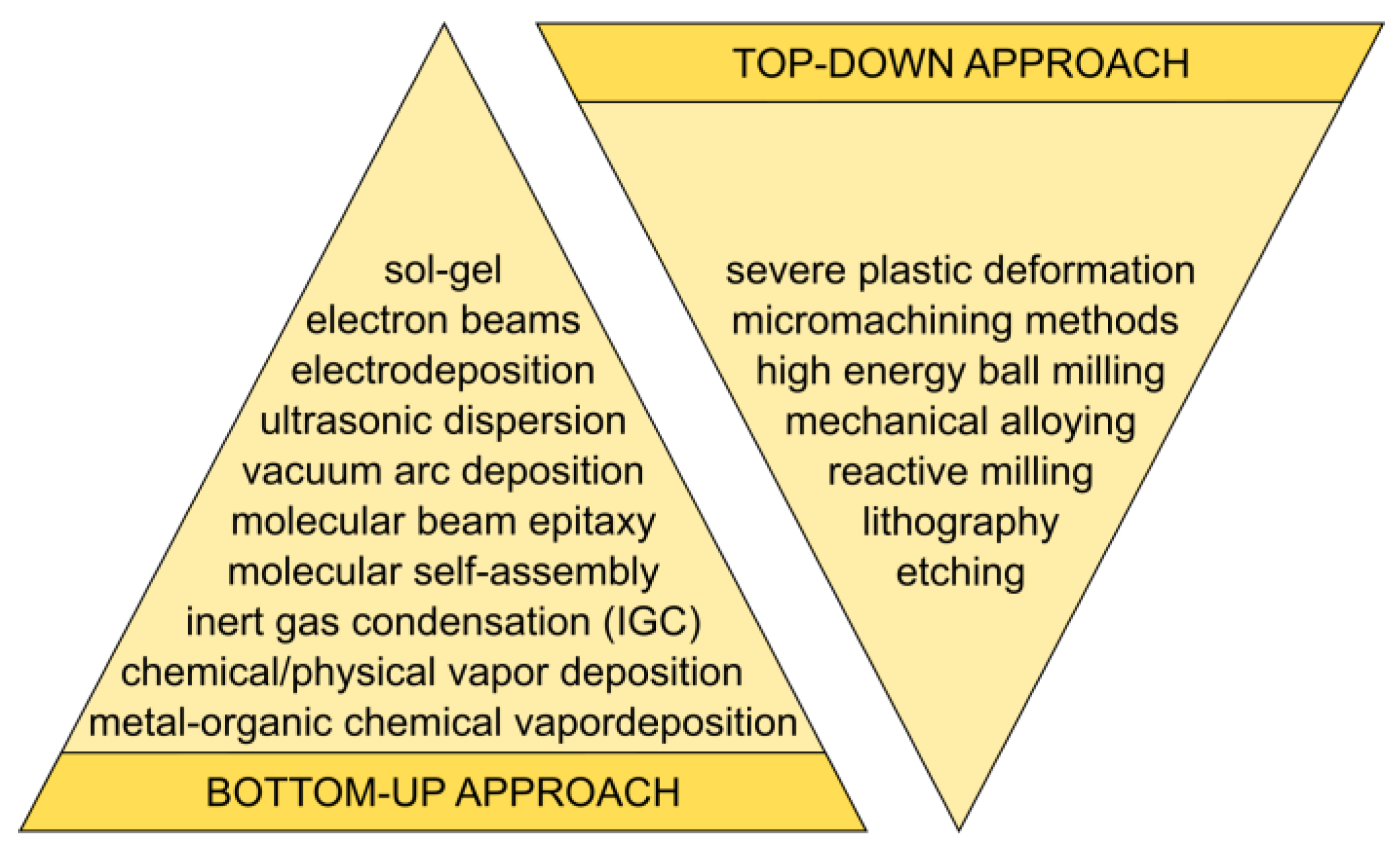
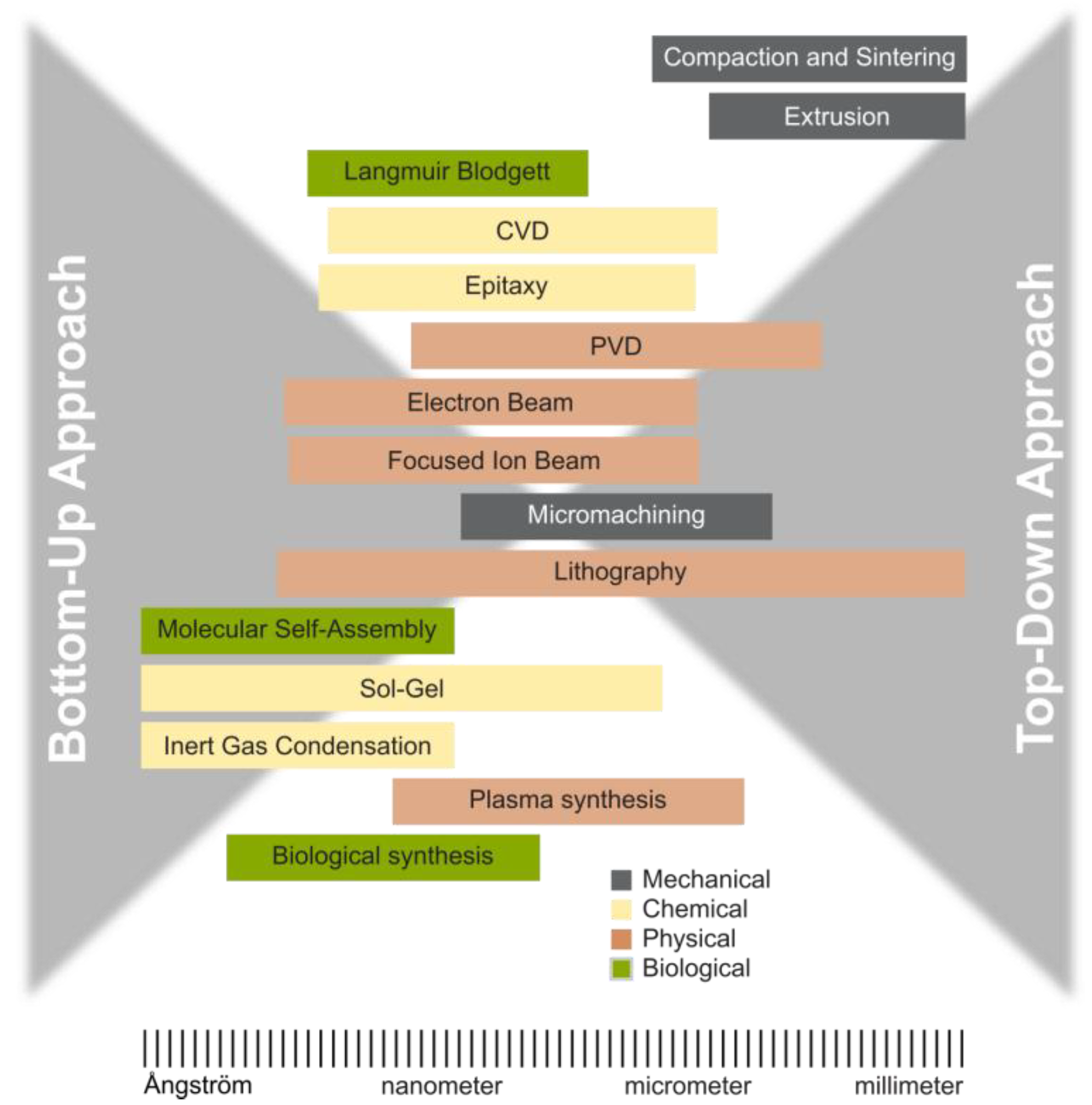
2. Classification of Synthesis Methods
3. Nanomaterial Synthesis Methods
3.1. Physical Methods
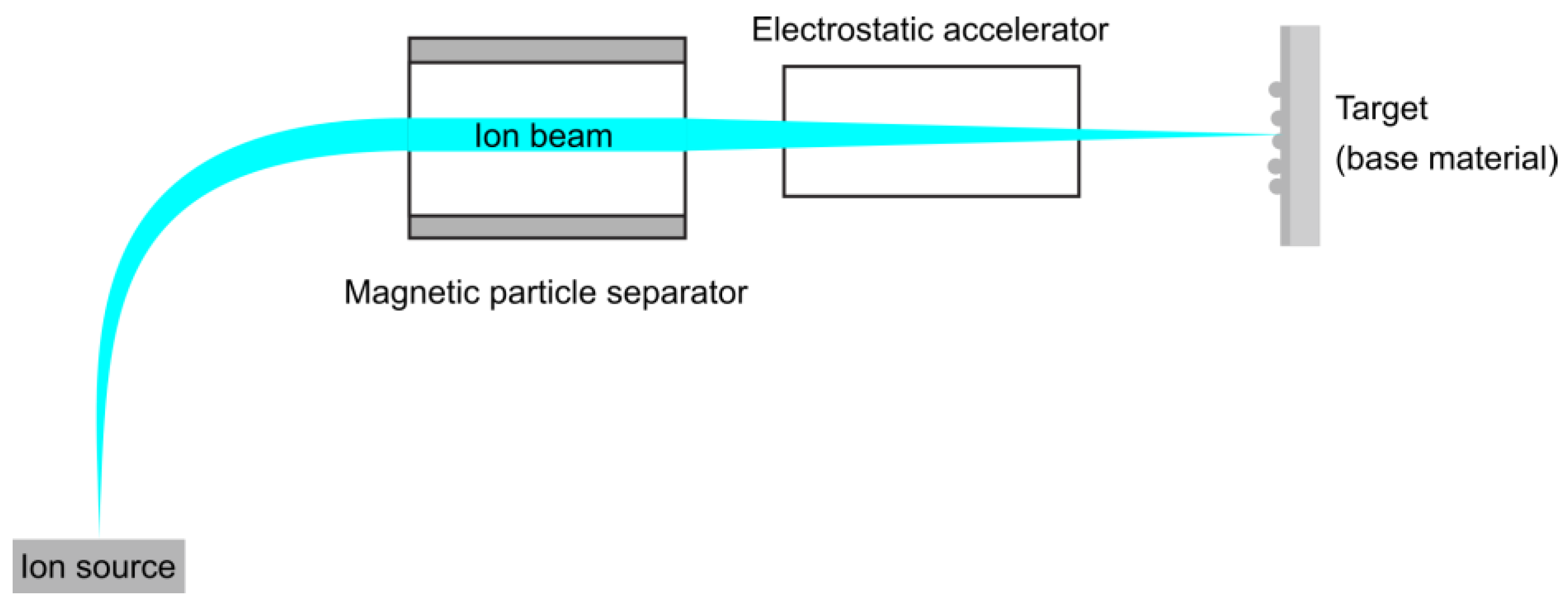
3.1.1. Ion Beam Techniques
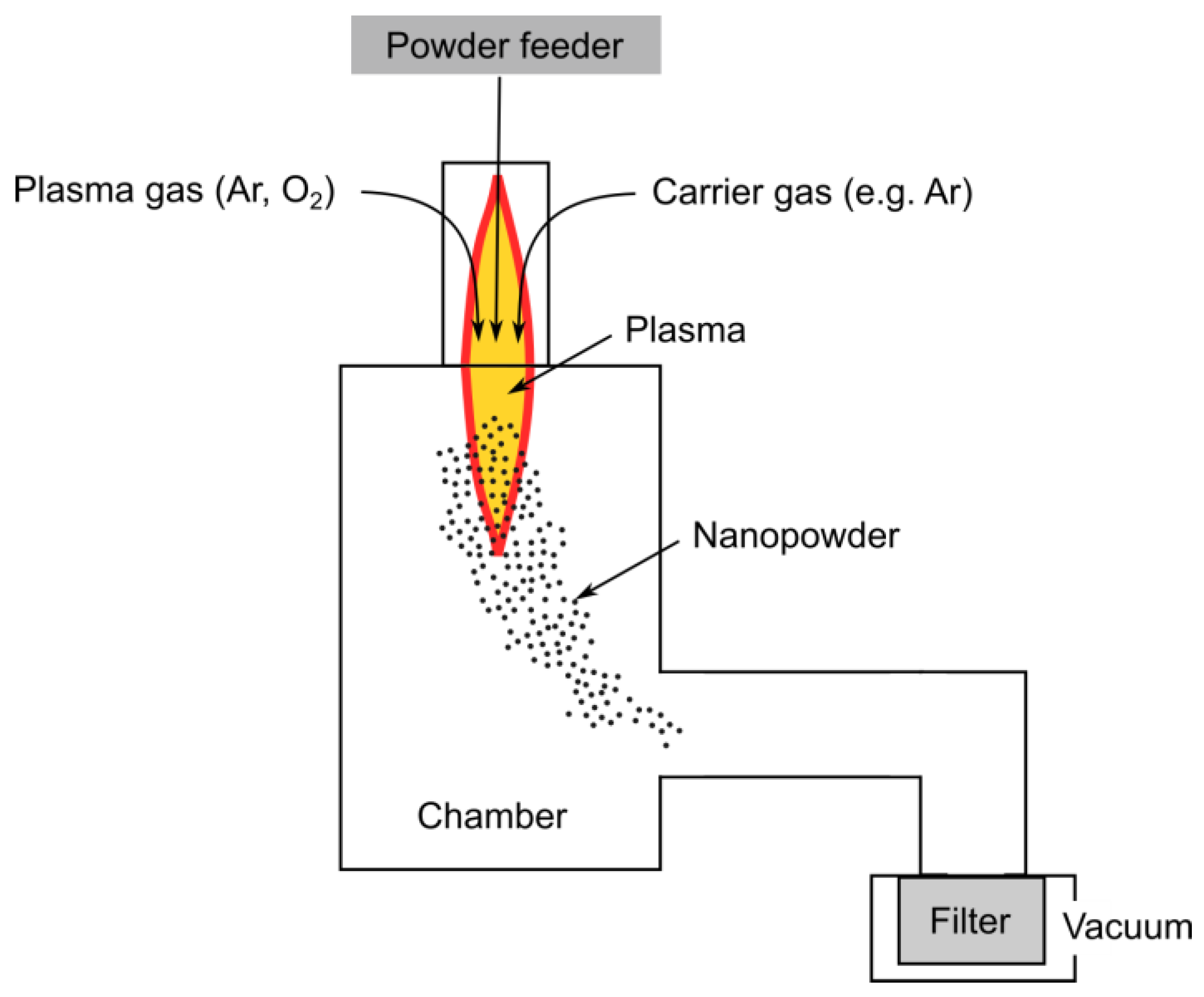
3.1.2. Plasma Synthesis
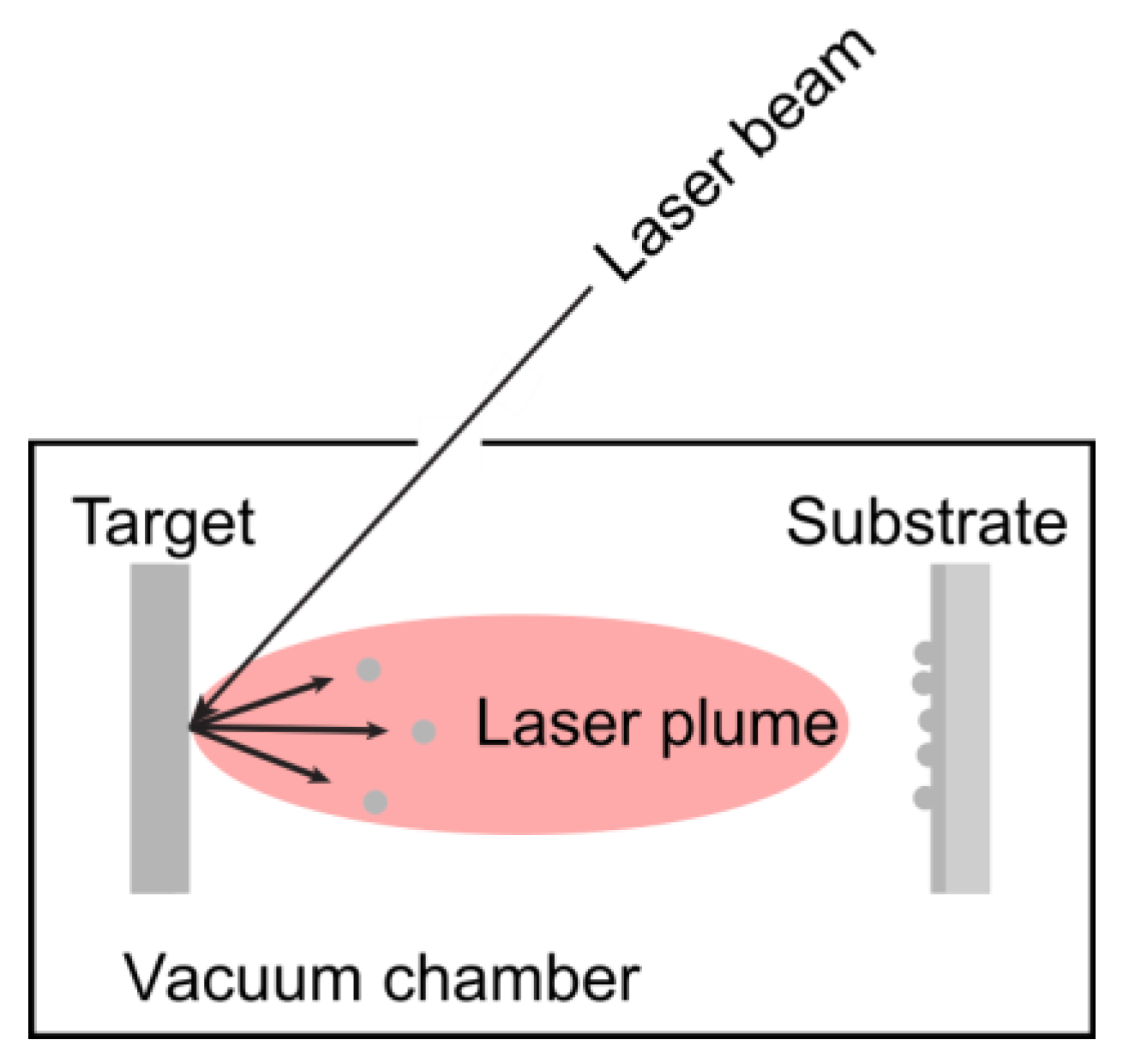
3.1.3. Pulsed Laser Deposition
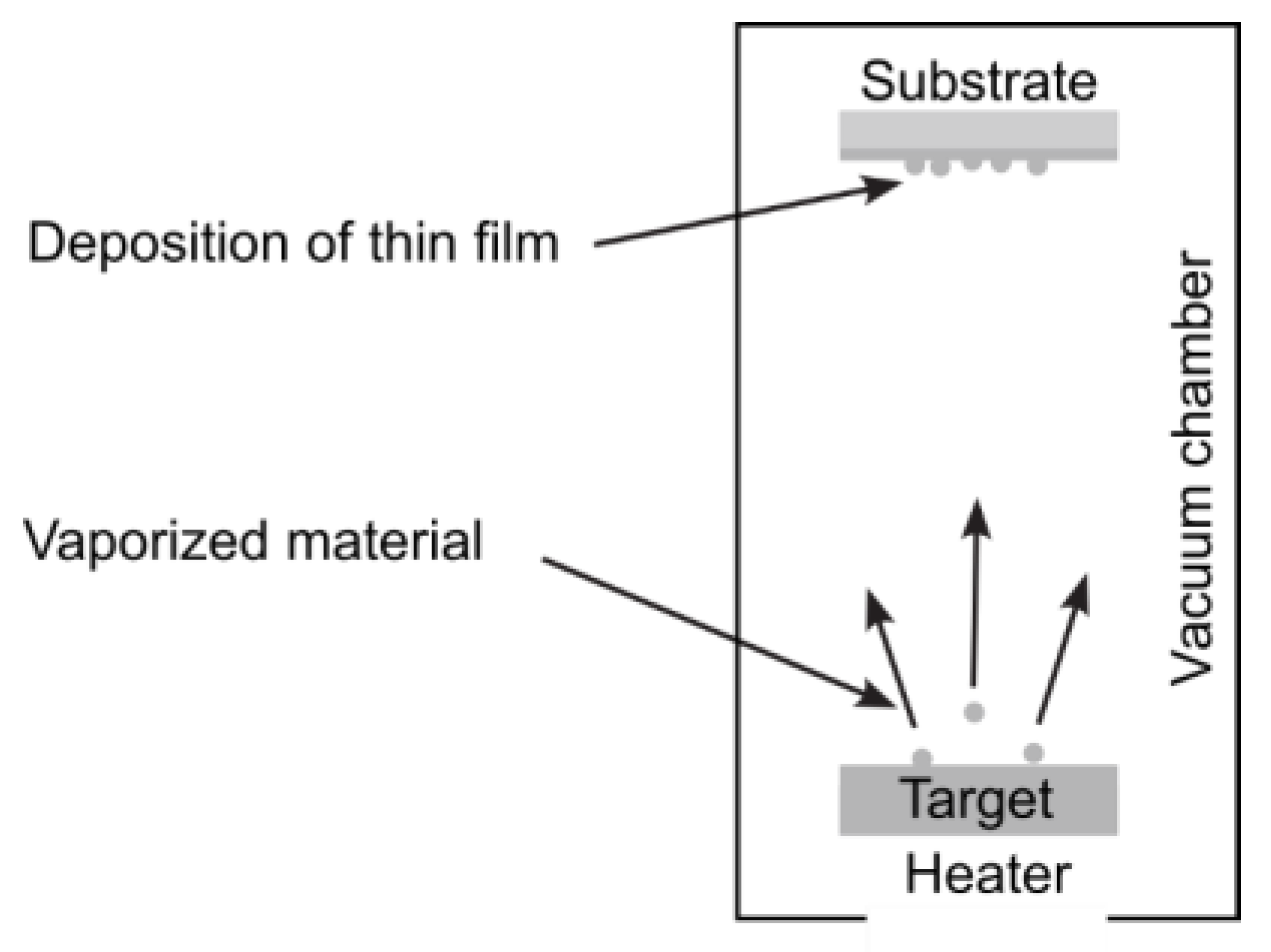
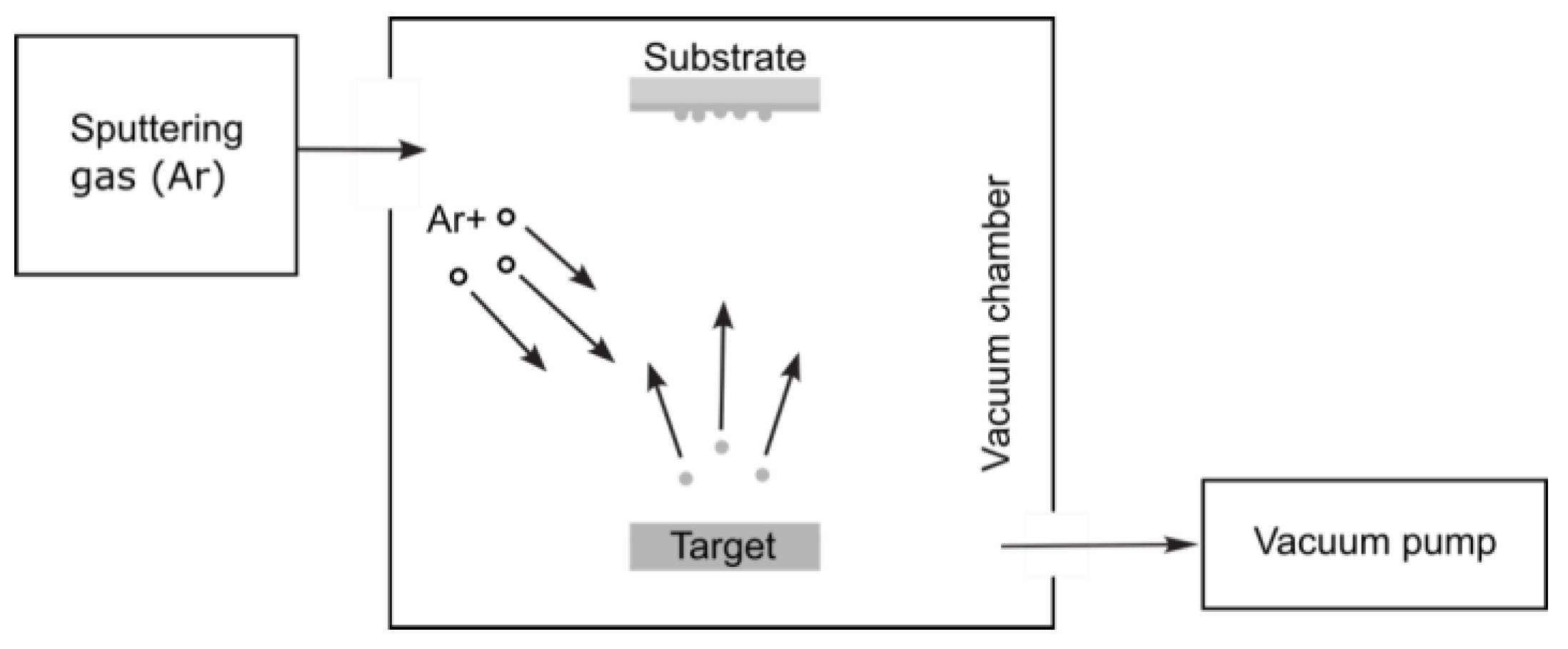
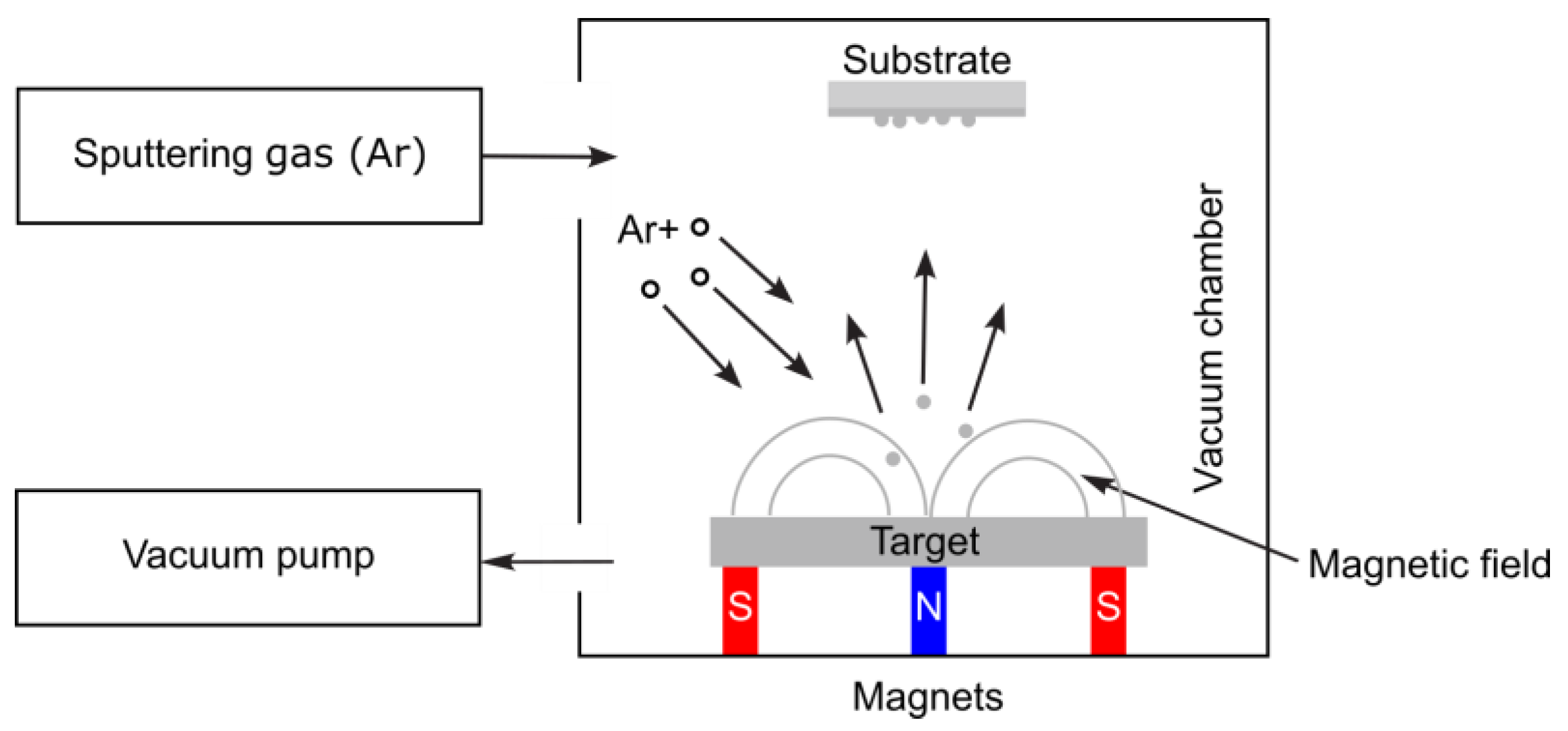
3.1.4. Physical Vapor Deposition
3.2. Chemical Methods
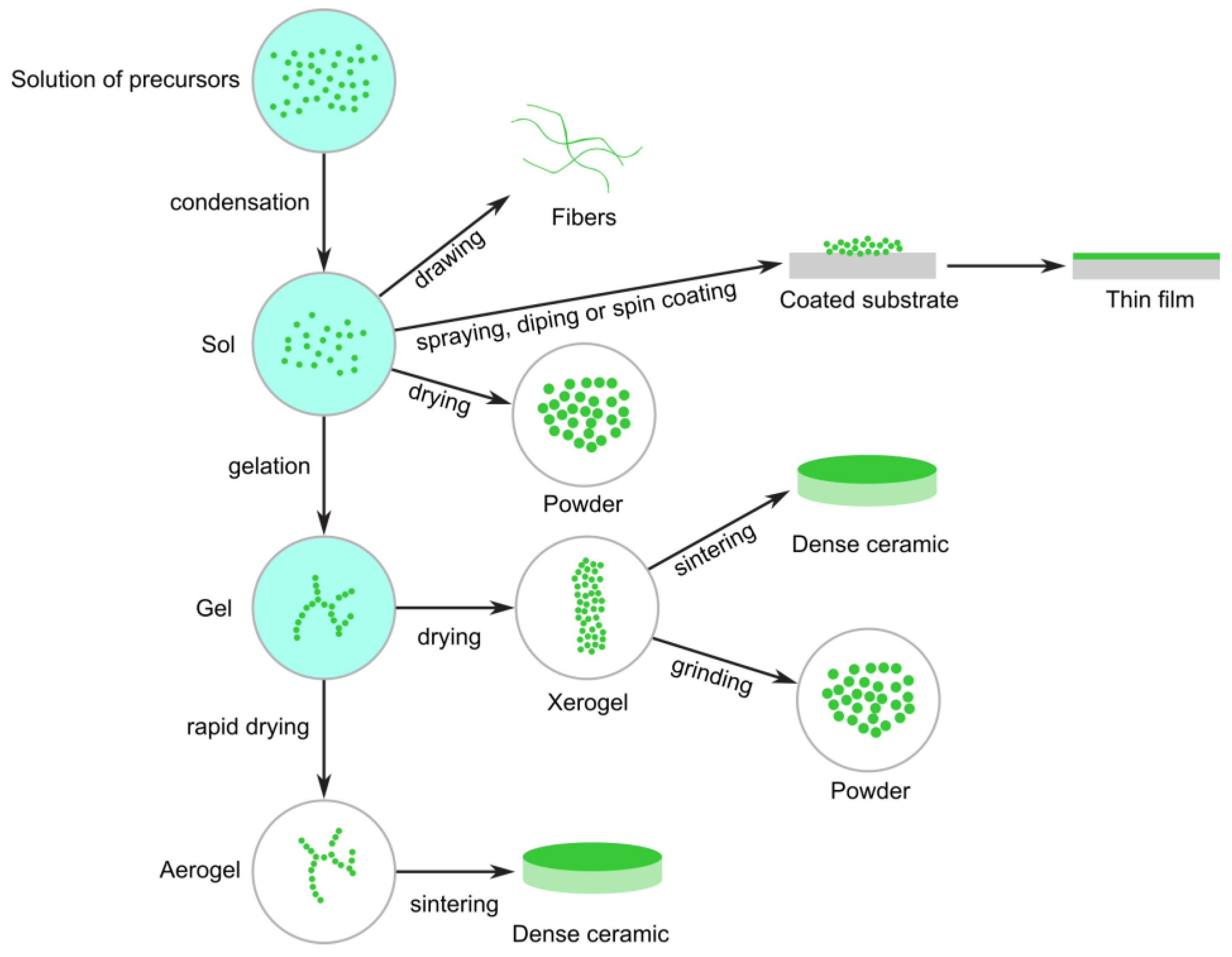
3.2.1. Sol–Gel
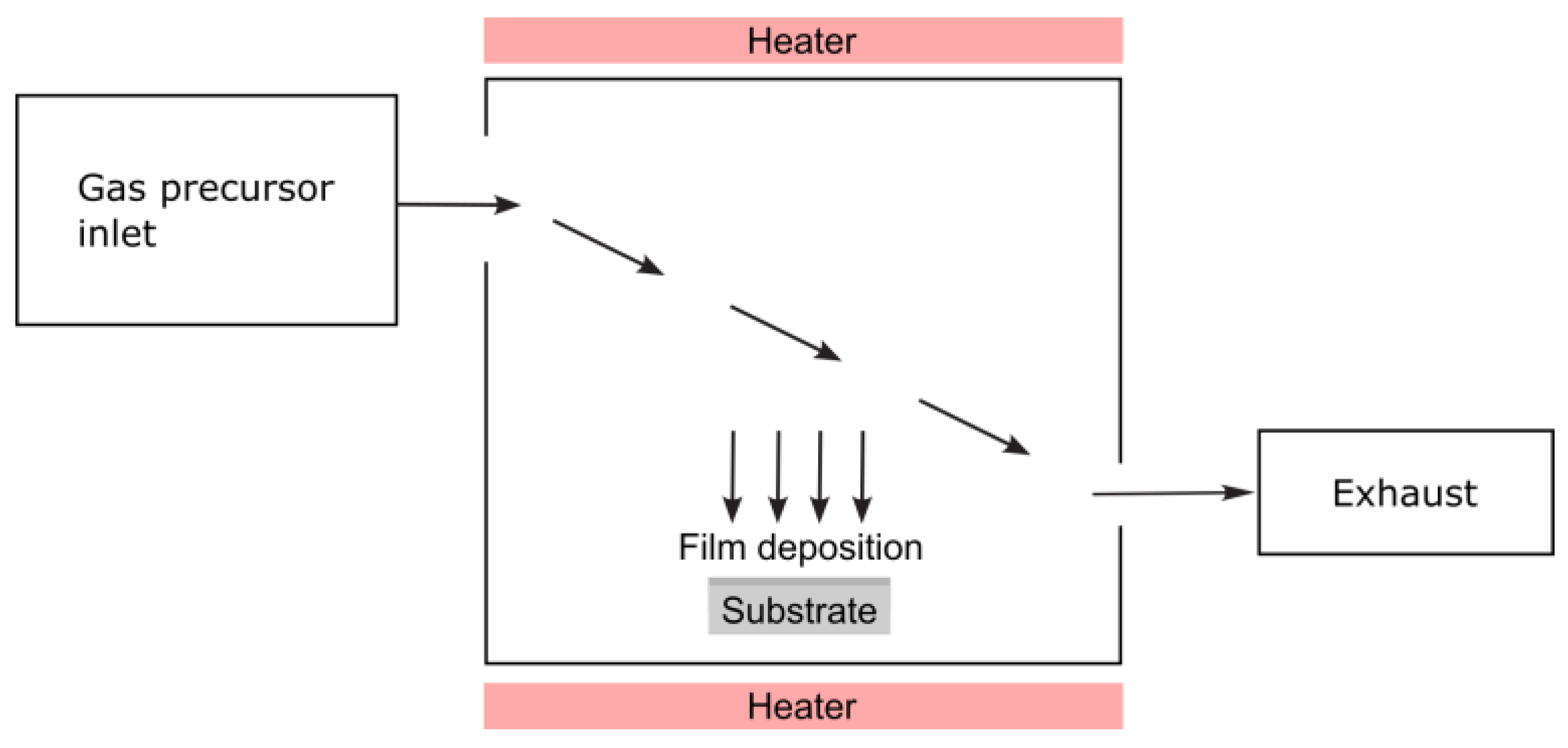
3.2.2. Chemical Vapor Deposition
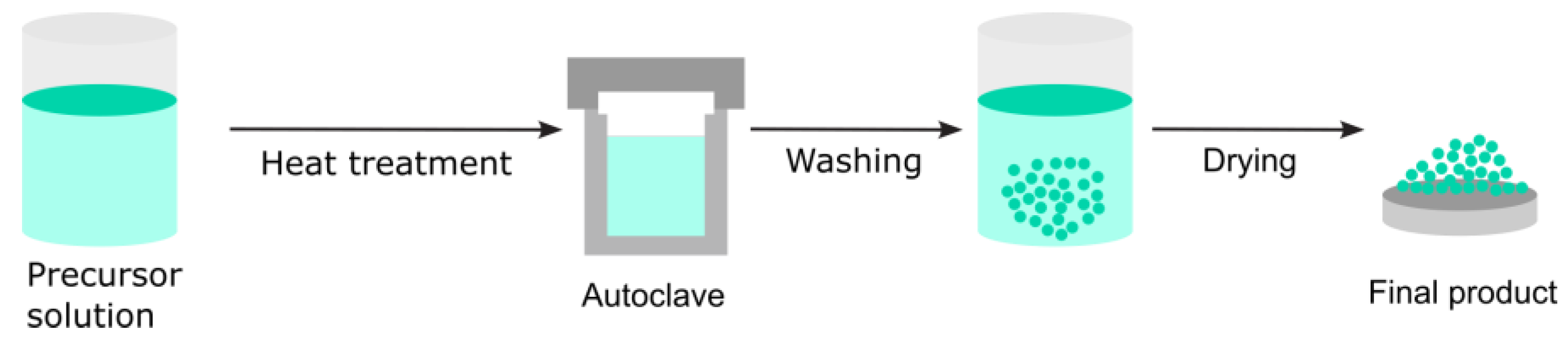
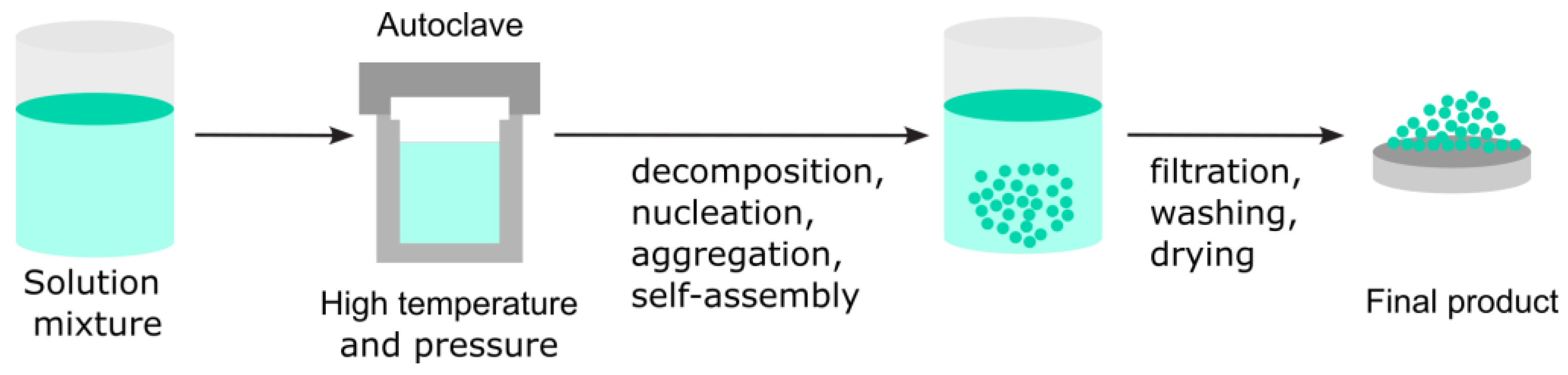
3.2.3. Hydrothermal Route
3.2.4. Epitaxial Growth
3.2.5. Polymer Route
3.2.6. Colloidal Dispersion
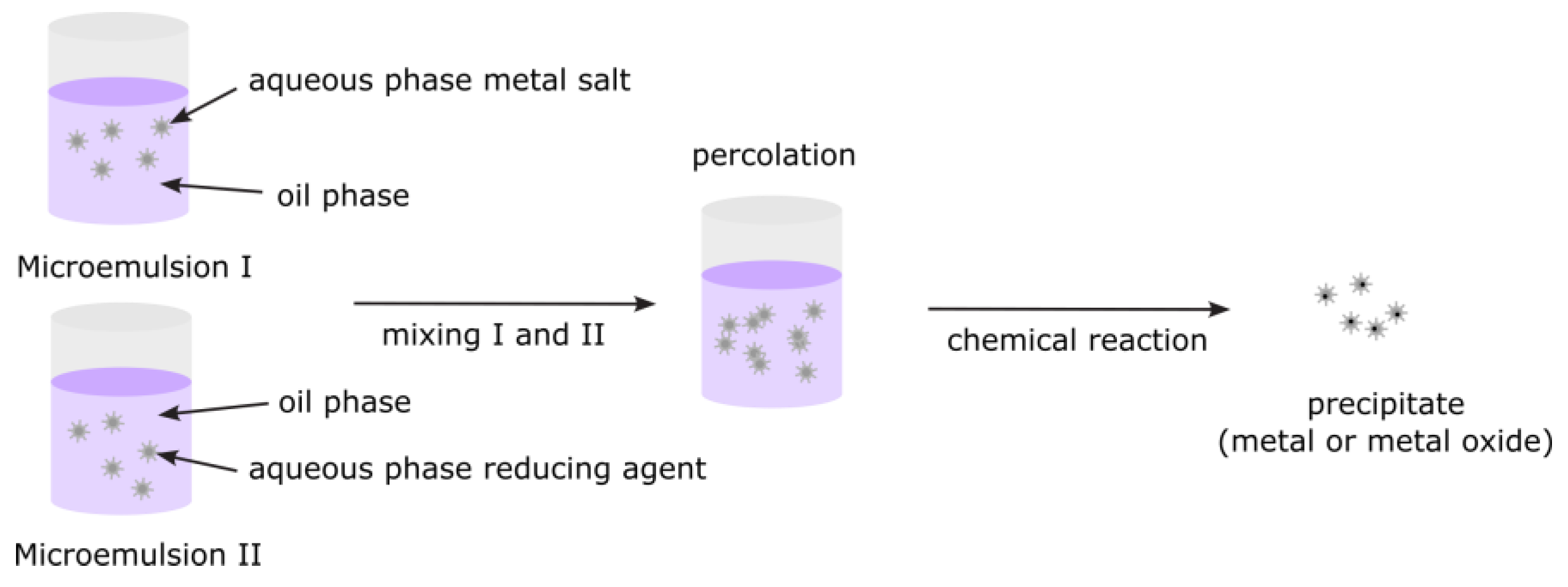
3.2.7. Microemulsions
3.2.8. Solvothermal Decomposition
3.3. Mechanical Methods
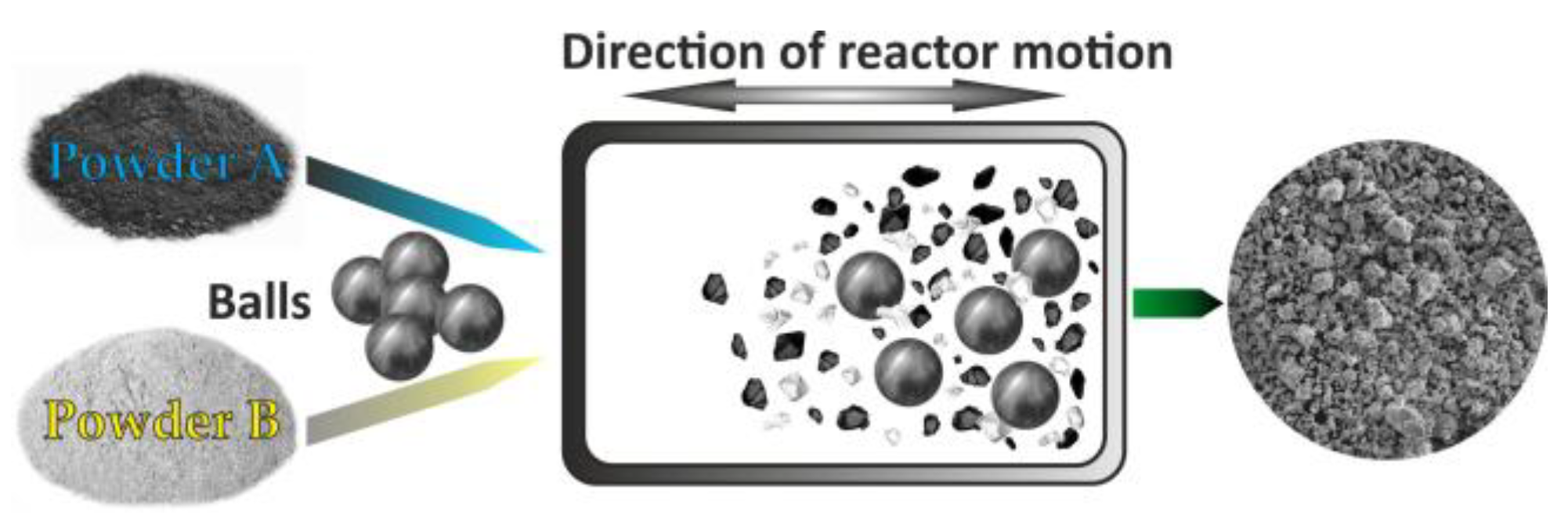
3.3.1. Milling Processes
3.3.2. High-Energy Ball Milling
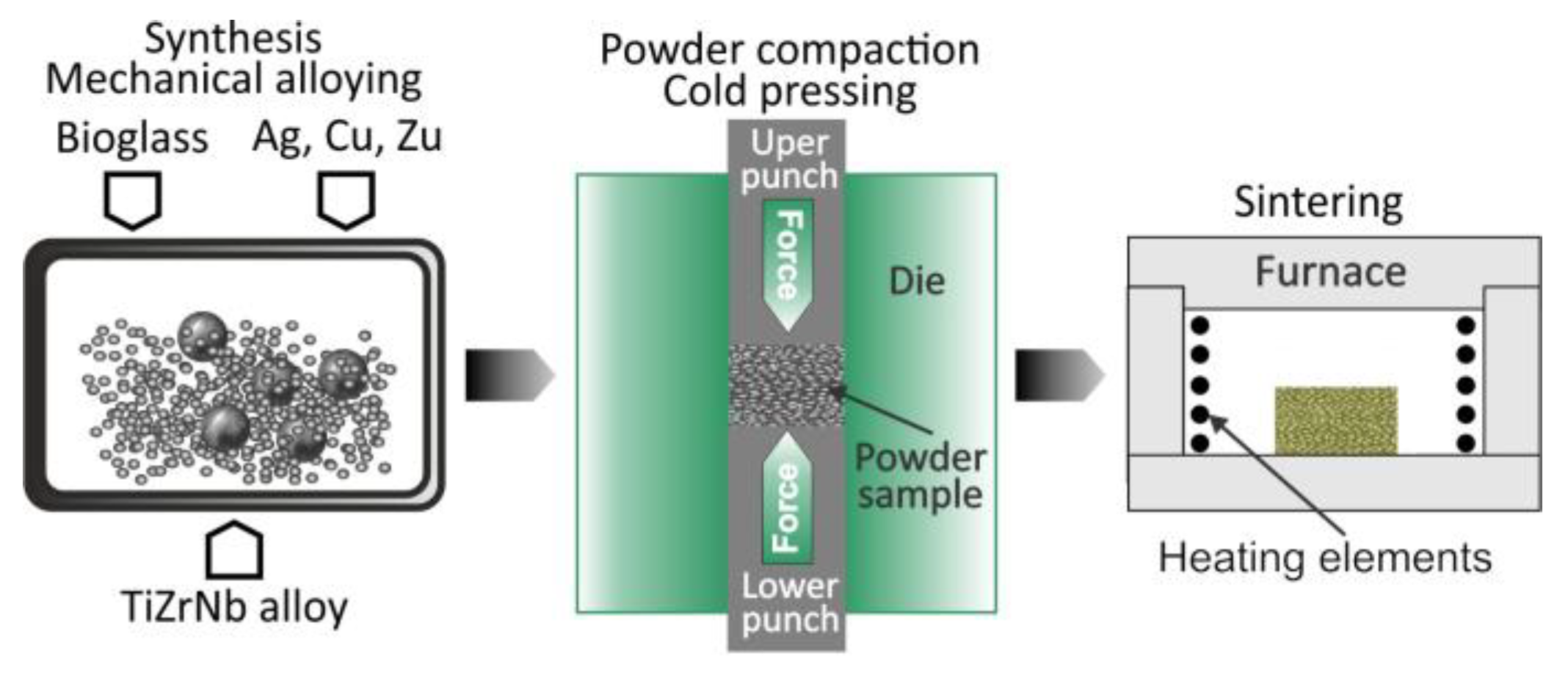
3.3.3. Mechanical Alloying
3.3.4. Mechanochemical Synthesis
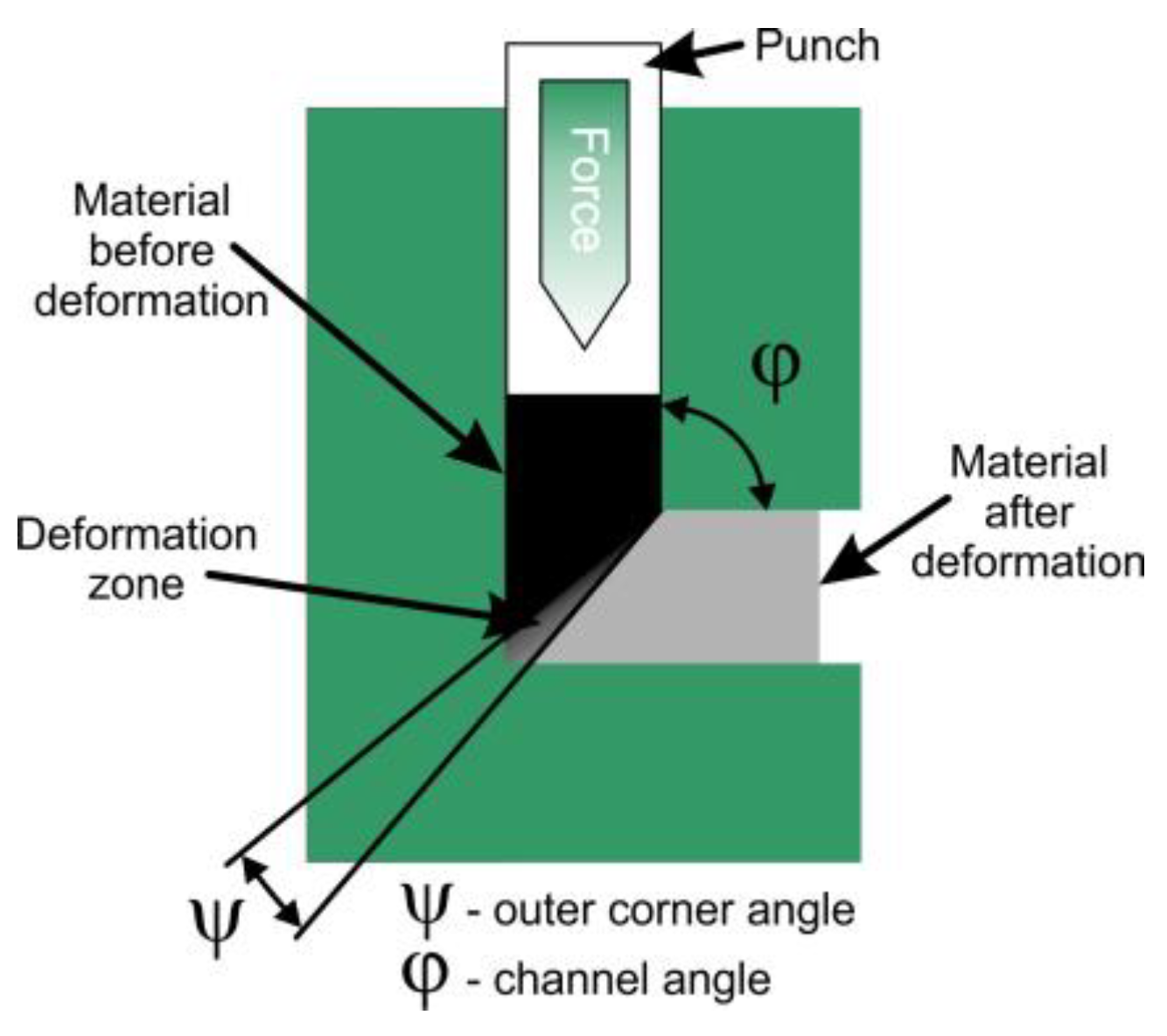
3.4. Severe Plastic Deformation
4. Consolidating the Techniques of Nanoparticles
4.1. Hot Pressing
4.2. Sinter Forging
4.3. Hot Isostatic Pressing
4.4. Transformation-Assisted Consolidation
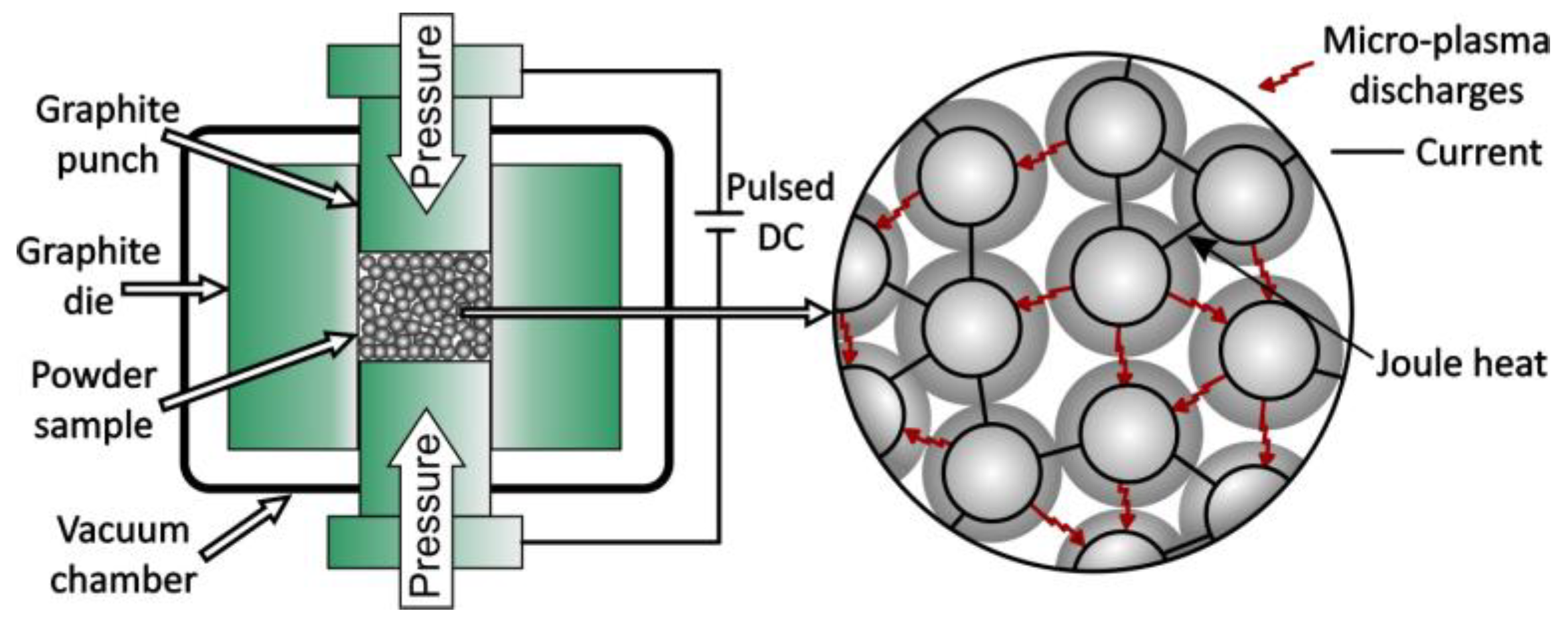
4.5. Spark Plasma Sintering
4.6. Plasma Pressure Compaction
4.7. Severe Plastic Torsional Straining
4.8. Microwave Sintering
4.9. Dynamic Consolidation Using Shock Waves
4.10. Plasma Forming
4.11. Laser-Based Techniques
5. Scaffolds
6. Surface Modification Methods for Titanium
6.1. Ion Beam-Assisted Deposition
6.2. Microplasma Surface Alloying
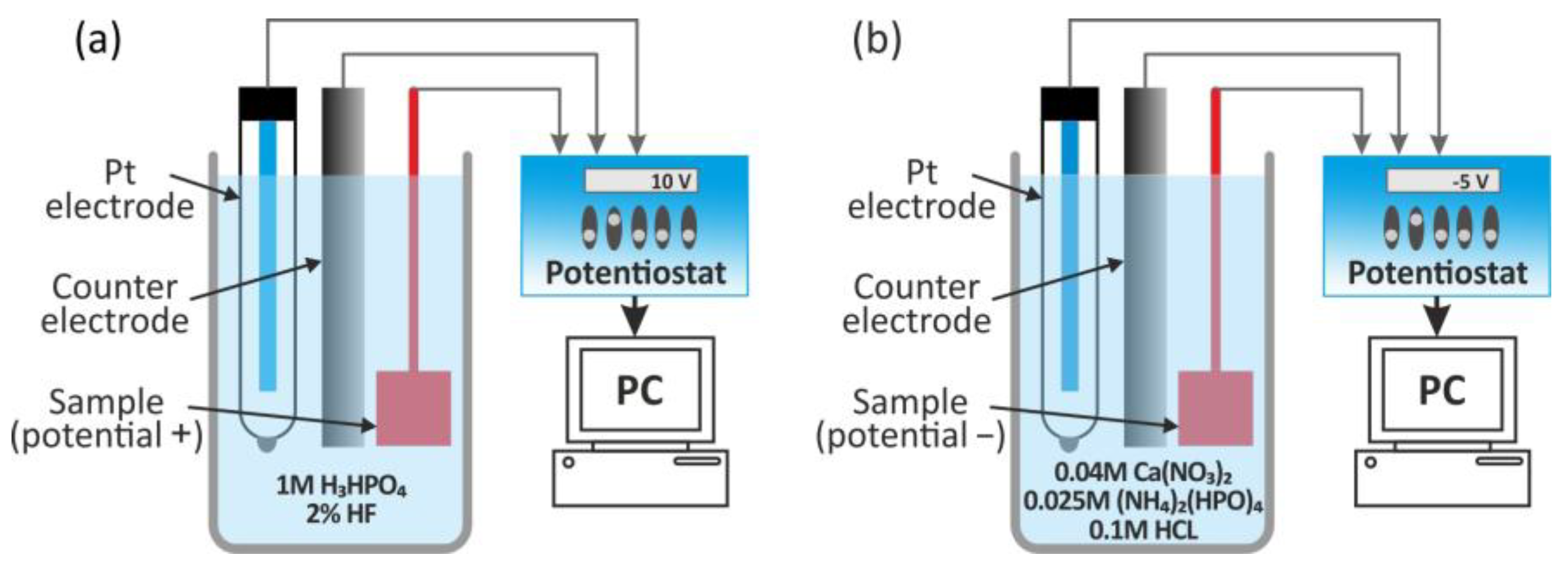
6.3. Anodization Process
6.4. Bioactive Foam Scaffolds
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABR | Accumulative Roll Bonding |
| AES | Auger Electron Spectroscopy |
| AFM | Atomic Force Microscopy |
| APS | X-Ray Photoelectron Spectroscopy |
| BET | Brunauer–Emmett–Teller Method |
| BIR | Bone Ingrowth Rate |
| BPR | Ball to Powder Ratio |
| Ca-P | Calcium Phosphate |
| CAD | Computer-Aided Design |
| CEC | Cyclic Extrusion Compression |
| CVD | Chemical Vapor Deposition |
| DIW | Distance-Controlled Direct Ink Writing |
| DC-DIW | Direct Ink Writing |
| DLS | Dynamic Light Scattering |
| DSC | Differential Scanning Calorimetry |
| NFs | Nanofibers |
| RHGF | Rapid Hot Gas Forming |
| SLM | Selective Laser Melting |
| EBSD | Electron Backscatter Diffraction |
| ECAP | Equal Channel Angular Processing |
| EDX/EDS | Energy Dispersive X-Ray Spectroscopy |
| ELI | Extra-Low Interstitials |
| FSP | Friction Stir Processing |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HA | Hydroxyapatite |
| HEBM | High Energy Ball Milling |
| HIP | Hot Isostatic Pressing |
| HPT | High Pressure Torsion |
| hTNTs | hexagonal Titanium dioxide Nanotubes |
| IBAD | Ion Beam Assisted Deposition |
| LEED | Low-Energy Electron Diffraction |
| MA | Mechanical Alloying |
| MAC/F | Multi Axial Compression/Forging |
| MAO | Micro-Arc Oxidation |
| MCS | Mechano-Chemical Synthesis |
| MDF | Multi Directional Forging |
| MMNCs | Metal Matrix Nanocomposites |
| MWCNTs | Multi-Walled Carbon Nanotubes |
| NPs | Nanoparticles |
| OA | Organo Apatite |
| OES | Optical Emission Spectroscopy |
| PAS | Plasma Activated Sintering |
| PLD | Pulsed Laser Deposition |
| PMMA | Polymethyl Methacrylate |
| PPC | Plasma Pressure Compaction |
| PVD | Plasma/Physical Vapor Deposition |
| RBS | Rutherford Backscattering Spectrometry |
| RCS | Repeated Corrugation and Straightening |
| RHEED | Reflection High-Energy Electron Diffraction |
| SAXS | Small-Angle X-Ray Scattering |
| SBF | Simulated Body Fluid |
| SCPL | Solvent Casting Particulate Leaching |
| SEM | Scanning Electron Microscopy |
| SIMS | Secondary Ion Mass Spectrometry |
| SPS | Severe Plastic Deformation |
| SPS | Spark Plasma Sintering |
| SPTS | Severe Plastic Torsional Staining |
| TAC | Transformation Assisted Consolidation |
| TE | Twist Extrusion |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| TMT | Thermomechanical Processing |
| UFG | Ultra-Fine Grains |
| UV-Vis | Ultraviolet–Visible Spectroscopy |
| XPS | X-Ray Photoelectron Spectroscopy |
| XRD | X-Ray Diffraction |
References
- The Global Number of People Aged 65 Years and Older Is Set to Double Within the Next Thirty Years. Available online: https://ourworldindata.org/data-insights/by-2060-the-number-of-people-aged-65-and-older-will-be-more-than-four-times-what-it-was-in-2000 (accessed on 23 July 2025).
- Spector, M. Biomedical Materials to Meet the Challenges of the Aging Epidemic. Biomed. Mater. 2018, 13, 030201. [Google Scholar] [CrossRef]
- Vaiani, L.; Boccaccio, A.; Uva, A.E.; Palumbo, G.; Piccininni, A.; Guglielmi, P.; Cantore, S.; Santacroce, L.; Charitos, I.A.; Ballini, A. Ceramic Materials for Biomedical Applications: An Overview on Properties and Fabrication Processes. J. Funct. Biomater. 2023, 14, 146. [Google Scholar] [CrossRef]
- Roco, M.C. International Strategy for Nanotechnology Research. J. Nanoparticle Res. 2001, 3, 353–360. [Google Scholar] [CrossRef]
- Webster, T.J.; Ejiofor, J.U. Increased Osteoblast Adhesion on Nanophase Metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef]
- Bionanomaterials for Dental Applications; Jurczyk, M., Ed.; Pan Stanford Publishing: Singapore, 2012; ISBN 9789814303835. [Google Scholar]
- Mansfield, E. (Ed.) Metrology and Standardization for Nanotechnology: Protocols and Industrial Innovations; Nanotechnology Innovation & Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinham, Germany, 2017; ISBN 978-3-527-34039-2. [Google Scholar]
- Lancaster, C.A.; Scholl, W.E.; Ticknor, M.A.; Shumaker-Parry, J.S. Uniting Top-Down and Bottom-Up Strategies Using Fabricated Nanostructures as Hosts for Synthesis of Nanomites. J. Phys. Chem. C 2020, 124, 6822–6829. [Google Scholar] [CrossRef]
- Dubadi, R.; Huang, S.D.; Jaroniec, M. Mechanochemical Synthesis of Nanoparticles for Potential Antimicrobial Applications. Materials 2023, 16, 1460. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, K.; Braegger, U.; Jurczyk, M. Application of nanotechnology for dental implants. In Handbook of Nanoethics; Jeswani, G., Van de Voorde, M., Eds.; De Gruyter: Berlin, Germany, 2021; pp. 75–94, Chapter 5. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on Microstructure, Mechanical Properties and Corrosion Behavior of Mg–Zn Alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical Properties of Biomedical Titanium Alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Baskar, N.; Anand Chairman, C.; Balasundaram, R. Mechanical Properties and Applications of Magnesium Alloy—Review. Mater. Today Proc. 2020, 27, 909–913. [Google Scholar] [CrossRef]
- Hussein, M.; Mohammed, A.; Al-Aqeeli, N. Wear Characteristics of Metallic Biomaterials: A Review. Materials 2015, 8, 2749–2768. [Google Scholar] [CrossRef]
- Kurowiak, J.; Kaczmarek-Pawelska, A.; Mackiewicz, A.; Baldy-Chudzik, K.; Mazurek-Popczyk, J.; Zaręba, Ł.; Klekiel, T.; Będziński, R. Changes in the Mechanical Properties of Alginate-Gelatin Hydrogels with the Addition of Pygeum Africanum with Potential Application in Urology. Int. J. Mol. Sci. 2022, 23, 10324. [Google Scholar] [CrossRef]
- Ali, N.A.; Hussein, S.I.; Asafa, T.B.; Abd-Elnaiem, A.M. Mechanical Properties and Electrical Conductivity of Poly(Methyl Methacrylate)/Multi-Walled Carbon Nanotubes Composites. Iran. J. Sci. Technol. Trans. Sci. 2020, 44, 1567–1576. [Google Scholar] [CrossRef]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Carnevale, G. Micro- and Nanostructured Biomaterials for Biomedical Applications and Regenerative Medicine. Nanomaterials 2024, 14, 1845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, J.; Narayan, R. Nanostructured Biomaterials for Regenerative Medicine: Clinical Perspectives. In Nanostructured Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 47–80. ISBN 978-0-08-102594-9. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium Alloys in Total Joint Replacement—A Materials Science Perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-є-Caprolactone Based Formulations for Drug Delivery and Tissue Engineering: A Review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural Origin Biodegradable Systems in Tissue Engineering and Regenerative Medicine: Present Status and Some Moving Trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Cotica, L.F.; Garcia, A.; Polli, A.D.; Bini, R.D.; de Chaves, T.; de Oliveira Junior, V.A.; Pamphile, J.A. Nanobiocomposites: Synthesis and Environmental Applications. In Fungal Nanobionics: Principles and Applications; Springer: Singapore, 2018; pp. 1–19. ISBN 978-981-10-8665-6. [Google Scholar]
- Bawa, R.; Audette, G.F.; Rubinstein, I. Handbook of Clinical Nanomedicine: Nanoparticles, Imaging, Therapy and Clinical Applications; Pan Stanford Series on Nanomedicine; Pan Stanford Publishing: Singapore, 2016; ISBN 978-981-4669-20-7. [Google Scholar]
- Jurczyk, K.; Miklaszewski, A.; Niespodziana, K.; Kubicka, M.; Jurczyk, M.U.; Jurczyk, M. Synthesis and Properties of Ag-Doped Titanium-10 Wt% 45S5 Bioglass Nanostructured Scaffolds. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 467–476. [Google Scholar] [CrossRef]
- Jurczyk, K.; Kubicka, M.M.; Ratajczak, M.; Jurczyk, M.U.; Niespodziana, K.; Nowak, D.M.; Gajecka, M.; Jurczyk, M. Antibacterial Activity of Nanostructured Ti–45S5 Bioglass–Ag Composite against Streptococcus Mutans and Staphylococcus Aureus. Trans. Nonferrous Met. Soc. China 2016, 26, 118–125. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Jurczyk, M.U.; Kaczmarek, M.; Paszel-Jaworska, A.; Romaniuk, A.; Lipińska, N.; Żurawski, J.; Urbaniak, P.; Jurczyk, M. Nanoscale Size Effect in in Situ Titanium Based Composites with Cell Viability and Cytocompatibility Studies. Mater. Sci. Eng. C 2017, 73, 525–536. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M.; Pecyna, P.; Ratajczak, M.; Gajecka, M.; Jurczyk, M.U. Effect of Hydroxyapatite and Ag, Ta2O5 or CeO2 Addition on the Properties of Ultrafine-Grained Ti31Mo Alloy. J. Alloys Compd. 2020, 823, 153749. [Google Scholar] [CrossRef]
- Marczewski, M.; Jurczyk, M.U.; Kowalski, K.; Miklaszewski, A.; Wirstlein, P.K.; Jurczyk, M. Composite and Surface Functionalization of Ultrafine-Grained Ti23Zr25Nb Alloy for Medical Applications. Materials 2020, 13, 5252. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, D.; Miklaszewski, A.; Jurczyk, M. Low-Temperature Hydrothermal Treatment Surface Functionalization of the Ultrafine-Grained TiMo Alloys for Medical Applications. Materials 2020, 13, 5763. [Google Scholar] [CrossRef] [PubMed]
- Marczewski, M.; Wieczerzak, K.; Maeder, X.; Lapeyre, L.; Hain, C.; Jurczyk, M.; Nelis, T. Microstructure and Mechanical Properties of Ti-Nb Alloys: Comparing Conventional Powder Metallurgy, Mechanical Alloying, and High Power Impulse Magnetron Sputtering Processes for Supporting Materials Screening. J. Mater. Sci. 2024, 59, 9107–9125. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of Nanotechnology in Medical Field: A Brief Review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Rotella, G.; Basile, V.; Carlone, P.; Col, J.D.; Filice, L.; Orazi, L.; Romoli, L.; Rubino, F.; Saffioti, M.R. Surface Functionalization of Metallic Biomaterials: Present Trend and Future Perspectives. In Lecture Notes in Mechanical Engineering; Springer Nature: Cham, Switzerland, 2024; pp. 295–341. ISBN 978-3-031-41162-5. [Google Scholar]
- Dhaka, A.; Chand Mali, S.; Sharma, S.; Trivedi, R. A Review on Biological Synthesis of Silver Nanoparticles and Their Potential Applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Ramsden, J. Nanotechnology: An Introduction; Micro and Nano Technologies Series; William Andrew/Elsevier: Oxford, UK; Waltham, MA, USA, 2011; ISBN 978-0-08-096447-8. [Google Scholar]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef]
- Dowling, A.P. Development of Nanotechnologies. Mater. Today 2004, 7, 30–35. [Google Scholar] [CrossRef]
- Zweck, A.; Bachmann, G.; Luther, W.; Ploetz, C. Nanotechnology in Germany: From Forecasting to Technological Assessment to Sustainability Studies. J. Clean. Prod. 2008, 16, 977–987. [Google Scholar] [CrossRef]
- VandenBerg, M.A.; Dong, X.; Smith, W.C.; Tian, G.; Stephens, O.; O’Connor, T.F.; Xu, X. Learning from the Future: Towards Continuous Manufacturing of Nanomaterials. AAPS Open 2025, 11, 7. [Google Scholar] [CrossRef]
- Wang, Z.L.; Liu, Y.; Zhang, Z. (Eds.) Handbook of Nanophase and Nanostructured Materials; Kluwer Academic/Plenum: New York, NY, USA, 2003; ISBN 978-0-306-46737-0. [Google Scholar]
- Komarneni, S.; Parker, J.C.; Wollenberger, H.J. Nanophase and Nanocomposite Materials II. In Proceedings of the MRS Symposium Proceedings, Boston, MA, USA, 2–5 December 1996. [Google Scholar]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-Derived Nanostructures: Types and Applications. Green Chem. 2016, 18, 20–52. [Google Scholar] [CrossRef]
- Ingale, A.G. Biogenic Synthesis of Nanoparticles and Potential Applications: An Eco-Friendly Approach. J. Nanomedic. Nanotechnol. 2013, 4, 1000165. [Google Scholar] [CrossRef]
- Alharthi, A. Development of Micro/Nanostructured–based Biomaterials with Biomedical Applications. Biocell 2023, 47, 1743–1755. [Google Scholar] [CrossRef]
- Li, W.; Zhan, X.; Song, X.; Si, S.; Chen, R.; Liu, J.; Wang, Z.; He, J.; Xiao, X. A Review of Recent Applications of Ion Beam Techniques on Nanomaterial Surface Modification: Design of Nanostructures and Energy Harvesting. Small 2019, 15, 1901820. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kim, K.-H. Ion Implantation of Titanium Based Biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Jurczyk, M.U.; Jurczyk, K.; Jurczyk, M. Plasma Surface Modification of Titanium by TiB Precipitation for Biomedical Applications. Surf. Coat. Technol. 2011, 206, 330–337. [Google Scholar] [CrossRef]
- Eason, R. (Ed.) Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-471-44709-2. [Google Scholar]
- Zhang, H.; Yang, J.; Wang, S.; Wu, Y.; Lv, Q.; Li, S. Film Thickness Dependence of Microstructure and Superconductive Property of PLD Prepared YBCO Layers. Phys. C Supercond. 2014, 499, 54–56. [Google Scholar] [CrossRef]
- Craciun, D.; Socol, G.; Lambers, E.; McCumiskey, E.J.; Taylor, C.R.; Martin, C.; Argibay, N.; Tanner, D.B.; Craciun, V. Optical and Mechanical Properties of Nanocrystalline ZrC Thin Films Grown by Pulsed Laser Deposition. Appl. Surf. Sci. 2015, 352, 28–32. [Google Scholar] [CrossRef]
- Sohail, M.T.; Wang, M.; Shareef, M.; Yan, P. A Review of Ultrafast Photonics Enabled by Metal-Based Nanomaterials: Fabrication, Integration, Applications and Future Perspective. Infrared Phys. Technol. 2024, 137, 105127. [Google Scholar] [CrossRef]
- Dhoble, S.J.; Kalyani, N.T.; Vengadaesvaran, B.; Arof, A.K. (Eds.) Solar Cell Technology. In Energy Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–60. ISBN 978-0-12-823710-6. [Google Scholar]
- Janarthanan, B.; Thirunavukkarasu, C.; Maruthamuthu, S.; Manthrammel, M.A.; Shkir, M.; AlFaify, S.; Selvakumar, M.; Reddy, V.R.M.; Park, C. Basic Deposition Methods of Thin Films. J. Mol. Struct. 2021, 1241, 130606. [Google Scholar] [CrossRef]
- Engoor, G.G.; Selvaraj, S.; Muthuvijayan, V.; Yadav, S.K.; Unni, S.N.; Vasa, N.J. ZnTiO3-Based Nanocomposite Coating Synthesized via Reactive Magnetron Co-Sputtering for Antibacterial Applications. Ceram. Int. 2025, 51, 35573–35581. [Google Scholar] [CrossRef]
- Nemati, A.; Saghafi, M.; Khamseh, S.; Alibakhshi, E.; Zarrintaj, P.; Saeb, M.R. Magnetron-Sputtered TixNy Thin Films Applied on Titanium-Based Alloys for Biomedical Applications: Composition-Microstructure-Property Relationships. Surf. Coat. Technol. 2018, 349, 251–259. [Google Scholar] [CrossRef]
- Innocenzi, P. Sol-Gel Processing for Advanced Ceramics, a Perspective. Open Ceram. 2023, 16, 100477. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA; San Diego, CA, USA; New York, NY, USA, 1990; ISBN 978-0-12-134970-7. [Google Scholar]
- Dobkin, D.M.; Zuraw, M.K. Principles of Chemical Vapor Deposition: What’s Going on Inside the Reactor; Dobkin, D.M., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; ISBN 978-1-4020-1248-8. [Google Scholar]
- Shimaoka, T.; Yamada, H.; Chayahara, A. Fabrication of Self-Standing Large (111) Single Crystal Diamond Using Bulk Growth of (100) CVD Diamond and Lift-off Process. Diam. Relat. Mater. 2024, 142, 110781. [Google Scholar] [CrossRef]
- Liu, M.; Shao, G.; Lv, P.; Li, J.; Zhu, Q. Effects of Nano-TiO2 Particles Addition on Fluidization of Flaky Fluorophlogopite Powder for the CVD Preparation of Pearlescent Pigments. Powder Technol. 2023, 421, 118414. [Google Scholar] [CrossRef]
- Bayki, S.; Mujumdar, S. Rapid Deposition of Superhydrophobic Organosilicon Films on Glass via DBD Plasma Jet-Enhanced CVD for Self-Cleaning and Optical Filtering Applications. Surf. Coat. Technol. 2025, 512, 132260. [Google Scholar] [CrossRef]
- Iyer, R.; Sharan, S. Chemical Vapor Deposition of Titanium from Titanium Tetrachloride and Hydrocarbon Reactants. US7268078B2, 9 November 2007. [Google Scholar]
- Tazim, T.Q.; Kawsar, M.; Sahadat Hossain, M.; Bahadur, N.M.; Ahmed, S. Hydrothermal Synthesis of Nano-Metal Oxides for Structural Modification: A Review. Next Nanotechnol. 2025, 7, 100167. [Google Scholar] [CrossRef]
- Erol, I.; Khamidov, G.; Hazman, Ö.; Çifci, C. Hydrothermal Synthesis of Novel Chitosan-Styrene-Based Nanocomposites Containing TiO2 Nanowires: Investigation of Thermal, Dielectric and Biological Properties. Mater. Today Commun. 2024, 41, 110726. [Google Scholar] [CrossRef]
- Kauffmann-Weiss, S.; Hahn, S.; Weigelt, C.; Schultz, L.; Wagner, M.F.-X.; Fähler, S. Growth, Microstructure and Thermal Transformation Behaviour of Epitaxial Ni-Ti Films. Acta Mater. 2017, 132, 255–263. [Google Scholar] [CrossRef][Green Version]
- Leys, M.R. Fundamental Growth Kinetics in MOMBE/CBE, MBE and MOVPE. J. Cryst. Growth 2000, 209, 225–231. [Google Scholar] [CrossRef]
- Ajayan, P.M. Bulk Metal and Ceramics Nanocomposites. In Nanocomposite Science and Technology; Wiley: Hoboken, NJ, USA, 2003; pp. 1–75. ISBN 978-3-527-30359-5. [Google Scholar]
- Du, Y.; Li, P.; Zhong, Z.-Y.; Chen, W.-X.; Liu, Y.-S.; Xie, Y.-L.; Zhou, L.-N.; Wang, K. A Universal Polymer-Assisted Route to Fabricate Zr-Doped TiO2 Nanocomposite: Boosting Reactive Desulfurization. Tungsten 2024, 6, 596–609. [Google Scholar] [CrossRef]
- Schramm, L.L. Dictionary of Nanotechnology, Colloid and Interface Science; Wiley-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-32203-9. [Google Scholar]
- Asgari, S.; Saberi, A.H.; McClements, D.J.; Lin, M. Microemulsions as Nanoreactors for Synthesis of Biopolymer Nanoparticles. Trends Food Sci. Technol. 2019, 86, 118–130. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Yan, H.-J.; Ye, X.-Y.; Fan, T.; Gui, Y.; Xie, B.; Li, X.-B.; Wu, L.-K.; Cao, F. A Novel and Facile Solvothermal Fluorination Approach for Boosting Oxidation Resistance of TiAl Alloy. Corros. Sci. 2025, 253, 113017. [Google Scholar] [CrossRef]
- Belew, A.A.; Assege, M.A. Solvothermal Synthesis of Metal Oxide Nanoparticles: A Review of Applications, Challenges, and Future Perspectives. Results Chem. 2025, 16, 102438. [Google Scholar] [CrossRef]
- Salhi, B.; Baig, N.; Abdulazeez, I. Air-Gap-Assisted Solvothermal Process to Synthesize Unprecedented Graphene-like Two-Dimensional TiO2 Nanosheets for Na+ Electrosorption/Desalination. npj Clean Water 2024, 7, 9. [Google Scholar] [CrossRef]
- Benjamin, J.S. Mechanical Alloying. Sci. Am. 1976, 234, 40–49. [Google Scholar] [CrossRef]
- Benjamin, J.S.; Volin, T.E. The Mechanism of Mechanical Alloying. Metall. Trans. 1974, 5, 1929–1934. [Google Scholar] [CrossRef]
- Suryanarayana, C. (Ed.) Non-Equilibrium Processing of Materials, 1st ed.; Pergamon Materials Series; Pergamon: Amsterdam, The Netherlands; New York, NY, USA, 1999; ISBN 978-0-08-042697-6. [Google Scholar]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Scheu, M.; Veefkind, V.; Verbandt, Y.; Galan, E.M.; Absalom, R.; Förster, W. Mapping Nanotechnology Patents: The EPO Approach. World Pat. Inf. 2006, 28, 204–211. [Google Scholar] [CrossRef]
- Rozhdestvenskiy, O.I.; Lalitha, Y.S.; Ikram, M.; Gupta, M.; Jain, A.; Verma, R.; Sood, S. Advances in Nanomaterials: Types, Synthesis, and Manufacturing Methods. E3S Web Conf. 2024, 588, 02005. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M. Development of β-Type Ti-x at. % Mo Alloys by Mechanical Alloying and Powder Metallurgy: Phase Evolution and Mechanical Properties (10 ≤ x ≤ 35). J. Alloys Compd. 2019, 776, 370–378. [Google Scholar] [CrossRef]
- Van de Voorde, M.; Tulinski, M.; Jurczyk, M. Engineered Nanomaterials: A Discussion of the Major Categories of Nanomaterials. In Metrology and Standardization of Nanotechnology; Wiley: Hoboken, NJ, USA, 2017; pp. 49–74. ISBN 978-3-527-34039-2. [Google Scholar]
- Jurczyk, M. (Ed.) Handbook of Nanomaterials for Hydrogen Storage; Pan Stanford Publishing: Singapore, 2018; ISBN 978-1-315-36444-5. [Google Scholar]
- Smardz, K.; Smardz, L.; Okonska, I.; Nowak, M.; Jurczyk, M. XPS Valence Band and Segregation Effect in Nanocrystalline Mg2NiMg2Ni-Type Materials. Int. J. Hydrogen Energy 2008, 33, 387–392. [Google Scholar] [CrossRef]
- Smardz, L.; Smardz, K.; Nowak, M.; Jurczyk, M. Structure and Electronic Properties of La(Ni,Al)5 Alloys. Cryst. Res. Technol. 2001, 36, 1385–1392. [Google Scholar] [CrossRef]
- Zadorozhnyi, V.Y.; Skakov, Y.A.; Milovzorov, G.S. Appearance of Metastable States in Fe-Ti and Ni-Ti Systems in the Process of Mechanochemical Synthesis. Met. Sci. Heat Treat. 2008, 50, 404–410. [Google Scholar] [CrossRef]
- Lowe, T.C.; NATO (Eds.) Investigations and Applications of Severe Plastic Deformation: Proceedings of the NATO Advanced Research Workshop on Investigations and Applications of Severe Plastic Deformation, Moscow, Russia, 2–7 August 1999; NATO science series Series 3, High technology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; ISBN 978-0-7923-6280-7. [Google Scholar]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk Nanostructured Materials from Severe Plastic Deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Richert, M.; Richert, J. Aluminum. A New Method Unltd. Deform. Met. Alloys 1986, 62, 604–607. [Google Scholar]
- Kim, H.S. Finite Element Analysis of High Pressure Torsion Processing. J. Mater. Process. Technol. 2001, 113, 617–621. [Google Scholar] [CrossRef]
- Beygelzimer, Y.; Varukhin, V.; Synkov, S.; Sapronov, A.; Synkov, V. New Techniques for Accumulating Large Plastic Deformations Using Hydroextrusion. Fiz. Tekhnika Vysok. Davlenii 1999, 9, 109–110. [Google Scholar]
- Kwon, Y.J.; Saito, N.; Shigematsu, I. Friction stir process as a new manufacturing technique of ultrafine grained aluminum alloy. J. Mater. Sci. Lett. 2022, 21, 1473–1476. [Google Scholar] [CrossRef]
- Fuloria, D.; Goel, S.; Jayaganthan, R.; Srivastava, D.; Dey, G.K.; Saibaba, N. Mechanical Properties and Microstructural Evolution of Ultrafine Grained Zircaloy-4 Processed through Multiaxial Forging at Cryogenic Temperature. Trans. Nonferrous Met. Soc. China 2015, 25, 2221–2229. [Google Scholar] [CrossRef]
- Tsuji, N.; Saito, Y.; Utsunomiya, H.; Tanigawa, S. Ultra-Fine Grained Bulk Steel Produced by Accumulative Roll-Bonding (ARB) Process. Scr. Mater. 1999, 40, 795–800. [Google Scholar] [CrossRef]
- Huang, J.Y.; Zhu, Y.T.; Jiang, H.; Lowe, T.C. Microstructures and Dislocation Configurations in Nanostructured Cu Processed by Repetitive Corrugation and Straightening. Acta Mater. 2001, 49, 1497–1505. [Google Scholar] [CrossRef]
- Mahmoodian, R.; Annuar, N.S.M.; Faraji, G.; Bahar, N.D.; Razak, B.A.; Sparham, M. Severe Plastic Deformation of Commercial Pure Titanium (CP-Ti) for Biomedical Applications: A Brief Review. JOM 2019, 71, 256–263. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Nagumothu, R.; Parfenov, E.; Valiev, R. Development of Nanostructured Titanium Implants for Biomedical Implants—A Short Review. Mater. Today Proc. 2021, 46, 1195–1200. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Prokofiev, E.A.; Kazarinov, N.A.; Raab, G.I.; Minasov, T.B.; Stráský, J. Developing Nanostructured Ti Alloys for Innovative Implantable Medical Devices. Materials 2020, 13, 967. [Google Scholar] [CrossRef]
- Janeček, M.; Čížek, J.; Stráský, J.; Václavová, K.; Hruška, P.; Polyakova, V.; Gatina, S.; Semenova, I. Microstructure Evolution in Solution Treated Ti15Mo Alloy Processed by High Pressure Torsion. Mater. Charact. 2014, 98, 233–240. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Semenova, I.P.; Latysh, V.V.; Shcherbakov, A.V.; Yakushina, E.B. Nanostructured Titanium for Biomedical Applications: New Developments and Challenges for Commercialization. Nanotechnol. Russ. 2008, 3, 593–601. [Google Scholar] [CrossRef]
- Jurczyk, K.; Miklaszewski, A.; Jurczyk, M.; Jurczyk, M. Development of β Type Ti23Mo-45S5 Bioglass Nanocomposites for Dental Applications. Materials 2015, 8, 8032–8046. [Google Scholar] [CrossRef]
- Wen, M.; Jiang, B.; Duan, X.; Xiang, D. Research Progress on Microstructure, Mechanical Properties, and Strengthening Mechanisms of In Situ-Synthesized Ceramic-Reinforced Titanium Matrix Composite Coatings via Laser Cladding. Coatings 2025, 15, 815. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Jurczyk, M. Mechanical Alloying and Electrical Current-Assisted Sintering Adopted for In Situ Ti-TiB Metal Matrix Composite Processing. Materials 2019, 12, 653. [Google Scholar] [CrossRef]
- Marczewski, M.; Miklaszewski, A.; Maeder, X.; Jurczyk, M. Crystal Structure Evolution, Microstructure Formation, and Properties of Mechanically Alloyed Ultrafine-Grained Ti-Zr-Nb Alloys at 36 ≤ Ti ≤ 70 (at. %). Materials 2020, 13, 587. [Google Scholar] [CrossRef]
- Sochacka, P.; Jurczyk, M.U.; Kowalski, K.; Wirstlein, P.K.; Jurczyk, M. Ultrafine-Grained Ti-31Mo-Type Composites with HA and Ag, Ta2O5 or CeO2 Addition for Implant Applications. Materials 2021, 14, 644. [Google Scholar] [CrossRef]
- Marczewski, M.; Jurczyk, M.U.; Żurawski, J.; Wirstlein, P.K.; Jurczyk, M. Studies of Normal Human Osteoblasts and Fibroblasts Growth on Composite Functionalized β-Type Ti23Zr25Nb (at%) Dental Implant Materials Containing 45S5 Bioglass and Silver. Bull. Mater. Sci. 2023, 46, 191. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Kowalski, K.; Jurczyk, M. Influence of the Processing Method on the Properties of Ti-23 at.% Mo Alloy. Metals 2019, 9, 931. [Google Scholar] [CrossRef]
- Seal, S.; Kuiry, S.C.; Georgieva, P.; Agarwal, A. Manufacturing Nanocomposite Parts: Present Status and Future Challenges. MRS Bull. 2004, 29, 16–21. [Google Scholar] [CrossRef][Green Version]
- Fang, Z.Z.; Wang, H. Densification and Grain Growth during Sintering of Nanosized Particles. Int. Mater. Rev. 2008, 53, 326–352. [Google Scholar] [CrossRef]
- Liao, S.-C.; Chen, Y.-J.; Mayo, W.E.; Kear, B.H. Transformation-Assisted Consolidation of Bulk Nanocrystalline TiO2. Nanostructured Mater. 1999, 11, 553–557. [Google Scholar] [CrossRef]
- Voisin, T.; Durand, L.; Karnatak, N.; Le Gallet, S.; Thomas, M.; Le Berre, Y.; Castagné, J.-F.; Couret, A. Temperature Control during Spark Plasma Sintering and Application to Up-Scaling and Complex Shaping. J. Mater. Process. Technol. 2013, 213, 269–278. [Google Scholar] [CrossRef]
- Santanach, J.G.; Weibel, A.; Estournès, C.; Yang, Q.; Laurent, C.; Peigney, A. Spark Plasma Sintering of Alumina: Study of Parameters, Formal Sintering Analysis and Hypotheses on the Mechanism(s) Involved in Densification and Grain Growth. Acta Mater. 2011, 59, 1400–1408. [Google Scholar] [CrossRef]
- Cho, K.C.; Woodman, R.H.; Klotz, B.R.; Dowding, R.J. Plasma Pressure Compaction of Tungsten Powders. Mater. Manuf. Proc. 2004, 19, 619–630. [Google Scholar] [CrossRef]
- Alexandrov, I.V.; Zhu, Y.T.; Lowe, T.C.; Islamgaliev, R.K.; Valiev, R.Z. Consolidation of Nanometer Sized Powders Using Severe Plastic Torsional Straining. Nanostructured Mater. 1998, 10, 45–54. [Google Scholar] [CrossRef]
- Ramanathan, A.; Krishnan, P.K.; Muraliraja, R. A Review on the Production of Metal Matrix Composites through Stir Casting—Furnace Design, Properties, Challenges, and Research Opportunities. J. Manuf. Process. 2019, 42, 213–245. [Google Scholar] [CrossRef]
- Meyers, M.A. Dynamic Behavior of Materials; Wiley: New York, NY, USA, 1994; ISBN 978-0-471-58262-5. [Google Scholar]
- Emelchenko, G.A.; Naumenko, I.G.; Veretennikov, V.A.; Gordopolov, Y.A. Shock Consolidation of Nanopowdered Ni. Mater. Sci. Eng. A 2009, 503, 55–57. [Google Scholar] [CrossRef]
- He, D.; Wang, M.; Bi, W.; Wang, L. Shock-Induced Plastic Deformation of Nanopowder Ti during Consolidation and Spallation. RSC Adv. 2024, 14, 8455–8463. [Google Scholar] [CrossRef]
- Patel, R.R.; Keshri, A.K.; Dulikravich, G.S.; Agarwal, A. An Experimental and Computational Methodology for near Net Shape Fabrication of Thin Walled Ceramic Structures by Plasma Spray Forming. J. Mater. Process. Technol. 2010, 210, 1260–1269. [Google Scholar] [CrossRef]
- Fang, J.C.; Xu, W.J. Plasma Spray Forming. J. Mater. Process. Technol. 2002, 129, 288–293. [Google Scholar] [CrossRef]
- Sampath, S.; Herman, H. Plasma Spray Forming Metals, Intermetallics, and Composites. JOM 1993, 45, 42–49. [Google Scholar] [CrossRef]
- Narayan, R.; Goering, P. Laser Micro- and Nanofabrication of Biomaterials. MRS Bull. 2011, 36, 973–982. [Google Scholar] [CrossRef][Green Version]
- Vilar, R. (Ed.) Laser Surface Modification of Biomaterials: Techniques and Applications; Woodhead Publishing Series in Biomaterials; Woodhead Publishing is an Imprint of Elsevier: Duxford, UK, 2016; ISBN 978-0-08-100883-6. [Google Scholar][Green Version]
- Zhou, W.; Bridges, D.; Li, R.; Bai, S.; Ma, Y.; Hou, T.; Hu, A. Recent Progress of Laser Micro-and Nano Manufacturing. ScienceJet 2015, 5, 228. [Google Scholar][Green Version]
- Hu, A. (Ed.) Laser Micro-Nano-Manufacturing and 3D Microprinting; Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland, 2020; Volume 309, ISBN 978-3-030-59312-4. [Google Scholar][Green Version]
- Günther, D.; Scharnweber, D.; Hess, R.; Wolf-Brandstetter, C.; Grosse Holthaus, M.; Lasagni, A.F. High Precision Patterning of Biomaterials Using the Direct Laser Interference Patterning Technology. In Laser Surface Modification of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–33. ISBN 978-0-08-100883-6. [Google Scholar][Green Version]
- Pérez, R.A.; Mateos-Timoneda, M.A. Scaffolds Fabrication Processes: From Classical to Advanced Techniques. In Stem Cell Biology and Regenerative Medicine; Springer International Publishing: Cham, Switzerland, 2023; pp. 305–315. ISBN 978-3-031-35831-9. [Google Scholar][Green Version]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various Manufacturing Methods and Ideal Properties of Scaffolds for Tissue Engineering Applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Vijayavenkataraman, S.; Cidonio, G. Biomaterials and Scaffolds for Tissue Engineering and Regenerative Medicine. BMC Methods 2024, 1, 2. [Google Scholar] [CrossRef]
- Chimerad, M.; Barazesh, A.; Zandi, M.; Zarkesh, I.; Moghaddam, A.; Borjian, P.; Chimehrad, R.; Asghari, A.; Akbarnejad, Z.; Khonakdar, H.A.; et al. Tissue Engineered Scaffold Fabrication Methods for Medical Applications. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 1455–1479. [Google Scholar] [CrossRef]
- Akampumuza, O.; Gao, H.; Zhang, H.; Wu, D.; Qin, X. Raising Nanofiber Output: The Progress, Mechanisms, Challenges, and Reasons for the Pursuit. Macromol. Mater. Eng. 2018, 303, 1700269. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Bosio, F.; Gilani, N.; Phutela, C.; Hague, R.J.; Tuck, C.J. Additive Manufacturing Processes for Metals. In Quality Analysis of Additively Manufactured Metals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 201–258. ISBN 978-0-323-88664-2. [Google Scholar]
- İyibilgin, O.; Gepek, E. Caracterización de CP-Titanio Producido Mediante Inyección Aglutinante y Pulvimetalurgia Convencional. Rev. Metal. 2021, 57, e205. [Google Scholar] [CrossRef]
- Odde, D.J.; Renn, M.J. Laser-Guided Direct Writing for Applications in Biotechnology. Trends Biotechnol. 1999, 17, 385–389. [Google Scholar] [CrossRef]
- Bandala, E.; Raymond, L.; Mitchell, K.; Rubbi, F.; Thella, J.; Osho, B.O.; Kushwaha, A.K.; Elahifard, M.; Su, J.; Zhang, X.; et al. Distance-Controlled Direct Ink Writing of Titanium Alloy with Enhanced Shape Diversity and Controllable Porosity. npj Adv. Manuf. 2025, 2, 4. [Google Scholar] [CrossRef]
- Shahriari-Khalaji, M.; Shafiq, M.; Cui, H.; Cao, R.; Zhu, M. Advancements in the Fabrication Technologies and Biomaterials for Small Diameter Vascular Grafts: A Fine-Tuning of Physicochemical and Biological Properties. Appl. Mater. Today 2023, 31, 101778. [Google Scholar] [CrossRef]
- Song, J.; Guan, R.; Xie, M.; Dong, P.; Yang, X.; Zhang, J. Advances in Electrospun TiO2 Nanofibers: Design, Construction, and Applications. Chem. Eng. J. 2022, 431, 134343. [Google Scholar] [CrossRef]
- Sfetsas, T.; Patsatzis, S.; Chioti, A. A Review of 3D Printing Techniques for Bio-Carrier Fabrication. J. Clean. Prod. 2021, 318, 128469. [Google Scholar] [CrossRef]
- Siyoum, Y.B.; Kindie, F.G.; Gebeyehu, M.A. A Review of Current Research and Prospects of Fused Deposition Modelling: Application, Materials, Performance, Process Variables, Parameter Optimization, and Numerical Study. Int. J. Adv. Manuf. Technol. 2025, 138, 1675–1711. [Google Scholar] [CrossRef]
- Liu, G.; Dang, K.; Wang, K.; Zhao, J. Progress on Rapid Hot Gas Forming of Titanium Alloys: Mechanism, Modelling, Innovations and Applications. Procedia Manuf. 2020, 50, 265–270. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Chohan, J.S. The Role of Additive Manufacturing for Biomedical Applications: A Critical Review. J. Manuf. Process. 2021, 64, 828–850. [Google Scholar] [CrossRef]
- Behymer, N.; Morsi, K. Review: Closed-Cell Metallic Foams Produced via Powder Metallurgy. Metals 2023, 13, 959. [Google Scholar] [CrossRef]
- Adamek, G.; Jakubowicz, J. Mechanoelectrochemical Synthesis and Properties of Porous Nano-Ti–6Al–4V Alloy with Hydroxyapatite Layer for Biomedical Applications. Electrochem. Commun. 2010, 12, 653–656. [Google Scholar] [CrossRef]
- Jurczyk, M.U.; Jurczyk, K.; Niespodziana, K.; Miklaszewski, A.; Jurczyk, M. Titanium–SiO2 Nanocomposites and Their Scaffolds for Dental Applications. Mater. Charact. 2013, 77, 99–108. [Google Scholar] [CrossRef]
- Singh, R.; Lee, P.D.; Dashwood, R.J.; Lindley, T.C. Titanium Foams for Biomedical Applications: A Review. Mater. Technol. 2010, 25, 127–136. [Google Scholar] [CrossRef]
- Cachinho, S.C.P.; Correia, R.N. Titanium Scaffolds for Osteointegration: Mechanical, in Vitro and Corrosion Behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 451–457. [Google Scholar] [CrossRef]
- Childerhouse, T.; Jackson, M. Near Net Shape Manufacture of Titanium Alloy Components from Powder and Wire: A Review of State-of-the-Art Process Routes. Metals 2019, 9, 689. [Google Scholar] [CrossRef]
- Dunand, D.C. Processing of Titanium Foams. Adv. Eng. Mater. 2004, 6, 369–376. [Google Scholar] [CrossRef]
- Jurczyk, K.; Adamek, G.; Kubicka, M.; Jakubowicz, J.; Jurczyk, M. Nanostructured Titanium-10 Wt% 45S5 Bioglass-Ag Composite Foams for Medical Applications. Materials 2015, 8, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Zweben, C.H.; Beaumont, P. 6.13 Additive Manufacturing of Multifunctional Nanocomposites and Composites. In Comprehensive Composite Materials II; Elsevier: Amsterdam, The Netherlands, 2018; pp. 380–407. ISBN 978-0-08-100534-7. [Google Scholar]
- Sun, D.; Gu, D.; Lin, K.; Ma, J.; Chen, W.; Huang, J.; Sun, X.; Chu, M. Selective Laser Melting of Titanium Parts: Influence of Laser Process Parameters on Macro- and Microstructures and Tensile Property. Powder Technol. 2019, 342, 371–379. [Google Scholar] [CrossRef]
- Qiu, C.; Panwisawas, C.; Ward, M.; Basoalto, H.C.; Brooks, J.W.; Attallah, M.M. On the Role of Melt Flow into the Surface Structure and Porosity Development during Selective Laser Melting. Acta Mater. 2015, 96, 72–79. [Google Scholar] [CrossRef]
- Mirzaali, M.J.; Moosabeiki, V.; Rajaai, S.M.; Zhou, J.; Zadpoor, A.A. Additive Manufacturing of Biomaterials—Design Principles and Their Implementation. Materials 2022, 15, 5457. [Google Scholar] [CrossRef]
- Butscher, A.; Bohner, M.; Hofmann, S.; Gauckler, L.; Müller, R. Structural and Material Approaches to Bone Tissue Engineering in Powder-Based Three-Dimensional Printing. Acta Biomater. 2011, 7, 907–920. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The Design of Scaffolds for Use in Tissue Engineering. Part II. Rapid Prototyping Techniques. Tissue Eng. 2002, 8, 1–11. [Google Scholar] [CrossRef]
- Marquez, C.; Mata, J.J.; Renteria, A.; Gonzalez, D.; Gomez, S.G.; Lopez, A.; Baca, A.N.; Nuñez, A.; Hassan, M.S.; Burke, V.; et al. Direct Ink-Write Printing of Ceramic Clay with an Embedded Wireless Temperature and Relative Humidity Sensor. Sensors 2023, 23, 3352. [Google Scholar] [CrossRef]
- Coffigniez, M.; Gremillard, L.; Balvay, S.; Lachambre, J.; Adrien, J.; Boulnat, X. Direct-Ink Writing of Strong and Biocompatible Titanium Scaffolds with Bimodal Interconnected Porosity. Addit. Manuf. 2021, 39, 101859. [Google Scholar] [CrossRef]
- Li, F.; Feng, Q.L.; Cui, F.Z.; Li, H.D.; Schubert, H. A Simple Biomimetic Method for Calcium Phosphate Coating. Surf. Coat. Technol. 2002, 154, 88–93. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, K. Biomimetic Coating of Calcium Phosphate on Biometallic Materials. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2005, 15, 1199–1205. [Google Scholar]
- Zhang, E.; Zou, C. Porous Titanium and Silicon-Substituted Hydroxyapatite Biomodification Prepared by a Biomimetic Process: Characterization and in Vivo Evaluation. Acta Biomater. 2009, 5, 1732–1741. [Google Scholar] [CrossRef]
- Nouri, A.; Hodgson, P.D.; Wen, C. Biomimetic Porous Titanium Scaffolds for Orthopedic and Dental Applications. In Biomimetics Learning from Nature; Mukherjee, A., Ed.; InTech: London, UK, 2010; ISBN 978-953-307-025-4. [Google Scholar] [CrossRef]
- Sarma, B.; Ravi Chandran, K.S. Recent Advances in Surface Hardening of Titanium. JOM 2011, 63, 85–92. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Tong, C. Surface Modification of Pure Titanium Treated with B4C at High Temperature. Surf. Coat. Technol. 2006, 200, 3016–3020. [Google Scholar] [CrossRef]
- Jakubowicz, J.; Jurczyk, K.; Niespodziana, K.; Jurczyk, M. Mechanoelectrochemical Synthesis of Porous Ti-Based Nanocomposite Biomaterials. Electrochem. Commun. 2009, 11, 461–465. [Google Scholar] [CrossRef]
- Wang, G.; Liu, X.; Ding, C. Phase Composition and In-Vitro Bioactivity of Plasma Sprayed Calcia Stabilized Zirconia Coatings. Surf. Coat. Technol. 2008, 202, 5824–5831. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Jurczyk, M.U.; Jurczyk, M. Microstructural Development of Ti–B Alloyed Layer for Hard Tissue Applications. J. Mater. Sci. Technol. 2013, 29, 565–572. [Google Scholar] [CrossRef]
- Jakubowicz, J.; Adamek, G. Preparation and Properties of Mechanically Alloyed and Electrochemically Etched Porous Ti–6Al–4V. Electrochem. Commun. 2009, 11, 1772–1775. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.S.; Aragão, F.J.L.; Cooper, L.F. Advancing Dental Implant Surface Technology—From Micron- to Nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef]
- Stanford, C. Surface Modifications of Dental Implants. Aust. Dent. J. 2008, 53, S26–S33. [Google Scholar] [CrossRef]
- Davarpanah, M.; Martinez, H.; Etienne, D.; Zabalegui, I.; Mattout, P.; Chiche, F.; Michel, J.F. A Prospective Multicenter Evaluation of 1583 3i Implants: 1- to 5-Year Data. Int. J. Oral Maxillofac. Implant. 2002, 17, 820–828. [Google Scholar]
- Coelho, P.G.; Suzuki, M. Evaluation of an IBAD Thin-Film Process as an Alternative Method for Surface Incorporation of Bioceramics on Dental Implants: A Study in Dogs. J. Appl. Oral Sci. 2005, 13, 87–92. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Zehetbauer, M.J.; Estrin, Y.; Höppel, H.W.; Ivanisenko, Y.; Hahn, H.; Wilde, G.; Roven, H.J.; Sauvage, X.; Langdon, T.G. The Innovation Potential of Bulk Nanostructured Materials. Adv. Eng. Mater. 2007, 9, 527–533. [Google Scholar] [CrossRef]
- Cao, W.; Hench, L.L. Bioactive Materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Rocci, M.; Rocci, A.; Martignoni, M.; Albrektsson, T.; Barlattani, A.; Gargari, M. Comparing the TiOblast and Osseospeed Surfaces. Histomorphometric and Histological Analysis in Humans. Oral Implant. 2008, 1, 34–42. [Google Scholar]
- Ellingsen, J.E. Pre-Treatment of Titanium Implants with Fluoride Improves Their Retention in Bone. J. Mater. Sci. Mater. Med. 1995, 6, 749–753. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Advancements in Titanium Dioxide Nanotube-Based Sensors for Medical Diagnostics: A Two-Decade Review. Nanomaterials 2025, 15, 1044. [Google Scholar] [CrossRef]
- Arkusz, K.; Paradowska, E.; Nycz, M.; Krasicka-Cydzik, E. Influence of Thermal Modification and Morphology of TiO2 Nanotubes on Their Electrochemical Properties for Biosensors Applications. J. Nanosci. Nanotechnol. 2018, 18, 3713–3721. [Google Scholar] [CrossRef]
- Singhatanadgit, W.; Toso, M.; Pratheepsawangwong, B.; Pimpin, A.; Srituravanich, W. Titanium Dioxide Nanotubes of Defined Diameter Enhance Mesenchymal Stem Cell Proliferation via JNK- and ERK-Dependent up-Regulation of Fibroblast Growth Factor-2 by T Lymphocytes. J. Biomater. Appl. 2019, 33, 997–1010. [Google Scholar] [CrossRef]
- Voltrova, B.; Hybasek, V.; Blahnova, V.; Sepitka, J.; Lukasova, V.; Vocetkova, K.; Sovkova, V.; Matejka, R.; Fojt, J.; Joska, L.; et al. Different Diameters of Titanium Dioxide Nanotubes Modulate Saos-2 Osteoblast-like Cell Adhesion and Osteogenic Differentiation and Nanomechanical Properties of the Surface. RSC Adv. 2019, 9, 11341–11355. [Google Scholar] [CrossRef] [PubMed]
- Arkusz, K.; Nycz, M.; Paradowska, E. Electrochemical Evaluation of the Compact and Nanotubular Oxide Layer Destruction under Ex Vivo Ti6Al4V ELI Transpedicular Screw Implantation. Materials 2020, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Nycz, M.; Paradowska, E.; Arkusz, K.; Pijanowska, D.G. Influence of Geometry and Annealing Temperature in Argon Atmosphere of TiO2 Nanotubes on Their Electrochemical Properties. Acta Bioeng. Biomech. 2020, 22, 165–177. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Von Der Mark, K.; Schmuki, P. Nanosize and Vitality: TiO2 Nanotube Diameter Directs Cell Fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Arkusz, K.; Jędrzejewska, A.; Siwak, P.; Jurczyk, M. Electrochemical and Mechanical Properties of Hexagonal Titanium Dioxide Nanotubes Formed by Sonoelectrochemical Anodization. Materials 2024, 17, 2138. [Google Scholar] [CrossRef]
- Jędrzejewska, A.; Pasik, K.; Nycz, M.; Arkusz, K. The Affinity for Dialysate Species of Thermally Modified Titania Nanotubes under Static and Dynamic Conditions. Acta Bioeng. Biomech. 2021, 23, 96–105. [Google Scholar] [CrossRef]
- Jędrzejewska, A.; Arkusz, K. Mechanism and Growth Kinetics of Hexagonal TiO2 Nanotubes with an Influence of Anodizing Parameters on Morphology and Physical Properties. Sci. Rep. 2024, 14, 24721. [Google Scholar] [CrossRef]
- Prakash, J.; Cho, J.; Ruzimuradov, O.; Fang, D. (Eds.) Titanium Dioxide-Based Multifunctional Hybrid Nanomaterials: Application on Health, Energy and Environment, 1st ed.; Engineering Materials; Springer Nature: Cham, Switzerland, 2025; ISBN 978-3-031-80625-4. [Google Scholar]
- Park, J.; Tesler, A.B.; Gongadze, E.; Iglič, A.; Mazare, A. Nanoscale Topography of Anodic TiO2 Nanostructures Is Crucial for Cell–Surface Interactions. ACS Appl. Mater. Interfaces 2024, 16, 4430–4438. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Murray, N.G.; Li, H.; Brinson, L.C.; Dunand, D.C.; Stupp, S.I. A Bioactive Titanium Foam Scaffold for Bone Repair. Acta Biomater. 2005, 1, 523–533. [Google Scholar] [CrossRef]
- Stupp, S.I.; Ciegler, G.W. Organoapatites: Materials for Artificial Bone. I. Synthesis and Microstructure. J. Biomed. Mater. Res. 1992, 26, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Stupp, S.I.; Mejicano, G.C.; Hanson, J.A. Organoapatites: Materials for Artificial Bone. II. Hardening Reactions and Properties. J. Biomed. Mater. Res. 1993, 27, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Stupp, S.I.; Hanson, J.A.; Eurell, J.A.; Ciegler, G.W.; Johnson, A. Organoapatites: Materials for Artificial Bone. III. Biological Testing. J. Biomed. Mater. Res. 1993, 27, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Stupp, S.I.; Braun, P.V. Molecular Manipulation of Microstructures: Biomaterials, Ceramics, and Semiconductors. Science 1997, 277, 1242–1248. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Stupp, S.I. Colonization of Organoapatite–Titanium Mesh by Preosteoblastic Cells. J. Biomed. Mater. Res. 2003, 67A, 960–969. [Google Scholar] [CrossRef]
- Hirota, M.; Hayakawa, T.; Yoshinari, M.; Ametani, A.; Shima, T.; Monden, Y.; Ozawa, T.; Sato, M.; Koyama, C.; Tamai, N.; et al. Hydroxyapatite Coating for Titanium Fibre Mesh Scaffold Enhances Osteoblast Activity and Bone Tissue Formation. Int. J. Oral Maxillofac. Surg. 2012, 41, 1304–1309. [Google Scholar] [CrossRef]
- Vehof, J.W.M.; Spauwen, P.H.M.; Jansen, J.A. Bone Formation in Calcium-Phosphate-Coated Titanium Mesh. Biomaterials 2000, 21, 2003–2009. [Google Scholar] [CrossRef]
- Yao, C.; Webster, T.J. Anodization: A Promising Nano-Modification Technique of Titanium Implants for Orthopedic Applications. J. Nanosci. Nanotechnol. 2006, 6, 2682–2692. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X. Research Progress on Surface Modification of Three-Dimensional Printing Porous Titanium Alloys. Digit. Med. 2023, 9, 1. [Google Scholar] [CrossRef]
- Woźniak, A.; Staszuk, M.; Reimann, Ł.; Bialas, O.; Brytan, Z.; Voinarovych, S.; Kyslytsia, O.; Kaliuzhnyi, S.; Basiaga, M.; Admiak, M. The Influence of Plasma-Sprayed Coatings on Surface Properties and Corrosion Resistance of 316L Stainless Steel for Possible Implant Application. Arch. Civ. Mech. Eng 2021, 21, 148. [Google Scholar] [CrossRef]
- Aissi, M.; Tayyaba, Q.; Er-Ramly, A.; Hermawan, H.; Merzouk, N. Improving the Clinical Performance of Dental Implants Through Advanced Surface Treatments: The Case of Ti and ZrO2 Coatings. Metals 2025, 15, 320. [Google Scholar] [CrossRef]
- Shahadat, M.; Teng, T.T.; Rafatullah, M.; Arshad, M. Titanium-Based Nanocomposite Materials: A Review of Recent Advances and Perspectives. Colloids Surf. B Biointerfaces 2015, 126, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wu, M.; Lu, P.; Ye, X. Preparation and Performance Study of Titanium-Based Nanocomposites in Selective Laser Melting: Microstructure Regulation and Optimization of Mechanical Properties. Adv. Eng. Mater. 2024, 26, 2301994. [Google Scholar] [CrossRef]
- Alonzo, S.M.M.; Ashie, M.D.; Pathiraja, G.; Bastakoti, B.P. A Novel Organic-Inorganic Hybrid Polymerization Strategy for Titanium Oxide/Polymer Nanocomposites with Supercapacitive Properties. Chem. Eng. J. 2025, 505, 159208. [Google Scholar] [CrossRef]
- Nawaz, H.; Umar, M.; Maryam, R.; Nawaz, I.; Razzaq, H.; Malik, T.; Liu, X. Polymer Nanocomposites Based on TiO2 as a Reinforcing Agent: An Overview. Adv. Eng. Mater. 2022, 24, 2200844. [Google Scholar] [CrossRef]
- Tamilselvan, R.; Magibalan, S.; Rajkumar, R.; Eswaran, S.; Senniangiri, N. Enhancement of Mechanical Properties in Hybrid Polymer Composites through Titanium Nanoparticles and Kenaf Fiber Integration. E3S Web Conf. 2024, 559, 02009. [Google Scholar] [CrossRef]
- Ammisetti, D.K.; Kruthiventi, S.S.H.; Vinjavarapu, S.; Babu, N.N.; Gandepudi, J.R.; Battula, S.K. A Review on Reinforcements, Fabrication Methods, and Mechanical and Wear Properties of Titanium Metal Matrix Composites. J. Eng. Appl. Sci. 2024, 71, 60. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Tyliszczak, B. Preparation of Polymer Materials Containing Titanium Compounds. Infrastruktura i Ekologia Terenów Wiejskich 2017, 1659–1672. Available online: http://dx.medra.org/10.14597/infraeco.2017.4.2.125 (accessed on 23 July 2025).
- Porras-Herrera, D.R.; Herrera-Hernández, H.; Miranda-Hernández, J.G.; Castillo-Robles, J.A.; Armendariz-Mireles, E.N.; Calles-Arriaga, C.A.; Rocha-Rangel, E. Innovative Bioceramic Based on Hydroxyapatite with Titanium Nanoparticles as Reinforcement for Possible Medical Applications. J. Manuf. Mater. Process. 2024, 8, 296. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Wu, H.; Liu, J.; Ren, Y.; Liang, L.; Yan, X.; Baker, I.; Liu, S.; Uglov, V.V.; et al. Enhanced Wear Resistance, Antibacterial Performance, and Biocompatibility Using Nanotubes Containing Nano-Ag and Bioceramics in Vitro. Bio-Des. Manuf. 2024, 7, 670–686. [Google Scholar] [CrossRef]
- Huang, C.-H.; Yoshimura, M. Biocompatible Hydroxyapatite Ceramic Coating on Titanium Alloys by Electrochemical Methods via Growing Integration Layers [GIL] Strategy: A Review. Ceram. Int. 2023, 49, 24532–24540. [Google Scholar] [CrossRef]
- Abdi Bastami, A.; Farnoush, H.; Sadeghi, A.; Aghazadeh Mohandesi, J. Sol–Gel Derived Nanohydroxyapatite Film on Friction Stir Processed Ti–6Al–4V Substrate. Surf. Eng. 2013, 29, 205–210. [Google Scholar] [CrossRef]
- Zamani Khalajabadi, S.; Haji Abu, A.; Ahmad, N.; Kadir, M.; Ismail, A.; Nasiri, R.; Haider, W.; Redzuan, N. Biodegradable Mg/HA/TiO2 Nanocomposites Coated with MgO and Si/MgO for Orthopedic Applications: A Study on the Corrosion, Surface Characterization, and Biocompatability. Coatings 2017, 7, 154. [Google Scholar] [CrossRef]
- Wang, L. Development and Application of Biomedical Titanium Alloys, 1st ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2018; ISBN 978-1-68108-620-0. [Google Scholar]
- Floriano, R.; Edalati, K.; Pereira, K.D.; Luchessi, A.D. Titanium-Protein Nanocomposites as New Biomaterials Produced by High-Pressure Torsion. Sci. Rep. 2023, 13, 470. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Abdul Kadir, M.R.; Izman, S.; Samavati, A.; Othaman, Z. Synthesis, Microstructure and Biodegradation Behavior of Nano-Si and Nano-ZnO/Si Coatings on a Mg/HA/TiO2/MgO Nanocomposite. Ceram. Int. 2015, 41, 11346–11358. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2023, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, R.; Khodabakhshi, F. Microstructural Evolution and Mechanical Properties of a Friction-Stir Processed Ti-Hydroxyapatite (HA) Nanocomposite. J. Mech. Behav. Biomed. Mater. 2018, 88, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, Y.; Srinivasan, A.; Hrishikesan, E. Nanomaterials for Medical Implants. In Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2021; pp. 297–315. ISBN 978-981-336-251-2. [Google Scholar]
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Functionalized TiO2 Based Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Youness, R.A.; Taha, M.A.; Ibrahim, M.A. Effect of Sintering Temperatures on the in Vitro Bioactivity, Molecular Structure and Mechanical Properties of Titanium/Carbonated Hydroxyapatite Nanobiocomposites. J. Mol. Struct. 2017, 1150, 188–195. [Google Scholar] [CrossRef]
- Valiev, R.; Gunderov, D.; Prokofiev, E.; Pushin, V.; Zhu, Y. Nanostructuring of TiNi Alloy by SPD Processing for Advanced Properties. Mater. Trans. 2008, 49, 97–101. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, L.; Xue, X.; Lu, W.; Qin, J.; Zhang, D. Microstructure Evolution and Mechanical Properties of a Ti–35Nb–3Zr–2Ta Biomedical Alloy Processed by Equal Channel Angular Pressing (ECAP). Mater. Sci. Eng. C 2013, 33, 4551–4561. [Google Scholar] [CrossRef]
- Niespodziana, K.; Jurczyk, K.; Jakubowicz, J.; Jurczyk, M. Fabrication and Properties of Titanium–Hydroxyapatite Nanocomposites. Mater. Chem. Phys. 2010, 123, 160–165. [Google Scholar] [CrossRef]


| Material Composite | Mechanical Strength | Biocompatibility | Cost & Economic Note |
|---|---|---|---|
| Ti-based nanocomposites | Very high strength, fatigue resistance | Excellent osseointegration, inert surface | High upfront cost; proven long-term value |
| Mg-based materials | Lower strength than Ti, close to bone modulus | Biodegradable, promotes healing, needs corrosion control | Base material cheap, implants pricier—but lifecycle savings |
| Mg–Ti alloys/hybrids | Improved strength & stability | Better corrosion control, biocompatible | Emerging; cost not well-established |
| Polymers/hybrids | Lower strength; better for non-load-bearing or soft tissue | Biocompatible; customizable | Usually low material cost; manufacturing affects price |
| Method | Approach | Key Materials | Applications |
|---|---|---|---|
| Mechanical milling | Top-down | Metals, ceramics | Nanopowders |
| Sol–gel | Bottom-up | Silica, bioactive glass | Bone regeneration |
| Self-assembly | Bottom-up | Lipids, DNA | Drug delivery, biosensors |
| Microbial synthesis | Biological | Ag, Au, ZnO | Antimicrobial agents |
| Plant extract synthesis | Biological | Metal oxide nanoparticles | Drug delivery, biosensing |
| Electrospinning | Hybrid | Biopolymer nanofibers | Tissue scaffolds |
| Method | Biological Agent | Reducing/Stabilizing Components | Examples of Nano Particles | Advantages | Challenges |
|---|---|---|---|---|---|
| Plant-mediated | Plant extracts (leaves, roots, etc.) | Flavonoids, phenols, alkaloids, terpenoids | Ag, Au, ZnO, Fe3O4 | Fast, scalable, eco-friendly | Variation in extract composition |
| Bacterial synthesis | Bacillus, Pseudomonas, etc. | Enzymes (e.g., nitrate reductase) | Ag, Au, Pd | Easy to culture, extracellular synthesis | Requires sterile conditions, slow growth |
| Fungal synthesis | Fusarium, Aspergillus, etc. | Extracellular enzymes, proteins | Ag, Au, TiO2 | High yield, good scalability | Post-synthesis purification needed |
| Algae-mediated | Chlorella, Spirulina, etc. | Proteins, pigments, polysaccharides | Ag, Au, ZnO | Abundant biomass, rapid growth | Less studied, consistency issues |
| Enzyme-mediated | Isolated enzymes (e.g., laccase) | Specific enzyme action | Ag, Au | High specificity, clean synthesis | Expensive, enzyme instability |
| Processing and Treatment Conditions | Ultimate Tensile Strength [MPa] | Yield Strength [MPa] | Elongation [%] | Reduction Area [%] | Fatigue Strength at 106 Cycles |
|---|---|---|---|---|---|
| Conventional Ti (as received) | 700 | 530 | 25 | 52 | 340 |
| nTi ECAP + TMT | 1240 | 1200 | 12 | 42 | 620 |
| Ti-6Al-4V ELI annealed | 940 | 840 | 16 | 45 | 530 |
| Technique | Synthesis Parameters | Characterization Methods | Reproducibility Checks |
|---|---|---|---|
| Ion Beam Techniques | Ion energy, dose, beam current, target material, vacuum level, temperature | TEM, RBS, SIMS, XPS, AFM | Beam current stability, dose calibration, repeated implantations |
| Plasma Synthesis | Gas type, pressure, power, precursor type, substrate temp, RF/microwave frequency | SEM, TEM, XRD, XPS, OES (plasma) | Plasma density monitoring, repeated depositions, gas flow reproducibility |
| Pulsed Laser Deposition (PLD) | Laser fluence, pulse rate, target-substrate distance, substrate temp, ambient gas (O2, Ar) pressure | XRD, AFM, SEM, TEM, EDX, Raman, Ellipsometry | Laser stability, uniform target erosion, substrate rotation |
| Physical Vapor Deposition (PVD) | Source material, vacuum pressure, deposition rate, substrate temp, target-substrate distance | XRD, SEM, AFM, Profilometry, XPS | Thickness monitoring, rate reproducibility, substrate prep standardization |
| Sol–Gel | Precursor type, pH, solvent, water/alkoxide ratio, aging time, drying/calcination temp | FTIR, TGA, XRD, SEM, TEM, BET surface area | Reagent concentration control, pH monitoring, drying uniformity |
| Chemical Vapor Deposition (CVD) | Precursor gas, carrier gas flow, pressure, temp, reactor design, substrate | XRD, SEM, TEM, Raman, AFM, EDX | Flow control calibration, temp uniformity, gas purity checks |
| Hydrothermal Route | Temp, pressure, pH, precursor concentration, time, autoclave material | XRD, SEM, TEM, FTIR, Raman, BET | Sealing integrity, batch homogeneity, repeated runs |
| Epitaxial Growth (e.g., MBE, CVD-epitaxy) | Substrate orientation, temp, flux/gas ratio, vacuum quality, growth rate | HRXRD, TEM, RHEED, AFM, LEED | Flux monitoring, substrate prep protocols, real-time growth monitoring |
| Polymer Route (e.g., polymer-assisted synthesis) | Polymer type/concentration, precursor-polymer ratio, heating/calcination temp | FTIR, TGA, SEM, XRD, DLS | Consistent polymer chain length, drying conditions |
| Colloidal Dispersion | Surfactant, pH, ionic strength, solvent, particle concentration | DLS, Zeta potential, TEM, UV-Vis, SAXS | pH buffer consistency, mixing time, filtration steps |
| Microemulsions | Surfactant/co-surfactant ratio, oil/water phase ratio, temp, mixing time | DLS, TEM, SAXS, UV-Vis | Droplet size stability, consistent emulsification protocol |
| Solvothermal Decomposition | Solvent, precursor, temp, pressure, duration, sealed reactor type | XRD, SEM, TEM, FTIR, TGA | Pressure/temp control, precursor purity, sealing reliability |
| High-Energy Ball Milling | Milling time, ball-to-powder ratio, speed (rpm), atmosphere (inert or air), vial material | XRD, SEM, TEM, BET, Particle size analysis | Milling cycle repeatability, vial/ball wear checks |
| Mechanical Alloying | Same as high-energy ball milling, with emphasis on repeated welding and fracturing | XRD (phase), SEM (morphology), Microhardness, EDX | Homogeneity sampling, repeat milling trials |
| Mechanochemical Synthesis | Grinding time, force (manual or automated), stoichiometry, moisture level | XRD, FTIR, SEM, Raman | Consistent grinding duration, repeat syntheses, moisture control |
| Equal Channel Angular Pressing (ECAP) | Die angle, number of passes, pressing temp, strain rate, sample size | TEM, EBSD, XRD, Microhardness, DSC | Process cycle control, die wear checks, orientation control |
| Method | Modified Layer | Objective |
|---|---|---|
| Physical | ||
| Plasma spray | Coatings such as HA, Bioglass, and boron-surface-modified layer | Improve wear-resistance, corrosion-resistance, and biological properties |
| Physical vapor deposition | TiN, TiC, TiCN, and thin film HA coating | Improve wear-resistance, corrosion-resistance, and biological properties |
| Ion implantation and deposition | Surface modification, layer, and thin film | Improve wear-resistance, corrosion-resistance, and bio-logical properties |
| Chemical | ||
| Sol–gel | Thin films of calcium, phosphate, TiO2, and silica. | Improve wear-resistance, corrosion-resistance, and biological properties |
| Chemical vapor deposition | TiN, TiC, TiCN, and thin film, HAp coating. | Improve wear-resistance, corrosion-resistance, and biological properties |
| Anodic oxidation | TiO2 layers with specific surface topographies | Improve wear-resistance, corrosion resistance, and biological properties |
| Cathodic deposition | Ca-P deposition | Improve corrosion-resistance and biological properties |
| Chemical treatments such as acidic, alkaline and with hydrogen peroxide | Oxide layers, sodium, titanate gel | Remove oxides and contaminations, improve biocompatibility |
| Biochemical methods | Coating deposition | Induce specific cell and tissue |
| Mechanical | ||
| Severe plastic deformation | Bulk ultrafine grain metals/alloys | Improve mechanical and biological properties |
| Mechanical alloying and powder metallurgy | Alloys with nanostructure and nanobiocomposites | Improve mechanical properties, corrosion-resistance, and biological properties |
| Synthesis Method | Biomaterial | Properties |
|---|---|---|
| sol–gel method | titanium-based organic–inorganic nanocomposites | good thermal and chemical stability |
| selective laser melting technology | titanium-based nanocomposites doped with 0.5 wt% graphene oxide and 7.0% nanomolybdenum | enhancing the hardness and tensile properties |
| organic–inorganic hybrid polymerization process | titanium oxide/carbon-based nanocomposite | intermediary properties of its organic and inorganic components |
| solution blending method | natural rubber and TiO2 NPs TiO2/PA6 NCs polyurethane/TiO2 composite TiO2 NPs with polyethylene | excellent 100% antibacterial activity against the treatment of E. coli |
| powder metallurgy technique | graphene nanoflakes as reinforcement for the preparation of Ti6Al4V MMCs graphene as a reinforcement to produce Ti MMCs hydroxyapatite with titanium NPs | enhancement of mechanical properties enhancement of mechanical properties enhancement of mechanical properties and stability when exposed to a physiological electrolyte |
| photopolymerization process | chitosan-based binders modified with titanium and titanium dioxide | good buffering properties in simulated body fluids, positive effect on the mechanical properties of the binder, increase in the hydrophobicity of the material |
| anodization, deposition, and spin-coating methods | nanotubes containing nano-Ag and bioceramics on Ti–35Nb–2Ta–3Zr | enhanced cell adhesion, proliferation, and alkaline phosphatase activity, good antibacterial activity |
| electrochemical methods via Growing Integration Layers | hydroxyapatite ceramic coating on titanium alloys | improved bioactivity and adhesion of the ceramic layer |
| sonoelectrochemical anodization and heat treatment | anatase nanotubes with a size of approximately 100 nm on the titanium | improved biocompatibility, good antibacterial properties, high biocompatibility and wettability, low roughness, excellent chemical stability, and large specific surface area |
| sonoelectrochemical anodization | hexagonal TNTs with diagonals in the range of 30–95 nm and heights in the range of 3500–4000 nm | lower impedance, higher electrical conductivity, decreased Young’s modulus with increasing diagonal size of the hTNTs |
| ECAP | cp-Ti (α + β) titanium alloys: Ti-6Al-4V and Ti-6Al-7Nb β-Ti alloys: Ti15Mo Ti–35Nb–3Zr–2Ta shape memory alloy NiTi | improvement of the strength and fatigue properties |
| high-pressure torsion | Ti-bovine serum albumin nanocomposite | high hardness, increased biocompatibility |
| friction-stir processing | Ti-HA nanocomposite | good combination of strength and ductility by refining the grain structure with a well-developed dimple-like structure on the fracture surface |
| MA and space-holder sintering process | Ti–10 wt.% SiO2 nanocomposites and their scaffolds nanostructured Ti-10 wt.% 45S5 Bioglass-Ag composite foams | more corrosion resistant than the cp-Ti, good in vitro cytocompatibility more corrosion resistant than the cp-Ti in Ringer solution, the cells well dispersed inside the pores, reduced bacteria adhesion (S. aureus) in comparison with cp-Ti |
| MA + powder metallurgy | Ti-HA nanocomposites (HA = 3, 10, 20 vol%) β-type Ti31Mo5HA-Ag or CeO2 biocomposites Ti-TiB MMNCs β-type Ti-Zr-Nb alloys modified with 45S5 Bioglass and silver | increased hardness and lowered Young’s modulus compared to cp-Ti, improved corrosion resistance elastic modulus lower than cp-Ti, two times higher hardness, significantly reduced number of S. aureus nanoscale size effect influences the mechanical properties and the cell viability and cytocompatibility lower Young’s modulus and better corrosion properties than the cp-Ti, improved biocompatibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tulinski, M.; Jurczyk, M.U.; Arkusz, K.; Nowak, M.; Jurczyk, M. Nanotechnology for Biomedical Applications: Synthesis and Properties of Ti-Based Nanocomposites. Nanomaterials 2025, 15, 1417. https://doi.org/10.3390/nano15181417
Tulinski M, Jurczyk MU, Arkusz K, Nowak M, Jurczyk M. Nanotechnology for Biomedical Applications: Synthesis and Properties of Ti-Based Nanocomposites. Nanomaterials. 2025; 15(18):1417. https://doi.org/10.3390/nano15181417
Chicago/Turabian StyleTulinski, Maciej, Mieczyslawa U. Jurczyk, Katarzyna Arkusz, Marek Nowak, and Mieczyslaw Jurczyk. 2025. "Nanotechnology for Biomedical Applications: Synthesis and Properties of Ti-Based Nanocomposites" Nanomaterials 15, no. 18: 1417. https://doi.org/10.3390/nano15181417
APA StyleTulinski, M., Jurczyk, M. U., Arkusz, K., Nowak, M., & Jurczyk, M. (2025). Nanotechnology for Biomedical Applications: Synthesis and Properties of Ti-Based Nanocomposites. Nanomaterials, 15(18), 1417. https://doi.org/10.3390/nano15181417










